Abstract
The HCO3−:Na+ cotransport stoichiometry of the electrogenic sodium bicarbonate cotransporter kNBC1 determines the reversal potential (Erev) and thus the net direction of transport of these ions through the cotransporter. Previously, we showed that phosphorylation of kNBC1-Ser982 in the carboxy-terminus of kNBC1 (kNBC1-Ct), by cAMP-protein kinase A (PKA), shifts the stoichiometry from 3:1 to 2:1 and that binding of bicarbonate to the cotransporter is electrostaticaly modulated. These results raise the possibility that phosphorylated kNBC1-Ser982, or other nearby negatively charged residues shift the stoichiometry by blocking a bicarbonate-binding site. In the current study, we examined the role of the negative charge on Ser982-phosphate and three aspartate residues in a D986NDD custer in altering the stoichiometry of kNBC1. mPCT cells expressing kNBC1 mutants were grown on filters and mounted in an Ussing chamber for electrophysiological studies. Enhanced green fluorescence protein (EGFP)-tagged mutant constructs expressed in the same cells were used to determine the phosphorylation status of kNBC1-Ser982. The data indicate that both kNBC1-Asp986 and kNBC1-Asp988, but not kNBC1-Asp989, are required for the phosphorylation-induced shift in stoichiometry. A homologous motif (D887ADD) in the carboxy-terminus of the anion exchanger AE1 binds to carbonic anhydrase II (CAII). In isothermal titration calorimetry experiments, CAII was found to bind to kNBC1-Ct with a KD of 160 ± 10 nm. Acetazolamide inhibited the short-circuit current through the cotransporter by 65 % when the latter operated in the 3:1 mode, but had no effect on the current in the 2:1 mode. Acetazolamide did not affect the cotransport stoichiometry or the ability of 8-Br-cAMP to shift the stoichiometry. Although CAII does not affect the transport stoichiometry, it may play an important role in enhancing the flux through the transporter when kNBC1-Ser982 is unphosphorylated.
Sodium bicarbonate cotransporters are membrane proteins which mediate the transport of Na+ and HCO3− (CO32-). The HCO3−:Na+ stoichiometry of cotransport is physiologically important as it determines the net direction of flux of these ions through the cotransporter. Thermodynamics suggest that a cotransporter with a 3 HCO3−:1 Na+ stoichiometry will mediate cellular efflux of these ions, while a 2:1 cotransporter will mediate cellular influx. The molecular/structural basis underlying the stoichiometry is poorly understood. Recently, we reported that the HCO3−:Na+ stoichiometry of the electrogenic sodium bicarbonate cotransporter kNBC1 shifted from 3:1 to 2:1 in response to phosphorylation of Ser982 in its carboxy-terminus (kNBC1-Ser982) by cAMP-dependent PKA (Gross et al. 2001b). The mechanism of that phosphorylation-induced shift in stoichiometry is not currently known.
In another study we found that the binding of bicarbonate to the cotransporter is voltage dependent with an electric coefficient of 0.2 (Gross & Hopfer, 1998). This finding raises the possibility that an increased negative charge on kNBC1-Ser982-phosphate shifts the stoichiometry by interfering with the binding of a bicarbonate anion to kNBC1 via modification of the electric field at or around the bicarbonate transport (binding) site(s). kNBC1 possesses an additional nearby negatively charged site, containing three aspartic acid residues (D986NDD) which could also interact with a putative positively charged bicarbonate binding site(s) on the transporter thus competing with bicarbonate for binding. In the current study, we determined whether these aspartate residues play a role in the shift in stoichiometry induced by kNBC1-Ser982 phosphorylation. This motif is of additional interest since a homologous site in the carboxy-terminus of AE1 has been recently shown to bind and functionally interact with CAII (Vince & Reithmeier, 2000; Sterling et al. 2001). Therefore we also determined whether kNBC1-Ct interacts with CAII. The functional importance of CAII inhibition in modulating the change in the stoichiometry of kNBC1 was also examined. A preliminary account of this work has been published in an abstract form (Gross et al. 2002).
Methods
Cell culture
Experiments were carried out with the mouse proximal convoluted tubule cell line mPCT 1296 (d). Cells were used at passages between 15 and 25.
Transfection
mPCT cells were stably transfected with kNBC1 constructs as previously described (Gross et al. 2001a). For transient transfection, cells were grown on filters in mouse renal tubular epithelium (mRTE) medium containing a 1:1 mixture of DMEM and Ham's F12 medium (Gibco BRL) and the following additives: 10 ng ml−1 EGF, 5 μg ml−1 insulin, 5 μg ml−1 transferrin, 4 μg ml−1 dexamethasone, 10 units ml−1 interferon-γ, 2 mm glutamine and 5 % fetal bovine serum. Cells were transfected with the corresponding plasmid using Effectene (Qiagen, Valencia, CA, USA) as per the manufacturer's protocol. Mock-transfected cells were transfected with the vector only. All plasmids were purified with the Endofree plasmid purification kit (Qiagen) prior to their use.
Mutagenesis
The coding regions of human kNBC1 were cloned into the Eco RI and Eco RV sites in the pcDNA3.1 vector (Clontech, Palo Alto, CA, USA). An N-terminal EGFP fusion protein was produced by inserting kNBC1 into the Eco RI and Apa I sites in the EGFP-C3 vector (Clontech). The QuikChange site-directed mutagenesis kit (Invitrogen, Carlsbad, CA, USA) was used to generate the mutant kNBC1 and EGFP-kNBC1 constructs. All mutations were verified by DNA sequencing.
Phosphorylation assays
EGFP-tagged wild-type (wt) or mutant kNBC1 was transiently expressed in mPCT cells. The EGFP-tagged proteins were immunoprecipitated using an anti-GFP antibody (Abcam, Cambridge, UK) and protein A-Sepharose 4B (Amersham Biosciences, Piscatawy, NJ, USA). The beads were washed and the EGFP-tagged proteins were eluted from the beads as described (Gross et al. 2001b). The EGFP-tagged proteins were phosphorylated in vitro using a PKA catalytic subunit (Promega, Madison, WI, USA) and [γ-32P]ATP as described (Zizak et al. 1999; Gross et al. 2001b) and were resolved on a 7.5 % polyacrylamide gel in the presence of sodium dodecyl sulphate. The proteins were electrotransferred onto PVDF membranes (Amersham Biosciences) and 32P incorporation was detected using Kodak X-Omat film (Fisher, Los Angeles, CA, USA).
Western blotting
Western blotting was performed using an affinity purified polyclonal anti-kNBC1 antibody (Bok et al. 2001) and carbonic anhydrase II antibody (Abcam). Secondary horseradish peroxidase-conjugated species-specific antibodies (Jackson Immunoresearch Labs, West Grove, PA, USA), an Enhanced Chemiluminescence kit (Amersham Biosciences) and a Hyperfilm-Enhanced ChemiLuminescence kit (Amersham Biosciences) were used for detection.
kNBC1-Ct expression and purification
The carboxy-terminus (amino acids 915-1035) of kNBC1 were cloned in the pRSET vector to generate a His-tagged construct and expressed in E. coli according to the manufacturer's protocol (Invitrogen). The proteins were extracted from the cells using BugBuster HT protein extraction reagent (Novagen, Madison, WI, USA) containing a complete protease inhibitor cocktail (Roche, Indianapolis, IN, USA). After centrifugation at 18 000 g for 20 min at 4 °C, supernatants were dialysed overnight at 4 °C against 500 volumes of 50 mm Tris-HCl (pH 7.5) and then loaded onto a DE-52 column (Whatman, Fairfield, NJ, USA) equilibrated with 50 mm Tris-HCl (pH 7.5). Peptides were eluted with 50 mm Tris-HCl, containing 50 mmNaCl. Fractions containing the protein were combined and loaded onto a Ni-NTA His-Bind Superflow column (Qiagen) and purified according to the manufacturer's protocol. The purified proteins were concentrated and transferred into phosphate-buffered solution (PBS) using an Amicon YM-3 centrifuge filter device (Millipore, Bedford, MA, USA).
Binding of CAII to kNBC1-Ct
CAII:kNBC1-Ct binding was studied with isothermal titration calorimetry (ITC) using a MicroCal OMEGA ultrasensitive titration calorimeter (MicroCal, Inc., Northampton, MA, USA). CAII and kNBC1-Ct were separately dialysed in 10 mm PBS for 12 h at 4 °C. All solutions were thoroughly degassed by stirring under vacuum before use. CAII (1 μm) was titrated with kNBC1-Ct (10 μm) at 30 °C, by injecting 35 identical 5 μl solutions at 3 min intervals from a syringe into the sample cell. The titrated solution was stirred at 400 r.p.m. by a stirring paddle mounted at the tip of the injection syringe. The areas under the peaks of the thermograms were integrated using the ORIGIN software (OriginLab, Northampton, MA, USA). The resulting isotherms were fitted by a non-linear least-squares algorithm yielding estimates of the binding constant (K), the calorimetric enthalpy change (ΔH) and the number of bound kNBC1 carboxy-termini per CAII (N).
Stoichiometry
The stoichiometry of the cotransporter was determined from Erev and eqn (1), as described previously (Gross et al. 2001b):
| (1) |
where n is the number of bicarbonate anions cotransported with each sodium cation, and the subscripts ‘i’ and ‘o‘ represent intra- and extracellular concentrations of the indicated ion. R, T and F have their usual meaning. Briefly, Erev of the cotransporter was determined from measurements of the current-voltage (I-V) relationships at several different sodium concentration gradients. Current through the cotransporter was defined as the dinitrostilbene disulfonate (DNDS)-sensitive current. Only transfected cell monolayers for which the DNDS-sensitive current was at least 10-fold larger then that of the corresponding mock-transfected cells were included in this study. About 30 % of all transfected cell monolayers met the inclusion criteria.
Materials
8-Br-cAMP, acetazolamide and all salts were purchased from Sigma Chemical Co. (St Louis, MO, USA). DNDS was obtained from Pfaltz & Bauer (Waterbury, CT, USA). Filters were purchased from Millipore (Bedford, MA, USA).
Statistics
Experiments were performed at least 3 times. The results for Erev and stoichiometry are presented as means ± s.e.m. Student's unpaired t tests were used for statistical analysis, with P < 0.05 considered significant.
Results
We recently reported that phosphorylation of kNBC1-Ser982 by PKA in mPCT cells, in response to treatment with 8-Br-cAMP, resulted in a shift in the HCO3−:Na+ stoichiometry from 3:1 to 2:1 (Gross et al. 2001b). To test the hypothesis that the shift in stoichiometry is associated with an added negative charge on kNBC1-Ser982, we replaced kNBC1-Ser982 with aspartate and expressed the S982D mutant in mPCT cells. Aspartate is commonly used to mimic the negative charge of a phosphorylated serine or threonine residues (Hoeffler et al. 1994; Kwak et al. 1999; Lin et al. 2000). As can be seen from Fig. 1, Erev of the S982D mutant in these cells was -40 mV, which under the conditions of the experiment corresponds to a HCO3−:Na+ stoichiometry of 2:1. These findings suggest that the effect of phosphorylation on the transport stoichiometry is associated with the added negative charge on kNBC1-Ser982.
Figure 1. Current-voltage relationships of kNBC1 mutants in mPCT cells.
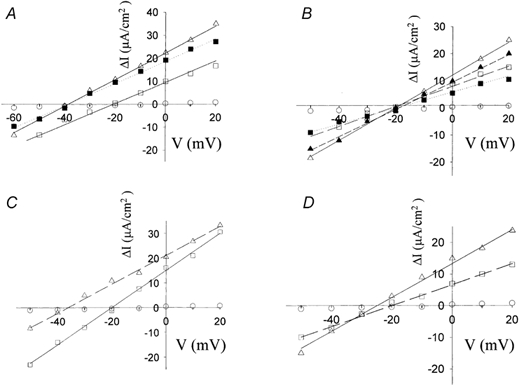
A, I-V relationships for wt-kNBC1 (□) and kNBC1-S982D mutant (▪) and wt-kNBC1-S982D in the presence (▵) of 8-Br-cAMP. B, kNBC1-NNNN mutant (open symbols) and EGFP-kNBC1-NNNN (filled symbols) in the absence (squares) or presence (triangles) of 8-Br-cAMP (100 μm). C, kNBC1-DNDN mutant in the absence (squares) or presence (triangles) of 8-Br-cAMP. D, kNBC1-NNDD mutant in the absence (squares) or presence (triangles) of 8-Br-cAMP. ○, I-V relationships of mock-transfected cells in A-D. All I-V relationships were measured at a 5-fold Na+ concentration gradient (basolateral/ apical = 50 mm/10 mm).
The nearby acidic cluster D986NDD might exert a similar electrostatic effect. To test whether the cluster is involved in the stoichiometry shift, we replaced the three aspartate residues with asparagines, and expressed the N986NNN mutant in mPCT cells for electrophysiological characterization. As can be seen from Fig. 1 and Table 1, 8-Br-cAMP did not have a significant effect on Erev of the mutant, nor on the stoichiometry, which remained at 3:1. This result suggests that the three aspartate residues, or a subset thereof, play an important role in the phosphorylation-induced shift in stoichiometry. To identify the critical residues we generated and functionally analysed additional mutants. The data are summarized in Table 1 and representative results are shown in Fig. 1. The results indicate that both Asp986 and Asp988, but not Asp989, are required for the phosphorylation-induced shift in stoichiometry to occur.
Table 1.
Reversal potential (Erev) and stoichiometry (n) of KNBC1 mutants (values for the corresponding EGFP-tagged constructs are in italics)
| DNDD | NNNN | DNNN | DNDN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | [Na+]AP/[Na+]BL | Erev | n | Erev | n | Erev | n | Erev | n |
| None | 10/50 | −20.1 ± 2 (6) | 3.1 ± 0.3 | −21.4 ± 2 (4) | 3.0 ± 0.3 | −22.9 ± 2 (4) | 2.8 ± 0.3 | −21.2 ± 2 (4) | 3.0 ± 0.3 |
| −19.4 ± 2 (4) | 3.2 ± 0.3 | −20.8 ± 2 (5) | 3.0 ± 0.3 | −23.5 ± 2 (5) | 2.8 ± 0.3 | ||||
| 50/10 | 23.2 ± 2 (5) | 2.8 ± 0.3 | 19.9 ± 2 (4) | 3.1 ± 0.3 | 23.2 ± 2 (4) | 2.8 ± 0.3 | 19.7 ± 2 (6) | 3.1 ± 0.3 | |
| 21.5 ± 2 (4) | 2.9 ± 0.3 | 20.2 ± 2 (4) | 3.1 ± 0.3 | 23.7 ± 2 (4) | 2.8 ± 0.3 | ||||
| 8-Br-cAMP | 10/50 | −38.2 ± 4 (7)* | 2.1 ± 0.2* | −22.5 ± 2 (6) | 2.9 ± 0.3 | −21.6 ± 2 (4) | 2.9 ± 0.3 | −36.2 ± 4 (5) | 2.1 ± 0.2* |
| −37.6 ± 4 (6)* | 2.1 ± 0.2* | −22.2 ± 2 (4) | 2.9 ± 0.3 | −20.9 ± 2 (4) | 3.0 ± 0.3 | ||||
| 50/10 | 37.5 ± 4 (8)* | 2.1 ± 0.2* | 19.8 ± 2 (4) | 3.1 ± 03 | 24.4 ± 2 (4) | 2.7 ± 0.3 | 34.7 ± 4 (4) | 22 ± 0.2* | |
| 44.2 ± 4 (5)* | 1.9 ± 0.2* | 23.2 ± 2 (5) | 2.8 ± 0.3 | 23.3 ± 2 (3) | 2.8 ± 0.3 | ||||
| ACTZ | 50/10 | −25.0 ± 2 (3) | 2.7 ± 0.2 | ||||||
| 8-Br-cAMP + ACTZ | 50/10 | −39.8 ± 4 (3)* | 2.1 ± 0.2* | ||||||
| DNND | NNDD | S989D | S982D/DNND | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | [Na+]AP/[Na+]BL | Erev | n | Erev | n | Erev | n | Erev | n |
| None | 10/50 | −22.5 ± 2 (6) | 2.9 ± 0.3 | −23.2 ± 2 (4) | 2.8 ± 0.3 | −39.2 ± 4 (5) | 2.1 ± 0.2* | −22.5 ± 2 (6) | 2.9 ± 0.3 |
| 50/10 | 21.7 ± 2 (7) | 2.9 ± 0.3 | 24.6 ± 2 (4) | 2.7 ± 0.3 | 41.0 ± 2 (4) | 2.0 ± 0.2* | 21.7 ± 2 (7) | 2.9 ± 0.3 | |
| 8-Br-cAMP | 10/50 | −20.8 ± 2 (4) | 3.0 ± 0.3 | −22.8 ± 2 (3) | 2.8 ± 0.3 | −38.9 ± 4 (4) | 2.1 ± 0.2* | −20.8 ± 2 (4) | 3.0 ± 0.3 |
| 50/10 | 18.7 ± 2 (5) | 3.2 ± 0.3 | 23.4 ± 2 (3) | 2.8 ± 0.3 | 37.7 ± 4 (3) | 2.1 ± 0.2* | 18.7 ± 2 (5) | 3.2 ± 0.3 | |
[Na+]AP/[Na+]BL, apical/basolateral sodium gradient. Number of experiments is shown in parentheses.
P < 0.05 compared with the corresponding wt-KNBC1 construct in untreated cells; ACTZ, acetazolamide.
The mutants N986NNN, D986NNN, D986NND and N986NDD had a stoichiometry of 3:1, which did not shift to 2:1 following treatment with 8-Br-cAMP (Table 1). It is possible that these mutations interfered with kNBC1-Ser982 phosphorylation following 8-Br-cAMP treatment. To test that hypothesis, we performed in vitro phopshorylation assays of 32P incorporation into EGFP-N-terminal-tagged protein constructs. Previously, we showed that 8-Br-cAMP treatment induces the phosphorylation of wt-EGFP-tagged kNBC1, expressed in mPCT cells, at kNBC1-Ser982 (Gross et al. 2001b). The EGFP tag did not affect the currents or the stoichiometry of the cotransporter significantly as compared to the corresponding untagged protein (Table 1). As can be seen in Fig. 2A, there was no effect of these mutations on kNBC1-Ser982 phosphorylation in response to 8-Br-cAMP treatment. The kNBC1 double mutant S982D/DNND exhibited a 3:1 stoichiometry in the presence or absence of 8-Br-cAMP (Table 1), in contrast to the 2:1 stoichiometry of the single site S982D mutant (Fig 1A). These results indicate that the increased negative charge induced by kNBC1-Ser982 phosphorylation or the S982D mutation is not sufficient per se to shift the stoichiometry, and further demonstrate the importance of Asp988 in this regard.
Figure 2. Phosphorylation of EGFP-tagged kNBC1 mutants by PKA and characterization of kNBC1:CAII binding.
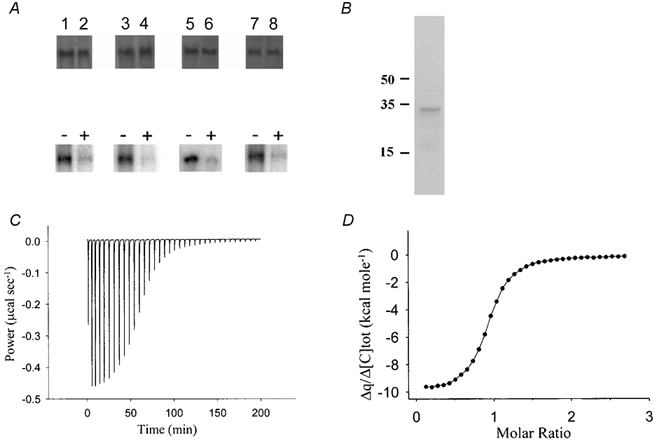
Upper panel in A, Western blots of kNBC1 used in in vitro phosphorylation assays. Treatment of the cells with 8-Br-cAMP did not affect the amount of protein expressed. Lower panel in A, in vitro phosphorylation assays of kNBC1-NNNN (lanes 1 and 2), kNBC1-DNNN (lanes 3 and 4), kNBC1-DNND (lanes 5, and 6) and kNBC1-NNDD (lanes 7 and 8) isolated from untreated mPCT cells (lanes 1, 3, 5 and 7) or cells treated with 100 μm 8-Br-cAMP (lanes 2, 4, 6 and 8) for 15 min. Immunoprecipitated EGFP-kNBC1 mutants were eluted from protein A-Sepharose beads and then phosphorylated in vitro using the PKA catalytic subunit and [γ-32P]ATP. Representative experiments are shown. B, Western blot of mPCT cells with anti-CAII showing the presence of CAII in these cells. Molecular weight labels are in kDa units. C, calorimetric titration of CAII with kNBC1-Ct. Heat changes associated with titration of CAII with kNBC1-Ct. D, binding isotherm and best-fit curve for peak area integrals shown in C. N (number of binding sites) = 1 ± 0.06; KD (dissociation constant) = 160 ± 10 nm.
The functional role of specific aspartate residues in the kNBC1-D986NDD motif is of particular interest, since a homologous site in the carboxy-terminus of AE1 (D887ADD) binds to basic residues in the amino-terminus of CAII (Vince & Reithmeier, 2000). Carbonic anhydrase II is highly expressed in proximal tubule cells (Lonnerholm & Ridderstrale, 1980), suggesting an analogous interaction with the cytoplasmic kNBC1-Ct. Figure 2B shows the expression of endogenous CAII in mPCT cells. To determine whether CAII interacts with kNBC1-Ct, we measured the binding of kNBC1-Ct (kNBC1-915-1035) to CAII in vitro using an ITC approach (Bradshaw et al. 1998). The data indicate that kNBC1-Ct contains a single binding site with a high affinity for CAII with a KD of 160 ± 10 nm (Fig. 2C and D).
The finding that CAII binds to kNBC1-Ct complements recent studies that have shown that functional CAII significantly stimulates bicarbonate flux through AE1, AE2 and AE3 (Sterling et al. 2001). Furthermore, Scozzafava & Supuran (2002) have shown that tetra-peptides incorporating the DADD sequence strongly activate CAII. To determine whether kNBC1-Ct:CAII binding has any functional consequences, mPCT cells stably transfected with wt-kNBC1 were pre-treated with 50 μm of the carbonic anhydrase inhibitor acetazolamide for 15 min. Acetazolamide caused a 65 % decrease in the flux through the cotransporter (Isc = 3.4 ± 0.3 μA cm−2 compared to 9.9 ± 1 μA cm−2 in the absence of acetazolamide, P < 0.05) but did not significantly change the stoichiometry of the transporter (Table 1). Increasing the concentration of acetazolamide to 100 μm did not significantly increase the inhibition of flux through the cotransporter, nor did it affect the phosphorylation-induced stoichiometry shift (not shown). In mPCT cells pre-treated with 8-Br-cAMP, the stoichiometry shifted to 2:1 (Table 1). Acetazolamide (50 μm) did not significantly change the flux through kNBC1 when added following pre-treatment of mPCT cells with 8-Br-cAMP (12.8 ± 3 μA cm−2 before compared to 10.2 ± 1 μA cm−2 after acetazolamide, P > 0.05). Furthermore, acetazolamide did not cause any shift in the stoichiometry in these cells, which remained at 2:1 (Table 1). In addition, acetazolamide did not block the 8-Br-cAMP-induced shift in stoichiometry when added 10 min prior to the addition of 8-Br-cAMP (Table 1). These results indicate that in the absence of functional CAII, kNBC1 can still function in the 3:1 mode, and can shift to a 2:1 stoichiometry following treatment with 8-Br-cAMP. Inhibition of carbonic anhydrase activity resulted in a significant decrease in kNBC1 flux only when the transporter operated in the 3:1 mode.
Discussion
The direction of sodium bicarbonate flux through the electrogenic sodium bicarbonate cotransporters is critically dependent on the HCO3−:Na+ cotransport stoichiometry. Previously, we demonstrated that cAMP-PKA-dependent phosphorylation of kNBC1-Ser982 in the carboxy-terminus of the protein shifts the stoichiometry from 3:1 to 2:1 (Gross et al. 2001b). In another study, we found that the binding of 3 HCO3− anions (or 1 CO32- and 1 HCO3−) to the cotransporter is voltage dependent with an electrical coefficient of 0.2 (Gross & Hopfer, 1998). This indicates that on average, bicarbonate senses ≈20 % of the membrane's electric field upon binding to the cotransporter, or that the binding site(s) for bicarbonate is located about one-fifth of the electrical distance into the membrane. This result raises the possibility that the binding of one or more bicarbonate anions to the cotransporter can be dynamically regulated by perturbing the local electric field at the transport site(s).
We hypothesized that the additional negative charge could interfere with the binding of bicarbonate to kNBC1 by either competing with it or by modifying the electric field profile around the anionic binding site, thereby shifting the stoichiometry from 3:1 to 2:1. The finding in the present study that the stoichiometry of the S982D mutant is also 2:1 in the presence or absence of 8-Br-cAMP suggests that the effect of phosphorylation is indeed electrostatic in nature. Detailed mutagenesis studies of the carboxy-terminal D986NDD motif, which indicated an important role for two additional charged residues, kNBC1-Asp986 and kNBC1-Asp988, further strengthens the electrostatic hypothesis. The data are consistent with a model wherein Asp986 and Asp988, electrostatically block a bicarbonate-binding site when kNBC1-Ser982 is phosphorylated (Fig. 3). Whether phosphorylation of Ser982 favours the electrostatic interaction of Asp986 and Asp988 with a bicarbonate-binding site by inducing a local conformational change in kNBC1-Ct remains to be determined. In this regard, it is noteworthy that conformational changes following PKC-dependent phosphorylation are associated with the removal of fast N-type inactivation of voltage-dependent potassium (Kv) channels (Antz et al. 1999). In a recent study, Muller-Berger et al. (2001) have found that elevation of intracellular Ca2+ in Xenopus oocytes expressing kNBC1 resulted in a shift of the HCO3−:Na+ stoichiometry of the cotransporter from 2:1 to 3:1. Whether elevation of intracellular Ca2+ alters the phosphorylation state of kNBC1 remains to be determined.
Figure 3. Main features of a hypothetical model of kNBC1 transport.
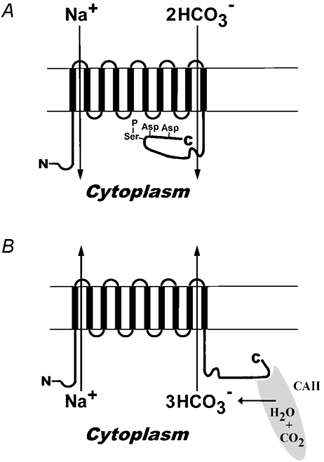
A, blockage of a putative bicarbonate transport site by Asp986 and Asp988 when kNBC1-Ser982 is phosphorylated and kNBC1 operates in the 2:1 mode. B, interaction of CAII with kNBC1-Ct enhances the flux through the transporter in the 3:1 mode.
The essential role for the aspartate residues in kNBC1-Ct in modulating the stoichiometry of the transporter is of particular interest since a homologous site in the carboxy-terminus of AE1 (D887ADD) has been shown to interact with histidine and lysine residues in the amino-terminus of CAII (Vince et al. 2000). In the present study, we demonstrated that kNBC1-Ct interacts with CAII. However, when the stoichiometry was 3:1, inhibition of carbonic anhydrase activity decreased the current through the transporter by ≈65 %. In the 2:1 mode, carbonic anhydrase inhibition did not affect the flux through the cotransporter significantly.
Previous studies confirm that carbonic anhydrase inhibition decreases the flux through the cotransporter when it operates in the 3:1 mode. Burckardt et al. (1984) reported that acetazolamide decreases the flux through the cotransporter when it operates in the 3:1 mode in vivo, but similar inhibition was not found in vitro when the cotransporter operates in the 2:1 mode (Seki & Fromter, 1992). Similar results have more recently been reported by Muller-Berger et al. (1997) and Kunimi et al. (2000). Muller-Berger et al. (1997) have suggested that the selective effect of acetazolamide indicates that the 3:1 mode is mediated by 1 CO32-:1 HCO3−:1 Na+, while the 2:1 mode is mediated by 2 HCO3−:1 Na+. Although this explanation cannot be ruled out, based on our current data, we propose an alternative model. Our model, depicted in Fig. 3, is based on the concept of a dynamic phosphorylation-dependent interaction of CAII with kNBC1-Ct. Specifically, when the transporter is functioning in the 3:1 mode (kNBC1-Ser982 unphosphorylated), CAII binds to kNBC1-Ct. Phosphorylation of kNBC1-Ser982 dissociates CAII from the transporter, which accounts for the decreased effect of CAII inhibition. The two models are not necessarily mutually exclusive. Experiments are currently in progress in our laboratory to measure the binding constant between kNBC1-Ct, phosphorylated in vitro, and CAII. A similar model has been proposed for phosphorylation-dependent binding of calmodulin to protein-43 (Hayashi et al. 1997). Our data indicate that functioning CAII plays no role in the stoichiometry or its shift, but rather acts to increase the flux through the transporter in the 3:1 mode, probably by increasing the local bicarbonate concentration adjacent to the transport site(s). Interaction between CAII and kNBC1 implies that the concept of a ‘transport metabolon’ is applicable to other members of the bicarbonate transporter superfamily (BTS), as suggested by Sterling et al. (2001).
Acknowledgments
This work was supported by grants from the American Heart Association and Cystic Fibrosis Foundation to E.G. and from the NIH (grants DK58563, DK07789), the Richard and Hinda Rosenthal Foundation, and the Fredericka Taubitz Foundation to I.K. N. Abuladze was supported by a training grant from the National Kidney Foundation of Southern California (J891002).
REFERENCES
- Antz C, Bauer T, Kalbacher H, Frank R, Covarrubias M, Kalbitzer HR, Ruppersberg JP, Baukrowitz T, Fakler B. Control of K+ channel gating by protein phosphorylation: structural switches of the inactivation gate. Nature Structural Biology. 1999;6:146–150. doi: 10.1038/5833. [DOI] [PubMed] [Google Scholar]
- Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. American Journal of Physiology - Renal Physiology. 2001;281:F920–935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- Bradshaw JM, Grucza RA, Ladbury JE, Waksman G. Probing the “two-pronged plug two-holed socket” model for the mechanism of binding of the Src SH2 domain to phosphotyrosyl peptides: a thermodynamic study. Biochemistry. 1998;37:9083–9090. doi: 10.1021/bi973147k. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Cassola AC, Fromter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. II. Exclusion of HCO3- effects on other ion permeabilities and of coupled electroneutral HCO3- transport. Pflügers Archiv. 1984;401:43–51. doi: 10.1007/BF00581531. [DOI] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. Journal of Physiology. 2001a;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Pushkin A, Abuladze N, Hopfer U, Sassani P, Dukkipati R, Kurtz I. Phosphorylation of Ser982 in kNBC1 shifts the HCO3-:Na+ stoichiometry from 3:1 to 2:1 in proximal tubule cells. Journal of Physiology. 2001b;537:659–665. doi: 10.1111/j.1469-7793.2001.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hopfer U. Voltage and co-substrate dependence of the Na-HCO3 cotransporter kinetics in renal proximal tubule cells. Biophysical Journal. 1998;75:810–824. doi: 10.1016/S0006-3495(98)77570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Pushkin A, Abuladze N, Fedotoff O, Kurtz I. kNBC1 stoichiometry shift: An essential role for Asp986 and Asp988. Journal of the American Society of Nephrology. 2002;13:61A. [Google Scholar]
- Hayashi N, Matsubara M, Titani K, Taniguchi H. Circular dichroism and 1H nuclear magnetic resonance studies on the solution and membrane structures of GAP-43 calmodulin-binding domain. Journal of Biological Chemistry. 1997;272:7639–7645. doi: 10.1074/jbc.272.12.7639. [DOI] [PubMed] [Google Scholar]
- Hoeffler WK, Levinson AD, Bauer EA. Activation of c-Jun transcription factor by substitution of a charged residue in its N-terminal domain. Nucleic Acid Research. 1994;22:1305–1312. doi: 10.1093/nar/22.7.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimi M, Muller-Berger S, Hara C, Samarzija I, Seki G, Fromter E. Incubation in tissue culture media allows isolated rabbit proximal tubules to regain in-vivo-like transport function: response of HCO3− absorption to norepinephrine. Pflügers Archiv. 2000;440:908–917. doi: 10.1007/s004240000361. [DOI] [PubMed] [Google Scholar]
- Kwak YG, Hu N, Wei J, George AL, Jr, Grobaski TD, Tamkun MM, Murray KT. Protein kinase A phosphorylation alters Kvbeta1. 3 subunit-mediated inactivation of the Kv1.5 potassium channel. Journal of Biological Chemistry. 1999;274:13928–13932. doi: 10.1074/jbc.274.20.13928. [DOI] [PubMed] [Google Scholar]
- Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO Journal. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnerholm G, Ridderstrale Y. Intracellular distribution of carbonic anhydrase in the rat kidney. Kidney International. 1980;17:162–174. doi: 10.1038/ki.1980.20. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Ducoudret O, Diakov A, Fromter E. The renal Na-HCO3−cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflügers Archiv. 2001;442:718–728. doi: 10.1007/s004240100592. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Nesterov VV, Fromter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. II. Change of Na-HCO3 cotransport stoichiometry and of response to acetazolamide. Pflügers Archiv. 1997;434:383–391. doi: 10.1007/s004240050411. [DOI] [PubMed] [Google Scholar]
- Scozzafava A, Supuran CT. Carbonic anhydrase activators: Human isozyme II is strongly activated by oligopeptides incorporating the carboxyterminal sequence of the bicarbonate anion exchanger AE1. Bioorganic and Medical Chemistry Letters. 2002;12:1177–1180. doi: 10.1016/s0960-894x(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Seki G, Fromter E. Acetazolamide inhibition of basolateral base exit in rabbit renal proximal tubule S2 segment. Pflügers Archiv. 1992;422:60–65. doi: 10.1007/BF00381514. [DOI] [PubMed] [Google Scholar]
- Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. Journal of Biological Chemistry. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- Vince JW, Carlsson U, Reithmeier RA. Localization of the Cl−/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry. 2000;39:13344–13349. doi: 10.1021/bi0015111. [DOI] [PubMed] [Google Scholar]
- Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry. 2000;39:5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. cAMP-induced phosphorylation and inhibition of Na+/H+ exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. Journal of Biological Chemistry. 1999;274:24753–24758. doi: 10.1074/jbc.274.35.24753. [DOI] [PubMed] [Google Scholar]


