Abstract
Physical exercise results in the appearance of heat shock protein (HSP) 72 in the circulation that precedes any increase in gene or protein expression in contracting skeletal muscle. In rodents, exercise increases liver HSP72 expression and the hepatosplanchnic viscera are known to release many acute phase proteins. In the present study, we tested the hypothesis that the splanchnic tissue beds release HSP72 during exercise. Seven male subjects performed 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake. Blood samples were obtained simultaneously from a brachial artery, a femoral vein and the hepatic vein prior to and at 30, 60 and 120 min of exercise. Leg blood flow (LBF) was measured by thermodilution in the femoral vein, and hepatosplanchnic blood flow (HBL) was measured using indocyanine green dye. Net leg and net hepatosplanchnic HSP72 balance were calculated as the product of LBF and femoral venous-arterial HSP72 difference and the product of HBF and hepatic venous-arterial HSP72 difference, respectively. Arterial plasma HSP72 was only detected in one subject at rest but progressively appeared in the arterial samples throughout exercise such that at 120 min it was detected in all subjects (0.88 ± 0.35 pg l−1; P < 0.05 compared with rest). The contracting muscle did not, however, contribute to this increase since there was no difference in the femoral venous-arterial HSP72 concentration at any time. Rather, the increase in arterial HSP72 was accounted for, at least in part, by release from the hepatosplanchnic viscera with values increasing (P < 0.05) from undetectable levels at rest to 5.2 ± 0.2 pg min−1 after 120 min. These data demonstrate that the splanchnic tissues release HSP72 during exercise and this release is responsible, in part, for the elevated systemic concentration of this protein during exercise.
It has been known for some time that heat shock proteins (HSPs) are present in the cells of all living organisms where their primary role is to bind to denatured proteins aiding in the assembly of protein complexes (for review see Kiang & Tsokos, 1998; Sharp et al. 1999; Hood, 2001). However, recent studies have identified that HSPs have important extracellular functions. Asea and colleagues have demonstrated that HSP72 (the inducible form of the 70 kDa family of HSPs) binds with high affinity to the cell surface receptors of human monocytes (Asea et al. 2000) and enters the cell via a specific signal transduction pathway (Asea et al. 2002). In addition, Multhoff et al. (1995) have identified HSP72 binding to the cell surface of vital tumour cell types in order to act as a target for natural killer cells, while neuronal cells can undergo cell-to-cell transfer (Tytell et al. 1986) where they can enhance neuronal stress tolerance (Guzhova et al. 2001). Recently, we demonstrated that physical exercise resulted in a marked increase in serum HSP72 in humans (Walsh et al. 2001). Interestingly, we observed the increase in circulating HSP72 to precede any increase in contracting muscle HSP72 gene or protein expression. In addition, even though HSP72 gene and protein expression are upregulated in human contracting skeletal muscle, it is not released from the contracting limb as measured by arterio-venous difference (Febbraio et al. 2002). Together, our recent data suggest that the contracting muscle is not responsible for the increase in systemic concentrations of HSP72 that is seen with exercise.
It is well documented that rodent liver cells can synthesise HSP72 in response to exercise (Salo et al. 1991; Kregal & Moseley, 1996). Although there is no documentation that HSPs are released by the liver, the hepatosplanchnic tissue bed can release many acute phase proteins during stress (De Feo & Lucidi, 2002). Hence, in the present study we tested the hypothesis that the hepatosplanchnic tissues release HSP72 during exercise.
Methods
Subjects
Seven healthy, active men (22.1 ± 3.8 years; 182.1 ± 5.5 cm; 81.0 ± 12.7 kg; maximal oxygen uptake (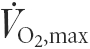 ) = 3.88 ± 0.34 l min−1; mean ± s.d.) participated in the study. The study was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before their written consent was obtained.
) = 3.88 ± 0.34 l min−1; mean ± s.d.) participated in the study. The study was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before their written consent was obtained.
Preliminary testing
Volunteers underwent a preliminary medical screening and were exempt from the study if they presented contra-indications. Following the medical screening, each subject underwent a 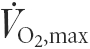 test on a semi-recumbent cycle ergometer. Semi-recumbent cycling was chosen to allow for the determination of leg blood flow using the thermodilution technique. From this test a workload was calculated which would elicit ≈65 % of each individual's
test on a semi-recumbent cycle ergometer. Semi-recumbent cycling was chosen to allow for the determination of leg blood flow using the thermodilution technique. From this test a workload was calculated which would elicit ≈65 % of each individual's 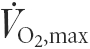 . Forty-eight hours prior to the experimental trial, subjects reported to the laboratory and completed 45 min of upright cycling exercise at a workload corresponding to 65 % of maximal heart rate. Thereafter, the subjects were provided with a food package which they consumed for the following days (15.6 MJ per day, 71 % CHO, 15 % protein, 14 % fat). During this period, subjects were asked to adhere to the diet and to refrain from strenuous exercise and the intake of alcohol, tobacco and caffeine. This procedure was adopted in order to reduce inter-subject variability.
. Forty-eight hours prior to the experimental trial, subjects reported to the laboratory and completed 45 min of upright cycling exercise at a workload corresponding to 65 % of maximal heart rate. Thereafter, the subjects were provided with a food package which they consumed for the following days (15.6 MJ per day, 71 % CHO, 15 % protein, 14 % fat). During this period, subjects were asked to adhere to the diet and to refrain from strenuous exercise and the intake of alcohol, tobacco and caffeine. This procedure was adopted in order to reduce inter-subject variability.
Experimental procedures
On the day of the experiment, the subjects reported to the laboratory at 07.30 h after a 12-14 h overnight fast. They voided, changed into appropriate exercise attire and rested in a supine position for 10 min. After this time a hepatic venous catheter was inserted (Nielsen et al. 2002). During the first four experimental trials, the hepatic venous catheter was introduced via the right median cubital vein and was guided with the subject supine. The position of the catheter was confirmed with fluoroscopy in the body position used during cycling. To ensure that ventilation (Ve) did not displace the catheter, the position was also confirmed after maximal voluntary Ve. Despite these efforts, the catheter dislodged during exercise in two of these experimental trials. Hence, we introduced the catheter via the right femoral vein in the subsequent three experiments and in these trials the catheter remained in the hepatic vein. Following this procedure a catheter was placed in the left brachial artery (1.0 mm i.d.; 20 gauge) and a third catheter (7 Fr diameter Cook, Denmark) was inserted into the left femoral vein ≈1-2 cm distal to the inguinal ligament. A thermistor for measurement of venous blood temperature was inserted through the catheter and advanced 8-10 cm proximal to the tip of the catheter. All catheters were kept patent by continuous infusion of isotonic saline (3 ml h−1) and were connected to a pressure monitoring kit (Baxter Healthcare, Maurepas, France) positioned at the level of the heart. The position of the femoral catheter and thermistor was checked after all experiments.
When the catheters were positioned, a constant infusion of indocyanine green (ICG; 0.18 ± 0.02 μmol l−1; Cardio-Green; Becton Dickinson, Cockeysville, MD, USA) was administered into a vein by a peristaltic roller pump (type 104; Ole Dich, Hvidovre, Denmark) (Nielsen et al. 2002) and was maintained for 30 min in order to secure a steady-state plasma concentration of ICG. After 30 min, blood samples were collected simultaneously from the brachial artery and hepatic vein every 5 min for the subsequent 30 min. These samples were analysed for ICG concentration. In addition, samples collected at 10 min intervals during this period were analysed for haemoglobin and haematocrit to estimate plasma volume shifts and determine hepatosplanchnic blood flow (HBF) from plasma flow measures.
After basal samples were collected for 30 min, subjects commenced a 5 min warm-up consisting of semi-recumbent cycling at 50 % 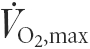 . On completion of the warm-up, the subjects cycled for a further 115 min at ≈65 %
. On completion of the warm-up, the subjects cycled for a further 115 min at ≈65 % 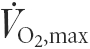 . During exercise, blood samples were collected simultaneously from the hepatic vein every 10 min for the measurement of ICG concentration and haemoglobin and haematocrit (Hct). In addition, immediately prior to exercise and at 30, 60 and 120 min during exercise, blood samples were simultaneously collected from the brachial artery and the femoral and hepatic veins for the measurement of serum HSP72. Immediately prior to sampling,
. During exercise, blood samples were collected simultaneously from the hepatic vein every 10 min for the measurement of ICG concentration and haemoglobin and haematocrit (Hct). In addition, immediately prior to exercise and at 30, 60 and 120 min during exercise, blood samples were simultaneously collected from the brachial artery and the femoral and hepatic veins for the measurement of serum HSP72. Immediately prior to sampling, 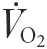 , respiratory exchange ratio (RER), heart rate (HR), mean arterial pressure (MAP) and leg blood flow were recorded. Subjects ingested 250 ml of artificially sweetened water at the onset of exercise and at 15 min intervals during exercise.
, respiratory exchange ratio (RER), heart rate (HR), mean arterial pressure (MAP) and leg blood flow were recorded. Subjects ingested 250 ml of artificially sweetened water at the onset of exercise and at 15 min intervals during exercise.
Hepatosplanchnic and leg blood flow
For description of hepatic blood flow and blood variables obtained from the hepatic vein, we used the term ‘hepatosplanchnic’ to indicate that blood from the hepatic vein also represents portal blood, whereas ICG is eliminated exclusively by the liver. HBF (Nielsen et al. 2002) and leg blood flow (LBF) (Andersen & Saltin, 1985; González-Alonso et al. 2000) measures were performed according to methods previously described.
Blood analysis
The ICG dye concentration was determined by high-performance liquid chromatography with a detection limit of 0.01 μmol l−1 (Ott, 1998). Paired samples of arterial and hepatosplanchnic venous blood were collected in heparinised syinges (QS50; Radiometer, Copenhagen, Denmark). Blood samples were kept on ice until analysis for haemoglobin and Hct using an ABL apparatus (model 615; Radiometer, Copenhagen, Denmark).
In order to determine serum HSP72 protein, 4 ml of blood was placed in a tube containing a clot-inducing plug (Vacutainer Systems Europe, France). This tube was inverted 6 times, left on ice for 30 min, then spun in a centrifuge at 1200 g at 4 °C. A highly sensitive, enzyme-linked immunosorbent assay (EIA) method (EKS-700 Stressgen, Victoria, BC, Canada) was used to determine the expression of HSP72 protein in serum as previously described (Walsh et al. 2001).
Physiological measures
Expired pulmonary 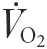 and carbon dioxide production (
and carbon dioxide production (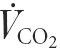 ) were measured on-line using a Medgraphics CPX/D metabolic cart (St Paul, MN, USA). HR and MAP were measured with the brachial artery catheter connected to a sterile disposable pressure transducer (Baxter, Uden, The Netherlands) interfaced with a pressure monitor (Danico Electronic-Dialogue 2000, Denmark). During each experiment HR and arterial blood pressure (ABP; i.e. mean, systolic (SBP) and diastolic (DBP) blood pressures) were acquired using a beat-to-beat customized software data acquisition system interfaced with a personnel computer.
) were measured on-line using a Medgraphics CPX/D metabolic cart (St Paul, MN, USA). HR and MAP were measured with the brachial artery catheter connected to a sterile disposable pressure transducer (Baxter, Uden, The Netherlands) interfaced with a pressure monitor (Danico Electronic-Dialogue 2000, Denmark). During each experiment HR and arterial blood pressure (ABP; i.e. mean, systolic (SBP) and diastolic (DBP) blood pressures) were acquired using a beat-to-beat customized software data acquisition system interfaced with a personnel computer.
Calculations and statistics
Net leg HSP72 balance was calculated by multiplying the femoral venous-arterial HSP72 difference by the net LBF. Similarly, the net hepatosplanchnic HSP72 balance was calculated by multiplying the hepatosplanchnic venous-arterial HSP72 difference by the net HBF. Comparative data are expressed as means ± s.e.m. A one-way analysis of variance (ANOVA) was used to compute the statistics using the Statistica computer software program (Statsoft Inc., Tulsa, OK, USA).
Results
Subjects exercised at a 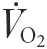 of 2.47 ± 0.15 l min−1, which was equivalent to 62 ± 2 % of
of 2.47 ± 0.15 l min−1, which was equivalent to 62 ± 2 % of 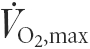 . As expected,
. As expected, 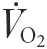 , RER and HR were all higher (P < 0.05) during exercise when compared with rest (Table 1).
, RER and HR were all higher (P < 0.05) during exercise when compared with rest (Table 1).
Table 1.
Oxygen consumption (V̇O2), respiratory exchange ratio (RER), heart rate (HR), and mean arterial pressure (MAP) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake
| 0 min | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| V̇O2(l min−1) | 0.27 ± 0.06 | 2.48 ± 0.17 * | 2.45 ± 0.15 * | 2.50 ± 0.15 * | 2.44 ± 0.15 * |
| RER | 0.81 ± 0.07 | 0.92 ± 0.05 * | 0.90 ± 0.05 * | 0.88 ± 0.05 * | 0.85 ± 0.05 * |
| HR (beats min−1) | 60 ± 1 | 144 ± 1 * | 148 ± 1 * | 149 ± 1 * | 150 ± 1 * |
| MAP (mmHg) | 96 ± 1 | 98 ± 1 | 97 ± 1 | 98 ± 1 | 96 ± 1 |
Denotes difference (P < 0.05) from 0 min. Data are expressed as means ± s.e.m. (n = 7).
The plasma volume had little effect on the serum HSP72 accumulation (data not shown). Likewise, whether the hepatosplanchnic data were expressed relative to plasma flow or blood flow had no effect on the results (data not shown). HSP72 protein was below the detection limit in the arterial serum in six subjects at rest. However, it progressively increased throughout exercise in all subjects, averaging 0.88 ± 0.35 pg l−1 at 120 min (P < 0.05 compared with rest) (Fig. 1). Contracting LBF increased (P < 0.05) from 0.5 ± 0.1 l min−1 at rest to an average of 6.1 ± 0.5 l min−1 during exercise (Fig. 2). HBF averaged 1.3 ± 0.1 l min−1 at rest. There was a small, but nonetheless significant, decrease (P < 0.05) in HBF at 120 min (1.0 ± 0.1 l min−1) compared with rest. A hepatosplanchnic venous-arterial difference for HSP72 was undetected at rest, but gradually increased such that the values at 60 and 120 min, were different (P < 0.05) compared with rest. This resulted in a net increase (P < 0.05) in hepatosplanchnic HSP72 release at all times during exercise, averaging 0.54 ± 0.18 pg min−1 at 120 min (Fig. 3). In contrast, a femoral venous-arterial difference for HSP72 was not observed at any point and there was neither a net uptake nor a net release of HSP72 across the contracting limb (Fig. 4).
Figure 1. Arterial heat shock protein 72 (HSP72) concentration before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake.
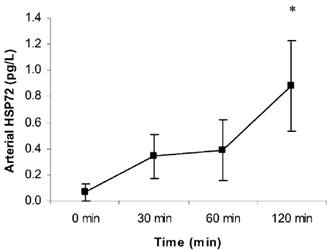
*Denotes difference (P < 0.05) from 0 min. Data expressed as means ± s.e.m. (n = 7).
Figure 2. Leg blood flow (A) and hepatosplanchnic blood flow (B) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake.
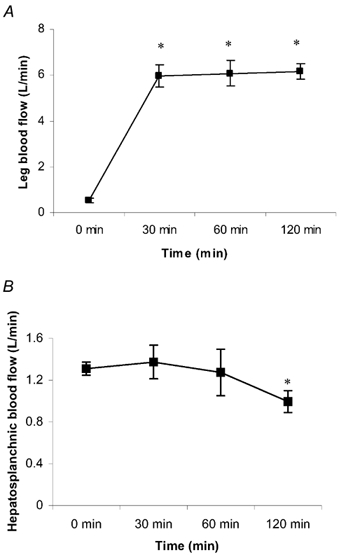
*Denotes difference (P < 0.05) from 0 min. Data expressed as means ± s.e.m. (n = 7 for leg blood flow, n = 5 for hepatosplanchnic blood flow).
Figure 3. Hepatic venous-arterial (hv-a) HSP72 concentration (A) and net hepatosplanchnic HSP72 release (B) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake.
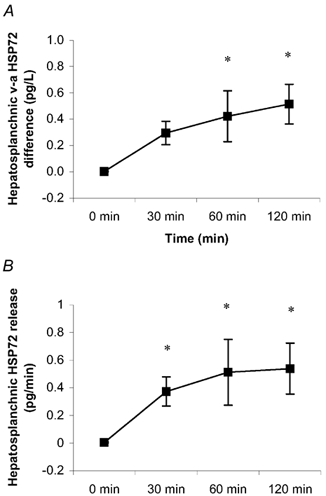
*Denotes difference (P < 0.05) from 0 min. Data expressed as means ± s.e.m. (n = 5).
Figure 4. Femoral venous-arterial (hv-a) HSP72 concentration (A) and net contracting limb HSP72 balance (B) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake.
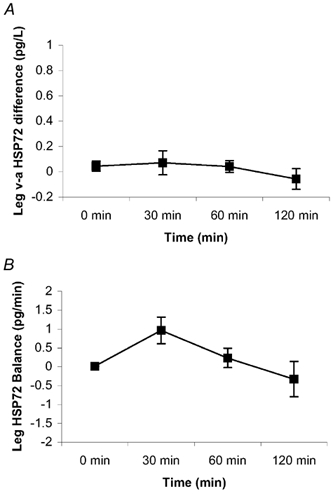
Data expressed as means ± s.e.m. (n = 7).
Discussion
This study is the first to demonstrate in vivo release of heat shock proteins from specific tissue beds in any species. The data confirm our earlier observations that exercise increases the systemic concentrations of HSP72 and that the contracting skeletal muscle does not release this protein (Walsh et al. 2001; Febbraio et al. 2002). In addition, since the liver is an organ known to release acute phase proteins and the type of exercise performed in this study was unlikely to cause lysis of hepatosplanchnic cells, we suggest that hepatosplanchnic tissue possesses a specific exocytosis pathway to release HSP72, allowing it to perform specific extracellular functions.
In the present study, we did not report contracting muscle HSP72 gene or protein expression. However, we have previously demonstrated that acute exercise increases HSP72 gene and/or protein expression in contracting human skeletal muscle approximately 120-240 min following the commencement of exercise (Febbraio & Koukoulas, 2000; Walsh et al. 2001; Febbraio et al. 2002). It is possible, therefore, that HSP72 gene and/or protein expression was elevated in the contracting muscles in the present study. Notwithstanding this, in our previous study (Febbraio et al. 2002) even though HSP72 gene and protein expression were upregulated in a contracting muscle which had lower than normal pre-exercise intramuscular glycogen content, we could not measure any HSP72 in the femoral venous effluent. The results from the present study are consistent with our previous work. In our previous study concentric knee-extensor exercise was employed, whereas in the present study semi-recumbent cycling was employed. Since the mode of exercise in both studies was predominantly concentric in nature, it was unlikely that major skeletal muscle damage was induced in either. Whether HSP72 is released from lysed muscle cells is yet to be determined; however, the present study supports the hypothesis that intact muscle cells are not able to release HSP72 into the circulation, but that stressed muscle cells synthesise HSP72 in order for them to participate in intracellular processes.
Since the present study was conducted in humans we were not able to obtain liver biopsy specimens. We cannot, therefore, determine whether HSP72 was released by the liver itself or that the gut contributed to the hepatosplanchnic release. This is difficult to determine in vivo in humans and is a limitation of the present study. However, given that the rodent liver markedly increases HSP72 protein expression in response to exercise, it is likely that the liver contributed to the hepatosplanchnic HSP72 release.
Based on the fact that the exercise was moderate in nature and that HSP72 was released at 30 and 60 min of exercise in the absence of any reduction in HBF at this time, it is unlikely that the procedure resulted in cellular necrosis secondary to ischaemia. In addition, one subject underwent a full blood examination 48 h following the experiment and there were no signs of liver damage or dysfunction (data not shown). We propose, therefore, that the hepatosplanchnic tissues release HSP72 to perform vital extracellular roles. In a recent report, Pittet et al. (2002) examined the serum HSP72 concentrations in patients following severe trauma. They observed that survival correlated with serum levels of HSP72. Although these authors were not able to demonstrate a causal link between elevated serum HSP72 and survival, their data nonetheless support the theory that extracellular HSP72 performs important roles designed to protect vital organs and/or cells. In the present study we were unable to determine the biological role of the rise in extracellular HSP72 and such a study warrants further investigation.
In summary, we have clearly demonstrated that the hepatosplanchnic tissues release HSP72 during exercise and contribute, in part, to the elevated circulating HSP72 observed with exercise. In addition, the type of exercise performed in this study, and the fact that HSP72 was produced in the presence of a maintained hepatosplanchnic blood flow, was unlikely to cause lysis of cells. We suggest, therefore, that HSP72 is released to perform specific and important extracellular functions during exercise.
Acknowledgments
We would like to thank the subjects for their extraordinary effort in this demanding study. We would also like to thank Ruth Rousing, Peter Nissen, Nina Schjerling, Ellen Dawson, Dr Rebecca Starkie, Natalie Hiscock and Hanne Willumsen for their excellent technical assistance. This study was supported by grants from The Danish National Research Foundation (504-14), and The Australian Research Council (DP0209570).
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft S, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nature Medicine. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. Journal of Biological Chemistry. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- De Feo P, Lucidi P. Liver protein synthesis in physiology and disease states. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:47–50. doi: 10.1097/00075197-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. Journal of Applied Physiology. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, Van Hall G, Saltin B, Pedersen BK. Reduced muscle glycogen availability is associated with elevated HSP72 in contracting human skeletal muscle. Journal of Physiology. 2002;583:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. Journal of Physiology. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kilyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Research. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Hood DA. Invited review: Contractile activity-induced mitochondrial biogenesis skeletal muscle. Journal of Applied Physiology. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry and physiology. Pharmacological Therapy. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Moseley PL. Differential effects of exercise and heat stress on liver HSP70 accumulation with aging. Journal of Applied Physiology. 1996;80:574–551. doi: 10.1152/jappl.1996.80.2.547. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Miller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 70 kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. International Journal of Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Clemmesen JO, Skak C, Ott P, Secher NH. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. Journal of Applied Physiology. 2002;92:1677–1683. doi: 10.1152/japplphysiol.00028.2001. [DOI] [PubMed] [Google Scholar]
- Ott P. Hepatic elimination of indocyanine green with special reference to distribution kinetics and the influence of plasma protein binding. Pharmacology and Toxicology. 1998;83(suppl. 2):1–48. doi: 10.1111/j.1600-0773.1998.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Pittet J-F, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie C. Serum levels of Hsp72 measured early after trauma correlate with survival. Journal of Trauma; Injury, Infection, and Critical Care. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Salo DC, Donovan CM, Davies KJA. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radical Biology and Medicine. 1991;11:239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat shock protein protection. Trends in Neurosciences. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Research. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress and Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


