Abstract
Cortical inhibitory systems play an important role in motor output. The motor cortex can be inhibited by intracortical mechanisms and by peripheral sensory inputs. We examined whether cortical inhibition from peripheral sensory input is mediated through previously identified intracortical inhibitory systems and how these inhibitory systems interact. Two types of intracortical inhibition were assessed by paired-pulse transcranial magnetic stimulation (TMS). Short-interval intracortical inhibition (SICI) was determined with a subthreshold conditioning stimulus (CS) followed by a test stimulus 2 ms later and long-interval intracortical inhibition (LICI) with suprathreshold conditioning and test stimuli 100 ms apart. Cortical inhibition from peripheral sensory input was induced by median nerve stimulation (MNS) of the right hand and followed by a suprathreshold TMS over the left motor cortex 200 ms later. The first set of experiments tested the effects of different test stimulus intensities on SICI, LICI and cortical inhibition induced by median nerve stimulation (MNSI). With higher test stimulus intensities, LICI and MNSI decreased whereas SICI showed a trend towards an increase. The extent of SICI, LICI and MNSI did not correlate. The second experiment assessed the interaction between MNSI and LICI. The results of applying MNSI and LICI simultaneously were compared with MNSI and LICI alone. MNSI was virtually abolished in the presence of LICI and LICI was also significantly decreased in the presence of MNSI. Thus, the effects of MNSI and LICI when applied together were much less than their expected additive effects when applied alone. The degree of interaction between MNSI and LICI was related to the combined strength of MNSI and LICI but not to the strength of LICI alone. The third experiment investigated the interaction between SICI and MNSI. MNSI and SICI were applied together and the results were compared with MNSI and SICI alone. SICI remained unchanged in the presence of MNSI. We conclude that MNSI is mediated by circuits distinct from those mediating LICI or SICI. The MNSI circuits seem to have an inhibitory interaction with the LICI circuits, whereas the SICI and MNSI circuits do not seem to interact.
Cortical excitatory and inhibitory mechanisms in humans can be assessed non-invasively by transcranial magnetic stimulation (TMS) using paired-pulse protocols. It has been shown that a subthreshold conditioning stimulus (CS) that precedes a suprathreshold test stimulus (TS) by 1-4 ms reduces the test motor-evoked potential (MEP) (Kujirai et al. 1993; Ziemann et al. 1996b). This mechanism will be referred to as short-interval intracortical inhibition (SICI). At interstimulus intervals (ISIs) of 8-15 ms the subthreshold CS increases the test MEP (Kujirai et al. 1993; Ziemann et al. 1996b). This mechanism will be referred to as intracortical facilitation (ICF). Another inhibitory phenomenon can be seen at longer ISIs (50-200 ms), where a suprathreshold CS inhibits the test MEP (Valls-Sole et al. 1992; Wassermann et al. 1996). This effect will be referred to as long-interval intracortical inhibition (LICI). There is evidence that SICI, ICF and LICI are of cortical origin (Fuhr et al. 1991; Inghilleri et al. 1993; Kujirai et al. 1993; Chen et al. 1998b, 1999b), but they seem to be mediated by different intracortical pathways (Ziemann et al. 1996a,b; Chen et al. 1998b). It has been suggested that SICI involves GABAA receptors (Hanajima et al. 1998), whereas LICI may be related to GABAB mechanisms (Werhahn et al. 1999; Sanger et al. 2001). Intracortical inhibition can be altered in various neurological diseases such as Parkinson's disease and dystonia (Ridding et al. 1995a; Chen et al. 1997) and in settings of brain plasticity such as following spinal cord injury and amputation (Chen et al. 1998a; Davey et al. 1998).
The effects of peripheral sensory stimulation on motor cortex excitability can be assessed by applying a peripheral sensory stimulus, such as median nerve stimulation (MNS) followed by a TS over the contralateral motor cortex. MEP inhibition induced by peripheral nerve stimulation has been reported for ISIs between 20 and 600 ms (Manganotti et al. 1997; Chen et al. 1999a; Tokimura et al. 2000). At an ISI of 200 ms the inhibition is probably of cortical origin since spinal cord excitability was unchanged (Chen et al. 1999a; Classen et al. 2000). Our preliminary study in 10 subjects found that between ISIs of 20 and 600 ms inhibition induced by median nerve stimulation (MNSI) is most consistent at an ISI of 200 ms.
While previous studies assessed the effect of sensory stimulation on MEP amplitude, there is evidence that peripheral sensory input interacts with cortical inhibitory mechanisms. Temporary deafferentation by ischaemic nerve block together with low frequency repetitive magnetic stimulation (Brasil-Neto et al. 1993; Ziemann et al. 1998) and long-term deafferentation following amputation of an extremity (Sanes et al. 1990; Chen et al. 1998a) reduces intracortical inhibition in animals and humans. Thus, interactions between sensory input and intracortical inhibitory circuits may play a role in cortical reorganization. Furthermore, focal dystonia may be related to abnormal sensory input (Hallett, 1995; Byl et al. 1996) and deficiencies in both intracortical inhibition (Ridding et al. 1995b; Chen et al. 1997) and MNSI at an ISI of 200 ms (Abbruzzese et al. 2001) have been reported in this condition. How the deficient peripheral sensory inhibition is related to changes in intracortical inhibition in this condition remains unclear.
The aim of this study was to assess whether MNSI is mediated via the same cortical inhibitory mechanisms as SICI or LICI and to investigate the interactions between these inhibitory systems.
METHODS
Subjects
We studied 15 healthy volunteers (9 men and 6 women, aged 40 ± 10.6 years). All subjects gave their written informed consent. The protocol was approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
Experimental set-up
Transcranial magnetic stimulation
TMS was performed with a 7 cm figure-of-eight coil, four Magstim 200 stimulators and three Bistim modules (The Magstim Company, Dyfed, UK). The outputs of each pair of Magstim 200 stimulators were directed to a Bistim module. The output of the two Bistim modules was then directed to the third Bistim module, which was connected to the TMS coil. The stimulator set-up allowed us to apply up to four consecutive TMS pulses of different stimulus intensities at short ISIs (Sanger et al. 2001).
The area for eliciting the best motor response (optimal position) in the right first dorsal interosseus muscle (FDI) was established over the left motor cortex with the coil held about 45 deg to the mid-sagittal line (approximately perpendicular to the central sulcus). The direction of the induced current was from posterior to anterior and was optimal to activate the motor cortex transynaptically (Werhahn et al. 1994; Kaneko et al. 1996). The optimal position was marked on the scalp to ensure identical placement of the coil throughout the experiment.
EMG recording
Surface electromyogram (EMG) was recorded from the right FDI muscle with Ag-AgCl electrodes placed over the belly of the muscle and near the metacarpal-phalangeal joint of the index finger. The EMG signal was monitored on a computer screen and via loudspeakers to provide feedback on the state of muscle relaxation. The signal was amplified (Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), filtered (band pass 2 Hz to 5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronics Design, Cambridge, UK) and stored in a laboratory computer for off-line analysis. The subjects relaxed throughout the study. Trials contaminated with voluntary muscle activities were rejected.
Median nerve stimulation
The median nerve was stimulated at the wrist with standard bar electrodes (0.2 ms square wave constant current pulses), with the cathode positioned proximally. Stimulus intensity was adjusted to produce a slight thumb twitch (Chen et al. 1999a; Abbruzzese et al. 2001).
Study design
We tested SICI, ICF, LICI, MNSI and their interactions. Each trial consisted of a suprathreshold TS that could be preceded by a conditioning stimulus (MNS or TMS). The timing of the pulses was controlled by the output features of the A/D converter (Micro 1401, Cambridge Electronics Design, Cambridge, UK). The CS elicited by TMS were delivered 2, 10 or 100 ms before the TS and are named CS2TMS, CS10TMS and CS100TMS. CS2TMS was used to elicit SICI (Kujirai et al. 1993) and CS10TMS for ICF (Ziemann et al. 1996b). These CS intensities were 80 % of the resting motor threshold. At this intensity the CS changes cortical excitability (Nakamura et al. 1997; Di Lazzaro et al. 1998) but does not influence subcortical or spinal excitability as tested by transcranial electric stimulation and H-reflex (Kujirai et al. 1993; Chen et al. 1998b).
A suprathreshold CS100TMS was used to produce LICI. A CS100TMS leads to reduced cortical excitability (Nakamura et al. 1997; Chen et al. 1999b) without changes in spinal excitability (Fuhr et al. 1991). MNS preceded the TS by 200 ms (CS200MNS). Previous studies showed that a CS200MNS also inhibits the test response without any changes in spinal excitability (Chen et al. 1999a).
SICI, ICF, LICI and MNSI were expressed as the ratio of the conditioned (with preceding CS) to the unconditioned (TS alone) MEP amplitudes. The ratio of the MEP produced by the CS2TMS-TS combination to that of the TS alone gave SICI. Similarly, the ratio of the MEP produced by the CS10TMS-TS combination to that of the TS alone gave ICF, the ratio of the MEP produced by the CS100TMS-TS combination to that of the TS alone gave LICI and the ratio of the MEP produced by the CS200MNS-TS combination to that of the TS alone gave MNSI.
The intensities of the suprathreshold stimuli for TS and CS100TMS were labelled according to their target MEP amplitudes. The minimum stimulus intensity that produced > 1 mV MEPs in at least five of 10 trials was named TS 1 mV. Stimulus intensities of TS 0.2 mV and TS 4 mV were defined in a similar way. In Experiment 2 we adjusted the test MEP to be about 1 mV in the presence of CS100TMS by increasing the TS intensity. This allowed us to match MEP amplitudes to produce a similar degree of corticospinal activation with and without a preceding CS100TMS. The TS that produced 1 mV MEPs in the presence of CS100TMS was termed TS 1 mV(CS100).
Experiment 1. Effects of different test stimulus intensities on SICI, ICF, LICI and MNSI
We tested different TS intensities while keeping the conditioning stimuli the same. If the inhibitory systems we tested are mediated by the same circuits, the effects of changes in TS intensities should be similar. TS intensities were set to achieve MEP amplitudes of 0.2 mV (TS 0.2 mV), 1 mV (TS 1 mV) and 4 mV (TS 4 mV). Each run consisted of five different conditions: TS alone, CS2TMS-TS, CS10TMS-TS, CS100TMS-TS and CS200MNS-TS. The test conditions were delivered in random order 6 s apart and repeated 10 times. The three TS intensities were studied in separate runs.
Experiment 2. Interactions between MNSI and LICI
We tested the interactions between MNSI and LICI by comparing the effects of applying MNSI and LICI together to that of MNSI or LICI alone. The test conditions are shown in Table 1. These conditions were delivered in random order and repeated 10 times. The first three conditions (2A-2C) assessed the inhibitory effect of CS100TMS (2B/2A) or CS200MNS (2C/2A) on a test MEP of 1 mV. The TS intensity was increased in conditions 2D-2G in order to produce a 1 mV MEP in the presence of a CS100TMS (TS 1 mV(CS100)). The experimental design allowed us to compare MNSI in the presence of LICI (2G/2E) to MNSI alone matched for MEP amplitude (2C/2A) and TS intensity (2F/2D).
Table 1.
Configuration of pulses in Experiment 2
| Condition | CS200MNS | CS100TMS | TS |
|---|---|---|---|
| 2A | — | — | 1 mV |
| 2B | — | 1 mV | 1 mV |
| 2C | + | — | 1 mV |
| 2D | — | — | 1 mV(CS100) |
| 2E | — | 1 mV | 1 mV(CS100) |
| 2F | + | — | 1 mV(CS100) |
| 2G | + | 1 mV | 1 mV(CS100) |
This experiment investigated the interactions between cortical inhibition induced by median nerve stimulation (MNSI) and long-interval intracortical inhibition (LICI). CS100TMS was set to achieve a 1 mV MEP. Conditions 2A to 2C assessed the inhibitory effects CS200MNS and CS100TMS on a 1 mV test MEP. In conditions 2D to 2G the test stimulus intensity was increased in order to produce a 1 mV test MEP in the presence of CS100TMS(1 mV(CS100)). CS200MNS is median nerve stimulation at an interstimulus interval (ISI) of 200 ms, CS100TMS is conditioning stimulus at ISI of 100 ms, and TS is test stimulus.
Experiment 3. Effect of increased CS100 on interaction between MNSI and LICI
In Experiment 2 we found that CS200MNS frequently inhibits the MEP evoked by the CS100TMS stimulus. Therefore, in this experiment we increased the CS100TMS intensity to compensate for this inhibitory effect while studying the interaction between MNSI and LICI. Test conditions are shown in Table 2. We adjusted the CS100TMS intensity in condition 3D to give CS100TMS MEPs of about 1 mV in the presence of CS200MNS (1 mV(CS200)). We compared MNSI alone (3C/3A) to MNSI in the presence of LICI with CS100TMS adjusted to compensate for the effects of CS200MNS (3D/3B).
Table 2.
Configuration of pulses in Experiment 3
| Condition | CS200MNS | CS100TMS | TS |
|---|---|---|---|
| 3A | — | — | 1 mV(CS100) |
| 3B | — | 1 mV | 1 mV(CS100) |
| 3C | + | — | 1 mV(CS100) |
| 3D | + | 1 mV(CS200) | 1 mV(CS100) |
The CS100TMS MEP was kept at 1 mV for conditions 3B and 3D. The stimulus intensity was increased for the CS100TMS pulse in condition 3D (with a preceding CS200MNS) to maintain a 1 mV MEP (1 mV(CS200)).
Experiment 4. Interactions between MNSI and SICI/ICF
In this experiment we examined the interactions between MNSI and SICI and between MNSI and ICF. The 10 test conditions are shown in Table 3. These conditions were delivered in random order and repeated 10 times. TS intensities were either set to achieve MEP amplitudes of 1 mV (TS 1 mV) or were adjusted to elicit 1 mV MEPs with a preceding MNS stimulus (TS 1 mV(CS200)). Conditions 4A-4D gave SICI (4B/4A), ICF (4C/4A) and MNSI (4D/4A) for a 1 mV test MEP. SICI (4F/4E), ICF (4G/4E) and MNSI (4H/4E) for a 1 mV(CS200) MEP were tested in conditions 4E to 4H. Condition 4I assessed the interactions between MNSI and SICI while condition 4J tested the interactions between MNSI and ICF. The experiment was designed to compare SICI (4I/4H) and ICF (4J/4H) in the presence of CS200MNS to SICI and ICF alone matched for test MEP amplitude (4B/4A for SICI, 4C/4A for ICF) and TS intensity (4F/4E for SICI, 4G/4E for ICF).
Table 3.
Configuration of pulses in Experiment 4
| Condition | CS200MNS | CS10TMS | CS2TMS | TS |
|---|---|---|---|---|
| 4A | — | — | — | 1 mV |
| 4B | — | — | 0.8 MT | 1 mV |
| 4C | — | 0.8 MT | — | 1 mV |
| 4D | + | — | — | 1 mV |
| 4E | — | — | — | 1 mV(CS200) |
| 4F | — | — | 0.8 MT | 1 mV(CS200) |
| 4G | — | 0.8 MT | — | 1 mV(CS200) |
| 4H | + | — | — | 1 mV(CS200) |
| 4I | + | — | 0.8 MT | 1 mV(CS200) |
| 4J | + | 0.8 MT | — | 1 mV(CS200) |
The set-up investigated the effect of MNSI on short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF). CS2TMS and CS10TMS were set at 80 % of the resting motor threshold. Conditions 2A to 2D assessed the effects of SICI, ICF and MNSI on a 1 mV test MEP. In conditions 2E to 2J the test stimulus intensity was increased in order to produce a 1 mV test MEP in the presence of CS200MNS(1 mV(CS200)).
Statistical analysis
Values were expressed as means ± standard deviation (s.d.). For Experiment 1, the effects of different test MEP amplitudes on SICI, ICF, LICI and MNSI were tested by repeated-measures ANOVA and Scheffe's post hoc testing. The effects of SICI, ICF and LICI in single subjects were compared with the effects of MNSI using Pearson's correlation coefficient.
For Experiment 2, we compared MNSI in the presence of LICI to MNSI alone matched for TS amplitude (TS 1 mV) and matched for TS intensity (TS 1 mV(CS100)) using Student's paired t test. Student's paired t test was also used to compare LICI in the presence of MNSI to LICI alone with the TS at 1 mV and 1 mV(CS100). ‘Changes in LICI’ are defined as the ratio of LICI in the presence of MNSI (2G/2F) to LICI alone (2E/2D). Similarly ‘changes in MNSI’ is defined as the ratio of MNSI in the presence of LICI (2G/2E) compared with MNSI alone (2F/2D). Since changes in LICI and changes in MNSI are identical (both equal to (2G × 2D/2E × 2F)), it will be termed ‘changes in LICI/MNSI’. In an attempt to determine factors that best correlate with changes in LICI/MNSI, we calculated the ‘magnitude of MNSI’ as (1 – MNSI (2F/2D)), the ‘magnitude of LICI’ as (1 – LICI(2E/2D)) and the combined effect of MNSI and LICI as ‘magnitude of MNSI × LICI’ (product of the magnitudes of MNSI and LICI). This transformation was done so that larger values represent greater inhibition and positive correlations can be expected. Pearson's correlation coefficient was used to examine the relationship between changes in LICI/MNSI to the magnitudes of MNSI, LICI and MNSI × LICI. Multiple regression was performed if more than one factor significantly correlated with changes in LICI/MNSI.
The inhibitory effect of CS200MNS on the CS100TMS MEP (CS100TMS MEP inhibition) was measured as a ratio between the CS100TMS MEP after CS200MNS (2G) and the CS100TMS MEP alone (2E). The relationship between CS100TMS MEP inhibition and changes in LICI/MNSI was also examined using Pearson's correlation coefficient.
For Experiment 3, MNSI with and without LICI was compared using Student's paired t test. For Experiment 4, paired t tests were used to compare SICI and ICF in the presence of MNSI to SICI and ICF alone. The threshold for significance was set at P < 0.05.
RESULTS
Experiment 1. Effects of test stimulus intensity on SICI, ICF, LICI and MNSI
Thirteen subjects participated in this study. Two subjects were excluded from the analysis because test MEPs over 1.5 mV could not be achieved due to high motor thresholds. The motor threshold of the remaining 11 subjects was 44.4 ± 7.0 % of stimulator output. TS intensities were 49.5 ± 9.3 % for the 0.2 mV condition, 56.3 ± 11.9 % for the 1 mV condition and 71.8 ± 9.4 % for the 4 mV condition. The amplitude for the test MEP alone was 0.37 ± 0.12 mV for the 0.2 mV condition, 1.15 ± 0.34 mV for the 1 mV condition and 3.26 ± 0.89 mV for the 4 mV condition.
Figure 1A illustrates typical MEPs showing LICI, SICI, ICF and MNSI in one subject. The results of all 11 subjects are shown in Figure 1B. SICI showed less inhibition for small test MEPs of about 0.2 mV than for test MEPs of about 1 or 4 mV. However, the effect of test MEP amplitudes on SICI was not significant (repeated-measures ANOVA). ICF tended to decrease with higher MEP amplitudes but the effect of MEP amplitude on ICF was also not significant (repeated-measures ANOVA). For LICI, the two lower target MEP amplitudes of 0.2 and 1 mV showed much greater inhibition than the target MEP of 4 mV. Repeated-measures ANOVA demonstrated a significant effect of MEP amplitudes on LICI (P < 0.0001). Post hoc testing showed significantly reduced LICI for the 4 mV condition compared with the 1 mV (P = 0.0005) and the 0.2 mV conditions (P < 0.0001), while the 1 and 0.2 mV conditions were not significantly different from each other. MNSI also decreased with higher test MEPs and repeated-measures ANOVA showed a significant effect of MEP amplitudes on MNSI (P = 0.006). Post hoc testing revealed that MNSI was significantly less for the 4 mV condition compared with the 0.2 mV condition (P = 0.005). There was no significant correlation between MNSI and the other parameters tested (SICI, ICF and LICI).
Figure 1. Effects of test stimulus intensity on SICI, ICF, LICI and MNSI.
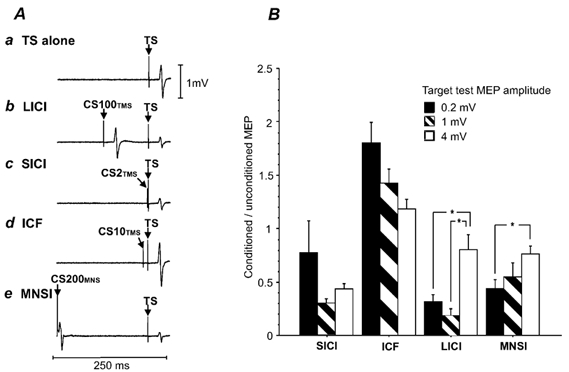
A, averaged MEPs from one subject in Experiment 1 (number of trials = 10). The test stimulus (TS) was set to produce MEPs of about 1 mV. a, TS alone. b, long-interval intracortical inhibition (LICI) elicited by a suprathreshold conditioning stimulus (CS100TMS) at 1 mV that precedes the TS by 100 ms. c, short-interval intracortical inhibition (SICI) elicited by a subthreshold conditioning stimulus (CS2TMS) that precedes the TS by 2 ms. d, intracortical facilitation (ICF) elicited by a subthreshold conditioning stimulus (CS10TMS) that precedes the TS by 10 ms. e, cortical inhibition induced by median nerve stimulation (MNSI) results from a median nerve stimulus preceding the TS by 200 ms (CS200MNS). B, the effects of different test stimulus intensities on SICI, LICI, ICF and MNSI in Experiment 1. The y-axis shows the ratio of the conditioned (TS with preceding CS) to the unconditioned (TS alone) MEP amplitude. Thus values < 1 represent inhibition of the conditioned TS. Error bars represent standard errors of the mean. Filled columns represent target MEP amplitudes of 0.2 mV, hatched columns target MEP amplitudes of 1 mV and open columns target MEP amplitude of 4 mV. Significant differences as shown by repeated-measures ANOVA and post hoc testing are indicated by asterisks.
Experiment 2. Interactions between MNSI and LICI
All 15 subjects participated in this experiment. TS intensities were 58.6 ± 10.6 % of stimulator output to elicit a 1 mV test MEP (conditions 2A-2C) and 71.6 ± 18.8 % for test MEPs of 1 mV(CS100) (2D-2G). The MEP amplitude for the 1 mV test MEP alone (2A) was 1.39 ± 0.51 mV, for the 1 mV(CS100) test MEP alone (2D) 2.63 ± 0.78 and for the CS100TMS-1 mV(CS100) pulse combination (2E) 1.43 ± 0.43 mV. Thus, the 1 mV test MEP (2A) and the CS100TMS-1 mV(CS100) pulse combination (2E) were matched for MEP amplitude. Although the experiment was not designed to compare LICI in the presence of MNSI with LICI matched for test MEP amplitude, the MEP amplitude for the CS200MNS-1 mV(CS100) pulse combination (2F) was 1.65 ± 0.81 mV and was only slightly higher than the 1 mV test MEP condition (2A). Therefore we also examined LICI in the presence of MNSI compared with LICI alone.
Figure 2A shows typical MEPs from one subject and the group results are shown in Figure 2B. MNSI for the 1 mV test MEP (2C/2A = 0.62 ± 0.34) and the 1 mV(CS100) test MEP (2F/2D = 0.68 ± 0.29) were not significantly different. MNSI was virtually abolished in the presence of LICI (2G/2E = 0.99 ± 0.34). Thus, MNSI had no additional effect in the presence of LICI. Student's paired t test revealed a significant reduction of MNSI in the presence of LICI (2G/2E) compared with MNSI in the absence of LICI matched for test MEP amplitude (2C/2A; P = 0.0039) and for TS intensity (2F/2D; P = 0.012). LICI was significantly (P < 0.0001) greater for the 1 mV test MEP (2B/2A = 0.22 ± 0.24) than for the 1 mV(CS100) test MEP (2E/2D = 0.58 ± 0.21). In the presence of MNSI, LICI had little additional inhibitory effect (2G/2F = 0.94 ± 0.60). LICI in the presence of MNSI was significantly reduced compared with LICI alone matched for TS intensity (2E/2D, paired t test; P = 0.020) and at a similar test MEP amplitude (2B/2A, paired t test; P = 0.0004). Thus, these results suggest that when MNSI and LICI were applied together the combined inhibitory effect was considerably less than their expected additive effects.
Figure 2. Interactions between MNSI and LICI.
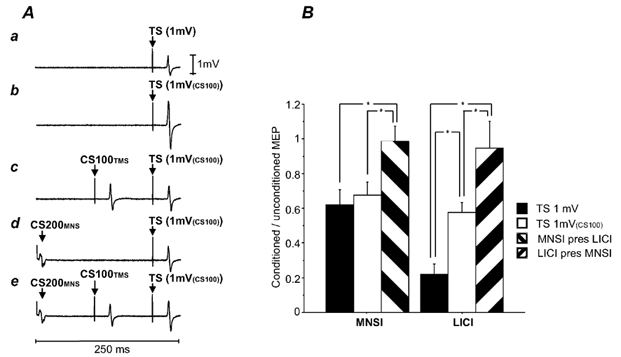
A, averaged MEPs of one subject in Experiment 2 (number of trials = 10). a, TS alone. The TS was adjusted to elicit MEPs of about 1 mV (TS 1 mV, condition 2A). b, TS 1 mV(CS100) alone. The TS was adjusted to produce test MEPs of 1 mV in the presence of a suprathreshold CS100TMS pulse (condition 2D). c, CS100TMS-TS 1 mV(CS100) pulse combination (condition 2E).The test MEP intensity matched the test MEP intensity in condition 2A. d, the effects of CS200MNS on the TS 1 mV(CS100) (condition 2F). e, effects of combining CS200MNS and CS100TMS (CS200MNS-CS100TMS-TS 1 mV(CS100) pulse combination, condition 2G). There is little further inhibition compared with c (LICI alone, condition 2E) and d (MNSI alone, condition 2F). B, interactions between LICI and MNSI. Error bars represent standard errors of the mean. The y-axis shows the ratio of the conditioned versus the unconditioned MEP. The left three columns show the results for MNSI: MNSI in the presence of LICI (MNSI pres LICI, hatched columns) was compared with the MNSI alone matched for test stimulus amplitude (TS 1 mV, filled columns) and test stimulus intensity (TS 1 mV(CS100), open columns). The right bars show LICI alone at 1 mV (filled columns) and 1 mV(CS100) (open columns) and LICI in the presence of MNSI (LICI pres MNSI, hatched columns). The asterisks indicate significant differences shown by Student's paired t test.
To further explore the interactions between MNSI and LICI, we examined the results of individual subjects. In two subjects, CS200MNS alone led to facilitation rather than inhibition of the test MEP (2F/2D = 1.18 and 1.16). Interestingly, in the presence of LICI, CS200MNS caused MEP inhibition in both subjects (2G/2E = 0.74 and 0.75). Furthermore, LICI in the presence of a CS200MNS pulse (2G/2F = 0.33 and 0.32) was more prominent than LICI alone (2E/2D = 0.54 and 0.50) in these subjects (Fig. 3A). Thus, when CS200MNS and CS100TMS pulses were applied together, these two subjects showed the opposite effect to the majority of subjects with increased rather than decreased inhibition. Another subject had no LICI (2E/2D = 1.06) and the addition of MNSI had essentially no effect on the CS100TMS pulse (2G/2F = 0.97). The other 12 subjects had both MNSI (2F/2D) and LICI (2E/2D) in the baseline conditions. When MNSI and LICI were applied together, all of these subjects had the same results as the group data with reduced MNSI in the presence of LICI compared with MNSI alone and also reduced LICI in the presence of MNSI compared with LICI alone. In four of the 12 subjects, the CS200MNS stimulus in the presence of LICI led to MEP facilitation (2G > 2E) and in two of these subjects the differences between 2E and 2G were significant (P = 0.026 and 0.002, Student's paired t test). In six subjects the CS100TMS stimulus in the presence of MNSI caused MEP facilitation (2G > 2F) and in one subject this difference was significant (P = 0.01, Student's paired t test).
Figure 3. Relationship between changes in LICI/MNSI and baseline variables.
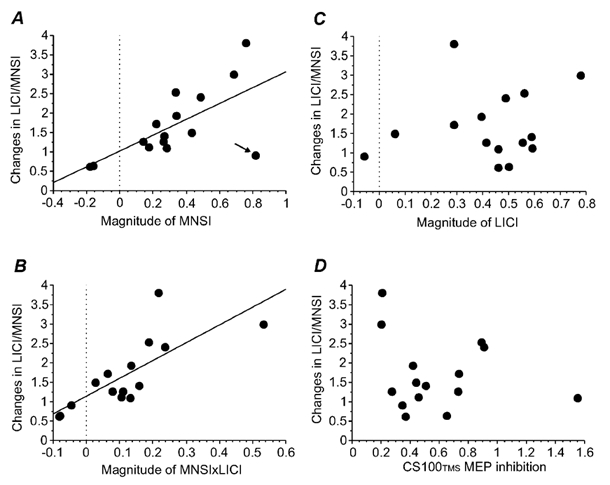
A, relationship between the ‘magnitude of MNSI’ and the ‘changes in LICI/MNSI’. Each point represents one subject (n = 15). The x-axis represents the magnitude of MNSI (1 – 2F/2D) and the y-axis the changes in LICI/MNSI calculated as a ratio of LICI in the presence of MNSI (2G/2F) to LICI alone (2E/2D). Thus ratios greater than 1 represent a decrease in LICI in the presence of MNSI. The changes in LICI/MNSI were correlated (r = 0.66, P = 0.007) with the magnitude of MNSI. The arrow points to the subject with no LICI. The two subjects in whom the CS200MNS stimulus alone led to MEP facilitation had negative values for magnitude of MNSI. B, relationship between the magnitude of MNSI × LICI and the changes in LICI/MNSI. Each point represents one subject (n = 15). The x-axis represents the magnitude of MNSI × LICI ((1 – 2F/2D) × (1 – 2E/2D)) and the y-axis the changes in LICI/MNSI. The changes in LICI/MNSI were correlated (r = 0.77, P = 0.0004) with the magnitude of MNSI × LICI. C, relationship between the magnitude of LICI (1 – 2E/2D) and the changes in LICI/MNSI (ratio of 2G/2E to 2F/2D). Each point represents one subject (n = 15). There was no significant correlation between the two measurements. D, relationship between the CS100TMS MEP inhibition and the changes in LICI/MNSI (n = 15). The x-axis represents the CS100TMS MEP inhibition as changes in CS100TMS MEP with and without preceding CS200MNS (CS100TMS MEP after CS200MNS (2G)/CS100TMS MEP alone (2D)). The y-axis represents changes in LICI/MNSI. There was no correlation between these two ratios.
These observations suggest that the interactions between MNSI and LICI may be related to the strength of baseline MNSI or LICI. Therefore we examined variables that may correlate with the changes in LICI/MNSI. Ratios greater than 1 represent less LICI/MNSI in the triple stimulation compared with baseline LICI or MNSI. The results are shown in Fig. 3. The changes in LICI/MNSI were correlated with the magnitude of MNSI (1 – MNSI) (r = 0.65, P = 0.007, Fig. 3A), the magnitude of MNSI × LICI (r = 0.77, P = 0.0004, Fig. 3B) but not with the magnitude of LICI (1 – LICI) (r = 0.16, n.s., Fig. 3C). Multiple regression analysis showed significant correlation between changes in LICI/MNSI and the magnitude of MNSI × LICI (P = 0.011) but not with magnitude of MNSI (P = 0.12). Thus, subjects with strong baseline MNSI and strong baseline MNSI × LICI had greater reduction of LICI in the presence of MNSI. However, the magnitude of baseline MNSI × LICI better predicts the interaction between MNSI and LICI than the strength of baseline MNSI alone.
CS200MNS often decreased the amplitude of the MEP elicited by the suprathreshold CS100TMS pulse. The CS100TMS MEP was 1.57 ± 0.49 mV without preceding CS200MNS and 0.91 ± 0.66 mV with preceding CS200MNS. The ratio of the CS100TMS MEP with preceding CS200MNS to the CS100TMS MEP alone was 0.58 ± 0.35. However, the extent of CS100TMS MEP inhibition by CS200MNS did not correlate with the changes in LICI/MNSI (r = 0.20; n.s.) as shown in Fig. 3D.
Experiment 3. Effect of increased CS100 on interaction between MNSI and LICI
Thirteen subjects participated in this experiment. TS intensities used were 58.9 ± 11.3 % of stimulator output for the 1 mV MEP and 71.6 ± 18.0 % for a 1 mV(CS100) MEP. The stimulus intensity that maintained the CS100TMS MEP at 1 mV in the presence of CS200MNS (3D) was 64.6 ± 14.1 %.
The MEP amplitude for 1 mV(CS100) pulse alone was 2.38 ± 0.77 mV (3A). LICI for the 1 mV(CS100) test pulse was 0.56 ± 0.20 (3B/3A). MNSI in the absence of LICI (0.71 ± 0.28, 3C/3A) was not significantly different from MNSI in the presence of LICI with CS100TMS adjusted (0.61 ± 0.36, 3D/3B).
Experiment 4. Interactions between MNSI and SICI/ICF
All 15 subjects participated. Data from three subjects were excluded because of technical problems (inadequate MNS in one subject, error in experimental set-up in two subjects). Therefore, the results from 12 subjects were analysed.
The resting motor threshold was 45.5 ± 7.2 % of stimulator output. TS intensities were 56.2 ± 12.6 % to elicit target MEPs of 1 mV (4A) and 62.3 ± 11.7 % for MEPs of 1 mV(CS200) (4E). The MEP amplitude was 1.44 ± 0.41 mV for the 1 mV test MEP (4A), 2.48 ± 1.06 mV for 1 mV(CS200) test MEP (4E) and 1.47 ± 0.44 mV for the CS200MNS-1 mV(CS200) pulse combination (4H). Thus, the amplitudes for the 1 mV test MEP (4A) and the CS200MNS-1 mV(CS200) (4H) test MEP were matched. Figure 4A shows typical MEPs for a single subject and the group results for SICI and ICF are shown in Fig. 4B. SICI and ICF for the 1 mV test MEP (for SICI, 4B/4A = 0.30 ± 0.12; for ICF, 4C/4A = 1.37 ± 0.45) and the 1 mV(CS200) test MEP (for SICI, 4F/4E = 0.37 ± 0.12; for ICF, 4G/4E = 1.24 ± 0.38) were not significantly different (Student's paired t test). There was a clear inhibitory effect of SICI in the presence of MNSI (4I/4H = 0.40 ± 0.22) which was not significantly different from SICI alone matched for test MEP amplitude (4B/4A) and TS intensity (4F/4E). ICF in the presence of MNSI (4J/4H = 1.61 ± 0.52) was not significantly different from ICF alone matched for test MEP amplitude (4C/4A), but was significantly higher than ICF alone matched for TS intensity (4G/4E; Student's paired t test, P = 0.005).
Figure 4. Interactions between MNSI and SICI/ICF.
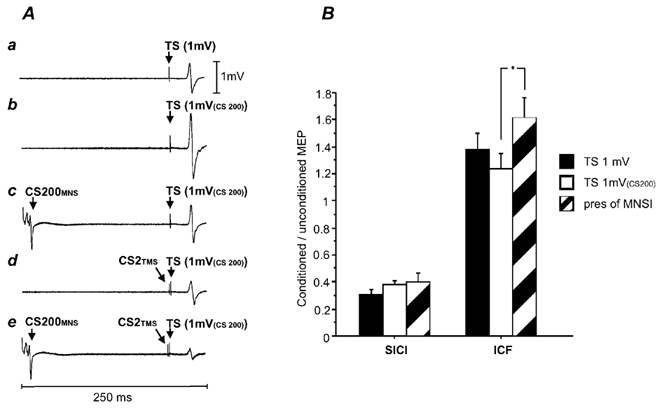
A, average MEPs of one subject in Experiment 4. a, TS alone. The TS was adjusted to elicit MEPs of about 1 mV (TS 1 mV, condition 4A). b, TS 1 mV(CS200) alone. The TS was adjusted to produce test MEPs of 1 mV in the presence of a CS200MNS (condition 4E). c, CS200MNS-TS 1 mV(CS200) pulse combination (condition 4H).The test MEP amplitude matched approximately the test MEP in condition 4A. d, CS2TMS-TS 1 mV(CS200) pulse combination (condition 4G).The subthreshold CS2TMS led to an inhibition of the TS 1 mV(CS200). e, effects of combining CS200MNS and CS2TMS (CS200MNS-CS2TMS-TS 1 mV(CS200) pulse combination, condition 4I). There is further inhibition compared with c (MNSI alone, condition 4H) and d (SICI alone, condition 4G). B, interactions between SICI, ICF and MNSI. Error bars represent standard errors of the mean. The y-axis shows the ratio of the conditioned versus the unconditioned MEP. Ratios < 1 represent inhibition, ratios > 1 represent facilitation. The left three columns show the results for SICI: SICI in the presence of MNSI (pres MNSI, hatched column) was compared with the SICI alone matched for test stimulus amplitude (TS 1 mV, filled column) and test stimulus intensity (TS 1 mV(CS200), open column). These conditions were not significantly different from each other. The right three columns show the results for ICF: ICF in the presence of MNSI (pres MNSI, hatched column) was not significantly different compared to ICF alone matched for test stimulus amplitude (TS 1 mV, filled column), but reached a significant difference for test stimulus intensity (TS 1 mV(CS200), open column; P = 0.005).
DISCUSSION
We examined the interactions between the cortical inhibition due to peripheral sensory stimulation and intracortical inhibition. We found that the inhibitory effects of LICI and MNSI were reduced when applied together, whereas SICI seemed unaffected by MNSI and their effects were additive.
Interaction between MNSI and LICI
Experiment 1 showed that both MNSI and LICI decreased with higher test MEP amplitude, although the effect was more marked for LICI than MNSI. Therefore, both MNSI and LICI had a greater effect on neurones activated at relatively low intensities than those activated at higher intensities. In Experiment 2, we studied the interaction between MNSI and LICI by applying them together. In order to produce a similar degree of corticospinal activation with and without LICI, we matched the test MEP amplitude by increasing the TS when preceded by the CS100TMS. MNSI in the presence of LICI was significantly reduced compared to MNSI whether matched for the TS intensity or test MEP amplitude. LICI also appeared to be reduced in the presence of MNSI.
Several possible mechanisms of interaction between MNSI and LICI are illustrated in Fig. 5. It should be noted that these models represent populations of neurones that are activated in certain experimental settings and they do not necessarily reflect functional populations in normal voluntary movement. In Model A, we hypothesize that MNSI and LICI are mediated via different cell populations that do not interact. Model B suggests that MNSI and LICI are both mediated via the same pathways with two possible scenarios: B1, LICI and MNSI activate the same neuronal population; B2, MNSI activates the neuronal population mediating LICI. Model C proposes that MNSI and LICI are mediated via independent cell populations, but may have excitatory or inhibitory interactions with each other.
Figure 5. Possible interactions between MNSI and LICI.
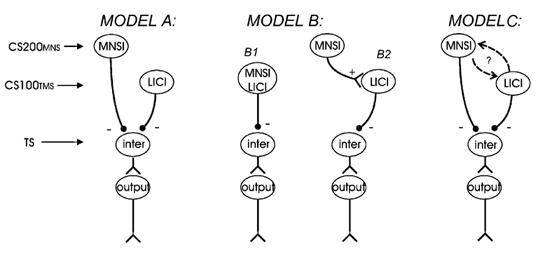
‘Inter’ indicates interneurones, whereas ‘output’ stands for output neurones. A, MNSI and LICI are independently mediated and do not interact; B1, MNSI and LICI are mediated simultaneously via the same interneurones; B2, MNSI is sequentially mediated via the LICI; C, MNSI and LICI are independently mediated, but interact via excitatory or inhibitory mechanisms.
It is unlikely that the interactions between MNSI and LICI can be explained with Model A. If these two mechanisms were mediated by independent pathways that do not interact, an additive effect of LICI and MNSI could be expected. However the results of Experiment 2 indicate that the effects of both MNSI and LICI were reduced in the presence of each other, suggesting there are interactions between these mechanisms. Since both MNSI and LICI predominately affect neurones activated at low intensities (Experiment 1), one possible explanation for this result is a saturation or occlusion effect. This possibility cannot be excluded from the results of the average data. However, there are several observations from single subject data that are not consistent with this mechanism. In four subjects, the effect of MNS changed from inhibition when applied alone to facilitation in the presence of LICI and in six subjects the CS100TMS pulse changed from inhibiting the test MEP to facilitation in the presence of MNSI. These facilitatory effects of either CS200MNS or CS100TMS in the triple pulse condition cannot be explained by the occlusion model. Moreover, the occlusion model predicts that the effect will be greater with greater baseline MNSI and LICI. We found that this is true for MNSI but not for LICI (Fig. 3).
The scheme depicted in Model B1 is unlikely since there was no correlation between the strength of LICI and MNSI at any of the target test MEP amplitudes studied in Experiment 1. If MNSI sequentially activates LICI pathways (Model B2), an additive effect of MNSI on LICI may be expected, which is different from the results in Experiment 2. A saturation effect should also be considered. One inhibitory mechanism alone might already activate the pathway to a high degree, so that another inhibitory mechanism has little additional effect. However, we have argued in the preceding paragraph that it is unlikely that a saturation effect can explain our finding.
Model C probably best explains our findings. Since Experiment 2 showed a reduced inhibitory effect of MNSI and LICI when the two mechanisms are combined, the interactions between MNSI and LICI are predominately inhibitory. This can be due to MNSI inhibiting LICI or LICI inhibiting MNSI. Since the interaction between MNSI and LICI is related to the strength of baseline MNSI but not baseline LICI, the most parsimonious explanation is that MNSI inhibits LICI rather than LICI inhibiting MNSI. This scheme is illustrated in Fig. 6A.
Figure 6. Proposed models for MNSI-LICI and MNSI-SICI interactions.
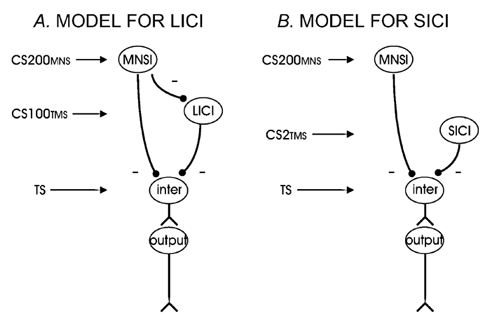
The interactions between MNSI and LICI/SICI that are consistent with our findings. A, MNSI and LICI are independently mediated, but MNSI has inhibitory influence on LICI. B, MNSI and SICI are independently mediated and do not interact.
However, LICI inhibiting MNSI can also occur if the CS100TMS stimulus simply ‘resets’ the whole system, removing any MNSI effect and leaving only the LICI effect. In this case the MEP amplitude of the CS200MNS- CS100TMS-TS pulse combination (2G) would be the same as the MEP amplitude of the CS100TMS-TS pulse combination (2E). This hypothesis is consistent with no overall effect of MNSI in the presence of LICI (2G/2E = 0.99) in the average data. While this hypothesis cannot be ruled out, several observations are more consistent with MNSI inhibiting LICI. First, in the two subjects who showed MEP facilitation with CS200MNS pulse alone, the CS200MNS pulse in the presence of LICI caused MEP inhibition in both subjects (2G/2E = 0.74 and 0.75). This can be explained by CS200MNS increasing LICI in these two subjects. If the CS100TMS stimulus simply resets the system by wiping out any effects of MNSI, the MEP amplitude for 2G and 2E should be the same. Second, one subject had no LICI (2E/2D = 1.06) but had strong MNSI (2F/2D = 0.19). If CS100TMS abolishes MNSI regardless of its inhibitory effect, the MEP in the triple pulse stimulation (2G) should be about the same size as the CS100TMS-TS MEP (2E) and considerably larger than the CS200MNS-TS MEP (2F). However, in this subject the MEP in 2G was essentially the same size as 2F (2G/2F = 0.97) and was much smaller than 2E (2G/2E = 0.17), indicating that the CS100TMS did not abolish MNSI in this subject. Third, in two subjects CS200MNS alone lead to MEP inhibition but CS200MNS in the presence of LICI caused significant facilitation (2G > 2E). Finally, the interactions between MNSI and LICI were better predicted by multiplying the inhibitory effects of MNSI and LICI than MNSI alone. This result can be expected if MNSI inhibits LICI, but if LICI abolishes MNSI then the interaction between MNSI and LICI would be best predicted by the strength of MNSI alone.
Another issue to consider is whether the inhibition of LICI by MNSI is related to the inhibition of the CS100TMS MEP that was observed in most subjects. The magnitudes of these effects are similar since increasing the strength of the CS100TMS pulse to compensate for the MEP inhibition in Experiment 3 abolished the effects of MNSI on LICI. However, they are probably mediated by different mechanisms since there was no correlation between the extent of LICI inhibition and CS100TMS MEP inhibition (Fig. 3D).
Interactions between SICI and MNSI
For the interactions between SICI and MNSI we will consider the same possible mechanisms as for the interactions between LICI and MNSI (see Fig. 5). It is unlikely that SICI and MNSI are mediated via the same pathways as illustrated in Model B. Experiment 1 showed that changing the TS intensity had opposite effects on MNSI and SICI. There was also no correlation between SICI and MNSI in any of the three TS intensities tested. These results are different from that of a previous study where a correlation between SICI and MNSI was found in six subjects (Trompetto et al. 2001). Model C for interactions between SICI and MNSI is also unlikely because we found no significant change in SICI in the presence of MNSI and the effects of SICI and MNSI seemed to be additive. Therefore, Model A showing that SICI and MNSI are mediated via independent mechanisms seems most consistent with our data (Fig. 6B).
A previous study showed that LICI inhibits SICI (Sanger et al. 2001). If MNSI inhibits LICI, it could potentially lead to facilitation of SICI. However, the absence of SICI facilitation by MNSI may be explained if there is little background LICI activity. LICI may be related to GABAB activity and it has been shown that GABAB receptors have little spontaneous background activity (Mott & Lewis, 1994). Although unlikely, we cannot completely exclude the possibility that MNSI inhibits SICI but the effect is counter-balanced by facilitation of SICI through inhibition of LICI.
Interactions between ICF and MNSI
ICF in the presence of MNSI is increased compared with MNSI alone when matched for TS intensity. However, the difference was not significant when matched for test MEP amplitude. Whether there is a weak interaction between MNSI and ICF needs to be confirmed in further studies.
Mechanisms of peripheral sensory stimulation
Neuro-imaging studies showed that peripheral sensory stimulation primarily activates the primary somatosensory cortex (S1), the second somatosensory area (S2) and the posterior parietal cortex (Korvenoja et al. 1999; Boakye et al. 2000). Temporal aspects of cortical activation after peripheral sensory stimulation were assessed by somatosensory evoked potentials (SEPs) and magnetoencephalographic somatosensory evoked fields. At shorter latencies (< 40 ms) the contralateral S1 (Allison et al. 1989; Forss et al. 1994) and contralateral S2 (Karhu & Tesche, 1999; Korvenoja et al. 1999) are primarily activated.
At longer latencies (> 40 ms) there is more widespread activation of sensory areas including S1, bilateral S2 (Hari et al. 1984; Allison et al. 1992) and the contralateral posterior parietal cortex (Forss et al. 1994).
It is likely that the peripheral sensory information contributing to MNSI is mediated from S1, S2 and the posterior parietal cortex and then projects to the motor cortex. Animal studies showed an extensive network of cortical connections from the S1 (Porter & Sakamoto, 1988; Burton & Fabri, 1995), S2 and the posterior parietal cortex (Ghosh et al. 1987) to the motor cortex. It has been suggested that the major sensory inputs to the motor cortex mainly terminate in superficial cortical layers (layers II-III). Cells in the superficial layers of the motor cortex respond to S1 stimulation with a consistent, short latency EPSP (Porter et al. 1990). The majority of sensory inputs seem to terminate at interneurones, that have modulatory effects on corticofugal neurones located in layers V and VI (Porter et al. 1990). These interneurones can be inhibitory or excitatory (Kosar et al. 1985; Porter et al. 1990), suggesting that sensory cortex stimulation can have both inhibitory and excitatory influences on pyramidal tract neurones. This may explain the findings of both increased (Deuschl et al. 1991; Komori et al. 1992) and decreased (Clouston et al. 1995; Manganotti et al. 1997; Tokimura et al. 2000) test MEP amplitudes at shorter latencies (< 50 ms) between MNS and TMS pulse.
However, a direct activation of the motor cortex via sensory afferents from the periphery cannot be excluded. A recent TMS study found inhibition of the motor cortex as early as 20 ms after a MNS and proposed a direct input from peripheral afferents to the motor cortex (Tokimura et al. 2000). Furthermore, the P22 peak of the short-latency SEP peaks may originate from the precentral motor area (Desmedt & Ozaki, 1991; Babiloni et al. 2001). However, motor cortex activation after MNS was not found in a PET study (Ibanez et al. 1995). Animal studies showed that the ventral posterior complex of the thalamus, the major sensory thalamic relay, only has minor direct projections to the motor cortex (Darian-Smith & Darian-Smith, 1993; Huffman & Krubitzer, 2001) and thus a structural correlate for direct motor cortex activation after peripheral sensory stimulation has not yet been found.
MNSI at short and long ISIs is likely to be mediated by different mechanisms. Ridding & Rothwell (1999) proposed a reduction of SICI in the presence of a peripheral sensory stimulus preceding the test pulse by 40 ms. MNSI at short and long ISIs also differs in pathological conditions. In patients with focal dystonia (Abbruzzese et al. 2001) the MNSI at an ISI of 200 ms is absent, whereas the MNSI at short ISIs was normal.
Interactions of peripheral stimulation and intracortical inhibition
The two inhibitory mechanisms detected by TMS (SICI and LICI) seem to be mediated via different inhibitory properties. Animal studies showed that early inhibitory postsynaptic potentials (IPSPs) (peaking around 10-20 ms) are mediated via GABAA and late IPSPs (peaking around 150-200 ms) are mediated via GABAB receptors (Davies et al. 1990; Kang et al. 1994; Deisz, 1999). The response pattern throughout the different layers of the motor cortex seems to be different for slow and fast IPSPs (Kang et al. 1994). Inhibitory interneurones producing slow IPSPs seem to be mainly distributed in layer II. This is also the layer where the main sensory input to the motor cortex arrives. Inhibitory interneurones producing fast IPSPs are distributed throughout almost all layers. It has been suggested that SICI may be mediated by GABAA receptors (Hanajima et al. 1998) and LICI by GABAB receptors (Werhahn et al. 1999; Sanger et al. 2001). If this is correct, this may explain why MNSI modulates LICI, but not SICI.
In conclusion, cortical inhibition from peripheral sensory stimulation at long ISIs (MNSI) seems to be mediated by circuits different from those mediating SICI and LICI. The MNSI circuit may inhibit LICI while MNSI and SICI circuits seem to be independent from each other.
Acknowledgments
We thank Dr Guillermo Paradiso for helpful comments, Lailoma Roshan and Carolyn Gunraj for technical assistance and Dr Peter Ashby for allowing us to use his equipment. This work was supported by the Canadian Institutes of Health, Canada Foundation for Innovation, Ontario Innovation Trust and the University Health Network Krembil Family Chair in Neurology.
REFERENCES
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC. The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalography and Clinical Neurophysiology. 1992;84:301–314. doi: 10.1016/0168-5597(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. Journal of Neurophysiology. 1989;62:694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Rosciarelli F, Rossini P, Arendt-Nielsen L, Chen A. Mapping of early and late human somatosensory evoked brain potentials to phasic galvanic painful stimulation. Human Brain Mapping. 2001;12:168–179. doi: 10.1002/1097-0193(200103)12:3<168::AID-HBM1013>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakye M, Huckins SC, Szeverenyi NM, Taskey BI, Hodge CJ., Jr Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. Journal of Neurosurgery. 2000;93:774–783. doi: 10.3171/jns.2000.93.5.0774. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, MacCabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. Journal of Comparative Neurology. 1995;355:508–538. doi: 10.1002/cne.903550404. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Jenkins WM. A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Experimental Brain Research. 1999a;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. Journal of Neuroscience. 1998a;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Experimental Brain Research. 1999b;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. Journal of Neurophysiology. 1998b;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Canos M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Experimental Brain Research. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Clouston PD, Kiers L, Menkes D, Sander H, Chiappa K, Cros D. Modulation of motor activity by cutaneous input: inhibition of the magnetic motor evoked potential by digital electrical stimulation. Electroencephalography and Clinical Neurophysiology. 1995;97:114–125. doi: 10.1016/0924-980x(94)00310-4. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Darian-Smith I. Thalamic projections to areas 3a, 3b, and 4 in the sensorimotor cortex of the mature and infant macaque monkey. Journal of Comparative Neurology. 1993;335:173–199. doi: 10.1002/cne.903350204. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Smith HC, Wells E, Maskill DW, Savic G, Ellaway PH, Frankel HL. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. Journal of Neurology, Neurosurgery and Psychiatry. 1998;65:80–87. doi: 10.1136/jnnp.65.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. Journal of Physiology. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA. GABA(B) receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Ozaki I. SEPs to finger joint input lack the N20-P20 response that is evoked by tactile inputs: contrast between cortical generators in areas 3b and 2 in humans. Electroencephalography and Clinical Neurophysiology. 1991;80:513–521. doi: 10.1016/0168-5597(91)90133-i. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Michels R, Berardelli A, Schenck E, Inghilleri M, Lucking CH. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Experimental Brain Research. 1991;83:403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Forss N, Hari R, Salmelin R, Ahonen A, Hamalainen M, Kajola M, Knuutila J, Simola J. Activation of the human posterior parietal cortex by median nerve stimulation. Experimental Brain Research. 1994;99:309–315. doi: 10.1007/BF00239597. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brinkman C, Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis) Journal of Comparative Neurology. 1987;259:424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Hallett M. Is dystonia a sensory disorder. Annals of Neurology. 1995;38:139–140. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- Hari R, Reinikainen K, Kaukoranta E, Hamalainen M, Ilmoniemi R, Penttinen A, Salminen J, Teszner D. Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalography and Clinical Neurophysiology. 1984;57:254–263. doi: 10.1016/0013-4694(84)90126-3. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Thalamo-cortical connections of areas 3a and M1 in marmoset monkeys. Journal of Comparative Neurology. 2001;435:291–310. doi: 10.1002/cne.1031. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Deiber MP, Sadato N, Toro C, Grissom J, Woods RP, Mazziotta JC, Hallett M. Effects of stimulus rate on regional cerebral blood flow after median nerve stimulation. Brain. 1995;118:1339–1351. doi: 10.1093/brain/118.5.1339. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. Journal of Physiology. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalography and Clinical Neurophysiology. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kang Y, Kaneko T, Ohishi H, Endo K, Araki T. Spatiotemporally differential inhibition of pyramidal cells in the cat motor cortex. Journal of Neurophysiology. 1994;71:280–293. doi: 10.1152/jn.1994.71.1.280. [DOI] [PubMed] [Google Scholar]
- Karhu J, Tesche CD. Simultaneous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. Journal of Neurophysiology. 1999;81:2017–2025. doi: 10.1152/jn.1999.81.5.2017. [DOI] [PubMed] [Google Scholar]
- Komori T, Watson BV, Brown WF. Influence of peripheral afferents on cortical and spinal motoneuron excitability. Muscle and Nerve. 1992;15:48–51. doi: 10.1002/mus.880150109. [DOI] [PubMed] [Google Scholar]
- Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva JM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ. Activation of multiple cortical areas in response to somatosensory stimulation: combined magnetoencephalographic and functional magnetic resonance imaging. Human Brain Mapping. 1999;8:13–27. doi: 10.1002/(SICI)1097-0193(1999)8:1<13::AID-HBM2>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosar E, Waters RS, Tsukahara N, Asanuma H. Anatomical and physiological properties of the projection from the sensory cortex to the motor cortex in normal cats: the difference between corticocortical and thalamocortical projections. Brain Research. 1985;345:68–78. doi: 10.1016/0006-8993(85)90837-6. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Zanette G, Bonato C, Tinazzi M, Polo A, Fiaschi A. Crossed and direct effects of digital nerves stimulation on motor evoked potential: a study with magnetic brain stimulation. Electroencephalography and Clinical Neurophysiology. 1997;105:280–289. doi: 10.1016/s0924-980x(97)00018-0. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. International Review of Neurobiology. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LL, Sakamoto K. Organization and synaptic relationships of the projection from the primary sensory to the primary motor cortex in the cat. Journal of Comparative Neurology. 1988;271:387–396. doi: 10.1002/cne.902710307. [DOI] [PubMed] [Google Scholar]
- Porter LL, Sakamoto T, Asanuma H. Morphological and physiological identification of neurons in the cat motor cortex which receive direct input from the somatic sensory cortex. Experimental Brain Research. 1990;80:209–212. doi: 10.1007/BF00228864. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell J. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Annals of Neurology. 1995a;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 1995b;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Experimental Brain Research. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. Journal of Physiology. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Di LV, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. Journal of Physiology. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompetto C, Buccolieri A, Abbruzzese G. Intracortical inhibitory circuits and sensory input: a study with transcranial magnetic stimulation in humans. Neuroscience Letters. 2001;297:17–20. doi: 10.1016/s0304-3940(00)01648-7. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and Clinical Neurophysiology. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Experimental Brain Research. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalography and Clinical Neurophysiology. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. Journal of Physiology. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. Journal of Neuroscience. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic Stimulation Study. Annals of Neurology. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. Journal of Physiology. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]


