Abstract
The cardiovascular response to exercise with several groups of skeletal muscle suggests that work with the arms may decrease leg blood flow. This study evaluated whether intense exercise with the legs would have a similar effect on arm blood flow (Q̇arm) and O2 consumption (V̇O2,arm). Ten healthy male subjects (age 21 ± 1 year; mean ± S.D.) performed arm cranking at 80 % of maximum arm work capacity (A trial) and combined arm cranking with cycling at 60 % of maximum leg work capacity (A + L trial). The combined trial was a maximum effort for 5-6 min. Q̇arm measurement by thermodilution in the axilliary vein and arterial and venous blood samples permitted calculation of V̇O2,arm. During the combined trial, Q̇arm was reduced by 0.58 ± 0.25 l min−1 (19.1 ± 3.0 %, P < 0.05) from the value during arm cranking (3.00 ± 0.46 l min−1). The arterio-venous O2 difference increased from 122 ± 15 ml l−1 during the arm trial to 150 ± 21 ml l−1 (P < 0.05) during the combined trial. Thus, V̇O2,arm (0.45 ± 0.06 l min−1) was reduced by 9.6 ± 6.3 % (P < 0.05) and arm vascular conductance from 27 ± 4 to 23 ± 3 ml min−1 (mmHg)−1 (P < 0.05) as noradrenaline spillover from the arm increased from 7.5 ± 3.5 to 13.8 ± 4.2 nmol min−1 (P < 0.05). The data suggest that during maximal whole body exercise in humans, arm vasoconstriction is established to an extent that affects oxygen delivery to and utilisation by working skeletal muscles.
In humans, it is not established whether sympathetic vasoconstriction influences blood flow in active skeletal muscles. It has been argued that because of local regulation of muscle blood flow during exercise sympathetic control is lost (Remensnyder et al. 1962; Donald et al. 1970). However, evidence of such sympathetic vasoconstriction in active muscle has been reported (Saito et al. 1992; Kagaya, 1993; Kagaya et al. 1994). In these studies calf blood flow was reduced when fatiguing handgrip exercise was added to ongoing plantar flexion exercise.
Perfusion of the quadriceps muscles or the whole leg has been studied extensively during exercise. A dynamic exercise knee-extensor model or cycling has been used to evaluate the perfusion of the thigh, i.e. the quadriceps muscles, or of the whole leg. Blood flow values that have been measured in the leg are in the range of 6-9 l min−1, which may represent a perfusion of 300-400 ml (100 g)−1 min−1 (Andersen & Saltin, 1985; Rowell et al. 1986; Richardson et al. 1993). If these muscle perfusion values were to be attainable for the whole human musculature, a net cardiac output (Q) of about 100 l min−1 (≈3 times as high as the highest Q measured) would be required to maximally perfuse all skeletal muscle during maximal whole body exercise. Since blood pressure always increases with exercise intensity, even when involving the whole body musculature as occurs during combined arm and leg exercise (Bevegård et al. 1966; Stenberg et al. 1967; Secher et al. 1977), it could be assumed that a certain degree of systemic vasoconstriction must take place. The intense sympathetic activity developed during heavy exercise results in differentiated vasoconstriction in, for example, the splanchnic circulation (Perko et al. 1998). However, it is not clear from the human data whether the increased sympathetic activity also affects working skeletal muscle perfusion. Secher et al. (1977) showed that when arm cranking is added to cycling (a combined exercise intensity of ≈77 % of maximal oxygen uptake (V̇O2,max), leg blood flow was reduced. However, subsequent studies that attempted to reproduce Secher's results did not show any evidence of neurogenic vasoconstriction during whole body exercise (Savard et al. 1989; Richter et al. 1992; Richardson et al. 1995; Bangsbo et al. 1997). Even though a reduction in leg vascular conductance was observed, there was no significant change in leg blood flow when arm exercise was added to leg exercise. Furthermore, an attempt to maximise sympathetic activation by static or ischaemic arm exercise affected leg vascular conductance, but not leg blood flow (Strange, 1999).
Data on arm blood flow (Q̇arm) and metabolism during arm exercise are limited. Ahlborg & Jensen-Urstad (1991a,b) measured Q̇arm and metabolism at rest and during arm cranking up to 78 % of V̇O2,max). They infused cardiogreen into the brachial artery and measured its concentration in blood withdrawn from the axillary vein as an expression of Q̇arm. Blood flow increased linearly with workrate and V̇O2 in most subjects, or it reached a plateau in some cases. Since the sympathetic activity, expressed as plasma levels of catecholamines during exercise, is proportional not only to the exercise intensity but also to the active muscle mass (Savard et al. 1989), it would be expected that the addition of leg to arm exercise would induce a greater increase in sympathetic activity than the addition of arm to leg exercise. Indeed, Secher et al. (1977) showed that the addition of cycling to arm cranking increased the oxygen extraction of the arm when working at the same work rate. Since an increased oxygen extraction is a response mechanism, aiming to maintain tissue oxygen uptake when delivery is limited, it also suggests a blood flow reduction was taking place. However, Secher et al. (1977) did not measure Q̇arm. The aim of the present study was to evaluate whether Q̇arm and V̇O2,arm decrease when leg exercise is added to arm exercise at a given work rate.
Methods
Following informed written consent ten healthy males (age 21 ± 1 year, height 183 ± 4 cm, weight 82 ± 3 kg, mean ± s.d.) participated in the study. The study was approved by the Copenhagen Ethics Committee (KF 01-314/97) and performed according to the Declaration of Helsinki. The subjects were physically active but not arm trained. All subjects were in the post-prandial phase when they reported to the laboratory and had been asked to refrain from any caffeine-containing products and intense physical activity on the previous day.
Experimental protocol
Following arterial and venous catheterisation of the non-dominant arm, the subjects performed in random order an A trial and an A + L trial for 5-6 min each. The maximum ≈5 min work capacities and V̇O2,max for the two exercise modes as well as for cycling had been determined in previous laboratory visits (A, 153 ± 10 W; cycling, 345 ± 20 W). Arm cranking was performed on a modified friction-braked cycle ergometer (Monark, Stockholm, Sweden), while cycling was performed on an electrically braked ergometer (Elema, Stockholm, Sweden). The arm cranking frequency was metronome paced at 70 revolutions min−1. The subjects were seated upright on the cycle ergometer with the arm cranking ergometer placed in front of them at a height and distance that ensured full arm extension in the horizontal position. Before the two trials, the subjects warmed up for 15 min using A + L at 40 % of the combined arm and leg work capacity. During the A trial the work intensity was 80 % of the arm work capacity. During the A + L trial, the work intensity for arm cranking was kept the same as in the A trial, while cycling at ≈60 % of work capacity was added. All subjects reached their limits of exercise tolerance in 5-6 min during the A + L trial that required ≈95 % of the subject's V̇O2,max. A recovery period of 15 min was used between the two trials. The subjects were asked to use their arms equally during cranking so that the blood flow measurement would be representative of both arms.
A bolus thermodilution technique was used to measure Q̇arm. Under local anaesthesia, a pulmonary artery catheter (Swan-Ganz 132F5, Baxter Healthcare Corporation, Irvine, CA, USA) was introduced into the vena basilica at the elbow and advanced so that the thermistor was lying in the axillary vein. It was important to verify that the catheter was advanced enough to register flow from both the basilic and cephalic veins, but not advanced into the caval vein. To confirm this placement the following procedure was performed. A 5 ml bolus of saline at room temperature was injected into another catheter introduced into the cephalic vein of both arms. When saline was injected into the same arm as the Swan-Ganz catheter the blood temperature changed, while a similar injection into the catheter of the other arm had no effect. Three millilitres of room temperature saline boluses were infused and the temperature change was monitored with an Explorer Cardiopulmonary Hemodynamic Monitor (Baxter Healthcare) to ensure a monophasic thermodilution curve with an exponential decay. The bolus method provides similar values of Q̇arm to those obtained by constant infusion of cooled saline (correlation coefficient, r = 0.89, P < 0.01, P. Krustrup, unpublished data).
During the first 3 min of each trial, measurements of Q̇arm were taken every 15 s. After the first 3 min of each trial, samples (3-10 ml) of arterial and venous blood were drawn anaerobically over 10-20 s, from the tip of the catheter and from a catheter placed in the radial artery of the same arm for blood-gas, metabolites, and plasma catecholamine analyses. Following blood sampling, additional flow measurements were taken until the completion of the trial. Thus, for each trial the arm blood flow was expressed as the average of 10-12 separate measurements. Even though there was no statistical difference between measurements taken before and after blood sampling, measurements from the first minute were excluded from the calculation to ensure that the initial phase of rapid blood flow elevation was not included in the averaging. Nevertheless, it should be emphasised that both exercise trials represent unsteady state conditions.
Blood variables
Haemoglobin, oxygen and carbon dioxide tensions (PO2 and PCO2), oxygen saturation (SO2), lactate, glucose, potassium, sodium and calcium were determined on an ABL blood analyser (model 700 Radiometer, Copenhagen, Denmark). Duplicate analyses from the same blood sample were made and the mean value of the two determinations was reported. Plasma adrenaline and noradrenaline (NA) levels were determined by a high-pressure liquid chromatography method with electrochemical detection. The rate of spillover of NA into plasma was determined as previously reported using flow rates, haematocrit, the difference in NA concentration between axilliary venous and arterial plasma, and the fractional extraction of adrenaline (Esler et al. 1990).
Central cardiovascular variables
The pulse contour method (Modelflow) was used to estimate the beat-to-beat changes in Q from the intra-radial arterial pressure input. This method uses a haemodynamic, non-linear, three-element model that relates the arterial pressure or pressure difference to a flow or volume via the impedance through which the flow is driven. The resultant flow waveform is integrated per beat to yield stroke volume that is multiplied by the heart rate (HR) to estimate Q (Wesselling et al. 1983). The model can be expressed as:
where Asys is the area under the systolic portion of the pressure wave and Zao the characteristic impedance of the aorta estimated from the age, mean pressure and heart rate. During exercise Q values obtained by the Modelflow method are comparable to those obtained by dye dilution (Ide et al. 1998).
Pulmonary gas exchange was measured with an Oxyscreen (CPX/D; Medical Graphics Corporation, St. Paul, Minnesota) metabolic cart and 15 s averaged values are reported. The HR was measured with a Vantage NV pulse watch (Polar Electro OY, Kempele, Finland). Mean arterial pressure (MAP) was obtained from the arterial catheter connected to a pressure monitoring kit (Baxter Healthcare Corporation, Irvine, CA, USA) positioned at the level of the heart with continuous infusion of isotonic saline (3 ml h−1). Arm vascular conductance was reported as the ratio between Q̇arm and MAP. For the calculation of arm vascular conductance, it was assumed that the influence of muscle contractions on blood flow was the same at a given work load and revolution rate.
Data are presented as mean ± s.e.m. for the whole exercise period. One-way ANOVA with repeated measures and Bonferroni post hoc test was used to assess differences between rest and the two exercise trials. For the meta-analysis of earlier relevant studies the Glass's effect size was used to compare results across studies. For continuous quantitative outcomes the standardised mean difference divided by the standard deviation was used as an index of effect size, as described by Hedges & Olkin (1985). A summary of weighted effect sizes and 95 % confidence intervals (CI) for all the studies were calculated. Statistical significance was set at P < 0.05.
Results
The arm workload was 122 ± 8 W during both trials while, during the A + L trial, the leg work load was 207 ± 12 W. The V̇O2 increased from 0.31 ± 0.01 l min−1 at rest to 2.36 ± 0.89 l min−1 during the A trial and to 3.85 ± 0.18 l min−1 during the A + L trial (≈95 % of V̇O2,max measured during combined exercise; 4.04 ± 0.28 l min−1).
Carbon dioxide (V̇CO2) and minute ventilation (V̇E) increased in a similar fashion from 0.24 ± 0.01 and 9 ± 3 l min−1, respectively, at rest to 2.59 ± 0.14 and 77 ± 6 l min−1, respectively, during the A trial. During the A + L trial V̇CO2 and V̇E rose even higher to 4.12 ± 0.21 and 115 ± 3 l min−1 respectively. Similarly, HR increased from 77 ± 4 beats min−1 at rest to 147 ± 6 beats min−1 during the A trial and to 187 ± 3 beats min−1 during the A + L trial. The MAP increased from 83 ± 1 mmHg at rest to 112 ± 2 mmHg during the A trial and fell to 103 ± 2 mmHg during the A + L trial. The Q was increased from 6.2 ± 0.3 l min−1 at rest to 14.1 ± 0.6 and 23.8 ± 0.7 l min−1 during the A and A + L trials, respectively.
At rest Q̇arm was 0.35 ± 0.04 l min−1 and it increased to 3.00 ± 0.46 l min−1 during the A trial. During the A + L trial, Q̇arm was 0.58 ± 0.25 l min−1 lower than during the A trial (19.1 ± 3.0 %). The arterio-venous oxygen difference increased from 59 ± 4 ml l−1 at rest to 122 ± 15 ml l−1 in the A trial and to 150 ± 21 ml l−1 during the A + L trial (Fig. 1). The arm vascular conductance was increased from 5 ± 0 ml min−1 (mmHg)−1 at rest to 27 ± 4 ml min−1 (mmHg)−1 during the A trial and to 23 ± 3 ml min−1 (mmHg)−1 during the A + L trial. Thus, V̇O2,arm was 0.45 ± 0.06 l min−1 during the A trial, but it was reduced by 9.6 ± 6.3 % to 0.40 ± 0.06 l min−1 during the A + L trial.
Figure 1. Central and regional cardiovascular and metabolic responses to arm (A) and combined arm and leg (A + L) exercise.
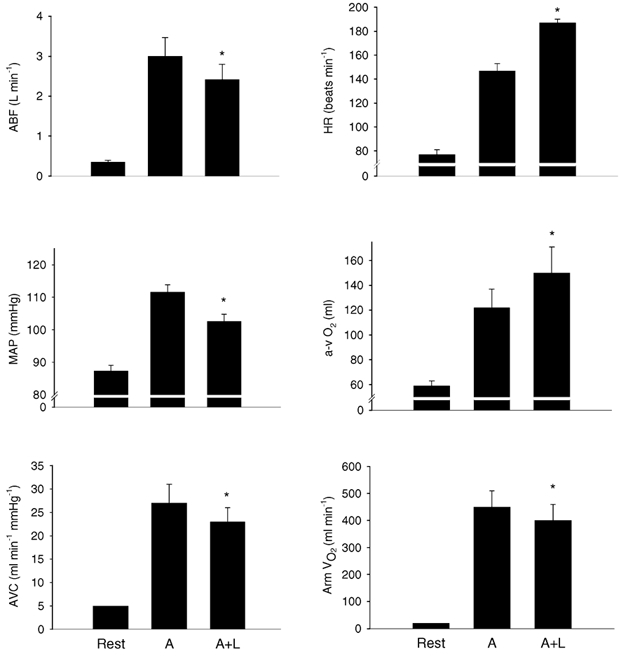
ABF, arm blood flow; HR, heart rate; MAP, mean arterial pressure; a-v O2, arterio-venous oxygen difference; AVC, arm vascular conductance, Arm V̇O2, arm oxygen consumption. Values are means ± s.e.m.; n = 10. * Different from A, P < 0.05.
Arterial pH decreased from 7.42 ± 0.01 at rest to 7.35 ± 0.02 during the A trial and to 7.31 ± 0.02 during the A + L trial (Table 1). Similarly, pH of blood from the axillary vein decreased from 7.35 ± 0.02 at rest to 7.21 ± 0.02 and to 7.16 ± 0.02 during the A and A + L trials, respectively. During the A + L trial there was a steep increase in lactate concentration in arterial blood compared with the A trial. However, the increase of the venous lactate concentration, even though significant, was moderate and lactate release from the arm decreased. Compared to the resting value, bicarbonate decreased in the A trial and this decrease was even more pronounced during the A + L trial. The PCO2 was not different between the two trials. The venous plasma potassium reached 6.1 ± 0.2 and 7.0 ± 0.4 mm during the A and A + L trials, respectively. Plasma glucose did not change significantly but the venous concentration was always higher than the arterial concentration, i.e. there was a net glucose release from the arm during both trials.
Table 1.
Arm metabolic responses to arm (A) and combined arm plus leg exercise (A+L)
| Rest | A | A + L | |
|---|---|---|---|
| O2 content, arterial (ml 1−1) | 200 ± 4 | 211 ± 5 | 214 ± 5 |
| Venous (ml 1 −1) | 128 ± 9 | 98 ± 5* | 73 ± 6*† |
| (a-v) diff. (ml 1−1) | 59 ± 4 | 122 ± 15* | 150 ± 21*† |
| pH, arterial | 7.42 ± 0.01 | 7.35 ± 0.02* | 7.31 ± 0.02*† |
| Venous | 7.35 ± 0.02 | 7.21 ± 0.02* | 7.16 ± 0.02*† |
| PCO2, arterial (kPa) | 4.69 ± 0.00 | 4.00 ± 0.00* | 4.00 ± 0.10* |
| Venous (kPa) | 6.19 ± 0.10 | 7.59 ± 0.00* | 7.90 ± 0.05* |
| HCO3−, arterial (mm) | 22 ± 0 | 17 ± 1* | 15 ± 1*† |
| Venous (mm) | 27 ± 0 | 24 ± 0* | 22 ± 1*† |
| Lac, arterial (mm) | 0.8 ± 0.1 | 7.0 ± 0.9* | 11.3 ± 1.2*† |
| Venous (mm) | 1.1 ± 0.1 | 10.1 ± 1.2* | 12.8 ± 1.4*† |
| (a-v) diff. (mm) | −0.1 ± 0.1 | −3.2 ± 0.5* | −1.7 ± 0.3*† |
| Release (mmol min−1) | 0.0 ± 0.0 | 7.4 ± 0.6* | 3.7 ± 0.6*† |
| Glu, arterial (mm) | 4.9 ± 0.3 | 4.7 ± 0.2 | 5.1 ± 0.2 |
| Venous (mm) | 5.2 ± 0.1 | 5.2 ± 0.2 | 5.4 ± 0.2 |
| (a-v) diff. (mm) | −0.3 ± 0.2 | −0.5 ± 0.2 | −0.2 ± 0.2† |
| Release (mmol min−1) | 0.1 ± 0.1 | 1.6 ± 0.8 | 0.7 ± 0.4 |
| K+, arterial (mm) | 3.7 ± 0.2 | 4.5 ± 0.3* | 5.4 ± 0.3*† |
| Venous (mm) | 4.5 ± 0.1 | 6.1 ± 0.2* | 7.0 ± 0.4*† |
| (a-v) diff. (mm) | −0.8 ± 0.1 | −1.6 ± 0.3* | −1.6 ± 0.3* |
| Release (mmol min−1) | 0.3 ± 0.0 | 4.5 ± 1.1* | 3.4 ± 0.7* |
| Na+, arterial (mm) | 140 ± 0 | 144 ± 1* | 145 ± 1* |
| Venous (mm) | 140 ± 1 | 145 ± 0* | 146 ± 0* |
| (a-v) diff (mm) | 1 ± 0 | −1 ± 0 | 0 ± 0 |
| Release (mmol min−1) | 0 ± 0 | 3 ± 1 | 1 ± 1 |
| Ca2+, arterial (mm) | 1.14 ± 0.02 | 1.19 ± 0.03 | 1.25 ± 0.03*† |
| Venous (mm) | 1.29 ± 0.01 | 1.39 ± 0.02* | 1.39 ± 0.02* |
| (a-v) diff (mm) | −0.14 ± 0.02 | −0.19 ± 0.04 | −0.14 ± 0.03 |
| Release (mmol min−1) | 0.02 ± 0.01 | 0.53 ± 0.16* | 0.31 ± 0.09* |
Values are means ± s.e.m.; (n = 10). (a-v) diff., arterial axillary venous oxygen difference; pH, H+ concentration; PCO2, carbon dioxide partial pressure; HCO3−, bicarbonate; Lac, lactate; Glu, glucose; K+, potassium; Na+, sodium; Ca2+, calcium.
Different from rest, P < 0.05
different from A, P < 0.05.
The arterial plasma ionised calcium increased only marginally (P = 0.18) during the A trial, but the increase became significant during the A + L trial (Table 1). In venous blood, plasma calcium was increased from the resting value, but there was no significant difference between the two trials. Plasma calcium was higher in venous compared with arterial blood. Plasma sodium increased from 140 ± 0 at rest to 145 ± 1 mm during both exercise trials. The NA spillover increased 37-fold from rest to the A trial and thereafter almost doubled during the A + L trial (Fig. 2).
Figure 2. Catecholamines and noradrenaline spillover at rest and during arm (A) and combined arm and leg (A + L) exercise.
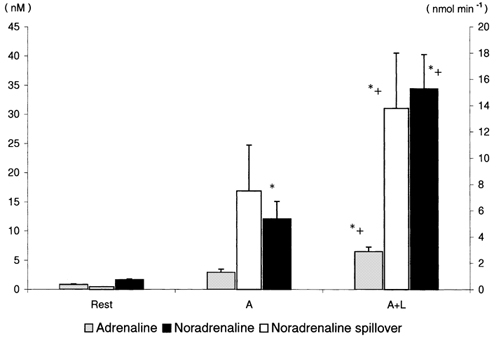
Values are means ± s.e.m.; n = 10. * Different from rest; + different from A, P < 0.05.
Discussion
The major new finding of this study is that during combined arm and leg exercise, arm blood flow was about 20 % lower than during arm exercise alone. Our data demonstrate local vasoconstriction in the exercising arm during maximal whole body exercise to an extent that affects oxygen delivery to and utilisation by the working muscles.
During dynamic exercise the traditional view of metabolic muscle vasodilatation is that it eliminates the effect of the increased sympathetic activity and thereby serves for adequate regional perfusion. This model, termed ‘functional sympatholysis’ (Remensnyder et al. 1962) suggests that muscle blood flow is locally regulated by the release of substances (e.g. potassium) which directly or indirectly impede the α-sympathetic tone (Hansen et al. 2000). An increase in exercise intensity is associated with increased sympathetically mediated systemic vasoconstriction in internal organs including the splanchnic area (Perko et al. 1998). Furthermore, during dynamic exercise, sympathetic vasoconstriction, also occurring in active skeletal muscle, has been shown both in animals (O'Leary et al.1997; Buckwalter & Clifford, 1999) and in humans (Joyner et al. 1992). Therefore, it seems that the perfusion of contracting skeletal muscle is the result of a dynamic balance between metabolic vasodilatation, sympathetic vasoconstriction and the effect of the muscle pump. The net result of this dynamic balance seems to be partially dependent on the overall exercise intensity that results from multiple contracting muscles (Secher et al. 1977; Saito et al. 1992; Kagaya, 1993; Kagaya et al. 1994) as well as on the ability to increase cardiac output (Pawelczyk et al. 1992). During low intensity exercise oxygen delivery, by means of vasodilatation, is prioritised, while during intense whole body exercise, as is the case in the present study, blood pressure regulation, and therefore vasoconstriction, becomes of importance.
Previous studies that investigated the effect of adding arm to leg exercise on the leg blood flow have contrasting results (Secher et al. 1977; Savard et al. 1989; Richter et al. 1992; Richardson et al. 1995; Bangsbo et al. 1997). In fact, all but Secher's study have failed to demonstrate such vasoconstriction. One explanation for the discrepancy between these reports may be the choice of relative workloads shared between the arms and the legs during the exercise protocol. It is likely that the exercise protocol used to investigate the effect of sympathetic vasoconstriction on the blood flow of active skeletal muscle should be strenuous enough to challenge the Q capacity, but not so intense that the exercise time is inadequate for the required measurements. During combined arm and leg exercise an elevated leg NA spillover was noted by Savard et al. (1989), Richter et al. (1992) and Richardson et al. (1995). From these data it can be argued that up to a given level, local vasodilatation is not overridden by sympathetic tone. It seems that net vasoconstriction ensues when the peripheral blood flow demand challenges the regulation of blood pressure, as demonstrated with an 8 % blood pressure reduction from the A to the A + L trial, in confirmation of data by Bevegård et al. (1966), Stenberg et al. (1967) and Secher et al. (1977).
Another explanation for the contrasting results amongst earlier studies may be the difference in experimental design and the lack of statistical power, due to a small sample size, to detect significant differences. Indeed, a meta-analysis of these studies including 32 subjects (Secher et al. 1977; Savard et al. 1989; Richter et al. 1992; Richardson et al. 1995; Bangsbo et al. 1997) revealed a mean effect size of 0.732 (95 % CI, 0.328-1.137) and a mean ± s.d. blood flow reduction of 11.0 ± 3.7%.
A decrease in flow with unchanged driving pressure would imply a decrease in arm vascular conductance. However, the 8 % reduction in MAP during the A + L trial suggests that the ≈20 % reduction in arm blood flow cannot be attributed only to the sympathetic vasoconstriction but is also caused by the reduced driving pressure. On the other hand, support for a sympathetic influence on arm vascular conductance comes from a two-fold increase in noradrenaline spillover, which is an index of sympathetic nerve activity. During the A + L trial the lowered blood pressure observed in our study may have triggered the arterial baroreceptors that would have to either increase Q or reduce the vascular bed in order to restore the blood pressure to the set point established during arm exercise. Indeed, Q was increased by 68 % from the A trial to the A + L trial. However, increasing Q was not a sufficient response to restore blood pressure, and a reduction of the vascular bed by means of vasoconstriction appeared to be required. Furthermore, preliminary data from ongoing studies in our laboratory suggest that the baroreceptor operating point may be reset lower during the A + L compared to A exercise. This finding could explain the lower blood pressure observed during the A + L trial.
Two mechanisms have been suggested to govern blood pressure and elicit the net vasoconstriction and reduced blood flow that we observed in our study: (a) an influence from the central nervous system ‘central command’ (Querry et al. 2001) and (b) a muscle chemoreflex (Gallagher et al. 2001). Vasodilatation in active muscle facilitates oxygen delivery for the increasing metabolic demand, while vasoconstriction regulates arterial pressure. The arterial baroreceptor response through the sympathetic branches of the autonomic nervous system aims to return arterial pressure to the established central nervous system operating point (Raven et al. 2002). The outcome of the baroreceptor response is reflex tachycardia and systemic vasoconstriction. In our study, the vasoconstriction required for the maintenance of arterial pressure seems to override the vasodilatation needed for oxygen delivery and even ultimately for oxygen utilisation. These data support the notion that the arterial pressure is the primary regulated variable during whole body dynamic exercise.
Secher et al. (1977) showed reduced leg blood flow by addition of arm exercise even though the combined exercise intensity was only 77 % of V̇O2,max, which seems unlikely to require a maximal Q. The results showed, however, that leg blood flow was only reduced when the arm intensity (% of arm V̇O2,max) exceeded 40 % of V̇O2 during the combined exercise. Therefore, it is possible that vasoconstriction in the legs was effected due to the fatiguing workload imposed on the arms, without Q being maximal. Support for the existence of this mechanism comes from the studies of Saito et al. (1992), Kagaya (1993) and Kagaya et al. (1994) who reported a reduction in calf blood flow during fatiguing forearm contractions. In addition, Harms et al. (1997) reported that respiratory muscle fatigue can induce vasoconstriction in the exercising legs. Therefore, a second mechanism responsible for sympathetic excitation and vasoconstriction may be the muscle chemoreflex originating from type III and IV afferents stimulated by fatiguing muscle contractions (Harms et al. 1997).
The novelty of our findings is that the magnitude of the sympathetic tone was so high that it induced not only a reduction in flow but also in V̇O2,arm. Even though oxygen extraction increased in the A + L trial, V̇O2,arm was not maintained due to the disproportionate reduction in blood flow. The effect of leg exercise on arm a-v O2 difference has been reported by Secher et al. (1977) where an increase was seen only during intense leg exercise. In our study the 23 % increase in the arm oxygen extraction during the A + L trial (with ≈60 % of work capacity) is in agreement with the previous data. The reduction in V̇O2,arm implies that ATP resynthesis from oxidative phosphorylation was impaired. Since power output was maintained constant, a proportionally greater reliance on non-oxidative ATP production might have occurred. The presumed increased energy contribution from anaerobic metabolism may have contributed to the increased lactate concentration in the venous blood during the A + L trial. However, the lactate (v-a) diff. suggests that the increase in arterial lactate concentration was not established by an increased lactate release from the arm muscles, which was lower, but possibly by a reduced lactate clearance from other organs. Indeed, the legs release lactate during combined arm and leg exercise compared with a lactate clearance when the arms are working alone (Secher et al. 1977). Furthermore, with increased systemic vasoconstriction, hepato-splanchnic blood flow decreases and the contribution of the Cori cycle to glucose production is minimised (Nielsen et al. 2002). Therefore, a reduction in the lactate uptake by the liver may have also contributed to the accumulation of lactate in arterial blood. The decreased net lactate release may be explained by lactate uptake and partial utilisation of lactate as substrate. The increased arterial lactate concentration during the combined exercise possibly enhanced muscle lactate uptake by the arms as previously suggested (Richter et al. 1988) and decreased lactate release through a reduction of the muscle to blood lactate gradient as implicated by Richardson et al. (1995). The trend of the glucose (v-a) difference, which was almost halved during the A + L trial compared with the A trial, confirms a glucose release from contracting muscle at the onset of intense exercise (Jorfeldt & Wahren, 1970). The increase in ionised calcium, parallel to the drop in pH, reflects the increased calcium release from albumin and the depression of calcium reuptake by the sarcoplasmic reticulum (Byrd et al. 1989). The two-fold increase in NA spillover was apparently effective in counteracting the elevated, e.g. potassium-dependent, vasodilating stimulus and producing a net vasoconstriction in the working arm.
It should be mentioned that the combination of workloads used during the A + L trial may not be a ‘realistic’ way to distribute the effort between arms and legs during whole body exercise. The exercise protocol was meant to be a model for the demonstration of a physiological mechanism. It is most likely that whole body exercise is performed at a lower intensity for the arms and the legs, i.e. at an intensity that does not elicit a mutual reduction in flow.
Acknowledgments
This research has been supported by a Marie Curie Fellowship of the European Community programme, Improving Human Potential under contract number HPMF-CT-2000-00526 and by the Danish National Research Foundation (504-14). We thank anaesthesia nurse Peter Nissen for expert technical assistance.
References
- Ahlborg G, Jensen-Urstad M. Arm blood flow at rest and during arm exercise. Journal of Applied Physiology. 1991a;70:928–933. doi: 10.1152/jappl.1991.70.2.928. [DOI] [PubMed] [Google Scholar]
- Ahlborg G, Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clinical Physiology. 1991b;11:459–468. doi: 10.1111/j.1475-097x.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. Journal of Physiology. 1997;504:489–499. doi: 10.1111/j.1469-7793.1997.489be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevegård S, Freyschuss U, Strandell T. Circulatory adaptation to arm and leg exercise in supine and sitting position. Journal of Applied Physiology. 1966;21:37–46. doi: 10.1152/jappl.1966.21.1.37. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. Alpha-adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. American Journal of Physiology. 1999;277:33–39. doi: 10.1152/ajpheart.1999.277.1.H33. [DOI] [PubMed] [Google Scholar]
- Byrd SK, McCutheon LJ, Hodgson DR, Gollnick PD. Altered sarcoplasmic reticulum function after high-intensity exercise. Journal of Applied Physiology. 1989;67:2072–2077. doi: 10.1152/jappl.1989.67.5.2072. [DOI] [PubMed] [Google Scholar]
- Donald DE, Rowlands DJ, Ferguson DA. Similarity of blood flow in the normal and sympathectomised dog limb during graded exercise. Clinical Research. 1970;26:185–199. doi: 10.1161/01.res.26.2.185. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. Journal of Physiology. 2001;533:871–880. doi: 10.1111/j.1469-7793.2001.t01-2-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. Journal of Physiology. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, Mcclaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. Journal of Applied Physiology. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL, USA: Academic press; 1985. [Google Scholar]
- Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiologica Scandinavica. 1998;162:13–20. doi: 10.1046/j.1365-201X.1998.0280f.x. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Human forearm muscle metabolism during exercise. V. Quantitative aspects of glucose uptake and lactate production during prolonged exercise. Scandinavian Journal of Clinical Laboratory Investigation. 1970;26:73–81. doi: 10.3109/00365517009049217. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. American Journal of Physiology. 1992;263:H1078–1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kagaya A. Relative contraction force producing a reduction in calf blood flow by superimposing forearm exercise on lower leg exercise. European Journal of Applied Physiology. 1993;66:309–314. doi: 10.1007/BF00237774. [DOI] [PubMed] [Google Scholar]
- Kagaya A, Saito M, Ogita F, Shinohara M. Exhausting handgrip exercise reduces the blood flow in the active calf muscle exercising at low intensity. European Journal of Applied Physiology. 1994;68:252–257. doi: 10.1007/BF00376774. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Clemmesen JO, Skak C, Ott P, Secher NH. Attenuated hepatosplachnic uptake of lactate during intense exercise in humans. Journal of Applied Physiology. 2002;92:1677–1683. doi: 10.1152/japplphysiol.00028.2001. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Robinson ED, Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? American Journal of Physiology. 1997;272:R386–391. doi: 10.1152/ajpregu.1997.272.1.R386. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Skak B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. Journal of Applied Physiology. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. Journal of Physiology. 1998;513:907–913. doi: 10.1111/j.1469-7793.1998.907ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Stromstad M, Ide K, Raven PB, Secher NH. Neural blockade during exercise augments central command's contribution to carotid baroreflex resetting. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H1635–1644. doi: 10.1152/ajpheart.2001.280.4.H1635. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Smith SA. The influence of central command on baroreflex resetting during exercise. Exercise and Sport Sciences Reviews. 2002;30:39–44. doi: 10.1097/00003677-200201000-00008. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during mascular activity: Observations on influence of carotid sinus on oxygen uptake. Circulation Research. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Kennedy B, Knight DR, Wagner PD. High muscle blood flows are not attenuated by recruitment of additional muscle mass. American Journal of Physiology. 1995;269:H1545–1552. doi: 10.1152/ajpheart.1995.269.5.H1545. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick K, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? Journal of Applied Physiology. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kiens B, Saltin B, Christensen NJ, Savard G. Skeletal muscle glucose uptake during dynamic exercise in humans: role of muscle mass. American Journal of Physiology. 1988;254:E551–561. doi: 10.1152/ajpendo.1988.254.5.E555. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen BJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? American Journal of Physiology. 1986;251:H1038–1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Saito M, Kagaya A, Ogita F, Shinohara M. Changes in muscle sympathetic nerve activity and calf blood flow during combined leg and forearm exercise. Acta Physiologica Scandinavica. American Journal of Physiology. 1992;146:449–456. doi: 10.1111/j.1748-1716.1992.tb09446.x. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: Role of muscle mass. American Journal of Physiology. 1989;257:H1812–1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiologica Scandinavica. 1977;100:288–297. doi: 10.1111/j.1748-1716.1977.tb05952.x. [DOI] [PubMed] [Google Scholar]
- Stenberg J, Åstrand P-O, Ekblom B, Royce J, Saltin B. Hemodynamic response to work with different muscle groups, sitting and supine. Journal of Applied Physiology. 1967;22:61–70. doi: 10.1152/jappl.1967.22.1.61. [DOI] [PubMed] [Google Scholar]
- Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. Journal of Physiology. 1999;514:283–291. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselling KH, De Wit B, Weber J A P, Smith NT. A simple device for the continuous measurement of cardiac output. Advances in Cardiovascular Physiology. 1983;5:16–52. [Google Scholar]


