Abstract
Endothelial cells secrete a range of procoagulant, anticoagulant and inflammatory proteins by exocytosis to regulate blood clotting and local immune responses. The mechanisms regulating vesicular exocytosis were studied in human umbilical vein endothelial cells (HUVEC) with high-resolution membrane capacitance (Cm) measurements. The total whole-cell Cm and the amplitudes and times of discrete femtoFarad (fF)-sized Cm steps due to exocytosis and endocytosis were monitored simultaneously. Intracellular calcium concentration [Ca2+]i was elevated by intracellular photolysis of calcium-DM-nitrophen to evoke secretion and monitored with the low-affinity Ca2+ indicator furaptra. Sustained elevation of [Ca2+]i to > 20 μm evoked large, slow increases in Cm of up to 5 pF in 1-2 min. Exocytotic and endocytotic steps of amplitude 0.5-110 fF were resolved, and accounted on average for ≈33 % of the total Cm change. A prominent component of Cm steps of 2.5-9.0 fF was seen and could be attributed to exocytosis of von-Willebrand-factor-containing Weibel-Palade bodies (WPb), based on the near-identical distributions of capacitance step amplitudes, with calculated estimates of WPb capacitance from morphometry, and on the absence of 2.5-9.0 fF Cm steps in cells deficient in WPb. WPb secretion was delayed on average by 23 s after [Ca2+]i elevation, whereas total Cm increased immediately due to the secretion of small, non-WPb granules. The results show that following a large increase of [Ca2+]i, corresponding to strong stimulation, small vesicular components are immediately available for secretion, whereas the large WPb undergo exocytosis only after a delay. The presence of events of magnitude 9-110 fF also provides evidence of compound secretion of WPb due to prior fusion of individual granules.
An important function of endothelium is to prevent intravascular coagulation under normal conditions, but to promote blood clotting and inflammation at sites of vessel damage (Mann, 1997). To achieve these opposing functions, endothelial cells secrete several proteins that regulate blood clotting, blood flow and local immune responses. They include procoagulant proteins (e.g. von Willebrand factor (vWf) and Factor VIII), anticoagulant proteins (e.g. tissue plasminogen activator (t-PA), tissue factor pathway inhibitor (TFPI) and Protein S (PS)), and vasoactive and inflammatory proteins (endothelin-1 (ET), calcitonin gene-related peptide (cGRP) and interleukin-8 (IL-8); Stern et al. 1986; Wagner, 1990; Schaumburg-Lever et al. 1994; Harrison et al. 1995; Lupu et al. 1995; Emeis et al. 1997; Ozaka et al. 1997; Russell et al. 1998a, b; Utgaard et al. 1998). All these are secreted by exocytosis, the common trigger being an increase in [Ca2+]i, (McNiff & Gil, 1983; Stern et al. 1986; Wagner, 1990; Eyden, 1993; Richardson et al. 1994; Lupu et al. 1995; Emeis et al. 1997; Russell et al. 1998b; Utgaard et al. 1998). To understand how the secretion of these differently acting mediators is regulated, it is necessary to determine the time course and dependence on[Ca2+]i of their release via exocytosis.
The procoagulant vWf is stored in large, specialised granules, the Weibel-Palade bodies (WPb), which are up to 3 μm in length and 0.1-0.2 μm in diameter (Weibel & Palade, 1964, Wagner, 1990). Other inflammatory or coagulant factors, including Factor VIII, cGRP, IL-8 and ET, may be co-localised with vWf in WPb, (Ueda et al. 1992; Schaumburg-Lever et al. 1994; Ozaka et al. 1997; Russell et al. 1998a, b; Utgaard et al. 1998). In contrast, anticoagulants including t-PA, PS and TFPI have been localised to separate and much smaller granules, typically less than ≈0.25 μm in diameter (Stern et al. 1986; Emeis et al. 1997; Lupu et al. 1997).
Due to their large size, exocytosis of individual WPb may be resolved with high-resolution membrane capacitance (Cm) measurements and may be distinguished from the exocytosis of other, much smaller non-WPb granules. The increase in Cm expected from WPb fusion, calculated from the WPb surface area and a specific Cm of 8 fF μm−2 (Zupancic et al. 1994), predicts a mean of 4-5 fF, which is readily detectable with good time resolution by whole-cell recording methods (Neher & Marty, 1982; Fernandez et al. 1984; Lindau & Neher, 1988). Anticoagulant-containing secretory granules, for which morphological data exists (Stern et al. 1986; Emeis et al. 1997; Lupu et al. 1997), would be expected to produce unitary events of typically less than 2 fF, close to the limit of resolution of whole-cell recording.
In the study presented here, conventional methods for Cm measurement have been improved to allow detection over a wide range, permitting simultaneous monitoring of total cell Cm and the amplitude and timing of discrete step events during sustained, high elevations of endothelial [Ca2+]i. This allows comparison of the time course and kinetics of WPb fusion with the total Cm change following a [Ca2+]i increase, to give the time course of WPb and non-WPb exocytosis and endocytosis.
Methods
Tissue culture
Human umbilical vein endothelial cells (HUVEC) were isolated and grown as described previously (Carter et al. 1988). Primary isolates of HUVEC were seeded on to 13 or 35 mm diameter glass coverslips in medium M199 supplemented with 10 % fetal calf serum (FCS), 10 % new born calf serum, 100 U ml−1 penicillin and 100 U ml−1 streptomycin, and incubated at 37 °C in an atmosphere of 95 % air-5 %CO2. Cells were used for electrophysiological experiments 4-96 h after isolation.
Immunocytochemistry and measurement of WPb lengths
Cells were washed once in phosphate-buffered saline (PBS; mm: NaCl 136, KCl 2.7, Na2HPO4 0.8, KH2PO4 1.28, CaCl2 2, MgCl2 0.5, pH 7.4) fixed for 20 min in 4 % paraformaldehyde in PBS at room temperature (RT; 25-28 °C), washed five times in PBS and permeabilised for 30 min with 0.1 % Triton-X100 in PBS supplemented with 10 % FCS. Cells were then incubated for 1 h with or without primary antibody (for vWf, 1:200 dilution of mouse anti-human vWf, DAKO; for t-PA, 1:200 dilution of sheep anti-human t-PA, Biogenesis) in PBS supplemented with 1 % FCS. For dual labelling, vWf and t-PA antibodies were added together (1:200 dilution). Cells were then washed five times in PBS and incubated for 1 h with a 1:200 dilution of secondary antibodies (for vWf, donkey anti-mouse Cy2, Jackson ImmunoResearch; for t-PA, donkey anti-sheep Cy3, Jackson ImmunoResearch), then washed five times in PBS and mounted in non-fluorescent aqueous mountant. Images were acquired with a ×100, 1.35 NA oil-immersion objective (U-PLAN-APO) and Photometrics CH350- cooled CCD camera. Twenty to 30 Z-plane sections at 0.2 μm intervals were taken and processed to remove out of focus light with a constrained iterative deconvolution algorithm (Deltavision softWoRx at http://www.api.com). WPb length measurements ‘L’ were made from the images of cells stained fluorescently for vWf, and were carried out in ObjectImage (an extended version of NIH image; ftp:/simon.bio.uva.nl/pub). WPb lying in the X-Y plane (selected by viewing sequential Z-plane images) were identified and lengths measured. The mean WPb diameter ‘D’ was measured in published electron micrographs (Weibel & Palade, 1964; Elgjo et al. 1975; Kagawa & Fujimoto, 1987). The surface area (S) was calculated assuming a cylinder diameter, D, and length, L, with hemispherical ends by the equation:
| (1) |
WPb Cm was calculated by multiplying S by a specific Cm of 8 fF mm−2 (determined in melanotrophes; Zupancic et al. 1994). The numbers of WPb granules per cell were counted using ObjectImage (projections of up to 20 0.2 mm sections) in deconvolved fluorescence images of cells stained for vWf. The operator marked WPb granules using a mouse pointer, and a total for the marked sites was stored on computer.
Electrophysiological recording
The whole-cell patch-clamp technique (Hamill et al. 1981) was used. Experiments were carried out in a Hepes-buffered physiological saline solution (mm: sodium gluconate 145, potassium gluconate 5, MgSO4 2, CaCl2 1.8, glucose 10, Hepes 20, pH 7.4) at RT. Pipette stray capacitance was minimised by coating with molten parafilm (50 % w/w in light mineral oil). Cl− ions were substituted with gluconate to improve resolution of Cm measurements by reducing Cm changes at high [Ca2+]i. The pipette solution contained (mm): potassium gluconate 110, Hepes 50, MgSO4 4, Na2 ATP 4, creatine phosphate 10, pH 7.3 with KOH. Cells were voltage clamped at -50 mV (unless stated otherwise) and current recorded with an Axon Instruments Axopatch 200A amplifier modified to telegraph series resistance and dither the whole-cell capacitance compensation (Axon DC-1 capacitance compensation dither). Data was acquired onto a PC computer using a 16 bit A/D converter (Cambridge Electronic Design CED 1401 plus; in some initial experiments a 12 bit 1401 plus was used). Patch pipettes filled with potassium gluconate solutions had resistances of 1-3 MΩ in external solutions, and access conductances in whole-cell recording were ≈150-400 nS.
Fluorescence measurements and flash photolysis
Changes in [Ca2+]i were measured with furaptra, as described previously (Konishi et al. 1991; Ogden et al. 1995). Briefly, furaptra (Magfura 2, Molecular Probes; 500 μm) and calcium-DM-nitrophen (synthesised by Gordon Reid, NIMR) were introduced into the cell by diffusion from the patch pipette. Microspectrofluorimetry was performed using a Nikon TMD microscope with a ×40, 1.3 NA objective. Excitation (400- 440 nm) was achieved using a 100 W quartz halogen lamp, and emitted light at > 470 nm was detected by a photomultiplier operated in photon-counting mode. Photon counts were converted to an analog signal by a discriminator and an integrating amplifier with correction for missed counts (Cairn Research) and digitised at 1 kHz (CED1401plus, 16 bit, AT bus computer). The method for converting the fluorescence change to free [Ca2+]i has been described in detail previously (see Ogden et al. 1995).
Calcium-DM-nitrophen solutions were prepared and flash photolysis carried out as described previously (Carter et al. 1998), with the modification that the flash light was directed into the epifluorescence port of the microscope via a 390 nm dichroic mirror and focussed by the objective to a spot of diameter ≈200 μm. DM-nitrophen concentration was measured using the published extinction coefficients (Kaplan & Ellis-Davies, 1988), and the equilibrium free [Ca2+] and [Mg2+] for a given Ca:DM-nitrophen ratio were calculated using the Ca2+ and Mg2+ dissociation constants for DM-nitrophen (Ellis-Davies et al. 1996), ATP (Sillén & Martell, 1971) and furaptra (Konishi et al. 1991). Solutions containing 4 mm DM-nitrophen, 4 mm Mg2+, 4 mm ATP and 500 μm furaptra, and either 4 mm or 0 mm Ca2+ were prepared and mixed to vary the Ca:DM-nitrophen ratio. To avoid sudden changes of [Ca2+]i following membrane rupture in whole-cell recording, internal solutions were prepared with Ca2+ and DM-nitrophen concentrations (2.4 mm Ca2+ and 4 mm DM-nitrophen) that give a free [Ca2+]i calculated as 88 nm, similar to the values measured by AM-ester loading of fura-2 or indo-1 in resting HUVEC (70-100 nm; Hamilton & Simms, 1987; Carter et al. 1988; Hallam et al. 1988).
A single flash applied to a cell loaded with the calcium-DM-nitrophen internal solution resulted in a transient elevation of free [Ca2+]i that rose to a peak within 1 ms and declined with a half time of ≈10 s to resting levels. To maintain [Ca2+]i at high levels, five or six sequential light pulses at 5 or 10 s intervals were applied. After the last light pulse, the [Ca2+]i declined approximately exponentially towards pre-flash resting levels. Photolysis light intensity in these experiments produced no bleaching of furaptra fluorescence, and no evidence was found of changes in fluorescence due to the photolysis of DM-nitrophen itself.
Cm measurement
To make a continuous record of capacitance steps and total cell capacitance, the whole-cell capacitance was recalculated at each time point from telegraphs of the series resistance (Rs) and capacitance offsets (CT). A two-phase lock-in amplifier, constructed to the circuit described by Lindau & Neher (1988), was calibrated to give a 1 V DC output on the two admittance outputs for a 1 V root mean square (r.m.s.) sine input. The zero phase angle of the lock-in amplifier was adjusted as described previously (Carter et al. 1998). For whole-cell recordings, 80 mV r.m.s. sinusoidal voltage at 1592 Hz was applied to the pipette. The resulting sinusoidal current was cancelled with the ‘slow’ transient cancellation circuit of the amplifier (Cslow). The extended Lindau & Neher (1988) algorithm (see also Gillis, 1995) was used to calculate the passive cell parameters Cm, membrance conductance (Gm) and access conductance (Ga) from: (1) the two outputs from the phase lock-in amplifier at 0 and -90 degrees (the real and imaginary components of admittance following partial capacitive current cancellation; YRe, YIm); (2) telegraphs of the Cslow and Rs capacitive current cancellation settings (CT, Rs); (3) the holding voltage (Vm); and (4) the DC current (Im). The operation of the Cslow and Rs telegraphs is described in Supplementary material on the website:http://www.jphysiol.org/cgi/content/full/544/3/741
The total YRe and YIm components of admittance were found by calculating the real and imaginary parts compensated for by the cancellation controls, telegraphed from the amplifier, and adding the result to the measured outputs of the lock-in amplifier. The total YRe and imaginary YIm parts were then used in the Lindau & Neher (L-N) parameter estimation algorithm (Lindau & Neher, 1988) to obtain the total capacitance and conductance. Data were sampled at 1 kHz by the program WinEDR written by Dr John Dempster (University of Strathclyde). Im, Vm, YRe and YIm were low-pass filtered at 100 Hz (-3 dB, 16 pole elliptic) prior to recording. The calculations necessary for parameter estimation and additional filtering were performed offline in MathWorks Matlab. A capacitance dither of 100 fF was applied to the compensation (via an Axon DC-1) to verify 0 deg phase angle and gain settings. The calculated Cm was additionally filtered at 100 Hz (2 × 50th order FIR filter) and DC current (IDC) at 1 Hz (2 × 50th order FIR filter) before step detection. All filtering was done both forward and backward in time to eliminate the distortions due to filter phase shifts.
The L-N algorithm for Cm estimation requires the zero-current potential, Vrev, for calculation of Gm. The effect of an erroneous Vrev estimate is negligible in low-resolution measurements, especially if Gm is low compared to Ga. However, with the extended L-N algorithm, if Vrev is not correctly estimated, a combination of high Gm and high lock-in amplifier gain can produce discontinuities in the Cm trace, and spurious changes of Gm and Cm. To minimise the error, an estimate of Vrev was obtained by iteratively adjusting the value of Vrev to minimise the difference between Cm values computed before and after a single compensation from a central section of the record. This approach eliminated most discontinuities and produced a substantial noise reduction in the Cm record.
Step detection
The step detection algorithm was essentially as described by Zupancic et al. (1994), with the addition of a modified procedure for background noise determination in each 20 s time period analysed; for example see Supplementary material on the website: http://www.jphysiol.org/cgi/content/full/544/3/741
Briefly, following low-pass filtering (2 × 50th order 100 Hz FIR digital filter), the capacitance record was converted point by point to time derivatives (dCm/dt). The peak-to-peak (p-p) noise levels, ±3 s.d., were determined as the Cm amplitude corresponding to ±3 s.d. of the dCm/dt. The procedure was verified empirically under our filtering conditions and assuming white noise, by generating a random noise signal, filtering it at 100 Hz (2 × 50th order FIR filter) and relating ±3 s.d. of amplitude to the ±3 s.d. of the dCm/dt. The ±3 s.d. of dCm/dt was obtained by fitting a Gaussian function to the dCm/dt distribution for each sequential 20 s time frame; only dCm/dt points within ±2 s.d. of the mean were fitted so as to exclude fast-changing values that might be part of distinct Cm steps. Events that exceeded ±3 s.d. of the Gaussian function fitted to dCm/dt signal were flagged on the Cm record, and inspected to exclude events associated with large Ga or Gm changes, patch-rupture or bit-level changes in the telegraphs. The amplitude of Cm steps was determined from a phase diagram of dCm/dt plotted with the amplitude of the Cm changes, as described previously (see Zupancic et al. 1994).
Artefacts due to bit-level inaccuracies in the telegraph inputs (CT and RS) were minimised by filtering the inputs at 1/50 times the sampling frequency, applying a 50th-order median filter twice to each channel in both directions (forwards and backwards in time), followed by upsampling to the original sampling rate. Further exclusion of artefactual steps was performed by observation during analysis. The false event rate for these records was estimated as 14 per 20 000 points (20 s) from simulated traces of white noise filtered at 100 Hz and processed by the step detection algorithm. The distribution of false steps has a characteristic shape, and amplitudes are much smaller than the nominal detection limit of the algorithm. A theoretical distribution of false-step amplitudes was calculated for each 20 s interval analysed, based on the noise estimate, and any steps falling into this range were excluded (see Zupancic et al. 1994).
With this approach, events that have a signal to noise ratio (SNR) of 1 (ratio of amplitude of signal to p-p noise of record) are detected with high reliability and amplitudes can be determined to within 5 % accuracy. The reliability of detecting and accurately estimating event amplitudes decreased with smaller SNRs (e.g. for an SNR of 0.5, 60 % of events are detected and their amplitudes overestimated by ≈10 %; see Zupancic et al. 1994). During an experiment the p-p noise may vary. The influence of changes in p-p noise on the resolution and the distribution of p-p noise for the recordings used to construct step-amplitude histograms (Fig. 4) are shown in the Supplementary material on the website:
Figure 4. Amplitude histograms for detected Cm steps in unstimulated and high [Ca2+]i stimulated cells.
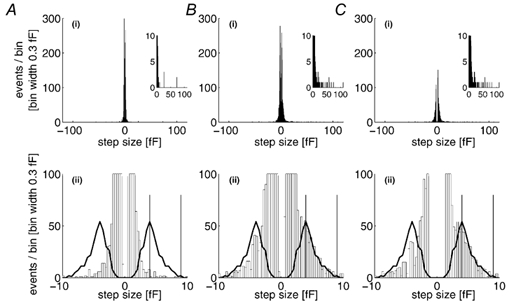
A(i) and B(i), the amplitude histograms for all exocytotic (positive) and endocytotic (negative) events detected during 1900 s of recording from four unstimulated cells (mean p-p noise 1.56 ± 0.39 fF; A), and 1920 s of recording during periods of stimulation by high [Ca2+]i in eight cells (mean p-p noise 1.56 ± 0.40 fF; B). The inset in each case shows the exocytotic events amplitude histogram on an expanded Y scale. A(ii) and B(ii), the same data on expanded X and Y scales, respectively, with the calculated distribution of WPb Cm superimposed on both the exocytotic and endocytotic distributions. The difference histogram (stimulated - unstimulated), displayed in the same way, is shown in C(i) and C(ii). The vertical lines on the lower panels indicate the region of the detected Cm steps used to calculate the frequency and kinetics of WPb exocytosis.
http://www.jphysiol.org/cgi/content/full/544/3/741
Data are expressed as means ± s.d. unless otherwise stated. Statistical analyses used Student's t test, and curve fitting was carried out with maximum likelihood, as described by Colquhoun & Sigworth (1995). Ethical committee approval was obtained for isolation of HUVEC.
Results
Electrical properties of HUVEC in culture
Small endothelial cells that were well separated from neighbouring cells were selected for Cm measurements. The mean ± s.d. Cm, Ga and Gm of the cells recorded was 13.8 ± 3.5 pF (n = 30), 264 ± 70 nS and 1.09 ± 0.9 nS, respectively.
Cm changes evoked by photolysis of calcium-DM-nitrophen in single HUVEC
The effects on Cm of raising [Ca2+]i are shown in Fig. 1. A single HUVEC of initial Cm ≈12 pF was perfused with calcium-DM-nitrophen and furaptra during whole-cell patch-clamp recording. Fig. 1A(i) shows the increase in [Ca2+]i during 17 sequential 1 ms near-UV flashes delivered over 125 s. [Ca2+]i increased immediately with each flash, declining slightly during the interval, to reach a steady level of ≈57 μm in eight flashes. [Ca2+]i remained at this level during the remainder of the train and declined to the initial level over 2 min when photolysis was stopped. The capacitance traces (Fig. 1A(ii)) show that initially, total Cm was declining slowly and the record of the net change due to all detected capacitance steps (Cdet) was constant. Following elevation of [Ca2+]i, Cm increased by 2.75 pF at a fairly constant rate at high [Ca2+]i, then declined as the free [Ca2+]i declined. Indicated on the record are the time points when the whole cell capacitence compensation was adjusted (†), and when the phase and gain settings were checked (*) by dithering the whole-cell compensation (100 fF).
Figure 1. High [Ca2+]i evokes large increases in membrane capacitance (Cm) that are composed of fast steps in Cm.
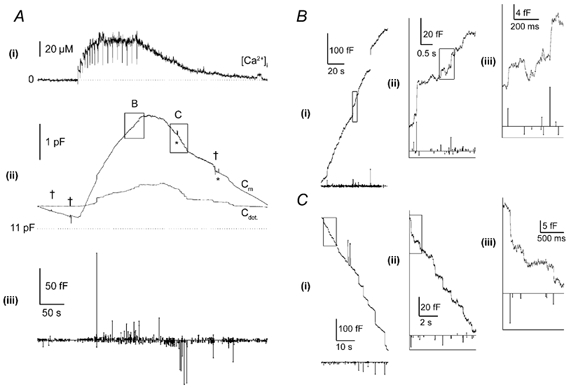
A, a continuous recording of [Ca2+]i (i) and Cm (ii) in a single human umbilical vein epithelial cell (HUVEC) that was voltage clamped at a holding potential of 0 mV, and equilibrated with an intracellular solution containing 500 μm furaptra and calcium-DM-nitrophen (2.4:4 mm). At points indicated by the deflections in the Ca2+ trace (i), brief (1 ms) pulses of near-UV light were applied (1500 μF, 200 V) from a xenon arc flash lamp. Points at which adjustments were made to the Cm and Rs compensation controls are shown by crosses, and 100 fF dithers of the Cm compensation control via the DC-1 dither module (Axon Instruments) are indicated by * (ii). Superimposed on the Cm trace is the net change in Cm produced by all detected exocytotic and endocytotic events (Cdet). The time and amplitude of all exocytotic Cm steps (upward lines) and endocytotic Cm steps (downward lines) are shown in (iii). B(i) and C(i), show regions of Cm and detected steps during a period of strong exocytosis (box B in A(ii)) and endocytosis (box C in A(ii)), respectively, on an expanded time scale. B or C (ii) is the outlined region in (i) expanded, and B or C (iii) is the outlined region in (ii) expanded. The mean peak-to-peak (p-p) noise during this record was 1.58 ± 0.23 fF.
Many fast steps of Cm attributable to exocytosis and endocytosis can be identified during the rise and fall of [Ca2+]i in the complete low-gain records shown in Fig. 1A(ii). High-gain Cm data in the boxed regions indicated are shown in Fig. 1B(i)-(ii) at sequentially higher gains and faster time scale for periods of rapid net exocytosis, and in Fig. 1C(i)-(iii) for periods of net endocytosis. These show numerous step increases and decreases of Cm, ranging in amplitude from ≈1 to 100 fF. The sum of all the detected Cm steps is shown as a line, Cdet, in Fig. 1A(ii). In this cell, resolved Cm steps account for approximately 25 % of the total Cm change, and the amount of membrane gained in resolved exocytotic steps is lost during the net endocytosis of recovery. Thus, most of the Cm change is due to small events (< ≈1 fF), unresolved in this recording. The amplitudes of the resolved Cm steps plotted with time are shown by the vertical lines in Fig. 1A(iii). The increase in Cm results from an increase in the frequency and size of exocytotic steps and is analysed in Fig. 7. In contrast, the frequency of endocytotic events increased little compared to baseline during the rising phase of the Cm record, but increased substantially as [Ca2+]i declined (see also Fig. 7).
Figure 7. Frequency and time course of granule exocytosis during stimulation by high [Ca2+]i.
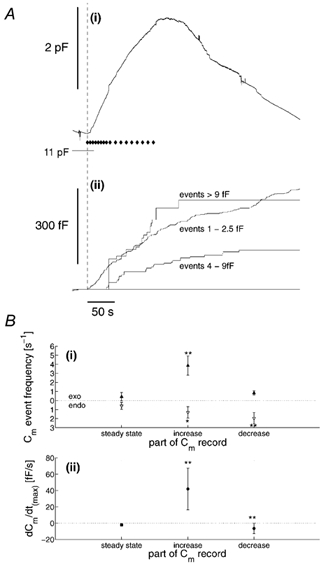
A(i), the Cm record for the cell in Fig. 1, with the time points at which light flashes were given indicated ♦. The time of the first flash is indicated by the dashed line. A(ii), cumulative plots of Cm step events 1-2.5, 4-9 and > 9-fF detected in this record. B(i), the mean frequency of all exocytotic (exo; ▴) and endocytotic (endo; ▵) events for eight cells, determined over a 15 s time window prior to stimulation (steady state), during the steepest part of the increase (increase) or decrease (decrease) in Cm during stimulation. B(ii), the mean slope of the Cm record from these regions in the eight cells studied. *P = 0.1, **P = 0.01 significance levels, Student's t test (determined with respect to the steady-state data).
Generation and subcellular distribution of WPb, vWf and t-PA
Following plating of dissociated HUVEC, the number of WPb per cell increased with time in culture. Figure 2A(i) shows fluorescence images of primary HUVEC labelled with a specific antibody against vWf at 4, 24, 48 and 72 h in culture. At 4 h after plating, few or no WPb could be identified; however, there was strong fluorescence staining due to vWf in perinuclear regions corresponding to endoplasmic reticulum and Golgi apparatus, and patchy staining at the cell/coverslip interface (Fig. 2A(i) and C(i)). At 24 h, WPb could be clearly identified, although there was considerable cell-to-cell variability in their numbers, and by 48-72 h in culture, the mean numbers of WPb had reached a steady level (Fig. 2A(ii)). Most experiments were made at 48-96 h in culture, although for comparison, some experiments were carried out at 4 h and 24 h (e.g. see Fig. 5). Under our culture conditions, t-PA and vWf were stored separately, vWf in large tubular WPb and t-PA in much smaller discrete punctate structures (Fig. 2C(i)-(iii)).
Figure 2. Time-dependent accumulation of Weibel-Palade bodies (WPb), distribution of WPb Cm, and subcellular localisation of von Willebrand factor (vWf) and tissue plasminogen activator (t-PA).
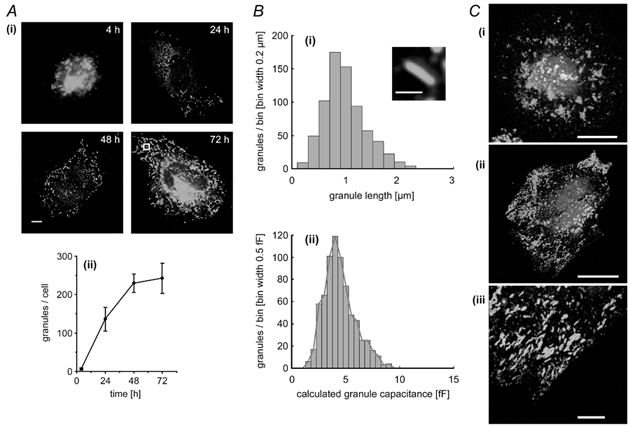
A(i), representative fluorescence images of fixed HUVEC that have been stained with a specific antibody to vWf at the times indicated. Images are projections of three to five, 0.2 μm optical sections, the scale bar indicates 5 μm. The mean number of vWf-immunopositive granules (WPb) counted in 12 cells at each of the indicated times is plotted in (ii) (means ± s.d.). B(i), the distribution of lengths of WPb measured from images such as those shown in (i) with an example (inset) of an average WPb from the 72 h cell in A(i) (indicated by the white box). The scale bar is 1 μm. The calculated distribution of WPb Cm derived from the data in B(i), assuming a specific Cm of 8 fF μm−2 is shown in (ii). A red line is drawn through the peak of each bin. C(i) and (ii), HUVEC at 4 and 24 h, respectively, labelled with specific antibodies to both t-PA (red) and vWf (green). An expanded region of the image in (ii; - 24 h cell) shows no co-localisation of t-PA and vWf. Images are projections of three, 0.3 μm optical sections, the scale bar being 20 μm in (i) and (ii) and 5 μm in (iii). Nuclear DNA was labelled with Hœchst 33342 (blue).
Figure 5. High [Ca2+]i does not evoke large Cm steps in cells lacking WPb.
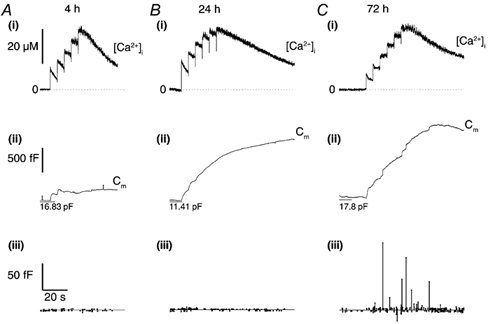
A(i), B(i) and C(i), continuous records of [Ca2+]i in cells at 4, 24 and 72 h post-isolation, respectively. Each cell was voltage clamped at a holding potential of -50 mV, and equilibrated with an intracellular solution containing 500 μm furaptra and calcium-DM-nitrophen (2.4:4 mm) and brief (1 ms) pulses of near-UV light applied (1500 μF, 200 V) at points indicated by deflections in the Ca2+ traces. The Cm records are shown in (ii) for each cell, and the amplitudes and times of detected Cm steps (exocytotic are represented by upward lines and endocytotic are represented by downward lines) shown in (iii). The mean p-p noise throughout each recording was 1.60 ± 0.23 fF (A), 1.63 ± 0.13 fF (B) and 1.91 ± 0.20 fF (C).
Calculation of the capacitance of WPb membranes
The Cm expected for structures of the size and shape of WPb, and their potential contribution to Cm changes was calculated on the basis of morphometric measurements of surface area and specific membrane capacitance. Cross sections of WPb (from electron microscope (EM) studies) are characterised by bundles of hollow tubes or rod-like structures, making their identification unambiguous (e.g. see Wagner, 1990). From 54 measurements of WPb diameters taken from published EM studies (Weibel & Palade, 1964; Elgjo et al. 1975; Kagawa & Fujimoto, 1987), the mean ± s.d. WPb diameter, D, was estimated as 154 ± 63 nm. The length L of 724 WPb was measured from high-resolution deconvolved fluorescence images of endothelial cells stained for vWf. The distribution of lengths is shown in Fig. 2B(i). The surface area was calculated (see Methods) and a specific membrane capacitance of 8 fF μm−2 (Zupancic et al. 1994) applied to yield the calculated distribution of granule capacitance shown in Fig. 2B(ii). The distribution ranges from ≈2 to 9.0 fF, with a mean of 4.4 ± 1.4 fF. At a Cm step detection resolution of 1.5 fF in the measurements here, > 95 % of WPb exocytosis would be resolved as discrete Cm steps. In contrast, it was calculated that granules containing anticoagulants, which have diameters < 0.25 μm (see Introduction for references), would produce Cm step sizes of less than 2 fF, which is close to, or below the limit of resolution of these recordings.
High [Ca2+]i triggers exocytosis of WPb
There is evidence to suggest that vWf secretion from WPb requires prolonged high elevated [Ca2+]i, > 10-20 μm (Scrutton & Pearson, 1989; Birch et al. 1994), concentrations that are readily achieved during hormone action in endothelial cells in culture and in situ (Carter & Ogden, 1994, 1997; Carter et al. 1998). We have shown previously that [Ca2+]i in this range evokes large Cm changes in single HUVEC (Carter et al. 1998) and it is of interest to see how individual WPb secretory events are related to the time course of [Ca2+]i and total Cm changes. Figure 3 shows recordings from two HUVEC of initial capacitance ≈11 pF. In the control cell there was no photolytic elevation of [Ca2+]i (epifluorescence excitation was shuttered; Fig. 3A). In the stimulated cell, [Ca2+]i was increased by photolysis of calcium-DM-nitrophen to levels of > 10 μm (Fig. 3B(i)). In the control condition, the total Cm (Fig. 3A(i)) declined over 6 min by ≈0.5 pF, 4 % of the initial level, and comprised exocytotic and endocytotic steps with approximately equal frequency, and mean amplitude 1.3 fF (Fig. 3A(ii)). In the stimulated cell there was a large, slow increase in Cm of > 1 pF, rising slowly immediately after the [Ca2+]i, and then after a delay more rapidly at high [Ca2+]i. An increase in the amplitude and frequency of exocytotic and endocytotic steps, and infrequent, large exocytotic Cm steps of 10-60 fF were seen following elevation of [Ca2+]i (Fig. 3B(ii) and (iii)). The data are summarised in the histograms shown in Fig. 3A(iii) for the control and Fig. 3B(iv) for the stimulated cell. More specifically, the histograms show a component of exocytotic and endocytotic steps in the range ≈2-9 fF in the stimulated cell (Fig. 3B(iv)) that is absent from control cells, as well as the very large amplitude steps seen in the stimulated cell. Amplitude histograms for exocytotic and endocytotic Cm step events derived from 1900 s of data from four control cells, and 1920 s recorded during periods of high [Ca2+]i in eight cells, are shown in Fig. 4A(i) and B(i), respectively. The exocytotic amplitude histograms, on a reduced ordinate (inset), show the appearance of infrequent and very large Cm steps in stimulated cells. Figure 4A(ii) and B(ii) shows, on expanded scales, the amplitude histograms for control and stimulated cells in the ranges predicted for WPb exocytosis. The continuous line shows the distribution of WPb capacitance calculated from the morphometric data of WPb surface areas superimposed on the distribution of Cm steps. There is overlap with a component of events, prominent particularly in stimulated cells. This is shown more clearly by the difference histograms (stimulated - control) plotted in Fig. 4C, which shows components of exocytotic and endocytotic events with sizes corresponding to WPb granule capacitance in cells stimulated with high [Ca2+]i.
Figure 3. A prominent component of Cm steps with amplitudes in the same range predicted for WPb exocytosis, evoked by high [Ca2+]i.
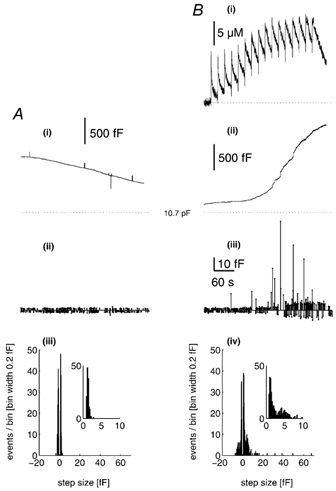
A(i), a continuous record of Cm from an endothelial cell of ≈11 pF, voltage clamped at -50 mV and dialysed with a solution containing 500 μm furaptra and calcium-DM-nitrophen (2.4:4 mm). The time and amplitude of discrete Cm steps (exocytotic are indicated by the upward lines, and endocytotic by the downward lines) are shown in A(ii). A(iii), the amplitude histogram of the detected exocytotic (positive) and endocytotic (negative) events, taken from a 360 s stretch of data. The inset is the exocytotic event amplitude histogram on an expanded scale. The mean p-p noise for the recording was 1.26 ± 0.14 fF. B(i) and (ii), continuous records of [Ca2+]i and Cm, respectively, from similarly sized endothelial cells voltage clamped at -50 mV and dialysed with a solution containing 500 μm furaptra and calcium-DM-nitrophen (2.4:4 mm). Brief (1 ms) pulses of near-UV light (1500 μF, 200 V) were applied at the times indicated by the deflections on the Ca2+ record in (i). The time and amplitude of discrete steps in Cm are shown in A(iii). A(iv), the amplitude histogram of the detected exocytotic and endocytotic steps, taken from a 360 s stretch of data during stimulation. The inset shows the exocytotic events amplitude histogram on an expanded scale. The mean p-p noise of the recording was 1.46 ± 0.36 fF.
To test whether the discrete Cm steps with amplitudes in the range predicted for WPb exocytosis were due to WPb, experiments were carried out in cells at early times in culture (4-24 h), when few or no WPb are detectable morphologically (see Fig. 2). At 4 and 24 h, high [Ca2+]i produced increases in total Cm but with few or no discrete steps in Cm in the range expected for WPb exocytosis (Fig. 5A and B, respectively). At longer times in culture, when cells have large numbers of WPb, discrete steps in Cm predicted for WPb exocytosis were seen (Fig. 5C). Since there are no other large secretory granules in endothelium that could account for these results, and compound fusion of smaller granules is unlikely to produce the distribution of Cm steps observed here, the data taken together suggest that the additional discrete Cm steps, in the range 2-9 fF are due to exocytosis of WPb.
Kinetics of WPb exocytosis
To investigate the kinetics and frequency of WPb exocytosis, events with amplitudes between 4 and 9 fF were identified in the records and attributed to individual WPb fusion based on comparison with the morphometric data shown in Fig. 2B(ii) (range indicated in Fig. 4B(ii) and C(ii) as vertical lines). The lower limit excludes the component of constitutive granule exocytosis that falls in the Cm range calculated for WPbs (Fig. 4A(ii)), and contributions from non-WPb-regulated exocytosis; the upper limit excludes the infrequent, very large events that were observed. The distribution of intervals between exocytotic events was measured for amplitudes in three ranges: WPb events of 4-9 fF (Fig. 6A(ii)), events of 2.5-4 fF, which are likely to comprise a mixture of constitutive non-WPb granule exocytosis and regulated WPb exocytosis (Fig. 6A(i)), and events > 9 fF (Fig. 6A(iii)). Each distribution was fitted by maximum likelihood with the sum of three exponential distributions, yielding the best-fit parameters (± -0.5 log likelihood intervals) summarised in Fig. 6A(iv) and (v). The similar rates for large granule (> 9 fF) and WPb (4-9 fF) exocytosis, seen by comparing best-fit time constants and areas, indicate that large events and WPb-sized events are components of the same granule population. On this basis, all events of magnitude > 4 fF were pooled (Fig. 6B(ii)) and their kinetics compared with events in the size range 1-2.5 fF, which represent predominantly small, non-WPb granule exocytosis (Fig. 6B(i)); fitted parameters are shown in Fig. 6B(iii) and (iv)). The time constants and areas differ substantially between the two size ranges, with no overlap of the -0.5 log likelihood regions (Fig. 6B(iii) and (iv)), indicating that they are due to different granule populations. Analysis of the distribution of intervals for events in the range 2.5-4 fF showed best-fit parameters intermediate between the small non-WPb events and those for large WPb > 4 fF (Fig. 6A(i) and B). These parameters may reflect the overlap of WPb exocytosis with constitutive (e.g. Fig. 4A(ii)) or non-WPb exocytosis in this size range.
Figure 6. Distribution of intervals between exocytotic events during stimulation by high [Ca2+]i.
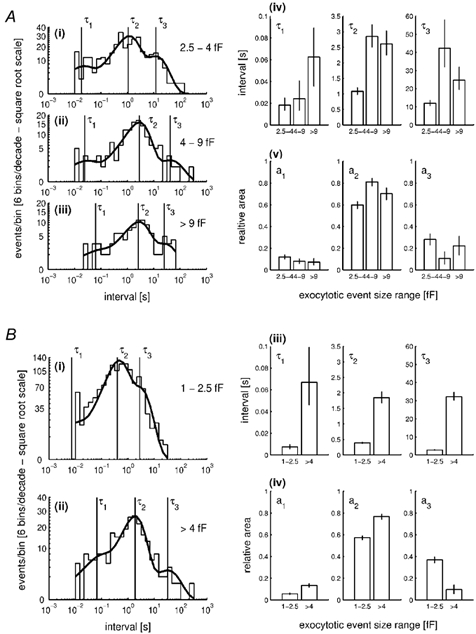
A, a comparison of interval distributions, plotted as the distribution of the logarithm of the time interval (Sigworth & Sine, 1987), for three subpopulations of exocytotic events: 2.5-4 fF (i), 4-9 fF (ii) and > 9 fF (iii). When generated in this way, an exponential probability density function (p.d.f.) is transformed so that it shows a peak at the value of the time constant. The distributions are limited on the lower end by the filtering bandwidth of 100 Hz (10 ms). Sums of three exponential distributions, with parameters of time constants (τ1-τ3) and relative areas (a1-a3), were fitted to the data so as to maximise the likelihood of the calculated distribution describing the actual distribution (Colquhoun & Sigworth, 1995). The continuous line shows a calculated sum of three exponential p.d.f. superimposed onto the distribution. Vertical lines indicate the positions of the three time constants. A(iv) and (v), a comparison of the parameters of the fitted distributions; time constants (τ1-τ3) and relative areas (a1-a3), respectively, among the three subpopulations. Vertical error bars correspond to ± -0.5 log likelihood limits. B(i) and (ii), the distribution of intervals for events of 1-2.5 and > 4 fF, respectively, produced in the same way as A(i)-(iii). B(iii) and (iv), a comparison of the parameters of the fitted distributions.
To exclude contributions due to non-WPb exocytosis, the time course of WPb exocytosis was determined using Cm steps in the size range 4-9 fF. This was compared to that for events > 9 fF, which may represent compound secretion of WPb (see below and Discussion), and for events of 1-2.5 fF. The time course of secretion attributed to these three components is shown in Fig. 7A, which analyses the data presented in Fig. 1. The exocytotic events in each size range are plotted as cumulative increments of capacitance (Fig. 7A(ii)), and compared with the net change of Cm (Fig. 7A(i)). In this cell there was a delay of 35 s after the increase in [Ca2+]i before the first WPb event occurred. A similar delay was seen in the appearance of events > 9 fF, 39 s. In contrast, there was only a short delay in this cell (141 ms) before an increase in total Cm, comprising small or unresolved events due to exocytosis of non-WPb granules. Overall, the delay in WPb and large granule exocytosis was 23.3 ± 7.1 s (n = 8 cells), and 25.2 ± 8.1 s (n = 8 cells), respectively.
Contribution of exocytotic and endocytotic events to total Cm changes
In control cells, Cm declined slowly at a rate of 2.2 ± 2.7 fF s−1 (n = 4 cells). The overall frequency of exocytotic and endocytotic events detected was 0.87 and 1.07 events s−1, respectively. Events falling in the range 4-9 fF, reflecting basal WPb exocytosis, occurred much less frequently (0-0.033 events s−1).
In cells with high [Ca2+]i due to photolysis, the frequency of Cm steps was determined for 15 s time windows in three parts of the response: (a) at rest just prior to stimulation, (b) during the steepest part of the increase in Cm and (c) during the steepest period of the Cm decrease. The results are summarised in Fig. 7B(i). Prior to stimulation, Cm declined at a mean rate of 2.0 ± 1.6 fF s−1, and the frequencies of exocytotic and endocytotic events were similar. Following elevation of [Ca2+]i, Cm increased with a mean rate of 41.8 ± 9.6 fF s−1, as shown in Fig. 7B(ii), coincident with a large increase in the frequency of exocytotic events and a smaller increase in frequency of endocytotic events (Fig. 7B(i)). During this period, the frequency of events of amplitude 4-9 fF, attributed to WPb exocytosis, increased to between 0.1 and 1.8 events s−1. Finally, in cells in which the Cm declined as [Ca2+]i declined to pre-flash levels, the frequency of exocytotic events decreased to close to pre-flash levels, whereas the frequency of endocytotic events increased (five cells; Fig. 7B(i)). These results indicate that most of the rapid increase in Cm during stimulation is due to a large increase in the frequency of exocytosis, and that increased endocytosis and decreased exocytosis result in the decline of Cm. The almost symmetrical distributions of exocytotic and endocytotic event amplitudes shown in the histogram of Fig. 4C(ii) show that the step sizes detected and the numbers in each bin range are similar for exocytosis and endocytosis, implying that similar membrane areas are gained and lost in single exocytotic and endocytotic events.
In eight cells, the mean contribution to the Cm change, after correction for detected endocytosis, from exocytosis of WPb granules in the 4-9 fF range was 6.0 %, and for granules > 9 fF, 14.4 %. For detected exocytotic events of magnitude < 4 fF thought to include both non-WPb and a proportion due to WPb, this was 14.5 %. The remaining 65 % comprises the net exocytosis and endocytosis of unresolved granules.
Exocytosis during brief elevations of [Ca2+]i
Following a single flash producing a rise of [Ca2+]i there was often a small (45-433 fF, n = 17) rapid increase in Cm, containing a few detected steps typically less than ≈2 fF in amplitude. The time course of this early increase comprises a delay in the range 13-174 ms (n = 17), a fast rise with a maximum rate of change of Cm of 337 fF s−1 (range 34-337 fF s−1, n = 17), followed by a slower decline. In the record shown in Fig. 8, Cm increased after a delay of 93 ms, peaked 1.59 s after the maximum [Ca2+]i, and subsequently declined to pre-flash levels over 13 s. The region marked between the asterisks in Fig. 8A(ii) was excluded from analysis because of large Im excursions that might have produced spurious changes in the Cm record. No Cm steps of the amplitude expected for WPb exocytosis were detected (Fig. 8A(iii)).
Figure 8. Brief elevation of [Ca2+]i to high levels does not evoke WPb exocytosis.
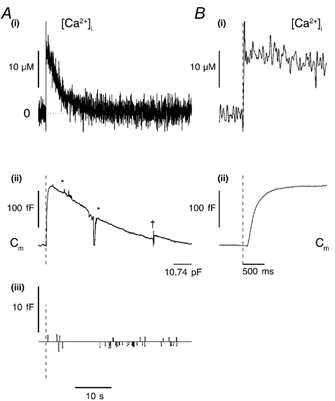
A, a continuous record of [Ca2+]i (i) and Cm (ii) from a cell that had been 72 h in culture, voltage clamped at -50 mV and dialysed with a solution containing 500 μm furaptra and calcium-DM-nitrophen (2.4:4 mm). A single, brief (1 ms) pulse of near-UV light (1500 μF, 200 V) was applied at the time indicated by the vertical dashed lines on the records in A(ii) and (iii) and B(i) and (ii). The detected Cm steps (exocytotic represented by upward lines, and endocytotic by downward lines) are shown in (iii). The area between the stars was excluded from step analysis due to a series of large Im fluctuations that might have produced spurious changes in Cm. B, the early part of the [Ca2+]i (i) and Cm (ii) response on an expanded time scale. The p-p noise was 0.88 ± 0.30 fF for the period shown. †, the point at which a CT and Rs compensation was applied.
Discussion
An elevation of [Ca2+]i is known to be a strong stimulus for secretion in vascular endothelial cells (Stern et al. 1986; Hamilton & Sims, 1987; Scrutton & Pearson, 1989; Birch et al. 1992, 1994; Kooistra et al. 1994; Frearson et al. 1995; Lupu et al. 1995; Carter & Ogden, 1997), and produces a large increase (up to 30 %) in endothelial Cm (Carter et al. 1998). In this study we show that the changes in endothelial Cm resulting from high [Ca2+]i comprise discrete step changes in Cm due to exocytosis and endocytosis. The resolution of these recordings permitted detection of Cm steps typically > 1.5 fF, sufficient to resolve more than 95 % of events due to fusion of WPb granules, as well as some events due to small non-WPb granule exocytosis, and further showing that a large proportion of the Cm change is due to secretory events < 1.5 fF in size. The results provide evidence that a major component of the distribution of Cm steps detected is due to exocytosis of WPb. Identification of this component shows a long delay, > 20 s, in the time course of WPb exocytosis evoked by [Ca2+]i elevation, and contrasts with the immediate Cm increase due to small granule exocytosis.
Ca2+ concentration and vascular secretion
In the experiments described here, exocytosis of WPb required free [Ca2+]i levels typically of 10-60 μm. Although high, these levels of free [Ca2+]i are comparable to those recorded during hormonally evoked responses. Secretion of anticoagulants such as t-PA occurs rapidly following stimulation, and is triggered by the first phase of the hormone-evoked [Ca2+]i elevation (Kooistra et al. 1994; Lupu et al. 1995), comprising a transient increase in [Ca2+]i to between 7 and 30 μm, due to InsP3-evoked Ca2+ release from internal stores (Carter & Ogden, 1994, 1997; Carter et al. 1998). Local [Ca2+]i in domains close to calcium-release sites are likely to be much higher than these averaged levels (Carter & Ogden, 1994, 1997). Brief increases of [Ca2+]i in this range produced by hormone, or by photolysis of caged InsP3 or caged Ca2+ evoked small increases of Cm (Carter et al. 1998). During this phase of the exocytotic response, only small discrete Cm events (less than 2.5 fF) can be resolved, indicating net exocytosis of small granules or vesicles only (Fig. 8). On the other hand, secretion of vWf from intact endothelial cells has been shown to require prolonged elevation of [Ca2+]i or frequent prolonged periods of Ca2+ spiking (Birch et al. 1994). In permeabilised endothelial cells, maximal vWf secretion was shown to require [Ca2+]i > 10-20 μm (Scrutton & Pearson, 1989; Birch et al. 1992). The data presented here and that reported by Carter et al. (1998) show that similar high levels of [Ca2+]i are required to evoke large overall increases in endothelial Cm.
Identification of Cm steps due to WPb exocytosis
On the basis of morphometric measurements of membrane area, WPb granules are predicted to contribute Cm steps of amplitude between ≈2 and 9 fF (mean 4.4 fF) if membrane fusion occurs in a concerted event. Here we show that the increase in Cm produced by maintained high [Ca2+]i contains steps ranging in amplitude from ≈1.0 to 100 fF, with a prominent component in the range ≈2-9 fF similar to that predicted for WPb fusion. Cm steps in this range of amplitudes are absent in recordings from cells at 4 h in culture that have no WPb, and are present in recordings from cells 1-3 days in culture that have developed immunocytochemically identified WPb. These results suggest strongly that the additional Cm steps in stimulated cells, in the range 2-9 fF, are due to WPb exocytosis. In resting cells, Cm events in the range 4-9 fF, corresponding to the upper range of WPb sizes, were infrequent (0-0.033 events s−1). Following stimulation, the frequency of events in this size range increased to a maximum of 1.8 events s−1, indicating that prolonged high [Ca2+]i evokes a large increase in WPb exocytosis, as predicted by biochemical studies of vWf secretion (Scrutton & Pearson, 1989; Birch et al. 1994).
Delays in small granule and WPb exocytosis
Following an abrupt increase of [Ca2+]i, there was a short delay (minimum 13 ms) before an increase in Cm occurred. During the early part of the increase, the few exocytotic steps of Cm detected were typically 1-2 fF in amplitude, indicating that exocytosis of small granules (< 0.25 μm in diameter) predominates early in the response. A further elevation of [Ca2+]i within a few seconds of the first resulted in an additional fast increase in Cm, again comprising small Cm steps (e.g. Figs 1, 5 and 7). These results indicate that WPb exocytosis does not occur early in the response to high [Ca2+]i and that small granules are readily available for exocytosis following a fast increase of [Ca2+]i produced by the first or a subsequent flash. The small granules comprising this phase of the Cm change may include those containing anticoagulants such as t-PA, TFPI and PS (Stern et al. 1986; Emeis et al. 1997; Lupu et al. 1997), which are known to be secreted rapidly in response to a rise in [Ca2+]i (Stern et al. 1986; Kooistra et al. 1994; Lupu et al. 1995). Evidence was also obtained that small granule secretion continues to predominate during prolonged [Ca2+]i elevations in which most of the Cm change (> 65 %) is due to small or unresolved exocytotic and endocytotic events.
In contrast to the small granule secretion, the delay between the rise of [Ca2+]i and the first large Cm steps attributable to WPb exocytosis was long, with a mean of 23 s. Furthermore, the overall rate of exocytosis of WPb is slow when compared with other tissues (Henkel & Almers, 1996), suggesting that multiple steps may be involved in WPb exocytosis. Further evidence of this was obtained here in the distribution of intervals between Cm steps. When partitioned into amplitude ranges 1-2.5, 2.5-4, 4-9 and > 9 fF, the distribution of intervals were in each case fitted with the sum of three exponential components, indicating that several steps in the secretory process are initiated by high [Ca2+]i. In the two ranges 4-9 and > 9 fF, the time constants and amplitudes of the components determined by maximum likelihood were similar, consistent with the expectation that large events (> 9 fF), arise from the WPb pool of granules. The slowest time constant in the distribution of intervals was similar to the delay seen in WPb and large granule exocytosis after rapid elevation of [Ca2+]i, consistent with a slow step of 20-30 s in the Ca2+ activation of WPb exocytosis. In contrast, events < 2.5 fF showed a substantially different distribution of intervals, indicating different processes in the calcium-evoked secretion of small non-WPb granules. In the range 2.5-4 fF, the distribution was intermediate between the small events and events > 4 fF, most probably comprising a mixture of regulated WPb exocytosis, and a component of basal or constitutive granule exocytosis. Histological studies indicate that anticoagulant-containing granules are typically < 0.25 μm in diameter (Stern et al. 1986; Emeis et al. 1997; Lupu et al. 1997); however, the lack of detailed analysis of the distribution of sizes of anticoagulant granules leaves open the possibility that some may be large enough to contribute to events in the range 2.5-4 fF. Evidence of a long delay in WPb exocytosis has been reported elsewhere. vWf-dependent platelet adhesion to venular endothelium in vivo occurs with a delay of 20-30 s following stimulation with histamine or ionophore (Andre et al. 2000). Using atomic-force microscopy (albeit in post-stimulation, fixed tissue), Schneider et al. (2000) reported numerous 200-500 nm diameter, ring-like structures in the plasmalemma, 30-50 s after stimulation with a protease-activated receptor 2 agonist, which they suggested reflected WPb exocytosis. The function of the slow step in producing the delay in WPb secretion may be to limit procoagulant or inflammatory-mediator release following brief stimulation, at the same time allowing the anticoagulant secretions from small granules and other non-vesicular mechanisms necessary for preventing intravascular coagulation, the main homeostatic function of endothelium.
Differential secretion from several granule populations in the same cell is achieved by many tissues. For example, neutrophils involved in regulating local immune responses contain several different storage granules that are exocytosed in a co-ordinated fashion. Secondary and tertiary granules are exocytosed in response to low levels of elevated [Ca2+]i, and peroxidase-positive, primary granules are released at much higher [Ca2+]i (Nusse et al. 1998). In neutrophils and other cells (Verhage et al. 1991), it has been suggested that different Ca2+ sensitivities of the granule populations may provide a basis for this regulation. The details of the mechanisms and their similarities with endothelium remain to be established.
Large Cm steps
A significant proportion of the Cm change evoked by high [Ca2+]i is attributable to very large (> 9 fF) step increases in Cm that cannot be accounted for by individual WPb or other endothelial secretory granules. Large Cm steps have been reported in a number of different cells during stimulation with high [Ca2+]i, and arise either because the individual secretory granules themselves are very large (e.g. the beige mouse mast cell; Breckenridge & Almers, 1987), or from the compound fusion of several smaller granules prior to or during exocytosis (e.g. eosinophil secretion; see Scepek & Lindau, 1993). In some cell types the nature of the exocytosed compartment is not clear, although one possible candidate is the lysosome (reviewed in Andrews, 2000).
Several lines of evidence suggest that the large steps seen here are due to compound fusion of WPb. First, the presence of large Cm steps at high [Ca2+]i correlates with the development of WPb; they were not seen in cells lacking WPb, appearing only in cells at > 24 h in culture when WPb are well established (e.g. Fig. 5C). Second, the large Cm events seen here have the same kinetics of secretion as the well-defined 4-9 fF WPb component of exocytotic steps, showing a long delay and similar time constants and amplitudes in the distribution of intervals (Fig. 6A). The long delay in WPb exocytosis (tens of seconds) would provide sufficient time for fusion of multiple WPb prior to exocytosis. Third, morphological studies suggest that following cell stimulation, WPb undergo compound fusion to form large vacuolar structures prior to exocytosis (Fujimoto, 1982; Richardson et al. 1994). Alternative explanations for these large Cm steps include the exocytosis of some other large compartment such as lysosomes (Andrews, 2000), or the compound fusion of many small non-WPb granules. Lysosomal exocytosis may occur in endothelium (De Bruyn & Cho, 1986), and recent results in other tissues have linked calcium-triggered lysosomal fusion or compound fusion of intracellular vesicles in the resealing of disrupted plasma membrane (McNeil & Steinhardt, 1997; Terasaki et al. 1997). However, in the experiments reported here it is unlikely that exocytosis of lysosomes or compound fusion of smaller bodies accounts for the large steps. Lysosomes are present in freshly isolated endothelial cells and at 24 h in culture, but large Cm steps were not seen in these cells, although overall net Cm changes in response to high [Ca2+]i were seen due to the cumulative secretion of small secretory bodies (e.g. Fig. 5A, B). The evidence presented here favours the compound fusion of WPb as an explanation of the large steps of Cm during high [Ca2+]i. Compound fusion may provide a mechanism by which it is possible to increase the efficiency of delivery of vWf, P-selectin and membrane to the cell surface under conditions of strong or prolonged stimulation.
Endocytosis
In resting cells, endothelial Cm declines slowly (at ≈2 fF s−1) during whole-cell recording. Similar observations have been made in many other cell types (Neher & Marty, 1982; Almers & Neher, 1987; Gillis & Miser, 1993; Burgoyne & Handel, 1994; Zupancic et al. 1994), and the reasons for the decline are not clear. Separation of detected exocytotic and endocytotic steps in resting endothelial cells showed a slightly higher frequency of endocytotic events (Fig. 7B), indicating that the slow decline is most probably due to a net endocytosis of membrane. Although care was taken to ensure that no large changes in resting [Ca2+]i occurred during patch-clamp recording, other factors such as the loss by dialysis of the small cell components required to maintain resting rates of exocytosis and endocytosis cannot be ruled out. However, evidence that the endocytotic machinery was not significantly disrupted under our experimental conditions was provided by the ability of many cells (5/8) to retrieve most if not all of the additional membrane inserted by exocytosis following stimulation.
Following elevation of [Ca2+]i there was an increase in the frequency of endocytotic events, often less pronounced during the early phase of the rise in [Ca2+]i, but increasing after periods of strong exocytosis as [Ca2+]i declined. The amplitude of endocytotic steps during the latter phase were often large (up to ≈60 fF), similar to those reported in a number of other cell types following periods of exocytosis triggered by high [Ca2+]i (reviewed in Henkel & Almers, 1996). This retrieval mechanism is thought to require high [Ca2+]i, and may involve a dynamin rather than a clathrin-mediated mechanism (Henkel & Almers, 1996). An increased frequency of endocytotic steps similar in amplitude to WPb exocytotic events in stimulated cells (Fig. 4C) raises the possibility that the WPb membrane may be retrieved by a cavity recapture (cavicapture) mechanism (see Henkel & Almers, 1996).
Conclusions
In endothelial cells, large changes in Cm evoked by prolonged high [Ca2+]i are composed of many discrete steps in Cm. A component of these Cm steps of amplitude 2.5-9 fF was identified as WPb exocytosis. The results indicate that ≈20 % of the membrane area increase is due to net WPb exocytosis, and > 65 % is due to small granule secretion. The time course of WPb exocytosis includes a delay of approximately 20 s with respect to the [Ca2+]i rise, whereas the secretion of small granules that may contain several important anticoagulant mediators, increases immediately. The delay in WPb exocytosis may provide a mechanism to prevent inflammatory mediator release during brief low-level stimulation, while allowing secretion of anticoagulants for the prevention of blood clotting. At sites of vascular injury or inflammation there is likely to be a strong or prolonged activation of endothelial cells, conditions favouring the exocytosis of WPb and the delivery of vWf and P-selection to promote blood clotting and an inflammatory response.
Acknowledgments
This work was funded by the British Heart Foundation and the MRC. We would like to thank Drs David Trentham and Gordon Reid for providing DM-nitrophen, and Dr Nicholai Kiskin for help with the equilibrium calculations for the calcium-DM-nitrophen solutions.
Supplementary material
The online version of this paper can be found at:
http://www.jphysiol.org/cgi/content/full/544/3/741
and contains supplementary material. It is a description of the method of analysis used for detection of discrete steps in Cm due to exocytosis and endocytosis, and the influence of p-p noise on the resolution of detection of Cm steps. It also includes a summary of the distributions of p-p noise for recordings used for the histogram construction of Fig. 4 of the paper.
References
- Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri CM, Wagner DD. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated vein. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- Andrews NW. Regulated secretion of conventional lysosomes. Trends in Cell Biology. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- Almers W, Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. Journal of Physiology. 1987;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch KA, Pober JS, Zavoico GB, Means AR, Ewenstein BM. Calcium/calmodulin transduces thrombin-stimulated secretion: studies in intact and minimally permeabilised human umbilical vein endothelial cells. Journal of Cell Biology. 1992;118:1501–1510. doi: 10.1083/jcb.118.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch KA, Ewenstein BM, Golan DE, Pober JS. Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. Journal of Cellular Physiology. 1994;160:545–554. doi: 10.1002/jcp.1041600318. [DOI] [PubMed] [Google Scholar]
- Breckenridge LJ, Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proceedings of the National Academy of Science of the USA. 1987;84:1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Handel SE. Activation of exocytosis by GTP analogues in adrenal chromaffin cells revealed by patch-clamp capacitance measurement. FEBS Letters. 1994;344:139–142. doi: 10.1016/0014-5793(94)00361-0. [DOI] [PubMed] [Google Scholar]
- Carter TD, Hallam TJ, Cusack NJ, Pearson JD. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. British Journal of Pharmacology. 1988;95:1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflügers Archiv. 1994;428:476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Kinetics of Ca2+ release by InsP3 in pig single aortic endothelial cells: evidence for an inhibitory role of cytosolic Ca2+ in regulating hormonally evoked Ca2+ spikes. Journal of Physiology. 1997;504:17–33. doi: 10.1111/j.1469-7793.1997.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Pearson JD. Regulation of prostacyclin synthesis in endothelial cells. News in Physiological Sciences. 1992;7:64–69. [Google Scholar]
- Carter TD, Zupančič G, Smith SM, Wheeler-Jones C, Ogden D. Membrane capacitance changes induced by thrombin and calcium in single endothelial cells cultured from human umbilical vein. Journal of Physiology. 1998;513:845–855. doi: 10.1111/j.1469-7793.1998.845ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single Channel Recording. 2. New York: Plenum; 1995. pp. 483–587. [Google Scholar]
- De Bruyn PP, Cho Y. In vivo exocytosis of lysosomes by the endothelium of the venous sinuses of bone marrow and liver: visualization at normal and low body temperature. American Journal of Anatomy. 1986;177:35–41. doi: 10.1002/aja.1001770105. [DOI] [PubMed] [Google Scholar]
- Elgjo RF, Henriksen T, Evensen SA. Ultrastructural identification of umbilical cord vein endothelium in situ and in culture. Cell Tissue Research. 1975;162:49–59. doi: 10.1007/BF00223261. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GCR, Kaplan JH, Barsotti JR. Laser photolysis of caged calcium: rates of calcium release by nitrophenyl-EGTA and DM-nitrophen. Biophysical Journal. 1996;70:1006–1016. doi: 10.1016/S0006-3495(96)79644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeis JJ, Van Den Eijnden-Schrauwen Y, Van Den Hoogen CM, De Priester W, Westmuckett A, Lupu F. An endothelial storage granule for tissue-type plasminogen activator. Journal of Cell Biology. 1997;139:245–256. doi: 10.1083/jcb.139.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyden BP. Ultrastructural observations on Weibel-Palade bodies suggesting exocytosis. Journal of Submicroscopic Cytology and Pathology. 1993;25:145–148. [PubMed] [Google Scholar]
- Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Frearson JA, Harrison P, Scrutton MC, Pearson JD. Differential regulation of von Willebrand factor exocytosis and prostacylin synthesis in electro-permeabilised endothelial cell monolayers. Biochemical Journal. 1995;309:473–479. doi: 10.1042/bj3090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S. Degranulation of endothelial specific granules of the toad aorta after treatment with compound 48/80. Anatomical Records. 1982;203:197–204. doi: 10.1002/ar.1092030202. [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Sakmann B, Neher E, editors. Single Channel Recording. 2. New York: Plenum; 1995. pp. 155–197. [Google Scholar]
- Gillis KD, Misler S. Enhancers of cytosolic cAMP augment depolarization-induced exocytosis from pancreatic B-cells: evidence for effects distal to Ca2+ entry. Pflügers Archive. 1993;424:195–197. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- Hallam TJ, Pearson JD, Needham L. Thrombin-stimulated elevation of endothelial cell cytoplasmic free calcium concentration causes prostacylin production. Biochemical Journal. 1988;257:243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. Journal of Clinical Investigation. 1987;79:600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison VJ, Barnes K, Turner AJ, Wood E, Corder R, Vane JR. Identification of endothelin-1 and big endothelin-1 in secretory vesicles isolated from bovine aortic endothelial cells. Proceedings of the National Academy of Science of the USA. 1995;92:6344–6348. doi: 10.1073/pnas.92.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Almers W. Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Current Opinion in Neurobiology. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- Kagawa H, Fujimoto S. Electron-microscopic and immunocytochemical analyses of Weibel-Palade bodies in the human umbilical vein during pregnancy. Cell Tissue Research. 1987;249:557–563. doi: 10.1007/BF00217327. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Ellis-Davies GC. Photolabile chelators for the rapid photorelease of divalent cations. Proceedings of the National Academy of Science of the USA. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Hollingworth S, Hawkins AB, Baylor SM. Myoplasmic Ca transients in intact frog skeletal muscle fibres monitored with the fluorescent indicator furaptra. Journal of General Physiology. 1991;97:271–302. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra T, Schrauwen Y, Arts J, Emeis JJ. Regulation of endothelial cell t-PA synthesis and release. International Journal of Hematology. 1994;59:233–255. [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Archiv. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lindau M, Nusse O, Bennet J, Cromwell O. The membrane fusion events in degranulating guinea pig eosinophils. Journal of Cell Science. 1993;104:203–210. doi: 10.1242/jcs.104.1.203. [DOI] [PubMed] [Google Scholar]
- Lupu C, Goodwin CA, Westmuckett AD, Emeis JJ, Scully MF, Kakkar VV, Lupu F. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomains/caveolae. Regulatory mechanism(s). of the anticoagulant properties of the endothelium. Arteriosclerosis Thrombosis and Vascular Biology. 1997;17:2964–2974. doi: 10.1161/01.atv.17.11.2964. [DOI] [PubMed] [Google Scholar]
- Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arteriosclerosis Thrombosis and Vascular Biology. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. Journal of Cell Biology. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiff JM, Gil J. Secretion of Weibel-Palade bodies observed in extra-alveolar vessels of rabbit lung. Journal of Applied Physiology. 1983;54:1284–1286. doi: 10.1152/jappl.1983.54.5.1284. [DOI] [PubMed] [Google Scholar]
- Mann KG. Thrombosis: theoretical considerations. American Journal of Clinical Nutrition. 1997;65:1657S–1664S. doi: 10.1093/ajcn/65.5.1657S. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proceedings of the National Academy of Science of the USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse O, Serrander L, Lew DP, Krause K -H. Ca2+-induced exocytosis in individual human neutrophils: high- and low-affinity granule populations and submaximal responses. EMBO Journal. 1998;17:1279–1288. doi: 10.1093/emboj/17.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D, Khodakhah K, Carter T, Thomas M, Capiod T. Analogue computation of transient changes of intracellular free calcium concentration with the low affinity Ca2+ indicator furaptra during whole cell patch clamp recording. Pflügers Archiv. 1995;49:587–591. doi: 10.1007/BF00704165. [DOI] [PubMed] [Google Scholar]
- Ozaka T, Doi Y, Kayashima K, Fujimoto S. Weibel-Palade bodies as a storage site of calcitonin gene-related peptide and endothelin-1 in blood vessels of the rat carotid body. Anatomical Records. 1997;247:388–394. doi: 10.1002/(SICI)1097-0185(199703)247:3<388::AID-AR10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Richardson M, Tinlin S, De Reske M, Webster S, Senis Y, Giles AR. Morphological alterations in endothelial cells with the release of von Willebrand factor after thrombin generation in vivo. Arteriosclerosis and Thrombosis. 1994;14:990–999. doi: 10.1161/01.atv.14.6.990. [DOI] [PubMed] [Google Scholar]
- Russell FD, Skepper JN, Davenport AP. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circulation Research. 1998a;83:314–321. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- Russell FD, Skepper JN, Davenport AP. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. Journal of Cardiovascular Pharmacology. 1998b;31:424–430. doi: 10.1097/00005344-199803000-00014. [DOI] [PubMed] [Google Scholar]
- Scepek S, Lindau M. Focal exocytosis by eosinophils - compound exocytosis and cumulative fusion. EMBO Journal. 1993;12:1811–1817. doi: 10.1002/j.1460-2075.1993.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg-Lever G, Gehring B, Kaiserling E. Ultrastructural localization of factor XIIIa. Journal of Cutaneous Pathology. 1994;21:129–134. doi: 10.1111/j.1600-0560.1994.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Schneider SW, Storck J, Reinhardt J, Niemeyer A, Ossig R, Oberleithner H. Dynamics of exocytosis imaged by atomic force and laser scanning microscopy. Pflügers Archiv. 2000;439:501–505. [Google Scholar]
- Scrutton MC, Pearson JD. Ca2+-driven prostacylin synthesis and von Willebrand factor secretion in electropermeabilised endothelial cells. British Journal of Pharmacology. 1989;97:420P. [Google Scholar]
- Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophysical Journal. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillén LG, Martell AE. Stability constants supplement 1. Special publication 25. London: The Chemical Society; 1971. pp. 650–654. [Google Scholar]
- Stern D, Brett J, Harris K, Nawroth P. Participation of endothelial cells in the Protein C-Protein S anticoagulant pathway: the synthesis and release of protein S. Journal of Cell Biology. 1986;102:1971–1978. doi: 10.1083/jcb.102.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. Journal of Cell Biology. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Doi Y, Sakamoto Y, Hamasaki K, Fujimoto S. Simultaneous localization of histamine and factor VIII-related antigen in the endothelium of the human umbilical vein. Anatomical Record. 1992;23:257–261. doi: 10.1002/ar.1092320210. [DOI] [PubMed] [Google Scholar]
- Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. Journal of Experimental Medicine. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- Wagner DD. Cell biology of von Willebrand factor. Annual Review of Cell Biology. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Palade GF. New cytoplasmic components in arterial endothelial. Journal of Cell Biology. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupančič G, Kocmur L, Veranic P, Grilc S, Kordas M, Zorec R. The separation of exocytosis from endocytosis in rat melanotroph membrane capacitance records. Journal of Physiology. 1994;480:539–552. doi: 10.1113/jphysiol.1994.sp020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


