One important feature of nociceptors is their ability to undergo sensitization in response to damage of their receptive field. This sensitization is an important component of hyperalgesia, defined as an increase in the magnitude of the nociceptive response after some sensitizing event such as inflammation. Numerous investigators have demonstrated that this sensitization is the result of the release of substances into the vicinity of the nerve endings in the periphery. These substances include prostaglandin and nerve growth factor (NGF) and they have been shown to enhance the sensitivity of nociceptive neurons.
The interesting paper by Zhang et al. (2002) in this issue of The Journal of Physiology is very timely because it explores cellular mechanisms of NGF- induced sensitization in dissociated DRG neurons that respond to capsaicin, i.e. that express the VR-1 receptor responsible for the response to noxious heat (Caterina & Julius, 2001). Although neurotrophins, a family of growth factors whose best-known member is NGF, act as survival factors and as stimulators of axonal growth, they can also have acute physiological actions such as sensitization and synaptic potentiation (Huang & Reichardt, 2001). Elucidation of the signalling mechanisms activated by NGF is complicated because it signals via two receptors, p75, the low affinity receptor, and trkA, the high affinity receptor (Kaplan & Miller, 2000) (Fig. 1).
Figure 1.
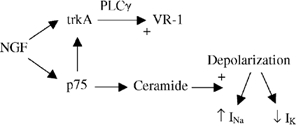
NGF activates p75 and trkA receptors in terminals of nociceptors which sensitizes their response to noxious heat (VR-1) and depolarization (↓IK and ↑INa), respectively.
Previous studies of the acute effect of NGF have demonstrated or assumed that trkA is the signalling pathway responsible for sensitization. NGF acutely enhances the inward current elicited by capsaicin in dissociated DRG cells (Shu & Mendell, 1999; 2001). This effect of NGF is blocked by K252a, which is suggestive of trkA involvement. Furthermore, sensitization cannot be elicited by the neurotrophin NT-3 suggesting that it is not mediated by p75 because p75 is activated by all neurotrophins. A role for trkA receptors in mediating the effects of NGF was demonstrated more definitively by Chuang et al. (2001) in oocytes expressing trkA and VR-1 with or without co-expression of p75. They demonstrated that the sensitizing effect of NGF depends on the interaction of the trk receptor and phospholipase C-γ (PLCγ). Mutations in the trkA receptor that prevented its association with PLCγ abolished the sensitizing effect of NGF on the response to capsaicin. Furthermore, an antibody to PLCγ also abolished the effect of NGF. The sensitizing effects of NGF could be observed in cells lacking p75 expression.
Zhang et al. manipulated the activity of ceramide, a second messenger liberated subsequent to activation of p75, and examined the resulting changes in membrane currents. NGF increased the magnitude of the response to depolarization of a TTX- insensitive INa that is characteristic of nociceptive neurons, and decreased the magnitude of delayed rectifier current (IK). The effects of NGF were mimicked by ceramide and blocked by inhibitors of ceramide. Both of these membrane effects would tend to facilitate neuronal discharges in response to stimulation and this was confirmed in the present study by recordings in current clamp. This suggests a role for p75 in NGF-induced sensitization of nociceptors. They also demonstrated that the inhibitory effects of ceramide on IK could be enhanced by subsequent treatment with PGE2.
An important question emerging from these findings is the role of the p75 receptor- activated system in sensitization and hyperalgesia, and whether it functions independently or in conjunction with the high affinity receptor (trkA) to elicit changes in membrane currents. The presently available data suggest that NGF has at least two independent actions, the first via trkA and PLCγ involving sensitization of the VR-1 receptor mediating among others the response to noxious heat, and the second via p75 involving enhanced response of voltage sensitive Na+ and K+ channels to changes in membrane potential (Fig. 1). NGF also regulates the number/density of TTX-resistant Na+ channels via trkA (Fjell et al. 1999), but it is not known whether activation of trkA acutely affects the physiology of ion channels. The interaction of low and high affinity receptors for NGF may be even more complex since other reports suggest that activation of p75 can influence the sensitivity of trkA to neurotrophins in regulating some trophic functions (Esposito et al. 2001).
The possibility for interaction either at the receptor level or intracellularly speaks to the need for methods to reliably study these actions independently by selectively blocking activation of the low and high affinity receptor. In interpreting such results it should be remembered that the results could depend on the function being studied (survival, ion channel function, etc.) since these involve different intracellular signalling pathways (Kaplan & Miller, 2000) whose regulation by these receptors could differ. Furthermore, the experiments of Zhang et al. were carried out on capsaicin-sensitive sensory neurons some of which express trkA (in addition to p75) while others do not (Michael & Priestly, 1999). It will be important to ascertain in future studies to what extent intracellular signalling pathways associated with p75 and trkA activation overlap and to what extent signalling pathways differ when activated by one or by both of these NGF receptors.
REFERENCES
- Caterina MJ, Julius D. Annual Review of Neuroscience. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chuang HH, et al. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Esposito D, et al. Journal of Biological Chemistry. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Fjell J, et al. Brain Research Molecular Brain Research. 1999;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Current Opinion in Neurobiology. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Journal of Neuroscience. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Neuroscience Letters. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Journal of Neurophysiology. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Zhang YH, et al. Journal of Physiology. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]


