Abstract
The gastrointestinal tract contains most of the body's 5-hydroxytryptamine (5-HT) and releases large amounts after meals or exposure to toxins. Increased 5-HT release occurs in patients with irritable bowel syndrome (IBS) and their peak plasma 5-HT levels correlate with pain episodes. 5-HT3 receptor antagonists reduce symptoms of IBS clinically, but their site of action is unclear and the potential for other therapeutic targets is unexplored. Here we investigated effects of 5-HT on sensory afferents from the colon and the expression of 5-HT3 receptors on their cell bodies in the dorsal root ganglia (DRG). Distal colon, inferior mesenteric ganglion and the lumbar splanchnic nerve bundle (LSN) were placed in a specialized organ bath. Eighty-six single fibres were recorded from the LSN. Three classes of primary afferents were found: 70 high-threshold serosal afferents, four low-threshold muscular afferents and 12 mucosal afferents. Afferent cell bodies were retrogradely labelled from the distal colon to the lumbar DRG, where they were processed for 5-HT3 receptor-like immunoreactivity. Fifty-six percent of colonic afferents responded to 5-HT (between 10−6 and 10−3 M) and 30 % responded to the selective 5-HT3 agonist, 2-methyl-5-HT (between 10−6 and 10−2 M). Responses to 2-methyl-5-HT were blocked by the 5-HT3 receptor antagonist alosetron (2 × 10−7 M), whereas responses to 5-HT were only partly inhibited. Twenty-six percent of L1 DRG cell bodies retrogradely labelled from the colon displayed 5-HT3 receptor-like immunoreactivity. We conclude that colonic sensory neurones expressing 5-HT3 receptors also functionally express the receptors at their peripheral endings. Our data reveal actions of 5-HT on colonic afferent endings via both 5-HT3 and non-5-HT3 receptors.
The primary source of 5-HT in the body is a population of enterochromaffin cells in the intestinal mucosa, from which it is released by meals, toxins and chemotherapeutic agents (Andrews et al. 1990; Bearcroft et al. 1998). 5-HT release activates vagal afferent endings in the upper gastrointestinal tract exclusively via 5-HT3 receptors (Blackshaw & Grundy, 1993b; Hillsley et al. 1998; Zhu et al. 2001). This contributes to 5-HT's role in triggering of nausea and vomiting and accounts for the efficacy of 5-HT3 receptor antagonists in treatment of these symptoms. 5-HT was also recently shown to activate cutaneous nociceptive primary afferents via 5-HT3 receptors, a mechanism which contributes to its role in inflammatory pain (Zeitz et al. 2002).
One of the commonest pain syndromes seen in the clinic is irritable bowel syndrome (IBS). IBS originates from the lower bowel and has no demonstrated organic basis. In patients with IBS, postprandial plasma 5-HT levels are abnormally high and correlate with lower abdominal symptoms (Bearcroft et al. 1998; Houghton et al. 2001). The source of 5-HT is also increased in many IBS patients in the form of an increased population of enterochromaffin cells in the colon (Spiller et al. 2000). 5-HT (5-HT3) receptor antagonists are effective in the treatment of IBS (Camilleri et al. 2000; Camilleri, 2001), however a crucial missing link in the treatment of symptoms in IBS is an understanding of the mechanism linking 5-HT release with activation of sensory pathways. Evidence from animal models of colorectal pain shows that 5-HT3 receptor antagonists have analgesic properties (Kozlowski et al. 2000), but their site of action is unknown. We have explored the hypotheses that, as in the upper gastrointestinal tract, 5-HT directly excites colonic primary afferents and that this is mediated via 5-HT3 receptors.
To determine the mechanisms of activation of colonic sensory neurones, we have established an isolated preparation in which the activity of rat lumbar splanchnic afferent fibres can be recorded during delivery of controlled stimuli to the colon (Lynn & Blackshaw, 1999; Berthoud et al. 2001). We classified three distinct types according to their mechanical sensitivity: high-threshold serosal afferents that respond to probing or distortion of the colon; low-threshold muscular afferents that respond to circular stretch; and low-threshold mucosal receptors that respond to probing and stroking of the mucosa, but not to stretch. Splanchnic afferents project to the lumbar spinal cord (Ness & Gebhart, 1988) and this pathway is considered important in the generation of symptoms of IBS in humans (Lembo et al. 1994). Neuronal cell bodies of lumbar splanchnic afferents are located mainly in L1 dorsal root ganglia (Ness & Gebhart, 1988).
The aims of this study were to determine if 5-HT excites afferent fibres when applied to their endings in the colon and to determine the role of 5-HT3 receptors in any observed effect. Furthermore, we aimed to establish if 5-HT3 receptors can be localized anatomically on these neurones using highly specific antibodies (Spier et al. 1999).
Methods
Animals
Adult Sprague-Dawley rats weighing 180-220 g were obtained from the Institute of Medicine and Veterinary Sciences, Adelaide. Animals were kept under standard laboratory conditions (22 ± 3 °C). All experiments were carried out with the approval of and according to guidelines of the Animal Ethics Committees of the Institute for Medical and Veterinary Science.
Dissection of the colon and nerve fibres
Dissection was carried out in female rats according to the protocol described in detail earlier (Lynn & Blackshaw, 1999). Briefly, after a midline laparotomy under pentobarbitone sodium anaesthesia (60 mg kg−1i.p.), 4-5 cm of distal colon was removed along with the lumbar colonic nerves and the bundle containing the inferior mesenteric ganglion (IMG), intermesenteric nerve (IMN), lumbar splanchnic nerves (LSN), inferior mesenteric artery and abdominal aorta, according to the classification of Baron et al. (1988) and transferred into cold, carbogenated modified Krebs bicarbonate buffer, comprising an equal mixture of serosal and mucosal solutions (see below). Animals were killed by exsanguination whilst still under anaesthetic. The distal colon was opened longitudinally, off-centre to the antimesenteric border in order to orientate lumbar colonic nerve insertions to lie along the edge of the opened preparation. Connective tissue was dissected away from the neural bundle; the bundle was cut and tied 2-15 mm rostral to the junction of the inferior mesenteric artery and abdominal aorta. The point of recording was thus approximately half way between the IMG and the lumbar dorsal root ganglia. The colon was then pinned flat in the perfusion chamber and the neurovascular bundle was passed through a small hole into an adjacent paraffin-filled recording chamber. The bundle lay over a mirror in a small blister of Krebs solution that was continuous with the solution in the colonic compartment. Under a dissecting microscope, afferent strands were teased away from the neurovascular bundle (which comprised lumbar splanchnic nerves and intermesenteric nerve) and placed on a platinum wire recording electrode (0.25 mm diameter). A reference electrode was positioned in the blister of solution in which the neurovascular bundle lay.
Perfusion chamber and perfusate
The organ bath consisted of two adjacent compartments machined from clear acrylic. One organ compartment was superfused with Krebs solution and the recording compartment containing the nerve and electrodes was filled with paraffin oil. The floor of both compartments was covered with a 5 mm layer of Sylgard (Dow Corning Corp., Midland, MI, USA). The serosal and mucosal surfaces of the colon were superfused with different media. This was achieved either by creating a basement chamber that was separated from the upper chamber by nylon mesh over which the colon was pinned, or by pinning the colon over a perforated tube through which Krebs solution was perfused. For the serosal chamber, modified Krebs solution containing (mm): 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4.7 H2O, 2.5 CaCl2 and 11.1 α-d-glucose was used. For the mucosal chamber, glucose was replaced by the short-chain fatty acids butyrate (2 mm) and acetate (20 mm). In all preparations the prostaglandin synthesis inhibitor indomethacin (3 μm) was added to suppress potential inhibitory actions of endogenous prostaglandins. The L-type calcium channel antagonist nifedipine (1 μm) was added to suppress smooth muscle activity that might otherwise give rise to indirect responses secondary to contractile effects of 5-HT. Nifedipine was also used to inhibit degranulation of mast cells and enterochromaffin cells that could similarly give rise to secondary effects. The perfusion media were bubbled with carbogen (95 % O2- 5 % CO2). After identification of receptive fields at room temperature, the perfusion solutions were warmed to between 32 and 34 °C in a heating coil and the flow rate was set at 15 ml min−1.
Protocol for recordings from colon
Receptive fields were identified by systematically probing with a blunt glass probe (tip contact area 6 mm2) with a transmural pressure of approximately 50 kPa. They were classified according to their responses to three distinct mechanical stimuli, as previously described. Briefly, afferents that responded to probing or distortion of the colon but not to stretch or mucosal stroking were classed as serosal receptors. Many of their receptive fields were located in the mesentery, which was often hidden under the colon as prepared. Afferents that responded to ≥ 5 mm circular stretch and probing but not to mucosal stroking were classed as muscular receptors. These all had receptive fields within the wall of the colon. Afferents that responded to mucosal stroking with a 0.1 mN von Frey hair and probing but not to stretch were classed as mucosal receptors. 5-HT and the selective 5-HT3 receptor agonist 2-methyl-5-HT were added to both superfusion media or were locally applied by means of a metal cylinder of 10 mm diameter placed around a specific receptive field. Care was taken to expose serosal receptors directly to drugs by reflecting the colon and applying locally or superfusing the drug. No differences in the strength and pattern of response were evident between the two application methods. For determination of responsiveness or unresponsiveness to agonists, at least 10−4m 5-HT and 10−3m 2-methyl-5-HT were given as these were maximal values on the respective concentration-response curves. Agonists were applied for approximately 2 min then washed out and normal superfusion resumed for at least 10 min before further drug treatment. Responses to maximal concentrations of agonists did not show desensitization on repeated tests when applied using this protocol. Effects of the 5-HT3 receptor antagonist alosetron on responses to agonists were assessed by co-administration following demonstration of a reproducible effect of the agonist alone. The concentration of alosetron used (200 nm) was chosen based on the maximum established from previous studies (Zhai et al. 1999).
Electrophysiological data collection and analysis
The signal from the platinum electrode was fed into a differential amplifier and filtered. The amplified signal was also used for the audio monitor and stored on tape for later use. The analog signal was sampled at a rate of 20 kHz in a 1401 data interface (CED Ltd, Cambridge, UK) and stored on the hard drive of an Apple Macintosh G3 or Pentium III PC, using Spike2 software (CED Ltd). Action potentials were analysed off-line using the Spike 2 wavemark function and discriminated as single units on the basis of distinguishable waveform, amplitude and duration. A maximum of two spontaneously active units on each recorded strand was allowed so as to avoid errors in discrimination. Otherwise strands were split further or discarded. The action potential discharge was assessed according to the mean rate over one minute before and the first minute during treatment with agonists. Responses to drugs were counted as having occurred if a reproducible > 25 % increase in discharge occurred with the maximum concentration used. Responses to mechanical stimuli are shown as instantaneous frequency because they were often very brief (1 s duration) and intense.
Retrograde labelling and immunohistochemistry
Following a midline laparotomy under isoflurane anaesthesia (2-4 % in O2:N2O) in adult male Sprague-Dawley rats weighing 180-220 g, Fast Blue tracer (1-5 % in saline) was injected subserosally into the distal colon in at least four separate sites in 2-5 μl aliquots. Animals were monitored closely for signs of postoperative complications, which were not encountered. After 10-14 days recovery, rats were killed by overdose of pentobarbitone sodium (120 mg kg−1i.p.) and dorsal root ganglia were removed from T10 to S2. The ganglia were fixed in 4 % paraformaldehyde for 4 h then placed in 30 % sucrose solution overnight, prior to snap freezing and storage at -80 °C. Sections (12 μm) were cut from frozen ganglia on a cryostat and thaw-mounted onto Vectabond-coated slides for visualization of fluorescent label and immunohistochemistry. For 5-HT3 receptor immunohistochemistry, sections were washed in 0.1 m phosphate-buffered saline (PBS; three times for 5 min each), then incubated in PBS supplemented with 10 % (v/v) normal goat serum (NGS, Vector Laboratories, USA), 0.3 % Triton X-100 for 60 min. After being rinsed twice in PBS, sections were incubated in primary antibody (anti-5-HT3 antiserum, raised in rabbit, supplied by Sarah Lummis, Department of Biochemistry, University of Cambridge, UK; see Spier et al. 1999 for details) at 1:20 000 in PBS/10 % NGS/0.3 % Triton for 48 h at 4 °C. Details of antibody work-up, Western blotting and controls are available in a prior publication (Spier et al. 1999). Sections were then rinsed twice and washed (three times for 5 min each) in PBS, before incubation in biotinylated anti-rabbit IgG (Vector Laboratories) at 1:1000 for 60 min. Sections were incubated in the presence of blocking buffer (supplied in the NEN Tyramide kit) for 30 min then in ABC (ABC Elite; Vector Laboratories) made up in PBS for 45 min, followed by tyramide (1:100 in kit buffer) for 10 min and finally in streptavidin-FITC (Vector Laboratories) at 1:1000 in PBS for 60 min before final washing, air-drying and coverslipping. Negative controls were prepared as above except that the primary antibody step was omitted. Specificity and selectivity of the anti-5-HT3 receptor antibody to native and recombinant receptors was established in a previous study (Spier et al. 1999). An antibody raised against a different peptide sequence within the 5-HT3 receptor revealed similar labelling patterns to the one used in this investigation (Hicks et al. 2001b; Mazzia & Clerc, 2001).
Sections were viewed with a Nikon microscope using epifluorescence with UV2A and appropriate filters for visualization of fast blue and FITC signals, respectively. Digital images were acquired with a Nikon CoolPix 900 digital camera and cross-sectional areas of fluorescently labelled cells calculated using image analysis software (NIH Imagetool). For assessment of which DRG levels contained labelled neurones, a simple expression of the number of labelled cells per section was used (since, due to the small number of labelled neurones, corrections for area of section containing neuronal profiles, total section area, or total cell number were found not to alter the data significantly). At least 20 sections from ganglia at each level were used for these calculations.
Results
Three types of colonic primary afferent fibres
Types of fibre could be clearly distinguished from one another by their responses to mechanical stimuli. Serosal receptors were reproducibly activated only by probing the tissue with pressures above 50 kPa applied via a glass rod of 6 mm2 tip diameter (Fig. 1A). Many of their receptive fields were located in the mesentery directly adjacent to the colon and showed much lower thresholds to probing when the colon was reflected to allow improved access to the receptive field. No differences were seen in the mechanical responsiveness of receptive fields on the surface of the colon and those in the mesentery. They were therefore treated as a single group, as has been the case in previous studies (Lynn & Blackshaw, 1999). Muscular receptors were optimally activated by a maintained circular stretch ≥ 5 mm, to which they responded with an excitation that adapted during the stimulus (usually 5 s, see Fig. 1B) as observed previously (Blumberg et al. 1983). Mucosal receptors were most sensitive to stroking of their receptive fields with a brush or a von Frey hair (Fig. 1C). Their thresholds were < 0.1 mN and could not be further determined because smaller hairs would not penetrate the surface tension of the superfusate. All three types of fibre were responsive to probing and displayed low or absent spontaneous activity (Fig. 1A-C). The majority of fibres encountered were serosal receptors, the remainder being split between muscular and mucosal receptors (Fig. 1D). The distribution of receptive fields was concentrated near the mesenteric border and none were found more than 180 deg around the colon from the mesenteric border (Fig. 1E) as shown previously (Lynn & Blackshaw, 1999), indicating that fibres project only within the anterior or posterior aspects of the colon and not around the full circumference. All fibres recorded had a single receptive field.
Figure 1. Three types of fibre were classified on the basis of their responses to mechanical stimuli.
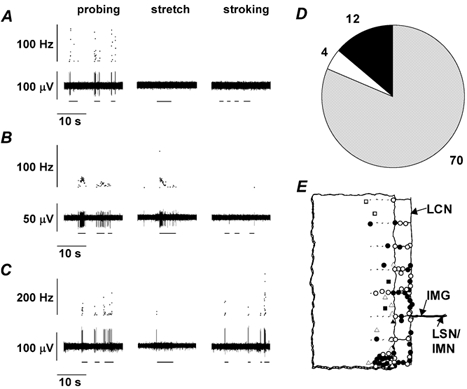
In A, B, and C upper traces show instantaneous frequency and lower traces show raw electrophysiological data. A, a serosal receptor is activated only by probing the tissue (horizontal bars). B, a muscular receptor is activated by probing and by a maintained circular stretch of 8 mm; the response adapts rapidly during the stimulus (horizontal bars). No increase above baseline activity occurred in response to mucosal stroking. C, a mucosal receptor responds to probing and to stroking of its receptive field with a von Frey hair giving a force of 0.1 mN. D, the majority of fibres encountered were serosal receptors (grey segment; numbers shown around pie chart), the remainder being split between muscular (open segment) and mucosal receptors (filled segment). E, the distribution of receptive fields was concentrated near the mesenteric border and on the mesentery itself. Circles, serosal afferents; squares, muscular afferents; triangles, mucosal afferents; filled symbols indicate responsive to 5-HT agonists, open symbols indicate unresponsive. LSN/IMN, lumbar splanchnic nerve/intermesenteric nerve; IMG, inferior mesenteric ganglion; LCN, lumbar colonic nerves.
Responses to 5-HT
All three types of afferent fibre were responsive to 5-HT. Responses were maintained throughout the period of superfusion, showing little if any adaptation during the 2 min exposure (Fig. 2A-C). Responses normally continued for approximately 2 min after washout before discharge returned to basal levels. Overall, 56 % of afferents were reproducibly responsive to 5-HT (Fig. 2E), with this proportion being approximately consistent within each fibre type: 40 % (4/10) of mucosal, 50 % (2/4) of muscular and 59 % (36/61) of serosal receptors, indicating that 5-HT receptors are not preferentially distributed in subtypes of colonic afferents. In seven experiments, serosal afferents were each tested with four concentrations of 5-HT, to which responses showed concentration dependence over the range 10−6-10−3m, with an EC50 of 2.9 μm (Fig. 2D).
Figure 2. Colonic primary afferents were activated by 5-HT.
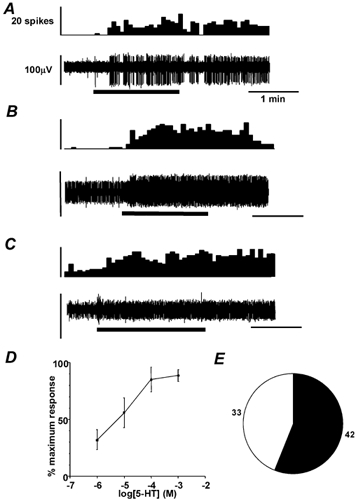
A, B and C show examples of afferent responses to 5-HT application to the receptive fields of serosal, muscular and mucosal afferents, respectively (thick horizontal bars). Upper panels show spike frequency histograms (5 s bin width) of single unit activity. Lower panels show raw electrophysiological data. In B, the unit of interest is the one with the larger spike amplitude. D, concentration-response function of 7 serosal afferents to 5-HT. Estimated EC50 was 2.93 × 10−6m. E, the number of fibres responsive to 5-HT (≥100 μm, filled segment, numbers adjacent), vs. unresponsive (open segment).
Responses to 2-methyl-5-HT
Overall, a smaller proportion (30 %) of fibres were responsive to the selective 5-HT3 receptor agonist 2-methyl-5-HT than to 5-HT itself (Fig. 3E). Specifically, 17 % (1/6) of mucosal, 0 % (0/3) of muscular and 35 % (11/31) of serosal receptors were responsive to 2-methyl-5-HT. These responses showed the same characteristics as those to 5-HT; i.e. they were slow- or non-adapting, continued after washout and were reproducible on subsequent stimulation (Fig. 3A-C). In 34 experiments, responses to both 5-HT (100 μm) and 2-methyl-5-HT (1 mm) were compared. Thirteen fibres responded to both, 12 responded to 5-HT only and nine responded to neither; none responded only to 2-methyl-5-HT. Responses of six serosal afferents tested were concentration dependent over the range 10−5-10−2m, with an EC50 of 28.3 μm (Fig. 3D), approximately ten times higher than that for 5-HT. The maximum response to 2-methyl-5-HT was 0.14 ± 0.04 action potentials s−1, whereas that to 5-HT was 0.90 ± 0.28 (P = 0.057, n = 5).
Figure 3. Colonic primary afferents were activated by the selective 5-HT3 receptor agonist 2-methyl-5-HT.
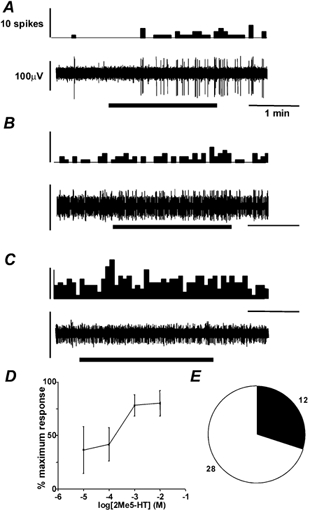
Legend for A, B and C as for Fig. 2. Spikes are per 5 s bin. Fibres recorded in A, B and C are the same as those in Fig. 2A-C. The muscular afferent in B did not show a reproducible increase in discharge, so the discharge shown was not considered as a response. D, concentration-response function of 6 serosal afferents to 2-methyl-5-HT. Estimated EC50 was 2.83 × 10−5m. E, the number of fibres responsive to 2-methyl 5-HT (1 mm, filled segment, numbers adjacent), vs. unresponsive (open segment).
Reversibility of responses with a 5-HT3 receptor antagonist
The highly selective and potent 5-HT3 receptor antagonist alosetron at a maximal concentration (200 nm) totally reversed the response to 2-methyl-5-HT (1 mm; Fig. 4B and C). On two occasions, half this concentration was tested and found to completely block the response to 1 mm 2-methyl-5-HT. The antagonist only partially reversed the response to 5-HT (100 μm; Fig. 4A-C). The amplitudes of responses to mechanical stimuli after alosetron were 100 % of those before in five serosal afferents tested (e.g. Fig. 4B).
Figure 4. Inhibition of responses to 5-HT receptor agonists by the selective 5-HT3 receptor antagonist alosetron.
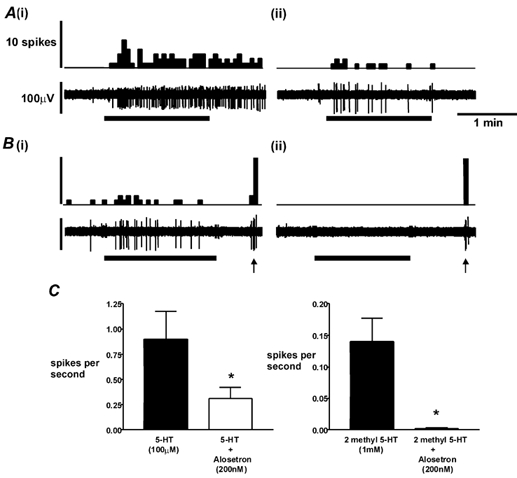
A, response of a serosal receptor to 100 μm 5-HT (horizontal bar) alone (i) and in the presence of 200 nm alosetron (ii). B, response of a serosal receptor to 1 mm 2-methyl-5-HT alone (i) and in the presence of 200 nm alosetron (ii). Note that response to probing (arrows) is unaffected. Spikes are per 5 s bin. C shows group data on effects of alosetron on responses to 5-HT and 2-methyl-5-HT. Both effects were significant (P < 0.05, n = 5). Maximal responses to 5-HT and 2-methyl 5-HT were not significantly different, although a trend was evident (P = 0.057).
Retrograde labelling and immunohistochemistry
Following Fast Blue injection into the distal colon, neuronal cell bodies in lumbosacral dorsal root ganglia (DRG) were labelled, with the most appearing in L1 and L6/S1 (Fig. 5A), which correspond to splanchnic and pelvic pathways, respectively. No DRG above T11 contained labelled cells, indicating that dye had not leaked into adjacent small intestine or stomach (see Ness & Gebhart, 1988). Double immunofluorescence of colonic splanchnic afferents in L1 DRG revealed that some of the retrogradely labelled cells were also immunoreactive for the 5-HT3 receptor (Fig. 5B). The proportion of these in eight ganglia from four rats was 26 % (Fig. 5C). There was no apparent difference in cell size (which may be indicative of specialized function) between 5-HT3 receptor-immunoreactive neurones and retrogradely labelled neurones (Fig. 5D).
Figure 5. 5-HT3 receptors are present on a proportion of dorsal root ganglion (DRG) neurones.
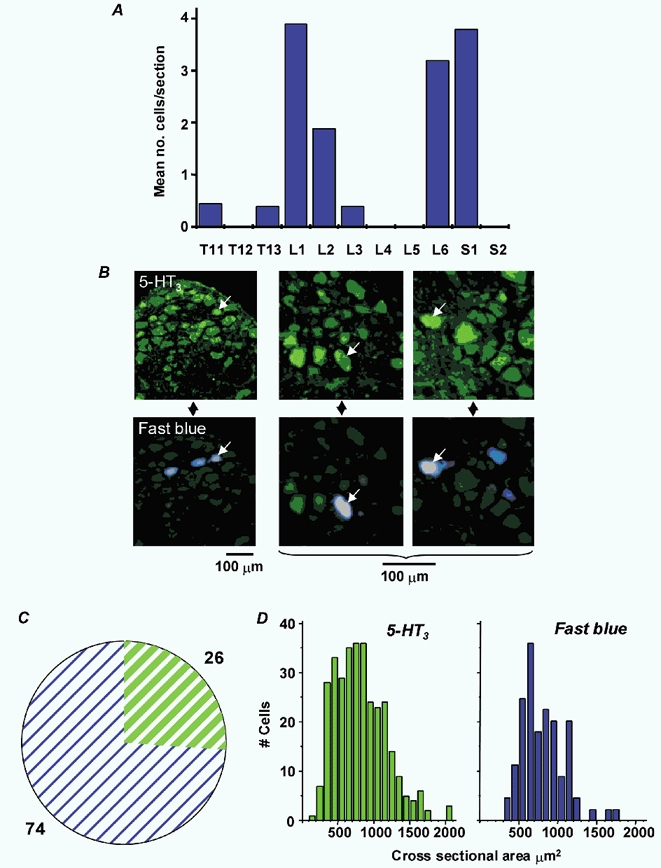
A, distribution of retrogradely labelled cells according to spinal segment. Data are expressed as mean number of labelled cells per section at each level. B, low- and medium-power fluorescence micrographs of L1 DRG showing 5-HT3 receptor immunohistochemistry in green (FITC) and retrograde label in blue (Fast Blue). Colocalization is indicated by the arrows in vertically paired images of the same section. C, pie chart showing percentages of retrogradely labelled cells immunoreactive for 5-HT3 receptors (green) vs. those lacking 5-HT3 receptor immunoreactivity (blue) in eight DRG from four animals. D, cell size distribution of 5-HT3 receptor-positive cells and retrogradely labelled cells.
Discussion
Our results demonstrate the existence of 5-HT3 receptors on sensory neurones in the rat lumbar DRG that are retrogradely labelled from the colon, and an excitatory effect of 5-HT3 receptor activation on colonic lumbar splanchnic afferent fibres. This pathway is involved in the signalling of symptoms including pain from the colon. An action of 5-HT3 receptors at this peripheral site is, therefore, a likely mechanism for some of the therapeutic effects of 5-HT3 receptor antagonists showing efficacy in the clinical treatment of pain and discomfort. In addition to the contribution of 5-HT3 receptors to excitation of colonic afferents, we have also revealed that of other subtypes of 5-HT receptor. We showed this firstly by finding afferents that were excited by 5-HT, but unaffected by the selective 5-HT3 receptor agonist 2-methyl 5-HT. Secondly, we could completely reverse effects of 2-methyl-5-HT on afferent fibres with a selective 5-HT3 receptor antagonist, but not the effects of 5-HT, which tended to be more potent. These other receptor subtypes present potential targets for future therapeutic intervention to reduce signalling from the colon.
Excitation of peripheral endings by 5-HT receptor agonists has long been established in vagal afferents (Paintal, 1954). More recent studies show that only mucosal afferents in the vagus respond directly to 5-HT, with distension-sensitive afferents responding indirectly following contractile responses (Blackshaw & Grundy, 1993a, b; Hillsley et al. 1998). As far as colonic afferents are concerned, there was until this report no evidence to indicate that they may express 5-HT receptors. We found that a proportion of all three types of lumbar splanchnic afferent responded to 5-HT. Responses to 5-HT and 2-methyl-5-HT were prolonged and reproducible, indicating a lack of desensitization, which is comparable with previous in vivo data on vagal mucosal afferent endings (Blackshaw & Grundy, 1993b; Hillsley et al. 1998). Responses of nerve cell bodies to 5-HT3 receptor activation, on the other hand, show rapid adaptation and marked desensitization (Zhai et al. 1999). Our findings indicate that 5-HT3 receptors are not involved in mechanotransduction in extrinsic afferents, because mechanosensitivity was not affected by alosetron. However, a role for other 5-HT (5-HT1P) receptors has been proposed for mechanotransduction from the mucosa to the submucous plexus, which involves intrinsic primary afferents of the gut (Kirchgessner et al. 1992; Pan & Gershon, 2000). We were unable to demonstrate a role for endogenous 5-HT in activation of colonic splanchnic afferents, unlike previous studies of vagal gastroduodenal afferents in vivo (Blackshaw & Grundy, 1993b; Hillsley et al. 1998), in which 5-HT3 receptor antagonists reduced ongoing discharge. This is possibly because of the very low rates of spontaneous activity we saw in most fibres. Although 5-HT is released from the colon in vitro (Kirchgessner et al. 1992; Pan & Gershon, 2000), it would not be likely to reach significant levels in our preparation due to the rapid removal by superfusion over the tissue. An important consideration is whether responses to 5-HT were direct or secondary to other events, such as muscle contraction or transmitter release. The involvement of such processes in our preparation was avoided as much as possible through the use of nifedipine to inhibit calcium entry via L-type channels.
The majority of the extrinsic afferent innervation of the distal colon arises from the pelvic and splanchnic nerves with cell bodies in the sacral and lumbar dorsal root ganglia, respectively (Ness & Gebhart, 1988). Our recordings were from fibres in the intermesenteric and lumbar splanchnic nerves, which together contain predominantly lumbar spinal primary afferents (Baron et al. 1988). As well as extrinsic fibres, the intermesenteric nerves also contain a small number of fibres that originate from enteric neurones within the gut wall and pass through the inferior mesenteric ganglion (Luckensmeyer & Keast, 1995). These are known as intestinofugal fibres and they project mainly to the more rostral prevertebral ganglia and not to the spinal cord. They do not, therefore, function as primary afferents. It is important to consider the possibility that intestinofugal fibres may represent a minority of the fibres that we sampled in our recordings. In the case of serosal and mesenteric afferents this is unlikely, due to the fact that the majority of fibres had individual receptive fields outside the colonic wall and no differences were seen between the responsiveness of fibres with endings within and outside the colon. Muscular and mucosal afferents all had receptive fields within the colon, so the likelihood of contributions from intestinofugal fibres to their responses must be considered further. Most intestinofugal neurones have Dogiel type I morphology and receive several synaptic inputs from intrinsic primary afferent neurones (Luckensmeyer & Keast, 1995; Furness et al. 2000). We encountered only afferents with single punctate receptive fields in this study, suggesting therefore that they are not intestinofugal. Beyond these circumstantial observations, however, we cannot exclude that some of our recordings may have been from intestinofugal fibres.
A major difference between expression of 5-HT receptors on colonic lumbar spinal afferents and on other pathways emerges from our data. A large proportion (46 %) of colonic afferents responsive to 5-HT did not respond to a 5-HT3 receptor-selective agonist, indicating that responses of these fibres were mediated via other, non-5-HT3 receptors. On the other hand, responses of vagal afferents are exclusively 5-HT3 receptor-mediated in ferret and rat (Blackshaw & Grundy, 1993b; Hillsley et al. 1998), as are responses of greater splanchnic nerve afferents of the cat (Fu & Longhurst, 1998) and those of intrinsic primary afferent neurones of the guinea pig myenteric plexus (Bertrand et al. 2000). This suggests that lumbar colonic spinal afferents may exhibit similar properties to spinal afferent cell bodies in the DRG in general; these respond to a range of agonists and express gene transcripts for several 5-HT receptors (Pierce et al. 1996; Chen et al. 1998; Cardenas et al. 1999), including 5-HT2, 5-HT4, 5-HT6 and 5-HT7 subtypes and their variants. These numerous excitatory subtypes of 5-HT receptor are all candidates for mediating colonic afferent responses. They are coupled via G-proteins, in contrast with 5-HT3 receptors, which are the only 5-HT receptors directly associated with ion channels. Pharmacology of the large family of G-protein-coupled 5-HT receptors is now becoming better established, both in expression systems and intact tissue. Determination of the role of these receptors in the effects of 5-HT that we observed is a considerable undertaking and therefore beyond the scope of the present study. Investigation of these will continue in our laboratory, using selective pharmacological tools as they become available. Our preliminary data from a parallel in vivo study show no effect of a 5-HT4 receptor agonist on colonic sensory pathways (Hicks et al. 2001a), but these data have not yet been confirmed in the current model.
In conclusion, our findings provide a simple mechanism by which 5-HT contributes to activation of sensory pathways from the colon by acting directly on afferent endings. We demonstrate not only the presence of 5-HT3 receptors functionally and anatomically on these neurones, but also that other mechanisms exist which may be of significance in the activation of sensory pathways by 5-HT.
Acknowledgments
Work in Adelaide was supported by NHMRC Australia grant number 104814 to L.A.B. Anti-5-HT3 receptor antibody was kindly provided by Dr Sarah Lummis, University of Cambridge, UK.
References
- Andrews PLR, Davis CJ, Bingham S, Davidson HIM, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Canadian Journal of Physiology and Pharmacology. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Baron R, Janig W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. Journal of Comparative Neurology. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;280:R1371–1381. doi: 10.1152/ajpregu.2001.280.5.R1371. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine (5-HT) on the discharge of vagal mechanoreceptors and motility in the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993a;45:51–59. doi: 10.1016/0165-1838(93)90361-w. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993b;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Haupt P, Janig W, Kohler W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflügers Archiv. 1983;398:33–40. doi: 10.1007/BF00584710. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–668. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. Journal of Physiology. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Vasko MR, Wu X, Staeva TP, Baez M, Zgombick JM, Nelson DL. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. Journal of Pharmacology and Experimental Therapeutics. 1998;287:1119–1127. [PubMed] [Google Scholar]
- Fu LW, Longhurst JC. Role of 5-HT3 receptors in activation of abdominal sympathetic C fibre afferents during ischaemia in cats. Journal of Physiology. 1998;509:729–740. doi: 10.1111/j.1469-7793.1998.729bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Koopmans HS, Robbins HL, Lin HC. Identification of intestinofugal neurons projecting to the coeliac and superior mesenteric ganglia in the rat. Autonomic Neuroscience. 2000;83:81–85. doi: 10.1016/S0165-1838(00)00159-4. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Clayton NM, Gaskin PJ, Kirkup AJ, Su X, Joshi S, Friedrich A, Connor HE, Cox B, Grundy D, Gebhart GF, Humphrey PPA. 5-HT4 receptor agonists stimulate small intestinal transit but do not have direct visceral antinociceptive effects in the rat. Gastroenterology. 2001a;120:A6. [Google Scholar]
- Hicks GA, Hurle M, Savage TJ, Mazzia C, Clerc NG, Humphrey PPA. Enterochromaffin cells of the human, rat and guinea-pig colon: lack of evidence for the presence of 5-HT3 receptors. Gastroenterology. 2001b;120:A171. [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. Journal of Physiology. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton LA, Whitaker P, Atkinson W, Whorwell PJ, Fricker J, Rimmer M, Jacques L, Mills J. Increased platelet stores of 5-hydroxytryptamine (5-HT) in female patients with diarrhoea predominant irritable bowel syndrome. Gastroenterology. 2001;120:A636. [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. Journal of Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Luckensmeyer GB, Keast JR. Distribution and morphological characterization of viscerofugal projections from the large intestine to the inferior mesenteric and pelvic ganglia of the male rat. Neuroscience. 1995;66:663–671. doi: 10.1016/0306-4522(94)00599-z. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. Journal of Physiology. 1999;518:271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzia CJ, Clerc NG. Localization of 5-HT3 receptor immunoreactivity in enteric nervous system of rat colon. Gastroenterology. 2001;120:A335. [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat. Journal of Neurophysiology. 1988;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- Paintal AS. The response of gastric stretch receptors and certain other abdominal and thoracic vagal receptors to some drugs. Journal of Physiology. 1954;126:271–285. doi: 10.1113/jphysiol.1954.sp005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. Journal of Neuroscience. 2000;20:3295–2309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Spier AD, Wotherspoon G, Nayak SV, Nichols RA, Priestley JV, Lummis SC. Antibodies against the extracellular domain of the 5-HT3 receptor label both native and recombinant receptors. Brain Research. Molecular Brain Research. 1999;67:221–230. doi: 10.1016/s0169-328x(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. Journal of Neuroscience. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Gershon MD, Walsh JH, Wong HC, Kirchgessner AL. Inward currents in neurons from newborn guinea pig intestine: mediation by 5-hydroxytryptamine type 3 receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;291:374–382. [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. Journal of Physiology. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


