Abstract
The properties of K+ channels in body wall muscle cells acutely dissected from the nematode Caenorhabditis elegans were investigated at the macroscopic and unitary level using an in situ patch clamp technique. In the whole-cell configuration, depolarizations to potentials positive to −40 mV gave rise to outward currents resulting from the activation of two kinetically distinct voltage-dependent K+ currents: a fast activating and inactivating 4-aminopyridine-sensitive component and a slowly activating and maintained tetraethylammonium-sensitive component. In cell-attached patches, voltage-dependent K+ channels, with unitary conductances of 34 and 80 pS in the presence of 5 and 140 mm external K+, respectively, activated at membrane potentials positive to −40 mV. Excision revealed that these channels corresponded to Ca2+-activated K+ channels exhibiting an unusual sensitivity to internal Cl− and whose activity progressively decreased in inside-out conditions. After complete run-down of these channels, one third of inside-out patches displayed activity of another Ca2+-activated K+ channel of smaller unitary conductance (6 pS at 0 mV in the presence of 5 mm external K+). In providing a detailed description of native K+ currents in body wall muscle cells of C. elegans, this work lays the basis for further comparisons with mutants to assess the function of K+ channels in this model organism that is highly amenable to molecular and classical genetics.
K+ channels play a key role in a great variety of cellular functions such as cell firing in neurones, secretion in endocrine cells and contraction in muscle cells. Open K+ channels drive the membrane potential to the negative value of the K+ equilibrium potential and, in this way, set the resting potential, shape action potentials and affect refractoriness and the rate of repetitive firing (Hille, 1992). Different K+ channels operate to fulfil these functions and K+ channels are known to represent the most diverse and largest class of ion channels (Coetzee et al. 1999). Functional as well as structural properties of K+ channels have turned out to be highly conserved in the animal kingdom. This encouraged biologists to take advantage of the apparent simplicity and the potential for molecular genetics of invertebrates to gain new insights into the molecular basis of K+ channel function. In this respect, the nematode Caenorhabditis elegans has become a preparation of prime interest. The genome of C. elegans has been completely sequenced and searches for homology have predicted more than 70 genes encoding putative K+ channels (Wei et al. 1996; Bargmann, 1998; The C. elegans Sequencing Consortium, 1998; Salkoff et al. 1999). In addition, the screening of large numbers of mutant nematodes has allowed the in vivo contribution of K+ channel genes to be unravelled. Mutations affecting C. elegans body wall muscle function have led to the identification of two genes expressed in muscle: twk-18, a gene encoding a K+ channel belonging to the two-P domain K+ channel family (Kunkel et al. 2000), and slo-1, a gene encoding a high conductance Ca2+-activated K+ channel (BK) (Wang et al. 2001). Additionally, C. elegans genome sequencing analysis has enabled the cloning of the slo-2 gene; this is also expressed in body wall muscle and encodes a Ca2+-activated K+ channel with an unusual Cl− sensitivity (Wei et al. 1996; Lim et al. 1999; Yuan et al. 2000). The functional properties of these K+ channels have been characterized using the Xenopus oocyte expression system. However, nothing is known about the properties of these K+ channels in their native cellular environment, when in fact it is possible that expression systems may distort ion channel behaviour. In situ electrophysiological studies at the cellular level in C. elegans have been greatly restricted by the difficulty in microdissecting this invertebrate and in exposing the cells of interest. To date, only macroscopic outward currents activated by depolarization and inhibited by 4-aminopyridine (4-AP) and tetraethylammonium (TEA) have been described using an in situ patch clamp technique in body wall muscle cells from C. elegans (Richmond & Jorgensen, 1999). However, detailed information on ion channel selectivity and voltage dependence is not available and K+ channel activity at the single-channel level has not been investigated.
In this study, using an in situ patch clamp technique on acutely dissected C. elegans, we investigate the properties of K+ channels in body wall muscle cells at the macroscopic as well as, for the first time, the unitary level. We provide a detailed description of ion selectivity and voltage dependence of depolarization-activated channels under whole-cell conditions. At the unitary level, we provide evidence for the presence of two types of Ca2+-activated K+ channel, one of which was also found to be sensitive to voltage and internal Cl− while the other, of much smaller conductance, was found to be sensitive only to internal Ca2+. This basic study on wild-type body wall muscle represents an essential step for further phenotypic characterization of mutant nematodes whose mutations affect muscle contractility or ion channel function.
METHODS
Dissection
Worm culture, handling and manipulations were performed according to the methods described by Brenner (1974). Experiments were performed on the N2 wild-type reference strain. The dissection technique was adapted from Richmond & Jorgensen (1999) and Goodman et al. (1998). Briefly, adult nematodes were immobilized by applying a cyanoacrylic glue (Histoacryl Blue, B. Braun, Melsungen, Germany) along one side of the body. An incision was made in the cuticle using a sharpened tungsten rod (Phymep, Paris, France). The viscera were cleared and the cuticle flap was pushed down with a glass rod held by a micromanipulator. Cellular surfaces were cleaned for 30 s using Ascaris Ringer solution containing 2 mg ml−1 collagenase (Sigma, Type 1). Recording pipettes were sealed on ventral body wall muscle cells. All experiments were carried out at room temperature (21-24 °C).
Electrophysiology
Macroscopic and single-channel currents were recorded using a patch clamp amplifier (model RK 400; Bio-Logic, Claix, France). The resistance of recording pipettes was within 2-3 MΩ for whole-cell experiments and 3-4 MΩ for single-channel recordings. Acquisition and generation of command voltage pulses was performed using Biopatch software (Bio-Logic) driving an A/D, D/A converter (Lab Master DMA board, Scientific Solutions Inc., Solon, OH, USA).
Recordings in the whole-cell configuration were performed 2 min after sealing the pipette on body wall muscle cells. Macroscopic currents were analysed using Microcal Origin software (Microcal Software Inc., Northampton, MA, USA). Linear resistive and capacitive components were not compensated. Cell capacitance was determined by integration of a control current trace obtained with a 10 mV depolarizing pulse from the holding potential. This capacitance was used to calculate the K+ current density (A F−1). Curves of the voltage dependence of the K+ current density were fitted with the following equation:
| (1) |
where I(V) is the measured density of the current, V is the test voltage pulse, Gmax is the maximum conductance, Vrev is the reversal potential, V0.5 is the half-activation voltage and k is a steepness factor.
Single-channel currents were recorded from either cell-attached or inside-out patches. Currents flowing into the pipette were considered to be positive. Currents were analysed with Biopatch software. Channel activity was determined from the average current (I) as NPo = I/i, where i is the single-channel current, N the number of channels in the patch and Po the channel open-state probability. I was measured after filtering at 300 Hz and sampling at 1 kHz over 2 s recording periods. Single-channel current amplitudes were determined with amplitude histograms.
Solutions and chemicals
For current recordings in the whole-cell configuration, pipettes were filled with (mm): 120 KCl, 20 KOH, 4 MgCl2, 5 Tes, 4 Na2ATP, 36 sucrose, 5 EGTA, pH 7.2 (high-Cl− solution) or 125 potassium aspartate, 15 KCl, 5 MgATP, 5 EGTA, 10 Hepes, pH 7.2 (low-Cl− solution) and the bath solution corresponded to a modified Ascaris Ringer solution containing (mm): 23 NaCl, 110 sodium acetate, 5 KCl, 6 CaCl2, 5 MgCl2, 11 sucrose and 5 Hepes, pH 7.2. In the 20 mm K+-containing solution, 15 mmNaCl of the modified Ascaris Ringer solution was replaced by 15 mm KCl. TEA chloride (TEACl), 4-AP, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and cadmium were diluted to the required concentrations, i.e. 20, 3, 0.5 and 0.5 mm, respectively, in the modified Ascaris Ringer solution.
For single-channel recordings, pipettes contained a Na+-rich external solution composed of (mm): 140 NaCl, 5 KCl, 6 CaCl2, 5 MgCl2, 5 Hepes, pH 7.2; or a K+-rich external solution containing (mm): 140 KCl, 10 Hepes, 5 MgCl2, 0.1 CaCl2, pH 7.2. In some experiments, 20 mm TEA was added to the Na+-rich external solution. The bath solution contained (mm): 140 KCl, 0.1 CaCl2, 5 MgCl2, 10 Hepes, pH 7.2. This solution was modified for recordings performed in the absence of calcium where 0.1 mm CaCl2 was removed and 1 mm EGTA was added, and for recordings performed in low chloride (10 mm) where 140 mm potassium acetate was substituted for 140 mm KCl.
Cells were exposed to different solutions by placing them in the mouth of a perfusion tube from which the rapidly exchanged solutions flowed by gravity.
Statistics
Linear and non-linear least-squares fits were performed using a Marquardt-Levenberg algorithm routine included in Microcal Origin. Data are presented as means ± s.e.m. Data were statistically analysed using Student's unpaired t test. Values were considered significant when P < 0.05.
RESULTS
Depolarization-activated outward currents
Under voltage clamp conditions, cells were depolarized from a holding potential of −70 mV by voltage steps of increasing amplitude and 200 ms duration. The depolarizing steps evoked outward currents which rose rapidly and then stabilized to an apparent plateau level (Fig. 1A). The rate of rise as well as the plateau value of the outward currents increased with depolarization to more positive potentials. The outward current waveform varied from one cell to another. In 19 cells out of 26 tested, the rising phase of the outward current was immediately followed by a plateau, as illustrated in Fig. 1A (upper panel). In the seven remaining cells, the outward current rose to a peak followed by a slower decay to a plateau level, as illustrated in Fig. 1A (lower panel). Little run-down of the depolarization-activated outward currents was observed during the course of the experiments.
Figure 1. Whole-cell currents elicited by positive and negative voltage pulses.
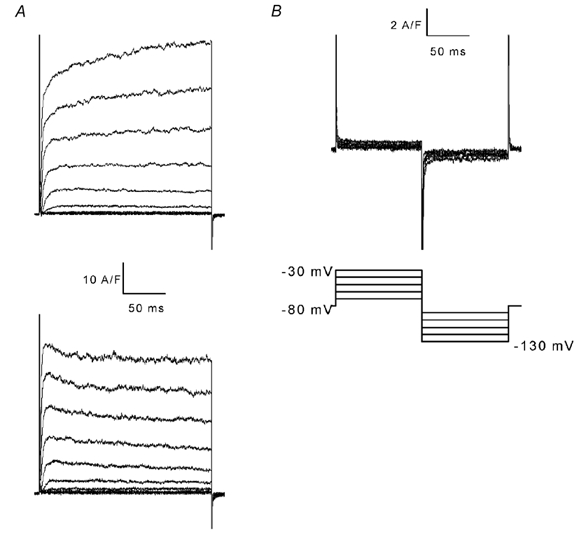
In A, whole-cell outward currents were elicited by applying voltage pulses of 200 ms duration in 10 mV increments from a holding potential of −70 mV. The two sets of current traces were obtained from two different cells and are representative of two typical outward current waveforms. In B, from a holding potential of −80 mV, a two-pulse protocol was applied: depolarizing pulses of 10 mV positive increments followed by hyperpolarizing pulses of 10 mV negative increments, as illustrated by the voltage protocol below the current traces.
A series of hyperpolarizing steps was also applied from a holding potential of −80 mV to test whether C. elegans muscle cells exhibited any inward rectification. Figure 1B shows that voltage steps between −130 and −30 mV induced negligible net membrane currents resulting from passive membrane properties. No evidence of inward rectification was found in the three cells tested under these experimental conditions.
Reversal potentials of depolarization-activated outward currents
To examine the ion selectivity of the outward currents elicited by depolarization, reversal potentials were estimated by measuring tail currents evoked during hyperpolarization over a large voltage range from a test potential of +20 mV, applied from a holding potential of −70 mV. Because in some cells the outward current displayed a peak phase followed by a plateau (see above), it seemed likely that this outward current consisted of two components. Therefore, tail currents were elicited during hyperpolarization applied at the peak of the outward current, i.e. 5 ms after the onset of depolarization (Fig. 2A, left panel), and after the current had reached a plateau level, i.e. 200 ms after the onset of depolarization (Fig. 2A, right panel). Reversal potentials were estimated by linear interpolation of tail current amplitudes obtained in each individual cell (Fig. 2B). The mean reversal potential was found to be −47 ± 0.7 mV for the peak current and −67.9 ± 3.2 mV for the plateau current (n = 4 cells). The same experiments conducted in the presence of 20 mm external K+ gave mean reversal potentials of −41.2 ± 0.2 mV for the peak current and −43.3 ± 0.4 mV for the plateau current (n = 4 cells; not shown). These values were significantly more positive than those obtained in the presence of 5 mm external K+ (Student's unpaired t test, P = 0.0002 and 0.0003 for the peak and the plateau currents, respectively). Because the equilibrium potential for K+ in the presence of 5 and 20 mm K+ and at the intracellular [K+] used here is −84 and −49 mV, respectively, the estimated reversal potentials for the plateau component of the outward current are consistent with K+ being the major ion carrying the outward current through the channels opened by membrane depolarization. Concerning the peak current, the mean value of −47 mV for the reversal potential in the presence of 5 mm K+ suggests that ions other than K+ may flow during the peak, although, as classically observed for K+ channels, the reversal potential moved closer to the K+ equilibrium potential when the external [K+] was increased to 20 mm. Two possible candidate ions able to move inward are Cl− and Ca2+, whose equilibrium potentials are both positive. A series of tail current experiments was therefore carried out in the presence of 500 μM Cd2+, a blocker of voltage-dependent Ca2+ current, since Ca2+ current is the main inward current observed early after seal establishment in the presence of external K+ channel blockers and internal Cs+ (not illustrated). In four cells, using the same protocol as in Fig. 2A, the mean reversal potential in the presence of Cd2+ was found to be −48.8 ± 1.6 mV, a value not significantly different from that obtained in the control (Student's unpaired t test, P = 0.3337; not shown); this result indicates that Ca2+ current did not contaminate the peak outward current. In contrast, the same experiments conducted in the presence of the Cl− channel inhibitor DIDS indicated a mean reversal potential of −57.5 ± 2.1 mV (n = 3 cells), significantly lower than that obtained in control (Student's unpaired t test, P = 0.0029; not shown). It is thus likely that a Cl− current contributes at least in part to the rapidly activating component of the outward current.
Figure 2. Tail currents and tail current-voltage relationships of the depolarization-activated outward currents.
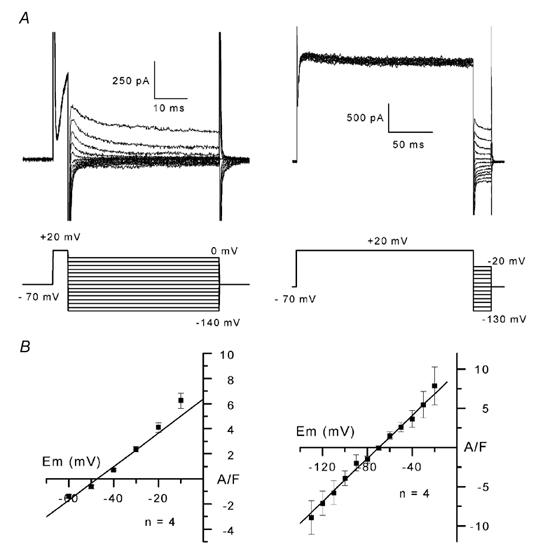
A, after depolarization to +20 mV from a holding potential of −70 mV, tail currents were evoked during membrane hyperpolarization to potentials between 0 and −140 mV (left panel) or between −20 and −130 mV (right panel). Hyperpolarizing voltage steps were presented 5 ms (left panel) or 200 ms (right panel) after the onset of the depolarization to +20 mV. The voltage protocol is illustrated below the current records. B, mean current-voltage relationships were established for tail currents recorded after a short (left panel) or a long (right panel) depolarizing pulse. For tail currents recorded after a short depolarizing pulse, measurements were only performed above −60 mV because of the low resolution of the rapidly deactivating inward current tails at hyperpolarized potentials.
Pharmacological separation of the outward currents and current-voltage relationships
The apparent two phases of the total outward current and the distinct estimated reversal potentials of the peak and plateau current suggested that the total outward current resulted from the presence of several types of K+ channel. To test this possibility, we examined the effects on depolarization-activated outward current of the pharmacological compounds 4-AP and TEA, which are known to preferentially block fast transient outward K+ currents and delayed outward rectifiers, respectively, in different cell types (Thompson, 1977; Rudy, 1988). Figure 3A shows outward currents elicited by depolarizing steps of 200 ms duration and of increasing amplitude from a holding potential of −70 mV in control, in the presence of 3 mm 4-AP and in the presence of 3 mm 4-AP plus 20 mm TEA. Application of 4-AP caused a marked reduction in the amplitude of the peak component of the current at all potentials (Fig. 3A, middle panel). The 4-AP-sensitive component of the total outward current was obtained by subtraction of the currents elicited in the same cell in the presence of 4-AP from those recorded in its absence (Fig. 3B, left panel). This 4-AP-sensitive component was characterized by a rapid rising phase to a transient peak followed by a slower decay to an apparent plateau level. Subsequent application of 20 mm TEA strongly inhibited the slow component remaining after addition of 4-AP (Fig. 3A, right panel). In the simultaneous presence of 4-AP and TEA, a small amplitude outward current characterized by a very slow rising phase persisted; this remaining outward current was not investigated further. Subtraction of the currents elicited in the presence of 4-AP plus TEA from those recorded in the presence of 4-AP revealed a TEA-sensitive component which activated slowly after the onset of the depolarizing step and rose gradually to plateau levels. Similar results were obtained in 11 cells, when either 4-AP or TEA was first applied.
Figure 3. Pharmacological separation of the outward currents and current-voltage relationships.
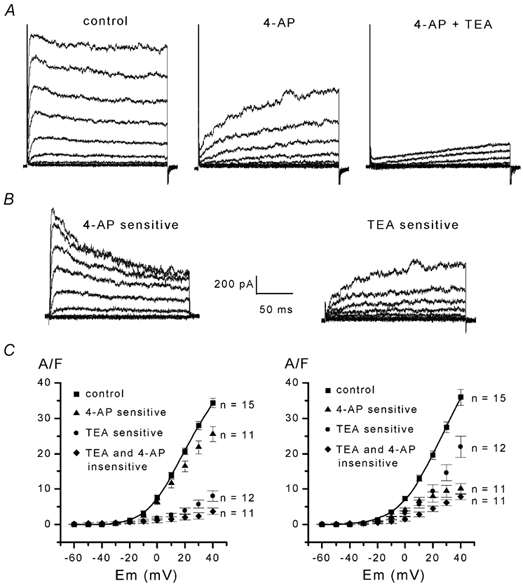
A, from a holding potential of −70 mV, voltage pulses of 200 ms duration in 10 mV increments were applied from −60 to +40 mV in control conditions (left panel), in the presence of 3 mm 4-AP (middle panel) and in the presence of 3 mm 4-AP plus 20 mm TEA (right panel). B, the 4-AP-sensitive component (left panel) was obtained by subtracting records in the presence of 4-AP from those in control conditions. The TEA-sensitive component was obtained by subtracting records in the presence of both drugs from those in the presence of 4-AP alone (right panel). C, current-voltage relationships for the control outward currents (▪), the 4-AP-sensitive component (▴), the TEA-sensitive component (•) and the 4-AP- and TEA-insensitive component (♦). In each case, measurements were performed 5 ms (left panel) or 200 ms (right panel) after the onset of depolarization. For control outward currents, the curves were fitted using eqn (1) with values for Vrev of −47.3 mV (left panel) and −69.7 mV (right panel).
Figure 3C shows the current-voltage relationships for the control outward currents, the 4-AP- and TEA-sensitive components, and for the current recorded in the presence of both drugs, 5 ms (left panel) and 200 ms (right panel) after the onset of depolarization. The threshold for the peak of the 4-AP-sensitive component, and that for the plateau level of the TEA-sensitive component, was −30 mV and both currents increased at more positive membrane potentials, displaying outward rectification. It can also be observed that the early peak phase of the total outward current consisted almost exclusively of the 4-AP-sensitive component while the plateau phase was dominated by the TEA-sensitive component but also consisted of the non-inactivating part of the 4-AP-sensitive component. The current-voltage relationships were fitted using eqn (1) (see Methods) and values of Vrev obtained from the tail current experiments. Mean values for Gmax, V0.5 and k were 423 S F−1, +6.6 mV and 13.1 mV for the peak and 399 S F−1, +16.7 mV and 15.7 mV for the end pulse current, respectively.
Single-channel activity in cell-attached patches
A series of experiments was then carried out in order to explore the kind of ion channel activity that could be detected at the unitary level in C. elegans muscle. Pipettes containing Na+-rich external solution were sealed on muscle cells bathed in K+-rich external solution in order to clamp the cells near 0 mV. Using the cell-attached configuration, single-channel activity was recorded at different membrane potentials. Figure 4A shows that single channels were spontaneously active at depolarizations above −40 mV. The amplitudes of the unitary currents were 1.1, 1.6, 1.9, 2.3, 2.7 and 3.1 pA at −30, −20, −10, 0, +10 and +20 mV, respectively. Figure 4B shows the mean amplitudes of unitary current through these channels plotted against membrane potential. The relationship was linear from −40 to +40 mV, while current amplitude saturated at higher membrane potentials. The best fit to the mean data indicated a slope conductance of 34 pS and the reversal potential was estimated by linear extrapolation to be −59.6 mV. In the presence of K+-rich solution containing 140 mm K+ in the pipette, the slope conductance increased to 80 pS and currents reversed at +2.8 mV (Fig. 4B). On the basis of these observations, it is likely that the channels recorded in cell-attached conditions were highly selective for K+. Figure 4A also clearly shows that channel opening increased with depolarization of the patch membrane; no channel opening could be observed at −40 mV and NPo was 0.04 at −30 mV and increased to 0.11, 0.58, 0.73, 1.52 and 2.17 at −20, −10, 0, +10 and +20 mV, respectively. Figure 4C illustrates the mean relationship between the activity (NPo) of the channels recorded in cell-attached patches and membrane potential. On average, channels began to open at −30 mV and channel activity gradually increased with depolarization without any apparent saturation up to +40 mV. Such channel activity was observed in all of the 17 patches tested under these experimental conditions. A series of cell-attached experiments was also performed in the presence of 20 mm TEA in the pipette. Depolarizations up to +50 mV never gave rise to channel opening in the eight cell-attached patches tested in the presence of the blocker, suggesting that these channels were inhibited by external TEA (not shown).
Figure 4. Single-channel currents recorded in cell-attached patches.

A, single-channel currents were recorded in cell-attached configuration at different membrane potentials indicated next to each current trace in the presence of Na+-rich solution in the pipette and K+-rich solution in the bath. B, mean current-voltage relationships were obtained in the presence of 5 mm K+ (•) or 140 mm K+ (○) in the pipette. Slopes indicate conductances of 34 and 80 pS in the presence of 5 and 140 mm K+, respectively. C, mean relationship between NPo and membrane potential obtained in the presence of Na+-rich solution in the pipette. In each patch, values of NPo were normalized to the value obtained at +50 mV.
Two kinds of Ca2+-sensitive channel in inside-out patches
The left panel of Fig. 5A shows single-channel activity in a cell-attached patch held at 0 mV in the presence of Na+-rich external solution in the pipette and K+-rich external solution with 0.1 mm Ca2+ in the bath. In this patch, no channel activity could be detected under cell-attached conditions at this membrane potential. However, channels exhibiting high activity spontaneously opened upon excision. The opening of these channels gradually decreased with time and, in this patch, 45 s after excision, dropped to 40 % of the initial activity (Fig. 5A, right panel). The fact that the channel abruptly opened upon excision suggests that loss of intracellular parameters or exposition of the cytoplasmic face to extracellular factors was responsible for the spontaneous opening of the channels. Indeed, we found that channel opening was completely and reversibly suppressed on perfusion of the cytoplasmic face of the patch with an internal solution containing EGTA and no added calcium. Additionally, when the internal [Cl−] was reduced from 150 to 10 mm, channel activity drastically and reversibly decreased to 2 % of the control. Such channel activity, revealed upon excision, and inhibited by removal of internal calcium and reduction of chloride, was observed in all of the 25 patches tested. When 20 mm TEA was present in the external pipette solution, this channel activity was never observed (n = 8), suggesting that these channels were blocked by external TEA (not shown).
Figure 5. Voltage- and Cl−-sensitive Ca2+-activated K+ currents in inside-out patches.
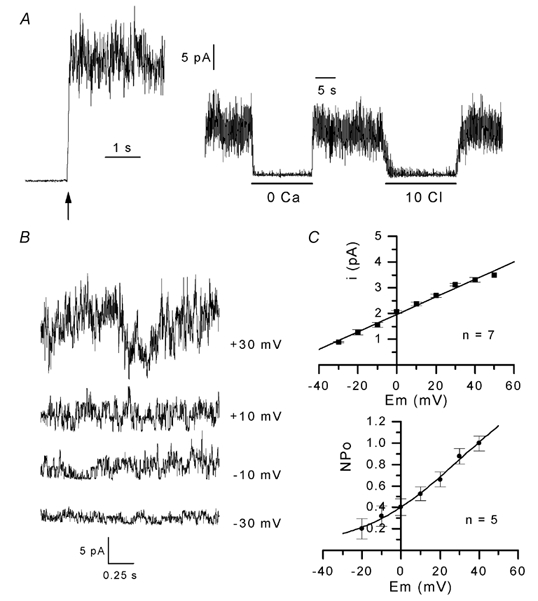
In A, left panel, the arrow indicates excision of a cell-attached patch held at 0 mV in K+-rich solution with 0.1 mm CaCl2. Na+-rich solution was present in the pipette. The right panel illustrates channel activity in the same patch 45 s after excision; bars indicate the periods during which solution containing EGTA and no added Ca2+ (0 Ca) and solution in which [Cl−] was reduced from 150 to 10 mm (10 Cl) were applied to the internal face of the patch. In B, traces correspond to segments of single-channel currents recorded in an inside-out patch at the different membrane potentials indicated next to each current trace. In C, the upper panel shows the current-voltage relationship. The slope indicates a conductance of 33.9 pS. The lower panel shows the relationship between NPo and membrane potential; in each patch, values of NPo were normalized to the value obtained at +50 mV. The curve was drawn by eye.
Figure 5B shows segments of single-channel currents through the channels active on excision at different membrane potentials in the presence of 140 mmCl− and 0.1 mm Ca2+ at the cytoplasmic face. Unitary currents had an amplitude of 0.86, 1.7, 2.3 and 3.1 pA at −30, −10, +10 and +30 mV, respectively. Figure 5C (upper panel) shows the amplitude of unitary currents through these channels plotted against membrane potential. The best fit to the mean data indicated a slope conductance of 33.9 pS and an extrapolated reversal potential of −58 mV. The current traces also clearly show that channel activity increased with depolarization of the patch membrane. In the patch illustrated in Fig. 5B, NPo increased from 1.04 at −30 mV to 1.28, 1.35 and 3 at −10, +10 and +30 mV, respectively. An increase in channel activity (NPo) with depolarization was observed in all the inside-out patches tested (Fig. 5C, lower panel). On the basis of these observations, it is very likely that the channels active on excision and sensitive to internal Ca2+ and Cl− correspond to the K+ channels activated by depolarization in cell-attached patches.
In order to evaluate the contribution of these channels active in cell-attached patches to the currents activated by depolarization in whole-cell recordings, a set of whole-cell experiments was performed in the presence of low internal chloride solution in the pipette. This condition was expected to repress the activity of the depolarization-activated K+ channels sensitive to internal Cl−. While in the presence of 128 mmCl− in the internal solution, outward currents elicited by depolarization routinely rose and stabilized to a plateau level (see upper panel of Fig. 1A), in the presence of low internal chloride (15 mm) solution, outward currents systematically exhibited a rapid peak phase followed by a marked decay to a plateau level (not illustrated). In the eight cells tested under low internal chloride conditions, at +40 mV, the ratio of the amplitude of the peak current to that of the end pulse current was 1.26 ± 0.03 compared to 0.93 ± 0.04 (n = 26) in the presence of high internal chloride solution, a statistically lower value (P = 0.0001). This result suggests that a current component involving channels sensitive to internal chloride contributes to the delayed part of the outward currents elicited by depolarization.
As shown above, in inside-out conditions, the channels spontaneously active on excision were subject to run-down, and openings vanished within a few minutes. In 6/17 inside-out patches tested, after the 34 pS channel had closed, openings of channels of much smaller conductance carrying outward currents at 0 mV were revealed. As illustrated in Fig. 6A, where at least four of these channels were active in a patch held at +30 mV, channel openings were reversibly inhibited by removal of internal Ca2+ but were not affected by a reduction in internal Cl−. While these channels closed in the absence of internal Ca2+, rare openings of channels carrying outward currents of higher amplitude persisted. This persistent channel activity observed in the absence of internal calcium was not investigated further. Figure 6B shows short segments of currents through one of these channels at different membrane potentials in the presence of 0.1 mm Ca2+ and 140 mmCl− at the cytoplasmic face. Unitary currents had an amplitude of 0.37, 0.83, 1.48 and 2.67 pA at −10, +10, +30 and +50 mV, respectively. Figure 6C shows the relationship between unitary current amplitude and membrane potential. The channels exhibited outward rectification, their mean conductance was 6 pS at 0 mV and the extrapolated reversal potential was estimated to be around −60 mV, indicative of a K+ selectivity of the channels. The activity of these channels was apparently not affected by voltage (not illustrated).
Figure 6. Small conductance Ca2+-activated K+ channels in inside-out patches.
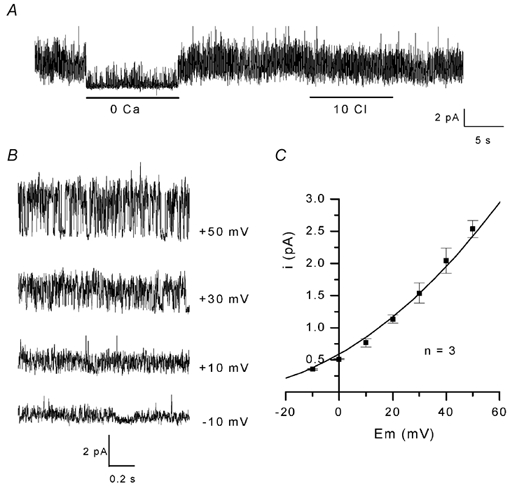
In A, channel activity was recorded at +30 mV in the presence of K+-rich solution with 0.1 mm CaCl2 at the internal face of the patch and Na+-rich solution in the pipette, 5.5 min after excision. Bars indicate the period during which 0 Ca and 10 Cl solutions (see legend to Fig. 5) were applied to the internal face of the patch. B, single-channel currents were recorded at the different membrane potentials indicated next to each current trace 3 min after excision of the patch. C, current-voltage relationship. The curve was drawn by eye.
DISCUSSION
In this study, using an in situ patch clamp technique on dissected nematodes, we give a detailed description of K+ currents in body wall muscle cells from C. elegans. Under whole-cell conditions, muscle cells did not display any apparent inward rectification while membrane depolarizations above −40 mV elicited net outward currents. On the basis of the differences observed in time and voltage dependencies, as well as in pharmacological sensitivity, these depolarization-activated outward currents could be separated into two distinct components: a rapidly activating and inactivating component inhibited by 4-AP and a slowly activating and maintained component inhibited by TEA. The reversal potentials for the rapid and the delayed components estimated from tail current experiments were consistent with these currents being predominantly carried by K+. Current-voltage relationships indicated that both components were voltage dependent, exhibiting about 15 mV per e-fold increase in K+ conductance. Similar depolarization-activated outward K+ currents have long been described in different cell types from invertebrates and vertebrates. A transient outward current which activates rapidly upon depolarization and inactivates in less than 100 ms, termed IA, has been characterized in neurones from molluscs and mammals, and in flight muscle from Drosophila (Connor & Stevens, 1971b; Salkoff & Wyman, 1983; Beluzzi et al. 1985). A 4-AP-sensitive current with comparable properties, termed Ito, has also been described in vertebrate cardiac cells (Coraboeuf & Carmeliet, 1982). The TEA-sensitive slowly activating outward current described here falls into the class of delayed rectifiers. Delayed K+ conductances that produce a maintained or slowly declining outward current have been recorded in neurones from molluscs and mammals, and in skeletal and cardiac muscle cells from vertebrates (Noble & Tsien, 1969; Adrian et al. 1970; Connor & Stevens, 1971a; Barrett et al. 1980). In neurones, the primary action of the delayed rectifiers is to repolarize the action potential while IA currents participate in setting the frequency of repetitive discharge and influence the threshold of firing (e.g. Rudy, 1988; Adams & Nonner, 1990). Body wall muscle cells in C. elegans are polyinnervated and receive multiple excitatory and inhibitory inputs from acetylcholine and γ-aminobutyric acid motoneurones, respectively (Lewis et al. 1980; McIntire et al. 1993a,b; Richmond & Jorgensen, 1999). The transient and delayed K+ conductances described in the present study probably operate to modulate muscle activation and to repress spreading of depolarizing signals ensuing from cholinergic receptor activation.
Our single-channel recordings revealed the presence of channels active at membrane potentials above −40 mV in cell-attached patches. Current-voltage relationships indicated that the channels were selective for K+; in addition, channel activity increased with membrane depolarization, indicative of a voltage dependence of the channel. Upon excision, K+ channels exhibiting high activity spontaneously opened, then underwent a progressive run-down probably resulting from the loss of intracellular factors following excision. The opening of these channels was found to be reversibly inhibited by the removal of internal Ca2+ as well as a reduction in internal [Cl−]. A K+ channel displaying Cl− dependence is unusual. The existence of such a Cl−-dependent K+ channel in C. elegans, termed SLO-2, was reported for the first time by Yuan et al. (2000). Identification of the slo-2 gene ensued from searches in the C. elegans genome for genes similar to the slo-1 gene, encoding high conductance Ca2+-activated K+ channels. Expression of slo-2 in Xenopus oocytes revealed that, in inside-out macropatches, SLO-2 produces large non-inactivating K+ currents that are sensitive to voltage and to internal Ca2+ and Cl− in a synergistic manner. Transforming nematodes with a slo-2:GFP gene indicated that SLO-2 was expressed in body wall muscles. In view of the properties displayed by the SLO-2 channel in the expression system, together with its expression pattern, it is likely that the K+ channel recorded here, in situ, in body wall muscle, corresponds to this SLO-2 channel.
We also observed that the channels active at depolarized membrane potentials in cell-attached patches displayed the same conductance as the Cl−-sensitive Ca2+-activated K+ channels. Additionally, the carried currents had similar reversal potentials, and both channels were voltage dependent and were inhibited by 20 mm external TEA. Taken together, these observations strongly suggest that these two K+ channels were one and the same channel. It thus implies that the Cl−-sensitive Ca2+-activated K+ channels may open in the presence of a native intracellular environment and could play a significant role during C. elegans muscle activation. Along this line, it is very probable that these channels had contributed, at least in part, to the depolarization-activated outward currents recorded under whole-cell conditions, although a high concentration of the Ca2+ chelator EGTA was present in the intracellular solution. Indeed, a reduction of the internal Cl− concentration from 128 to 15 mm led to a decrease in the ratio of the amplitude of the peak current to the amplitude of the end pulse current of voltage-activated whole-cell currents, suggesting that a current component involving the Cl−-sensitive Ca2+-activated K+ channels contributes significantly to the delayed component of the outward macroscopic currents. This is further supported by the fact that the Cl−-sensitive Ca2+-activated K+ channels were inhibited by 20 mm external TEA, a concentration inducing strong inhibition of the maintained component of the voltage-activated outward currents. The variable proportion of Cl−-sensitive Ca2+-activated K+ currents to total outward whole-cell currents might also explain the variations in the waveform of the outward currents observed from one cell to another, as illustrated in Fig. 1A. However, it is likely that other channel types may contribute to the total macroscopic current elicited by depolarization but whose unitary events cannot be detected under our experimental conditions.
The Cl−-sensitive Ca2+-activated K+ channel described in this study differed from the well-characterized high conductance Ca2+-activated K+ channels, also termed BK, found at high density in mammalian skeletal muscle (Allard et al. 1996; Jacquemond & Allard, 1998; Mallouk et al. 2000). The unitary conductance of BK channels in mammalian muscle is around 70 pS in the presence of 5 mm external K+, while the Ca2+-activated K+ channels sensitive to Cl− had a conductance of 34 pS under corresponding conditions. Additionally, in our hands, we have never observed any effect of a reduction in internal [Cl−] on mammalian BK channel activity (B. Allard, unpublished observations). On the whole, out of the 25 excised patches tested from C. elegans body wall muscle cells, we never detected any activity of a channel that could be identified as a BK channel. Yet, a gene showing a high level of identity to the slo-1 gene encoding mammalian BK channels has been cloned in C. elegans (Wang et al. 2001). However, immunolocalization of SLO-1 in C. elegans indicated that high conductance Ca2+-activated K+ channels are expressed in body wall muscle but in a sparse manner. Moreover, while removal of SLO-1 improved locomotion in mutants displaying synaptic transmission defects, a drive expression of a rescuing SLO-1 construct in body wall muscle of these nematode mutants had no detectable effect on locomotion.
In 35 % of the inside-out patches tested, after the Cl−-sensitive Ca2+-activated K+ channel had closed, another Ca2+-activated K+ channel was identified. This channel had a much smaller conductance, 6 pS at 0 mV in the presence of 5 mm external K+, exhibited an outward rectification and was apparently not sensitive to voltage. This small conductance channel may fall into the class of SK channels found in various cell types from vertebrates (Latorre et al. 1989; McManus, 1991; Vergara et al. 1998), as sequence analysis has revealed the presence of at least three SK genes in the C. elegans genome (Wei et al. 1996; Bargmann, 1998; Salkoff et al. 1999). In view of its small conductance and apparent low density, it is likely that this channel has little influence on C. elegans muscle excitability compared to the apparently greater expressed and larger Cl−-sensitive Ca2+-activated K+ channel.
In conclusion, this paper provides a detailed description of the K+ channels that operate in wild-type C. elegans body wall muscle cells. In this way, this work lays the basis for further comparisons with mutants to assess the function of K+ channels in this model organism that is highly amenable to molecular and classical genetics.
Acknowledgments
We are grateful to Robert Bonvallet, Christophe Chouabe and Vincent Jacquemond for helpful discussion while the manuscript was in preparation. This study was supported by funds from the Centre National de la Recherche Scientifique, the Ministère de la Recherche (Action Concertée Incitative Project 2000), the Université Claude Bernard Lyon 1 and the Association Française contre les Myopathies.
REFERENCES
- Adams DJ, Nonner W. Voltage-dependent potassium channels: gating, ion permeation and block. In: Cook NS, editor. Potassium Channels, Structure, Classification, Function and Therapeutic Potential. Chichester, UK: Ellis Horwood Limited; 1990. pp. 40–69. [Google Scholar]
- Adrian RH, Chandler WK, Hodgkin AL. Voltage clamp experiments in striated muscle fibres. Journal of Physiology. 1970;208:607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Bernengo J-C, Rougier O, Jacquemond V. Intracellular Ca2+ changes and Ca2+-activated K+ channel activation induced by acetylcholine at the end-plate of mouse skeletal muscle fibres. Journal of Physiology. 1996;494:337–349. doi: 10.1113/jphysiol.1996.sp021496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Crill WE. Voltage-sensitive outward currents in cat motoneurones. Journal of Physiology. 1980;304:231–249. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O, Sacchi O, Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. Journal of Physiology. 1985;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de, Miera E, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. Journal of Physiology. 1971a;213:1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. Journal of Physiology. 1971b;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E, Carmeliet E. Existence of two transient outward membrane currents in sheep cardiac Purkinje fibers. Pflügers Archiv. 1982;392:352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Hall DH, Avery L, Lockery SR. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–772. doi: 10.1016/s0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates; 1992. Potassium channels and chloride channels; pp. 115–140. [Google Scholar]
- Jacquemond V, Allard B. Activation of Ca2+-activated K+ channels by an increase in intracellular Ca2+ induced by membrane depolarization in mouse skeletal muscle fibres. Journal of Physiology. 1998;509:93–102. doi: 10.1111/j.1469-7793.1998.093bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MT, Johnstone DB, Thomas JH, Salkoff L. Mutants of a temperature-sensitive two-P domain potassium channel. Journal of Neuroscience. 2000;20:7517–7524. doi: 10.1523/JNEUROSCI.20-20-07517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annual Review of Physiology. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5:967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Lim H-H, Park B-J, Choi H-S, Park C-S, Eom SH, Ahnn J. Identification and characterization of a putative C. elegans potassium channel gene (Ce-slo-2) distantly related to Ca2+-activated K+ channels. Gene. 1999;240:35–43. doi: 10.1016/s0378-1119(99)00398-4. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993a;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993b;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- McManus OB. Calcium-activated potassium channels: regulation by calcium. Journal of Bioenergetics and Biomembranes. 1991;23:537–559. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Mallouk N, Jacquemond V, Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proceedings of the National Academy of Sciences of the USA. 2000;97:4950–4955. doi: 10.1073/pnas.97.9.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. Journal of Physiology. 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nature Neuroscience. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Kunkel MT, Wang Z-W, Butler A, Yuan A, Nonet M, Wei A, De Miera E, Rudy B. The impact of the Caenorhabditis elegans genome project on potassium channel biology. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences. 1999;868:233–285. [Google Scholar]
- Salkoff LB, Wyman RJ. Ion currents in Drosophila flight muscles. Journal of Physiology. 1983;337:687–709. doi: 10.1113/jphysiol.1983.sp014649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Thompson S. Pharmacological distinct potassium channels in molluscan neurones. Journal of Physiology. 1977;265:465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Current Opinion in Neurobiology. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wang Z-W, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Yuan A, Dourado M, Butler A, Walton N, Wei A, Salkoff L. SLO-2, a K+ channel with an unusual Cl− dependence. Nature Neuroscience. 2000;3:771–779. doi: 10.1038/77670. [DOI] [PubMed] [Google Scholar]


