Abstract
The inward rectifier potassium channel Kir2.1 is more sensitive to the weakly voltage-dependent block by extracellular Mg2+ () than Kir2.2 and Kir2.3. We identified Glu125 in an extracellular loop before the pore region of Kir2.1 as a site responsible for this sensitivity to block, based on the observations that the Glu125Gln (E125Q) mutation strongly decreased the sensitivity, while a mutation to Glu at the corresponding sites of Kir2.2 and 2.3 led to an increase. The negative charge proved to be crucial since the Glu125Asp (E125D) mutant showed similar properties to the wild type (WT). A similar weakly voltage-dependent block was also caused by extracellular Ca2+ and La3+ in Kir2.1 WT but not in the E125Q mutant. The sensitivity to block by extracellular Ba2+ () was also decreased in the E125Q mutant, although the voltage dependency of half-inhibition concentration was not changed, as reported previously. We additionally observed that the speed of block and recovery was decelerated by the presence of in WT, but not in the E125Q mutant. The sensitivity to the block by was increased by lowering extracellular K+ (), suggesting a competitive interaction of and . The single-channel conductance of the WT in 140 mm K+ was 39.6 pS (0 mm ) and 11.5 pS (10 mm), while that of the E125Q mutant was 26.0 pS (0 mm) and 19.6 pS (10 mm). These results demonstrate that Mg2+ competes with K+ permeation in the WT and that E125 is required for efficient K+ permeation in the absence of . We conclude that E125 in an extracellular loop of Kir2.1 is a site which facilitates K+ permeation and entry of Ba2+ toward a deeper plugging site, and that competes with and at this site.
It is known that the inward rectifier K+ channel has a high sensitivity to the block by cytoplasmic Mg2+ () (Matsuda et al. 1987; Vandenberg, 1987) and polyamines (Ficker et al. 1994; Lopatin et al. 1994). The high sensitivity of this channel to block by various extracellular cations has also been reported in skeletal muscle cells (Standen & Stanfield, 1978, 1980), tunicate eggs (Fukushima, 1981), cardiac myocytes (Biermans et al. 1987; Matsuda et al. 1989; Shioya et al. 1993), aortic endothelial cells (Elam & Lansman, 1995) and heterologous expression systems using cloned cDNA (Sabirov et al. 1997; Shieh et al. 1998; Owen et al. 1999; Alagem et al. 2001). From the analysis of voltage dependency of half-inhibition concentration (Ki) values, and were shown to be blockers at a deep site, whereas blocks the channel at a superficial site of the electric field (Shioya et al. 1993).
After the initial cDNA cloning of ROMK1 (Kir1.1) (Ho et al. 1993) and IRK1 (Kir2.1; Kubo et al. 1993), many members of the inwardly rectifying K+ channel family have been isolated (Isomoto et al. 1997; Jan & Jan, 1997; Nichols & Lopatin, 1997). Recently, we have isolated three cDNAs from tunicate tadpoles that encode inwardly rectifying K+ channels (TuIRK, TuGIRK-A and -B) (Murata et al. 2001a,b). We observed that TuIRK encodes a simple inward rectifier expressed in epidermal cells of tadpoles at a high level (Murata et al. 2001a), while TuGIRKs encode subunits of G protein-coupled channels (Murata et al. 2001b). TuIRK was clearly less sensitive to block by than Kir2.1. Based on the difference in primary structure between Kir2.1 and TuIRK, we initially focused on the three amino acid residues after the GYG selectivity filter, and observed that this region does not determine the sensitivity to the block. We then focused on Glu (E) 125, an amino acid residue located in the extracellular loop between the first transmembrane region (M1) and the pore (P) region of Kir2.1 (Fig. 1), to examine whether it served as a determinant. By mutagenesis study, it was shown that E125 explains the high sensitivity of Kir2.1 to block by (Murata et al. 2001a). However, whether the negative charge of E125 is critical or not remains to be elucidated. Because introduction of Glu to the corresponding site of TuIRK did not increase the sensitivity, it is necessary to expand this study to other members of the Kir2 family. As Kir2.2 and 2.3 have Gln (Q) and His (H) at the corresponding sites, respectively (Fig. 1), we examined in this study the sensitivity to bock by of these WT and mutant channels with a Glu substitution at these positions. In addition, it remains to be determined whether other negatively charged amino acids in the extracellular loop (Fig. 1) could also contribute to the block sensitivity.
Figure 1. Positions of negatively charged amino acid residues in the extracellular loop of the Kir2.1 channel, and the comparison of the corresponding amino acid residues among members of the Kir2 family.

A, schematic representation of the structure of Kir2.1 channel based on the initial model (Kubo et al. 1993) with modification by later observations in terms of the inner vestibule structure (Yang et al. 1995; Kubo & Murata, 2001). E125 locates in the extracellular loop between the M1 and P regions. Other negatively charged amino acid residues in the extracellular loop, D112 and D114 between the M1 and P regions, and D152 and D153 between the P and M2 regions are also depicted. B, alignments of the amino acid sequences of the extracellular loop regions. * Amino acids shown in A. The original reports used for the alignment are as follows: Kir 2.1, Kubo et al. (1993); Kir 2.2, Koyama et al. (1994) and Kir2.3, Morishige et al. (1994).
It was recently reported that the identical site, E125, critically determines the sensitivity of the Kir2.1 channel to block by (Alagem et al. 2001). According to the report, E125 is an intermediate site for Ba2+ before finally binding to the plugging site, T141, in the pore. Based on the results of the experiments in which sensitivity of block to extracellular pH (pHo) was examined, E125 was proposed to play a role in dehydration of before entering the pore (Alagem et al. 2001).
MacKinnon's research group reported both the structure of the KcsA channel pore and the image of permeating K+ ions (Doyle et al. 1998; Morais-Cabral et al. 2001; Zhou et al. 2001), and concluded that there are two sites outside the selectivity filter where K+ ions are stably present (Zhou et al. 2001). As this region is above the entrance of the pore, it is still not known how K+ ions can be present here stably or with a high probability.
In this study, our objective was to investigate the functional significance of E125. Taking the results of our previous study and other reports together, we hypothesized that E125 was a superficial site where and interact with each other physiologically, and where also interacts competitively. We therefore designed experiments to analyse the interaction of these three ions.
Methods
In vitro mutagenesis
The single point mutants were made using Sculptor Kit (Amersham, Piscataway, NJ, USA) or QuikChange Kit (Stratagene, La Jolla, CA, USA). Introduction of the mutation was confirmed by sequencing the mutation primer and the surrounding regions with the ABI 377-18 or the ABI 310 DNA sequencer (Applied Biosystems, Foster City, CA, USA). cRNA was prepared from the linearized plasmid DNA with an RNA transcription kit (Stratagene).
Two-electrode voltage clamp recordings in Xenopus oocytes
The African clawed toads were anaesthetized by immersion in water containing 0.15 % tricaine, and oocytes were isolated surgically. After the surgical operation, the toads were put in fresh water to allow recovery from the anaesthesia. The toads were raised for at least 3 months before the next surgical operation. After the third or fourth operation, the anaesthetized toads were killed by decapitation. Isolated oocytes were treated with collagenase (2 mg ml−1, type 1, Sigma, St Louis, MO, USA), and approximately 50 nl cRNA solution was injected. The oocytes subjected to injection were incubated for 2-4 days at 17 °C in frog Ringer solution (Kubo et al. 1993) supplemented with 20 mm KCl. Macroscopic current was recorded under two-electrode voltage clamp using a ‘bath-clamp’ amplifier (OC-725B-HV or OC-725C, Warner Co., Hamden, CT, USA). A method for taking into account the error due to series resistance was as described previously (Kubo & Murata, 2001). Stimulation, data acquisition and analysis were performed on a Pentium-based computer using Digidata 1322A and pCLAMP8 (Axon Instruments, Union City, CA, USA) software. Intracellular glass microelectrodes were filled with 3 m potassium acetate with 10 mm KCl (pH 7.2). The microelectrode resistance ranged from 0.1 to 0.6 MΩ. Two Ag-AgCl pellets (Warner Co.) were used to pass the bath current and to sense the bath voltage. The standard bath solution contained (mm); 86 KCl, 3 MgCl2, 4 KOH and 5 Hepes (pH 7.4), supplemented with 200 μm niflumic acid, a Cl− channel blocker endogenously expressed in oocytes (Sabirov et al. 1997). Niflumic acid was indispensable for the recording in 0 mm to minimize the leakage Cl− current. [Mg2+]o, [Ba2+]o and [La3+]o were changed by simply adding or reducing the amount of MgCl2, BaCl2 or LaCl3 without adjusting the osmolarity. [K+]o was changed by replacing KCl with N-methyl d-glucamine. All experiments were carried out at room temperature (23 ± 2 °C).
Analysis of the dose-block relationship
The block by occurred almost instantaneously, and there was little time-dependent component. To analyse the instantaneous block, the current amplitudes at the beginning of various step pulses in the absence and presence of were measured, and the ratio was calculated as a fraction of unblocked current. In the case of block, the current amplitude at the end of 1 s pulses was used as the block proceeded in a time-dependent manner. After 1 s, the block almost reached a steady state, but it was incomplete especially when the concentration was low. However, as too long step pulses increased the leak component, we opted to take the value at 1 s from the beginning. The fractions of unblocked current at various membrane potentials were plotted against [Mg2+]o or [Ba2+]o, and fitted with the following Hill equation:
| (1) |
The dissociation constant (Ki) and the Hill coefficient (nH) were calculated from the fit, and the Δ value, a fractional distance in the electric field, was calculated using the following equation as described previously (Woodhull, 1973; Sabirov et al. 1997; Alagem et al. 2001):
| (2) |
It is generally accepted that the Δ value calculated by the analysis of block in the steady state gives the fractional distance of the binding site of blockers in the electric field, if the pore has a low occupancy property (Hille, 2001). If the pore has a high occupancy or multi-ion property as in the case of K+ channels, the Δ value is affected, e.g. by the locking-in of blocking ions by K+ ions (Hille, 2001). Therefore, it should be understood that a Δ value for K+ channel does not simply reflect the depth of the binding site. Taking this point into consideration, we calculated the Δ value as a reference parameter as reported previously (Sabirov et al. 1997; Alagem et al. 2001).
Kinetics analysis
For the block, the speed of block and unblock was analysed by fitting with an exponential function, and the time constants, τblock and τrecovery, were obtained. When the [Ba2+]o was too high, the speed could not be analysed reliably due to its very fast behaviour, and when too low, the contribution of leak current hindered accurate analysis. As the sensitivity to the block differed significantly between the WT and the E125Q mutant, and depended on [Mg2+]o, we used data of suitable concentrations of to achieve reliable analyses of blocking kinetics. We used 3 and 10 μm for the WT in 0 mm , 10 and 30 μm for the WT in 10 mm , 30 and 100 μm for the E125 mutants.
| (3) |
| (4) |
The kon values can be calculated as the slope of a linear plot of 1/τblockversus [Ba2+]o. The koff values can be calculated as the intercept with the y-axis, but reliable analyses of koff values could not be performed. At depolarized potentials:
| (5) |
As it is known that koff is dominant at depolarized potentials, 1/τrecovery almost reflects the koff value as reported previously (Alagem et al. 2001). By these calculations, we estimated kon and koff values of Ba2+ block of the WT and the E125Q mutant in 0 and 10 mm . Results are expressed as means ± s.d. unless otherwise stated.
Single channel recordings in Xenopus oocytes
For single channel recordings, the vitelline membrane was peeled off by bathing oocytes in a high osmolarity solution (Kubo et al. 1993) for 5-10 min. The patch pipettes were prepared from borosilicate glass (Warner Co.) using a P-97 horizontal puller (Sutter, Novato, CA, USA) and a fire polisher (Narishige, Japan). Recordings were obtained under cell-attached configuration using an AxoPatch 1D amplifier (Axon Instruments). The current recordings were filtered at 1 kHz by a Bessel filter built into the amplifier, and was digitized at 5 kHz. The recordings shown in Fig. 10 were digitally filtered at 200 Hz. The pipette (extracellular) and the bath solution contained (mm): 136 KCl, 10 Hepes, 4 KOH and various concentrations of MgCl2 or CaCl2 (pH 7.4). The patch pipette resistance ranged from 5 to 15 MΩ.
Figure 10. Analysis of single channel properties.

A and B, the WT and the E125Q mutant Kir2.1, respectively, were expressed in Xenopus oocytes, and single channel recordings were obtained in the cell-attached configuration of patch clamp. The recordings at -120 mV in 0 mm or (1st row), 3 mm (2nd row), 10 mm (3rd row), 3 mm (4th) and 10 mm (5th) are shown. Extracellular and patch pipette solutions contained 140 mm K+. C and D, plots of single channel current-voltage relationship in various and concentrations, respectively. The data of representative cases are shown.
Results
Comparison of the sensitivity of Kir2 members to the block by
Macroscopic current recordings under two electrode voltage clamp of Kir2.1 expressed in Xenopus oocytes are shown in Fig. 2. The current amplitude was strongly reduced by increasing [Mg2+]o from 0 to 1, 3 and 10 mm (Fig. 2A and B). In contrast, Kir2.2 and Kir2.3 showed a much lower sensitivity (Fig. 2C and D). By fitting the dose-block relationship plot (Fig. 2E), we estimated the Ki at -120 mV, and obtained the values of 10.6 ± 3.2 mm (n = 8; Kir2.1) and 43.9 ± 9.4 mm (n = 8; Kir2.2). The Ki value of Kir2.3 could not be estimated reliably due to the sensitivity being too low. From these data and our previous results on TuIRK (Murata et al. 2001a), we focused on the difference of amino acid residues at extracellular loops, E125 of Kir2.1 (Fig. 1A). Kir2.2 and 2.3 have Gln and His, respectively, at the corresponding site (Fig. 1B).
Figure 2. Sensitivity to the block by of Kir2 family members expressed in Xenopus oocytes.

A, macroscopic current recordings under two-electrode voltage clamp of WT Kir2.1 in 0 mm (left) and 10 mm (right) solution. The concentration was 90 mm. The holding potential was 0 mV, and step pulses from +20 to -150 mV were applied by 10 mV decrements. B, current-voltage (I-V) relationships at the beginning of step pulses in various concentrations of . C and D, I-V plots of Kir2.2 and Kir2.3, respectively. E, dose-block relationship plot of the data in B-D. The plots are of Kir2.1 (lower set), Kir2.2 (middle set) and Kir2.3 (upper set). Multiple symbols in one set represent values from -100 (top) to -150 mV (bottom). The plots of Kir2.1 and 2.2 were fitted with the Hill equation, and the fitted curves were also drawn.
To examine whether the presence of a negatively charged amino acid at this position is critical for the sensitivity to the block by of the Kir2 family members, we introduced single point mutations into the latter. The E125Q mutant of Kir2.1 was almost insensitive to block (Fig. 3A and B). The introduction of Glu at corresponding sites of Kir2.2 (Q126E) and Kir2.3 (H116E) induced a high sensitivity (Fig. 3C and D), suggesting that these sites play a critical role in the determination of block sensitivity among Kir2 family members. As Mg2+ is a divalent cation, it is natural to expect that the negative charge of E125 is critical for the interaction. To examine this point, we made mutants in which E125 is mutated to Asp (D; Fig. 3E), Asn (N; Fig. 3F) and Lys (K; Fig. 3G). The E125D mutant, but not the E125K or the E125N mutant showed a clear sensitivity to the block. The dose- block relationship of the WT and these mutants at -120 mV were plotted (Fig. 4A), and the data of block-sensitive channels were fitted with the Hill equation (Fig. 4A). The Ki values were 10.6 ± 3.2 (n = 8; Kir2.1 WT), 13.9 ± 6.0 (n = 3; Kir2.1 E125D), 43.9 ± 9.4 (n = 3; Kir2.2 WT), 1.6 ± 0.6 (n = 3; Kir2.2 Q126E) and 3.0 ± 1.8 mm (n = 3; Kir2.3 Q116E). The Δ values were calculated from the voltage dependency of Ki (Fig. 4B). The values were 0.11 ± 0.02 (n = 8; Kir 2.1 WT), 0.10 ± 0.02 (n = 3; Kir 2.2 WT), 0.23 ± 0.04 (n = 3; Kir 2.1 E125D), 0.12 ± 0.02 (n = 3; Kir 2.2 Q126E), and 0.13 ± 0.02 (n = 3; Kir 2.3 H116E). The apparent increase in the E125D mutant was thought to be, at least partly, due to a downward curvature of I-V relationship observed in the absence of (Fig. 3E).
Figure 3. Effects of mutations of Kir 2.1 E125 and corresponding sites of Kir2.2 and Kir2.3 on the sensitivity to the block by .

A, macroscopic current recordings under two-electrode voltage clamp of the E125Q Kir2.1 mutant in 0 (left) and 10 mm (right) solutions. The concentration was 90 mm. The holding potential was 0 mV, and step pulses from +20 to -150 mV were applied in 10 mV decrements. B-D, I-V plots of the E125Q Kir2.1, Q126E Kir2.2 and H116E Kir2.3 mutants, respectively. The current amplitudes were measured at the beginning of step pulses in various concentrations of . E-G, I-V plots of the E125D, E125N and E125K Kir2.1 mutants, respectively.
Figure 4. dose-block relationships of the WT and mutants of Kir2 members and the voltage dependency of Ki values.
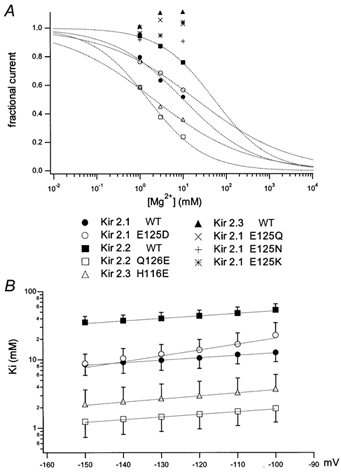
A, plots of the fraction of unblocked current in 1, 3 and 10 mm with respect to the value in 0 mm at -120 mV. For the block sensitive channels (Kir2.1 WT, Kir2.1 E125D, Kir2.2 WT, Kir2.2 Q126E, Kir2.3 H116E), the plots were fitted with the Hill equation and the fitted curves were also drawn. For the almost insensitive channels such as Kir2.1 E125Q, E125N, E125K and WT Kir2.3, reliable fittings could not be performed. B, the Ki values at each voltage were obtained by fitting with the Hill equation and the values were plotted against the membrane potential. The symbols are the same as in A. The Δ values which reflect the depth of the binding site in the electric field were calculated from the slope, using the equation described in Methods.
Inward rectifier K+ channels in the cardiac myocytes are known to be blocked not only by but also by (Shioya et al. 1993). To examine whether sensitivity of Kir2.1 and the difference between the WT and the E125Q mutant is specific to or not, we analysed the sensitivity to block by and (Fig. 5). The WT Kir2.1 was blocked by and in a weakly voltage-dependent manner (Fig. 5A and C), which is similar to block by (Fig. 2B), while E125Q was almost insensitive. The fractions of unblocked current at -100 mV by 10 mm were 0.66 ± 0.054 (n = 5; WT) and 0.93 ± 0.033 (n = 6; E125Q). Those by 3 mm were 0.69 ± 0.034 (n = 5; WT) and 0.91 ± 0.033 (n = 5; E125Q). The values of the WT and the E125Q mutant differ significantly. These results show that the weakly voltage-dependent block of WT Kir2.1 determined by E125 is not specific to , but is also caused by and .
Figure 5. Sensitivity to block by and of the WT and the E125Q mutant Kir2.1.

A and C, I-V relationships at the beginning of step pulses of the WT and B and D the E125Q Kir2.1 in A and B, various [Ca2+]o and C and D [La3+]oare shown.
Effects of other mutations in the extracellular loops of Kir2.1 to the block
In the extracellular loops of the M1-P and P-M2 regions of the Kir2.1 channel, there are four negatively charged amino acids other than E125, namely D112, D114, D152 and E153 (Fig. 1). With the hypothesis that one of these sites may determine the block sensitivity co-operatively with E125, we made single point mutants in which one of these four sites were neutralized. All these mutants showed functional expression, and were blocked by with a sensitivity similar to WT (n = 3, data not shown). These results indicate that these four negatively charged amino acids are not critically involved in the block, and E125 alone possibly determines the sensitivity in Kir2.1 channels. Therefore, we focused on the E125 site in the further analyses, as described below.
Effects of E125Q mutation on the block by
Alagem et al. (2001) observed that E125 of Kir2.1 affects the sensitivity to the block by , and concluded that E125 was a superficial site where Ba2+ interacts before entering into a deep pore-plugging site of T141. By performing similar experiments (Fig. 6A and B), we confirm that the dose-block relationship plot is shifted to the right in Kir2.1 E125Q mutant (Fig. 6C and D). Ki values at -120 mV were 1.38 ± 0.48 μm (n = 3; WT) and 6.32 ± 1.70 μm (n = 3; E125Q). The Δ values obtained from the voltage dependency of Ki were 0.39 ± 0.04 (n = 3; WT) and 0.44 ± 0.05 (n = 3; E125Q mutant; Fig. 6E). In contrast to the observations of Alagem et al. (2001), our results show that the E125 mutation clearly changes the sensitivity to the block by , but not the Δ value remarkably.
Figure 6. Comparison of the sensitivity to block of WT and the E125Q mutant Kir2.1.

A and B, I-V relationships for Kir2.1 WT and E125Q mutant, respectively. The current amplitudes were measured at the end of 1 s pulses where the current almost reached a steady level. The external solution contained 90 mm K+ and 3 mm Mg2+ with various concentrations of Ba2+. C and D, the fractions of unblocked current of the WT and the E125Q mutant, respectively, were calculated by dividing the amplitudes at the end of the 1 s pulse by those at the beginning. Plots were fitted with the Hill equation, and the fitted curves are shown as continuous curves. The data sets are for -70 to -120 mV, from top to bottom. E, the Ki values obtained by fitting with the Hill equation were plotted as a function of voltage.
Effects of on the block by in the WT and the E125Q mutant
Next, we analysed the effect of on kon values of block in the WT (Fig. 7A, C, E and G) and E125Q (Fig. 7B, D, F and H), to examine our hypothesis that and compete for E125. We obtained the inverse of the time constants of block process at various membrane potentials (Fig. 7A and B), and plotted the values in 0 (Fig. 7C and D) or 10 mm (Fig. 7E and F) as a function of [Ba2+]o. The slopes of this plot, which reflect the koff values of block at each potential as described in Methods, were plotted in Fig. 7G and H. The values at -120 mV were 0.65 ± 0.037 (n = 3; WT in 0 mm ), 0.17 ± 0.004 (n = 4; WT in 10 mm ), 0.082 ± 0.014 (n = 3; E125Q in 0 mm ) and 0.075 ± 0.009 μm−1 s−1 (n = 3; E125Q in 10 mm ). It is clear that: (1) the kon values of the E125Q mutant were significantly lower than those of the WT, and (2) the kon values of the WT decreased significantly with increasing , in contrast to E125Q whose kon values were almost unaffected by the change of .
Figure 7. Comparison of the rates of block in 0 and 10 mm of the WT (A, C, E and G) and the E125Q mutant (B, D, F and H) Kir2.1.

A and B, the time course of the block was fitted with exponential functions and the time constants (τ) were obtained, and 1/τ (= kon[Ba2+]o + koff) of the WT (A) and the E125Q mutant (B) were plotted as a function of the voltage. As the sensitivity to the block differed significantly, results of suitable concentrations of were used in each case for the analyses of blocking kinetics. C and D, 1/τ values of A and B in 0 mm were plotted against [Ba2+]o, and the symbols are for the data from -160 (top) to -90 mV (bottom). E and F, similar analysis to C and D. 1/τ values in 10 mm were plotted. G and H, the kon values at each potential were obtained as a slope in C-F as described in Methods. The values were plotted as a function of the voltage.
We also analysed the kinetics of recovery from block by 30 μm Ba2+ at depolarized potentials by a similar method described previously (Shieh et al. 1998; Alagem et al. 2001) (Fig. 8A), and observed that the recovery proceeded at a clearly slower rate in the E125Q mutant (Fig. 8B and C). To confirm our hypothesis that and compete for E125, we analysed the effect of on the recovery speed from a block. As shown in Fig. 8B and C, an increase in [Mg2+]o decelerated the recovery from a block in the WT, but not in the E125Q mutant (Fig. 8B and C). The values of 1/τrecovery at 0 mV were 0.98 ± 0.13 (n = 3; WT in 0 mm ), 0.64 ± 0.026 (n = 3; WT in 10 mm ), 0.31 ± 0.068 (n = 3; E125Q in 0 mm ) and 0.32 ± 0.044 s−1 (n = 4; E125Q in 10 mm ). The most straightforward interpretation of these results is that E125 is crucial for efficient entry and exit of Ba2+ into and out of the deep plugging site, and that hinders this step by competing with for this site.
Figure 8. Comparison of recovery of block in 0 and 10 mm of the WT and the E125Q mutant Kir2.1.

A, the time course of the recovery from block at depolarized potential was analysed. In this example, the WT Kir2.1 was in 3 mm with 30 μm , and the potential at which the block recovered was +60 mV. Voltage commands (upper) and current recordings (lower) are shown. The duration of recovery phase was changed as depicted in the graph. B, the time course of the recovery from the block was measured as an increase in the current amplitude by applying depolarizing step pulses for various duration. This example shows the recovery at +30 mV. C, the time constant of the recovery from the block (τrecovery) was obtained from the fittings in B, and the 1/τrecovery values which mainly reflect the koff values as described in Methods were plotted against voltage. The mean and standard deviation (n = 3-4) are shown.
Effects of on the block by
We speculated that might also interact with . We analysed the block of the WT Kir2.1 by in 4 mm K+ solution (Fig. 9A and B), and compared the intensity of the block with that in 90 mm solution, shown in Fig. 2. The plots of the dose-block relationship at -120 mV were fitted with the Hill equation (Fig. 9C), and the calculated Ki values were 10.6 ± 3.2 (n = 8; in 90 mm ) and 6.1 ± 0.9 mm (n = 8; in 4 mm ). Ki values at various potentials are plotted in Fig. 9D. Ki values in 4 mm are significantly lower than those in 90 mm . Taken together, the results show that decreases the sensitivity to the Mg2+ block of the WT Kir2.1 without changing the location of the depth of the binding site in the electric field remarkably. It was not possible to carry out similar studies using the E125Q mutant, because the block by was not apparent.
Figure 9. Effect of to the block by .

A, recordings of current in 4 mm K+ with 0 mm (left) and 10 mm (right) of Kir2.1 expressed in Xenopus oocytes. The holding potential was 0 mV, and step pulses from +20 to -160 mV were applied in 10 mV decrements. B, I-V relationship in various [Mg2+]o. C, comparison of dose-block relationship at -120 mV. The plots were fitted with the Hill equation, and the fitted curves are also shown. D, Ki values of the block at various potentials were calculated by fitting with the Hill equation, and plotted against the voltage. The mean and standard deviation (n = 8) are shown.
Effects of on the single channel conductance
Shioya et al. (1993) reported that reduces the single channel conductance of the inward rectifier K+ channel of the cardiac ventricular cells. They interpreted that this is due to an extremely fast block of K+ permeation beyond the bandwidth of the recording. We compared the single channel recordings of the WT and the E125Q mutant Kir2.1 in various and concentrations (Fig. 10). The gating properties did not show remarkable differences between the WT and the E125Q mutant, and were not apparently affected by or by . The values of single channel conductance in the absence of and were 39.6 ± 2.5 (n = 3; WT) and 26.0 ± 1.6 pS (n = 3; E125Q; Figs 10C and D, 11A and B). The values in the presence of were 21.1 ± 2.0 (n = 3; WT in 3 mm ), 11.5 ± 1.4 (n = 4; WT in 10 mm ), 24.5 ± 0.5 (n = 3; E125Q in 3 mm ) and 19.6 ± 1.2 pS (n = 3; E125Q in 10 mm ; Fig. 10C and Fig. 11A). Those in the presence of were 31.3 ± 1.4 (n = 3; WT in 3 mm ), 18.9 ± 2.0 (n = 3; WT in 10 mm ), 25.4 ± 2.4 (n = 3; E125Q in 3 mm ) and 20.8 ± 1.0 pS (n = 3; E125Q in 10 mm ; Fig. 10D and Fig. 11B). The decrease in single channel conductance by increasing or was more evident in Kir2.1 WT than in E125Q, as expected from the macroscopic recording. Interestingly, the single channel conductance of the E125Q mutant in 0 mm and was smaller than that of the WT. This result suggests that E125 is utilized also by while it travels through the channel efficiently.
Figure 11. Comparison between single channel conductances.

A and B, single channel conductance of the WT and the E125Q Kir2.1 in various and concentrations, respectively, were calculated from the plots of Fig. 10C and D. The mean and the standard deviation (n = 3-4) were plotted. C, single channel conductance of the WT and the Q126E mutant Kir2.2 in various concentrations. The mean and standard deviation (n = 3-4) are plotted.
From the macroscopic recording of the WT (Q126) and the Q126E mutant Kir2.2, we demonstrated that the site corresponding to E125 of Kir2.1 was also critical in Kir2.2. Here, we analysed the effect of on the single channel conductance of the WT and the Q126E mutant Kir2.2 (Fig. 11C). The values were 42.5 ± 1.8 (n = 3; Kir2.2 WT in 0 mm ), 26.7 ± 1.5 (n =3; WT in 3 mm ), 21.1 ± 0.3 (n = 3; WT in 10 mm ), 51.2 ± 1.6 (n = 3; Q126E in 0 mm ), 11.4 ± 0.8 (n = 3; Q126E in 3 mm ) and 6.0 ± 1.0 pS (n = 4; Q126E in 10 mm ). As expected, the presence of a negatively charged amino acid affected the change of single channel conductance in various , in Kir2.2 as well, in the same way as in Kir2.1.
Discussion
In this study, we focused on the difference of sensitivity to block by among the Kir family members, and identified E125 of Kir2.1 in the extracellular loop between M1 and P region as a critical site for the determination of the sensitivity. We also observed that E125 affected the sensitivity to the block by and the single channel conductance.
Functional role of E125 at the extracellular loop
From the analysis of the single channel conductance and the voltage dependency of block, Shioya et al. (1993) reported that causes an extremely fast open-channel block beyond the recording bandwidth at a very shallow site in the electric field. In this study, we focused on E125, a negatively charged amino acid residue in the extracellular loop.
We observed that the E125Q mutant of Kir 2.1 has a diminished sensitivity to block by . The importance of this residue was confirmed by the fact that the introduction of Glu to the corresponding sites of Kir2.2 and Kir2.3 with only a weak sensitivity gave rise to a high sensitivity. It was also confirmed from the results of various mutations, that not Glu itself but a negative charge was crucial from the results of various mutations. It was reported that E125 was also critical for the block (Alagem et al. 2001). These authors determined that E125 was in a shallow and intermediate site, and that T141 was in a deep plugging site. They also speculated that E125 was a critical site where dehydration occurs. We confirmed that E125Q mutation increases the Ki values, and that it decelerates both the block at hyperpolarized potential and the recovery from the block at depolarized potential, i.e. both the kon and the koff of the block were decreased by this mutation. We speculate that E125 increases the local concentration or the probability of the presence of at the entrance of the pore, and increases the kon values. Upon depolarization, the presence of the negatively charged site might facilitate the release from the deep pore plugging site by providing an intermediate binding site. The E125Q mutation also affected the single channel conductance in the absence of and . The value of E125Q was significantly lower than that of the WT, showing that E125 is critical also for efficient permeation of K+. A similar principle that increases the kon and the koff for the block might apply for K+ permeation (Fig. 12). Taken together, the results show that E125 is involved in the block by and by , as well as in K+ permeation.
Figure 12. Significance of E125 in the Kir2.1 channel.

Schematic diagrams depicting the WT (A) and the E125Q mutant (B), and explaining the competition of with the block by (left), and the effect of on the permeation of K+ (right). We speculate that E125 is a superficial site on the extracellular side which plays the role of increasing the local concentration of cations, and both and , as well as , compete for this site.
Interaction between , and
The following findings imply the interaction of , and . (1) The kon values of block at hyperpolarized potential and the koff values of recovery from block at depolarized potential decreased significantly by increasing in the WT but not in the E125Q mutant (Fig. 7 and Fig. 8). (2) The block was more enhanced in low solution than in high solution in the WT (Fig. 9). Owen et al. (1999) also observed a similar phenomenon. (3) Single channel conductance was decreased when the was increased, and this effect was more evident in the WT than in E125Q mutant (Fig. 10 and Fig. 11). These results suggest that E125 is a superficial site for which and compete in physiological conditions, and that and also compete for this site. In the E125Q mutant, the interpretation of the results is that the competition no longer occurs, and the various features described above were unaffected in this case. Alagem et al. (2001) also described the effect of pHo on the block, and reported that increase in [H+]o decreases the speed of recovery from block. This result suggests the possibility that also interacts with and physiologically in WT Kir2.1. Shieh et al. (1998) analysed the interaction between and intracellular K+ at a deep binding site and showed that intracellular K+ decreases the entrance rate of .
What are the molecular mechanisms for the ‘binding’ and the ‘competition’ of cations at E125? We observed that not only but also and block WT Kir2.1 in a similar weak voltage-dependent manner (Fig. 5). These results suggest that the ‘binding’ of Mg2+ to E125 does not occur in a specific manner. It is possible that the negatively charged environment provided by E125 plays a role in increasing the local concentration or the probability of presence of K+ ions at the entrance of the channel pore, and that the latter is diminished by non-specific screening effects of the negatively charged environment by divalent and trivalent cations (Fig. 12). The decrease in the single channel conductance by divalent cations in the previous study (Shioya et al. 1993) and in the present study (Fig. 10 and Fig. 11) could also be interpreted as being due to the decrease of the probability of the presence of K+ at the pore entrance, rather than the extremely fast block beyond recording resolution.
Physiological significance of the presence of E125
What is the physiological significance of the presence of E125 in WT Kir2.1? As this negatively charged amino acid is not conserved in other members of the Kir family, e.g. Kir2.2 and Kir2.3, it is reasonable to expect that the presence of E125 is not indispensable for the basic functioning of the inward rectifier K+ channel such as permeation of K+. A basically intact function as an inward rectifier K+ channel of E125Q mutant supports this idea. However, it is possible that E125 regulates the current amplitude and the cell excitability depending on [pH]o (Alagem et al. 2001) and [Mg2+]o.
Under physiological conditions, the resting membrane potential is mostly above the equilibrium potential for potassium (EK), and Kir2.1 allows small but significant outward current which is critical for the regulation of the resting membrane potential. Therefore, we examined the blocking effect of and on the outward current above EK of the WT and the E125Q mutant Kir2.1 (Fig. 13). The fractions of unblocked outward current at -20 mV in the presence of 10 mm were 0.44 ± 0.073 (n = 5; WT) and 0.81 ± 0.024 (n = 5; E125Q). The values in the presence of 30 μm were 0.30 ± 0.015 (n = 4; WT) and 0.52 ± 0.026 (n = 5; E125Q). From these results, we confirmed that similar blocking properties with respect to inward current are observed also for outward current at membrane potentials in the physiological range.
Figure 13. Sensitivity of the outward current of the WT and the E125Q mutant Kir2.1 to block by and .

The external solution contained 10 mm K+, and the holding potential was -50 mV. I-V relationships at the end of 200 ms step pulses of the WT (A and C) and the E125Q Kir2.1 (B and D) in various (A and B) and (C and D) concentrations are shown.
Structure of the outer mouth of the pore
As Mg2+ and Ba2+ are divalent cations, it is reasonable to expect that two negatively charged amino acids may interact with them. One possibility is that there are two interacting sites on the same subunit. As there are four other negatively charged amino acids in the extracellular loop region, we mutated each of them, one at a time, and observed that none of the four residues affects the sensitivity to block by . Therefore, we speculate that the sensitivity to block by is determined solely by E125 in Kir2.1, and that E125 residue(s) form (an) interacting site(s) for , and .
The structure of the transmembrane regions and the pore region of KcsA, a two transmembrane bacterial K+ channel was solved (Doyle et al. 1998), and the principle of high selectivity and optimal conduction of K+ was proposed (Zhou et al. 2001). The image of permeating K+ ions and water was also presented by high resolution X-ray analysis using the KcsA channel-Fab complex crystal (Zhou et al. 2001). Two K+ ions were unexpectedly observed at extracellular sites above the selectivity filter. One K+ at an upper site was present with water, and another K+ at a lower site was dehydrated. We speculate that E125 of Kir2.1 corresponds to one of these two sites where K+ is observed with a high probability. It is possible that E125 might correspond to the upper one of the two sites, and D152 and/or E153 of Kir2.1 might be the lower one, close to the selectivity filter. The reasons why mutations at D152 and E153 did not affect block may be that , in contrast to K+, interacts only with the upper site (E125) and does not access the lower site.
Sabirov et al. (1997) reported that R148 at the extracellular end of the selectivity filter functions as a barrier for various blocking ions. They showed that, the binding site for is slightly shifted by R148H mutation to a deeper position in the electric field. It is possible that R148 affects the position of E125, and R148H mutation change the distance of E125 from the membrane surface.
Acknowledgments
The authors would like to thank Dr Y. Kurachi for providing us with Kir2.2 and Kir2.3 cDNA clones; Dr S. H. Heinemann for invaluable suggestions; Drs S. Oiki, Y. Okamura, H. Okado, T. Misaka, H. Abe and K. Takahashi for discussion; Ms R. Watanabe for technical assistance; and Drs L. Guo and K. Nakajo for comments on the manuscript. This work was supported partly by research grants from the Ministry of Education, Science, Sports, Culture and Technology of Japan and from foundations of Mitsubishi, Toyota, Uehara, Umami, Kowa, Novartis, and Salt Science.
References
- Alagem N, Dvir M, Reuveny E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. Journal of Physiology. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermans G, Vereecke J. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflügers Archiv. 1987;10:604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Elam TR, Lansman JB. The role of Mg2+ in the inactivation of inwardly rectifying K+ channels in aortic endothelial cells. Journal of General Physiology. 1995;105:463–484. doi: 10.1085/jgp.105.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Single channel potassium currents of the anomalous rectifier. Nature. 1981;294:368–371. doi: 10.1038/294368a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA, USA: Sinauer Associates Inc.; 2001. [Google Scholar]
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Japanese Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. Journal of Physiology. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Morishige K, Takahashi N, Zanelli JS, Fass DN, Kurachi Y. Molecular cloning, functional expression and localization of a novel inward rectifier potassium channel in the rat brain. FEBS Letters. 1994;341:303–307. doi: 10.1016/0014-5793(94)80478-8. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Murata Y. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2. 1 K+ channel. Journal of Physiology. 2001;531:645–660. doi: 10.1111/j.1469-7793.2001.0645h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Matsuura H. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. Journal of Physiology. 1989;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- Morishige K, Takahashi N, Jahangir A, Yamada M, Koyama H, Zanelli JS, Kurachi Y. Molecular cloning and functional expression of a novel brain-specific inward rectifier potassium channel. FEBS Letters. 1994;346:251–256. doi: 10.1016/0014-5793(94)00483-8. [DOI] [PubMed] [Google Scholar]
- Murata Y, Okado H, Katsuyama Y, Okamura Y, Kubo Y. Primary structure, developmental expression and functional properties of an inward rectifier K+ channel of the tunicate. Receptors and Channels. 2001a;7:387–399. [PubMed] [Google Scholar]
- Murata Y, Okado H. Characterization of heteromultimeric G protein-coupled inwardly rectifying potassium channels of the tunicate tadpole with a unique pore property. Journal of Biological Chemistry. 2001b;276:18529–18539. doi: 10.1074/jbc.M009644200. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annual Review of Physiology. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Owen JM, Quinn CC, Leach R, Findlay JB, Kubo Y. Effect of extracellular cations on the inward recfying K+ channels Kir2. 1 and Kir3.1/3.4. Experimental Physiology. 1999;84:471–488. [PubMed] [Google Scholar]
- Sabirov RZ, Tominaga T, Miwa A, Okada Y, Oiki S. A conserved arginine residue in the pore region of an inward rectifier K channel (IRK1) as an external barrier for cationic blockers. Journal of General Physiology. 1997;110:665–677. doi: 10.1085/jgp.110.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh RC, Chang JC, Arreola J. Interaction of Ba2+ with the pores of the cloned inward rectifier K+ channels Kir2. 1 expressed in Xenopus oocytes. Biophysical Journal. 1998;75:2313–2322. doi: 10.1016/S0006-3495(98)77675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioya T, Matsuda H. Fast and slow blockades of the inward-rectifier K+ channel by external divalent cations in guinea-pig cardiac myocytes. Pflugers Archiv. 1993;422:427–435. doi: 10.1007/BF00375067. [DOI] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. Journal of Physiology. 1978;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. Journal of Physiology. 1980;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proceedings of the National Academy of Sciences of the USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995;14:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2. 0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]


