Abstract
Myocardial ischaemia activates blood platelets and cardiac sympathetic afferents, which mediate chest pain and cardiovascular reflex responses. We have demonstrated that activated platelets stimulate ischaemically sensitive cardiac sympathetic afferents. Platelets absorb and release 5-hydroxytryptamine (5-HT) when they are activated. In the present study we hypothesized that, by releasing 5-HT, activated platelets stimulate cardiac afferents during ischaemia through a 5-HT3 receptor mechanism. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were obtained from cats. Activation of platelets in PRP was induced by thrombin (5 units ml−1) or collagen (2 mg kg−1). Using high-performance liquid chromatography, we observed that the concentration of 5-HT was increased significantly in suspensions of platelets activated with thrombin (PRP+thrombin, 28 ± 1.7 μm) or collagen (PRP+collagen, 27 ± 2.5 μm) compared with suspensions of unactivated platelets (PRP+saline, 2.3 ± 0.8 μm) and PPP. During myocardial ischaemia and reperfusion, tirofiban, a specific inhibitor of platelet glycoprotein (GP) IIb-IIIa receptors (100 μg kg−1, I.V., followed by 5 μg kg−1 min−1), significantly reduced the increase in the concentration of 5-HT in cardiac venous plasma from ischaemic region. Nerve activity of single-unit cardiac afferents was recorded from the left sympathetic chain (T2-T5) in anaesthetized cats. Eighty ischaemically sensitive and seven ischaemically insensitive cardiac afferents were identified. Tirofiban reduced the ischaemia-related increase in activity of seven cardiac sympathetic afferents by 50 %. Injection of 1.5 ml of PRP+collagen or PRP+thrombin into the left atrium (LA) increased activity of 16 cardiac afferents. Tropisetron (300 μg kg−1, I.V.), a selective 5-HT3 receptor antagonist, eliminated the afferent's responses to platelets activated with collagen or thrombin. Moreover, LA injection of 5-HT (20-40 μg kg−1) and PBG (100 μg kg−1), a 5-HT3 receptor agonist, stimulated nine ischaemically sensitive cardiac sympathetic afferents, significantly increasing the activity of these afferents. However, injection of α-M-5-HT (100 μg kg−1, LA), a 5-HT2 receptor agonist, stimulated only two of the nine ischaemically sensitive cardiac afferents, and thus did not significantly alter impulse activity of this group of afferents. Both the 5-HT1 (5-CT, 100 μg kg−1, LA) and 5-HT4 receptor agonists (SC53116, 100 μg kg−1, LA) did not stimulate any of the nine afferents tested. Tropisetron (300 μg kg−1, I.V.) also eliminated the response of seven ischaemically sensitive cardiac afferents to exogenous 5-HT and attenuated the ischaemia-related increase in activity of nine cardiac sympathetic afferents by 41 %. Conversely, LA injection of 5-HT (40 μg kg−1) did not stimulate any of seven ischaemically insensitive cardiac afferents, although this group of afferents consistently responded to bradykinin (3 μg, LA). These data indicate that during myocardial ischaemia the activated platelets stimulate cardiac sympathetic afferents, at least in part, through a 5-HT3 receptor mechanism.
Myocardial ischaemia is associated with both chest pain and cardiovascular reflex responses originating from the heart. Our laboratory and others have documented that myocardial ischaemia stimulates cardiac sympathetic afferents (Uchida & Murao, 1974; Tjen-A-Looi et al. 1998; Fu & Longhurst, 2002). It generally is accepted that cardiac sympathetic afferents are the primary pathway transmitting nociceptive information from the heart to the central nervous system to elicit the perception of cardiac pain and initiate excitatory cardiovascular reflex responses including hypertension and tachyarrhythmias (White, 1957; Malliani, 1990; Meller & Gebhart, 1992). Activation of platelets during myocardial ischaemia occurs in patients with unstable angina, spontaneous angina or myocardial infarction (Grande et al. 1990; Flores & Sheridan, 1994) and in experimental animal preparations undergoing coronary artery occlusion (Oei et al. 1983; Flores & Sheridan, 1994). Recently, we have suggested that activated platelets contribute to excitation of cardiac sympathetic afferents during myocardial ischaemia (Fu & Longhurst, 2002). However, the mechanisms underlying the stimulating effects of activated platelets on this afferent system have not been elucidated.
Platelets contain a number of small molecules and ions, including ATP, ADP, 5-hydroxytryptamine (5-HT, i.e. serotonin), histamine, calcium, inorganic diphosphate and inorganic phosphate, that are stored in platelet dense granules (Meyers et al. 1982; Stormorken, 1986) and released when platelets are activated by agonists or by various natural and artificial surfaces. In addition, during platelet aggregation, cyclic endoperoxide products from arachidonic acid are converted to thromboxane A2 (TxA2), which is highly labile and is released into the medium of the vascular bed (Hamberg et al. 1975). Of the platelet mediators, TxA2, ATP and biogenic amines, including 5-HT and histamine, potentially play a role in platelet-mediated excitation of sensory nerve endings. Previous studies have shown that TxA2 is capable of stimulating both somatic and vagal afferents and sensitizing these afferents to the action of other mediators (Karla et al. 1992; Kenagy et al. 1997). Pelleg and colleagues (Pelleg et al. 1993; Pelleg & Hurt, 1996) observed that ATP evokes pulmonary-cardiac depressor reflex responses through direct stimulation of vagal afferents. We have documented that endogenous serotonin and histamine stimulate ischaemically sensitive abdominal visceral afferents (Fu et al. 1997b; Fu & Longhurst, 1998). These studies led us to postulate that activated platelets likely stimulate cardiac sympathetic afferents through multiple mediator mechanisms.
Recent studies have demonstrated that activation of platelet GP IIb-IIIa receptors is the final common pathway leading to platelet activation (Lefkovits et al. 1995; Topol et al. 1999). For instance, the GP IIb-IIIa receptor or ‘αIIbβ3′ (integrin nomenclature) is expressed only in megakaryocytes and platelets and so is uniquely adapted to its role in platelet physiology. Vessel damage, adhesion itself and shear forces initiate signals that transform the GP IIb-IIIa receptor into a high affinity state that binds plasma-borne adhesive proteins such as fibrinogen and von Willebrand factor (vWF). This binding reaction leads to platelet aggregation irrespective of any of the agonists that stimulate platelets or of the stimulus-response-coupling pathway (Lefkovits et al. 1995; Coller, 1997). Furthermore, a number of studies have documented that the specific inhibitors of the platelet GP IIb-IIIa receptor, including tirofiban, abciximab and eptifibatide, completely inhibit platelet activation induced by a number of platelet agonists, including ADP, thrombin, collagen, and TxA2, in several animal models (Lefkovits et al. 1995; Coller, 1997; Hiramatsu et al. 1997). Clinical trials of GP IIb-IIIa antagonists have shown a high degree of inhibition of platelets leading to a significant reduction in the number of ischaemic events in patients with myocardial infarction and unstable angina (Hiramatsu et al. 1997; Phillips & Scarborough, 1997; Lincoff et al. 2000).
In the present study, we focused on the 5-HT mechanism since 5-HT is a major platelet mediator and likely is responsible, at least in part, for the stimulating effect of activated platelets on ischaemically sensitive cardiac sympathetic afferents. In this regard, a previous study demonstrated that the concentration of free 5-HT in human whole blood increases from 36 to 836 nm following activation of platelets with collagen (Joseph et al. 1992). Adding thrombin to platelet-rich plasma from rats and humans also leads to the release of 5-HT from platelets (Belville et al. 1979; Verhoeven et al. 1984). Additionally, the concentration of 5-HT is increased in coronary sinus blood of patients with angina and after brief coronary occlusion with angioplasty (Van den Berg et al. 1989; Golino et al. 1994). Nishi et al. (1977) have observed that exogenous 5-HT stimulates cardiac sympathetic Aδ afferents. We recently have shown that 5-HT stimulates ischaemically sensitive abdominal sympathetic afferents through a 5-HT3 receptor mechanism (Fu & Longhurst, 1998). However, there is no information on the role of platelet-derived 5-HT and the mechanism by which this chemical mediator may stimulate cardiac sympathetic afferents during myocardial ischaemia.
The effects of 5-HT potentially could be mediated by a large family of receptors which have been divided into seven types designated from 5-HT1 to 5-HT7 (Hoyer et al. 1994). However, pharmacological data suggest that four of the 5-HT receptor subtypes, 5-HT1, 5-HT2, 5-HT3 and 5-HT4, are involved in activation of the sensory nervous system (Hoyer et al. 1994) since selective agonists and antagonists to these 5-HT receptor subtypes recently have become available. In this regard, direct activation of 5-HT1A receptors on the primary afferents produces hyperalgesia (Taiwo & Levine, 1992). Activation of 5-HT2 receptors induces nociception at the spinal level through stimulation of somatic C-fibres (Hoyer et al. 1994). Stimulation of 5-HT4 receptors depolarizes the cervical vagus nerve (Rhodes et al. 1992). It also has been observed that through 5-HT3 receptors exogenous 5-HT stimulates vagal mucosal chemosensitive afferents (Grundy et al. 1994). We recently have found that endogenous 5-HT stimulates abdominal visceral afferents through 5-HT3, but not through 5-HT1 or 5-HT2, receptors (Fu & Longhurst, 1998). Thus, although several 5-HT receptor subtypes may be active in neuronal tissue the bulk of evidence suggests that 5-HT3 receptors are potentially important.
The general aim of the present study was to investigate the possibility that activated platelets, at least in part, excite ischaemically sensitive cardiac sympathetic afferents through a 5-HT mechanism. To approach the problem we employed four tactics. First, we activated platelets exogenously and examined the effect of such activation on platelet-mediated serotonin production and the influence of these activated platelets on ischaemically sensitive cardiac afferents. In these studies, we also examined the specific receptor subtype mechanism by which serotonin stimulates this sub-population of ventricular afferents. Second, we measured the concentration of serotonin in cardiac venous plasma from the ischaemic region in the absence and presence of tirofiban, an inhibitor of platelet GP IIb-IIIa receptors, to determine if activated platelets constitute the source of 5-HT during brief (5 min) myocardial ischaemia and reperfusion. Third, we observed the effect of a platelet GP IIb-IIIa receptor inhibitor on myocardial ischaemia-mediated activation of cardiac afferents. Fourth, we examined the influence of specific serotonin receptor agonists and antagonists on the activity of ischaemically sensitive and ischaemically insensitive cardiac afferents at baseline and during brief ischaemia.
Part of this work was presented as a preliminary communication at Experimental Biology 2000 in San Diego and, as such, was published as an abstract (Fu & Longhurst, 2000).
Methods
Surgical preparation
Fasted adult cats were anaesthetized with ketamine (20-30 mg kg−1, i.m., Phoenix Scientific, Inc., St Joseph, MO, USA) and anaesthesia was maintained with α-chloralose (40-50 mg kg−1, i.v.). Supplemental doses of α-chloralose (5-10 mg kg−1, i.v.) were given as necessary to maintain an adequate depth of anaesthesia that was assessed by observing the absence of a conjunctival reflex. The trachea of the animal was intubated and respiration was maintained artificially (Harvard pump, model 661, Ealing, South Natick, MA, USA). The cat was ventilated by air supplemented with 100 % O2 through the respirator. The femoral vein was cannulated to administer drugs and fluid. Another catheter (PE 90) was introduced into the left atrium (LA) through the appendage for intra-cardiac injection of solutions. Arterial blood pressure was measured by a pressure transducer (Statham P23 ID, Gould) connected to the femoral arterial catheter. Arterial blood gases were assessed frequently by a blood gas analyser (Radiometer ABL-5, Copenhagen, Denmark) and maintained within physiological limits (PO2 > 100 mmHg, PCO2 = 28-35 mmHg, pH 7.35-7.45) by adjusting the respirator rate or tidal volume, or by intravenous administration of 2-3 ml of 1 m NaHCO3 (8.4 % w/v). Body temperature was monitored by a rectal thermistor and was maintained at 36-38 °C with a circulating water heating pad and a heat lamp. At the end of the experiment, animals were killed by administration of saturated potassium chloride into the femoral vein under deep anaesthesia that was ensured by giving an additional dose of α-chloralose (50 mg kg−1). Surgical and experimental protocols used in this study were approved by the Animal Use and Care Committee at the University of California at Irvine. The studies conformed to American Physiological Society Guidelines and Principles Involving Animals.
Activation of platelets with agonists
Activation of platelets was assessed by observing aggregation of platelets as described previously (Fu & Longhurst, 2002). Briefly, 15 ml of blood from a donor cat was collected into a polystyrene tube containing 3.8 % sodium citrate (9:1 v/v) and was centrifuged at 150 g for 10 min at 22 °C (Allegra 06R Centrifuge, Beckman Coulter, Fullerton, CA, USA). The supernatant platelet-rich plasma (PRP) was placed in plastic tubes and the remaining blood samples were centrifuged at 2500 g for 10 min at 22 °C to obtain platelet-poor plasma (PPP). In each case, the number of platelets in PRP was adjusted to (480-780) × 109 platelets l−1. Platelets were activated immediately by incubating PRP (37 °C, 10 min) with 2 mg ml−1 collagen (Type I, bovine Achilles’ tendon, Sigma, St Louis, MO, USA) or 5 units ml−1 thrombin (Calbiochem, La Jolla, CA, USA), respectively, followed by agitation for 30 s on a vortex genie mixer (Fisher Scientific, USA (Verhoeven et al. 1984). The numbers of platelets were determined in the samples of PPP as well as in activated and unactivated PRP. Supernatants from the suspensions were obtained by centrifugation of PPP and activated or unactivated PRP at 2500 g for 10 min at 22 °C, respectively. These supernatants then were stored in crushed ice or in a refrigerator at -20 °C until needed.
Collection of cardiac venous plasma
A midline sternotomy was performed, and the second-eighth left ribs, along with the upper and middle lobes of the left lung, were removed. The pericardium was incised longitudinally, and a suture was placed at the apex of the heart to elevate it for manipulation of various vessels. This procedure did not alter arterial pressure or heart rate. A loop snare was placed around the proximal left anterior descending (LAD) artery. The cardiac vein was cannulated for collection of blood in all animals after administration of heparin (500 units kg−1, i.v.). Plasma was obtained by centrifugation of venous blood samples at 2500 g for 10 min at 22 °C and then stored at -20 °C until needed.
Chromatography for measurement of 5-HT
The concentration of 5-HT in PPP, activated and unactivated PRP, and plasma was measured according to our previous description (Fu et al. 1997a). Briefly, a 100 μl PRP, PPP, or plasma sample was added to an equal volume of 5 % 5-sulfosalicylic acid (Sigma), and then centrifuged at 3000 g at 4 °C for 15 min. The supernatant was analysed immediately thereafter for 5-HT and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). The mobile phase for isocratic elution of 5-HT and 5-HIAA consisted of 30 mm NaH2PO4 (pH 3.5), 7 % methanol and 1.5 % (v/v) isopropyl alcohol, which was run through an RP-8 7 μm guard column (15 mm × 3.2 mm, Applied Biosystems, Palo Alto, CA, USA) and a Spherisorb pS Phase Separations ODS2, 5 μm high-performance liquid chromatography (HPLC) analytical column (Phase Separations Inc.). The mobile phase was pumped (510 pump, Waters, Milford, MA, USA) at a flow rate of 1.2 ml min−1. Both 5-HT and 5HIAA were detected coulometrically by a Coulochem II detector (ESA, Chelmsford, MA, USA) with a 5020 guard cell and a 5010 analytic cell (ESA). The applied working potentials were 700 mV for the guard cell and 200 mV (E1) and 650 mV (E2) for the analytic cell. The detection limit for this assay is 5 pg for 5-HT. Confirmation of peak identity was accomplished by concurrently running samples with known standards.
Cardiac sympathetic afferent recording
Single-unit activity of cardiac sympathetic afferents was recorded as described previously (Tjen-A-Looi et al. 1998; Fu & Longhurst, 2002). Briefly, a midline sternotomy was performed and the first-seventh left ribs and the left lung were removed. The left paravertebral sympathetic chain was isolated, then draped over a Plexiglass platform and covered with warm mineral oil. Small nerve filaments were dissected gently from the chain between T2 and T5 under an operating microscope (Zeiss, Germany) and the rostral ends were placed across one pole of the recording electrode. The other pole of the recording electrode was grounded with a cotton thread to the animal. The recording electrode was attached to a high impedance probe (model HIP511, Grass Instruments, Quince, MA, USA), and the signal was amplified (model P511 Preamplifier, Grass, USA) and processed through an audioamplifier (AM8B, Audiomonitor, Grass, USA) and an oscilloscope (model 2201, Tektronix, Beavertown, OR, USA). The signal was recorded on a chart recorder (TA 4000B, Gould, Cleveland, OH, USA) with an IBM compatible Pentium II computer through an analog-to-digital interface card (R.C. Electronics Inc., Santa Barbara, CA, USA) for subsequent off-line analysis. The discharge frequency of afferents was analysed by using data acquisition and analysis software (EGAA, version 3.02, R.C. Electronics Inc.) and a histogram was created for each afferent.
The precise location of the afferent nerve ending was confirmed by placing a stimulating electrode directly on the surface of the myocardium to evoke the afferent's action potential. Conduction velocity of each afferent fibre was calculated by dividing conduction distance by the conduction time. The conduction time and the conduction distance were determined as described previously (Fu & Longhurst, 2002). C- and Aδ-fibre afferents were classified as those with conduction velocities (CV) of < 2.5 and 2.5-30 m s−1, respectively. In the present study, each afferent had a single receptive field that could be located precisely.
Myocardial ischaemia was induced by complete occlusion of the appropriate coronary artery supplying the regional receptive field of the cardiac afferent nerve with a thread placed around the vessel (Fu & Longhurst, 2002). An afferent was considered to be ischaemically sensitive if its discharge activity during 3-5 min of myocardial ischaemia was increased at least twofold above baseline activity (Tjen-A-Looi et al. 1998; Fu & Longhurst, 2002).
Experimental protocols
Activation of platelets by collagen and thrombin
PRP and PPP were collected from 32 cats including 16 for PRP activated with collagen (PRP+collagen, 2 mg ml−1) and 16 for PRP activated with thrombin (PRP+thrombin, 5 units ml−1). Activation of platelets was verified by observing the formation of platelet clumps and a large decrease in the number of platelets (from (483-770) × 109 to < (1-66) × 109 platelets l−1) in the PRP using microscopy with a haemacytometer (Fisher Scientific). Supernatants from activated PRP (collagen and thrombin), unactivated PRP (PRP+saline) and PPP (collagen and thrombin) were used in the measurement of 5-HT concentration and in the cardiac afferent study.
Measurement of 5-HT in PRP and PPP samples
We utilized HPLC with coulometric detection to measure 5-HT concentration in 16 samples of PRP+collagen, PRP+saline and PPP+collagen, as well as in another 16 samples of PRP+thrombin, PRP+saline and PPP+thrombin.
Effects of tropisetron on responses of afferents to platelets activated with collagen or thrombin
This protocol was used to examine the effect of 5-HT3 receptor antagonism with 3-tropanl-indole-3-carboxylate (ICS-205-930 or tropisetron, 300 μg kg−1, i.v.; RBI, Natick, MA, USA) on the response of ischaemically sensitive afferents to platelets activated with collagen or thrombin. In 16 cats (8 for collagen, 8 for thrombin), after identification of an ischaemically sensitive unit following 5 min of regional myocardial ischaemia, we injected 1.5 ml of PRP+collagen, PRP+thrombin, PPP+collagen or PPP+thrombin into the left atrium (LA) while activity of the afferent was recorded. We repeated injection of PRP+collagen or PRP+thrombin, 15-20 min after its initial injection including at least 15 min following treatment with tropisetron (300 μg kg−1, i.v.). On the day of each experiment, tropisetron was dissolved in 0.9 % NaCl (w/v) and diluted to a concentration of 2 mg ml−1. After treatment with tropisetron, we administered bradykinin (BK, 3 μg) into the LA to establish responsiveness of the afferent nerve ending.
To differentiate between variations in afferent responses related to drug effects and time related effects, 16 separate animals (8 for collagen and 8 for thrombin) were studied as time controls, since the elimination half-life following intravenous administration of tropisetron is 7.3 h (Lee et al. 1993). After identification of an ischaemically sensitive unit, each animal was treated in an identical manner except that 0.9 % NaCl was used in place of tropisetron.
Measurement of 5-HT in cardiac venous plasma
Myocardial ischaemia was induced by reversible occlusion of the LAD coronary artery with a loop snare for 5 min in two groups of cats. In the first group of animals (n = 7), sham ischaemia and myocardial ischaemia were induced randomly, maintaining at least 60 min of recovery between both interventions. Tirofiban (100 μg kg−1, i.v. bolus, followed by 5 μg kg−1 min−1, i.v. infusion,for 25-30 min) was administered in the second group of animals (n = 7). Myocardial ischaemia was induced 15 min after the bolus dose of tirofiban (Merck, Rahway, NJ, USA) and during the period of infusion. Cardiac venous blood samples from the ischaemic region of myocardium were collected and blood flow was determined during pre-ischaemia, ischaemia and the early reperfusion period. Plasma was obtained immediately by centrifugation of blood samples and HPLC was used to measure the concentration of 5-HT.
Effects of inhibition of platelet GP IIb-IIIa receptors on responses of afferents to ischaemia
This protocol was used to examine the effects of platelet GP IIb-IIIa receptor inhibition with tirofiban (100 μg kg−1, i.v. bolus, followed by 5 μg kg−1 min−1 infusion, for 25-30 min) on the afferent responses to 5 min of ischaemia in eight animals. After locating the receptive field of an afferent, 5 min of ischaemia was induced by occluding the coronary artery supplying this region with a thread placed around the vessel. If the afferent responded to ischaemia, this intervention was repeated 30-40 min after the first period of ischaemia, 15 min after tirofiban bolus and during tirofiban infusion. In the occasional circumstance where afferent activity was suppressed completely, we stimulated the receptive field with an electrode to establish the viability of the nerve ending.
To differentiate between variations in afferent response related to drug effect and time-related effects, seven additional ischaemically sensitive afferents were examined to determine repeatability of the afferent response to ischaemia. In this protocol, after identification, each afferent fibre was treated in an identical manner but was not subjected to tirofiban.
Responses of ischaemically sensitive afferents to 5-HT and specific 5-HT receptor agonists
This protocol consisted of subjecting nine cats to 5 min of myocardial ischaemia followed by 2-3 min of reperfusion. Based on the responses of afferents to ischaemia, we classified cardiac afferents as either ischaemically sensitive or ischaemically insensitive (see next protocol). In the ischaemically sensitive afferent group, 5-HT (20-40 μg kg−1), 5-carboxamido-tryptamine (5-CT, 100 μg kg−1, 5-HT1 receptor agonist), α-methylserotonin (α-M-5-HT, 100 μg kg−1, 5-HT2 receptor agonist), phenylbiguanide (PBG, 100 μg kg−1, 5-HT3 receptor agonist) or SC53116 (100 μg kg−1, 5-HT4 receptor agonist) was injected into the LA. These drugs, purchased from Research Biochemicals International (RBI), were dissolved in physiological saline to a concentration of 1 mg ml−1 and were prepared fresh daily. The five agonists were injected randomly, maintaining at least 20 min of recovery between injections to avoid tachyphalaxis. The afferent activity was recorded as described previously (Fu & Longhurst, 2002). The chosen doses of these drugs have been shown to effectively stimulate visceral afferents in cats (Fu & Longhurst, 1998). We measured nerve conduction velocity as noted above.
Responses of ischaemically insensitive cardiac afferents to 5-HT and BK
In the ischaemically insensitive afferent group (n = 7), 5-HT (40 μg kg−1) was injected into the LA 30-40 min after recording the activity of cardiac afferent during 5 min of ischaemia and 2-3 min of reperfusion. We also injected BK (3 μg, LA) to establish the responsiveness of these afferents.
Effect of tropisetron on response of afferents to 5-HT
In this protocol, the effect of tropisetron (300 μg kg−1, i.v.) on the response of seven ischaemically sensitive afferents to 5-HT was investigated. After identification of an ischaemically sensitive unit, we injected 5-HT (40 μg kg−1) into the left atrium while recording afferent activity. We repeated application of 5-HT (40 μg kg−1, LA) 15-20 min after its initial injection, including at least 15 min following treatment with tropisetron. After treatment with tropisetron, we administered BK (3 μg) into the LA to establish the responsiveness of the afferent nerve ending.
To determine the reproducibility of afferent responses to 5-HT, eight additional animals were studied as time controls. After identification of an ischaemically sensitive unit, each animal in this group was treated identically except that saline (2-3 ml, i.v.) was used in place of tropisetron.
Effects of 5-HT3 receptor blockade on responses of afferents to ischaemia
After locating the receptive field of an afferent on the heart, discharge activity was measured during 5 min of regional myocardial ischaemia. If the afferent responded to ischaemia, a second period of ischaemia was repeated 30-40 min later in the presence of tropisetron (300 μg kg−1, i.v.) to block 5-HT3 receptors (Fu & Longhurst, 1998). The control group used in tirofiban protocol served as time control for comparison.
Data analysis
Discharge activity of cardiac sympathetic afferents was expressed in impulses s−1 and was averaged during 5 min of pre-ischaemia, 5 min of ischaemia and the initial 2 min of reperfusion (Tjen-A-Looi et al. 1998; Fu & Longhurst, 2002). We measured the responses of the afferents to 5-HT, specific 5-HT receptor agonists, PRP+thrombin, PPP+thrombin, PRP+collagen, PPP+collagen and PRP+saline by averaging discharge rates of the afferents during the entire period of response, defined as the time during which sustained activity exceeded baseline activity by 20 %. Baseline activity was determined over the 5 min period immediately preceding ischaemia.
Data are expressed as means ± s.e.m. Student's paired t test was used to evaluate the effects of each 5-HT receptor agonist on the discharge activity of cardiac afferents. Changes in 5-HT concentration, cardiac venous blood flow in the absence and presence of tirofiban, and the effects of repeated injection of 5-HT, repeated myocardial ischaemia, tirofiban on responses of the afferents to ischaemia, and activated PRPs and PPPs on the activity of cardiac afferents were compared individually using one-way repeated-measures analysis of variance (ANOVA) with Tukey's post hoc test. These tests also were used to examine the influence of the tropisetron on responses of the afferents to myocardial ischaemia, 5-HT and activated PRP (collagen or thrombin). If data were not normally distributed, as determined by the Kolmogorov-Smirnov test, they were compared using Friedman's analysis of variance of ranks with Dunnett's post hoc test. All statistical calculations were performed with SigmaStat software (SPSS Science, Chicago, IL, USA). Values were considered to be significantly different when P < 0.05.
Results
Platelet 5-HT
Consistent with our previous observations (Fu & Longhurst, 2002), we found that in samples of PRP+thrombin or PRP+collagen, platelets clumped and the numbers of platelets decreased dramatically (from (594 ± 28) × 109 to (29 ± 7) × 109 platelets l−1 for collagen and from (605 ± 22) × 109 to (26 ± 4) × 109 platelets l−1, for thrombin). Conversely, the concentrations of platelets in PRP+saline were unaltered ((609 ± 24) × 109 vs. (597 ± 22) × 109 platelets l−1, before vs. after).
Figure 1A shows a typical chromatogram of a standard solution of tryptophan, 5-HT and its metabolite 5-HIAA obtained by HPLC with coulometeric detection. Figure 1B displays a chromatogram of a thrombin-activated platelet sample extract (PRP+thrombin) containing 5-HT and tryptophan. The amounts of 5-HT released from PPP, unactivated platelets (PRP+saline), and activated platelets (PRP+collagen and PRP+thrombin) are summarized in Table 1. Compared with unactivated PRP (PRP+saline) and the corresponding PPP solutions (PPP+collagen and PPP+thrombin), collagen and thrombin stimulated platelets to release large amounts of 5-HT.
Figure 1. Concentration of 5-HT in sample of platelets activated with thrombin.
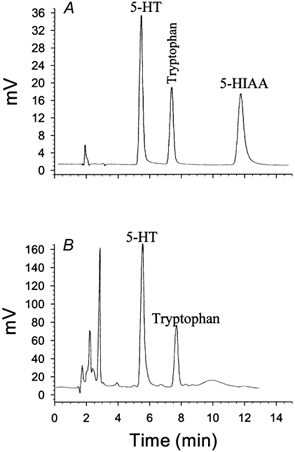
A, representative chromatograph of 5-HT, 5-HIAA and tryptophan from standards (5 ng each). B, chromatograph of 5-HT and tryptophan from 0.1 ml of plasma containing platelets activated by thrombin (5 units ml−1). Detection was accomplished coulometrically. Recovery of 5-HT, 93 ± 1.5 %. Limit of detection of 5-HT, 5 pg.
Table 1.
Concentrations of 5-HT in PRP or PPP
| n | 5-HT(μm) | n | 5-HT(μm) | ||
|---|---|---|---|---|---|
| PPP+collagen | 16 | 2.72 ± 0.68 | PPP+thrombin | 16 | 1.69 ± 0.46 |
| PRP+saline | 16 | 1.93 ± 0.79 | PRP+saline | 16 | 2.26 ± 0.86 |
| PRP+collagen | 16 | 26.6 ± 2.60*† | PRP+thrombin | 16 | 27.6 ± 1.42*† |
PPP, platelet-poor plasma; PRP, platelet-rich plasma
P < 0.01 compared with the corresponding PPP suspensions
P < 0.01 compared with PRP+saline.
Effects of tropisetron on the responses of afferents to platelets activated with collagen
Antagonism of 5-HT3 receptors with tropisetron in a group of eight ischaemically sensitive cardiac afferents (one Aδ-fibre, CV = 4.58 m s−1; seven C-fibres, CV = 0.63 ± 0.13 m s−1) on the response of the afferents to platelets activated with collagen (PRP+collagen) is summarized in Fig. 2B. Injection of PRP+collagen (1.5 ml, LA) stimulated the eight ischaemically sensitive afferents resulting in an increase in their activity from 0.63 ± 0.13 to 1.69 ± 0.17 impulses s−1. Conversely, PPP+collagen (1.5 ml, LA) did not stimulate any of the afferents (0.51 ± 0.09 vs. 0.50 ± 0.08 impulses s−1, before vs. after). Tropisetron (300 μg kg−1, i.v.) eliminated this response (Fig. 2B), although these afferents still responded to LA injection of 3 μg of BK (0.44 ± 0.12 vs. 1.88 ± 0.32 impulses s−1, before vs. after, P < 0.05). In a time control group, we observed consistent responses of the eight separate ischaemically sensitive afferents (two Aδ-fibres, CV = 3.98 and 3.06 m s−1; six C-fibres, CV = 0.57 ± 0.10 m s−1) to repeated injections of PRP+collagen (Fig. 2A). The location of each of these afferent nerve endings is shown in Table 3. Representative tracings of an ischaemically sensitive cardiac sympathetic afferent responding to injection of PRP+collagen before and after tropisetron are shown in Fig. 2C. Injection of PRP+collagen into the LA increased the discharge activity of this cardiac afferent (Fig. 2Ca), a response that was eliminated by tropisetron (Fig. 2Cb).
Figure 2. Responses of ischaemically sensitive cardiac afferents to PRP+collagen before and after the blockade of 5-HT3 receptors.
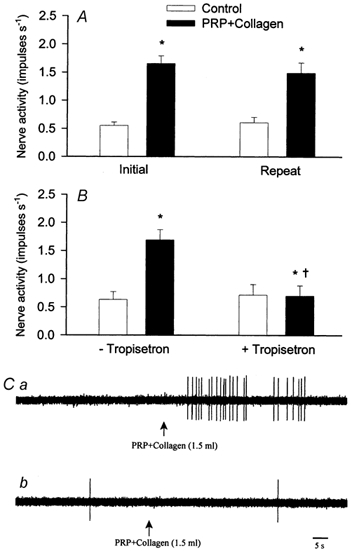
A, discharge frequencies of nine cardiac sympathetic afferents before (□) and after (▪) injection of PRP+collagen (1.5 ml, LA). B, effect of PRP+collagen on mean activity of eight separate cardiac afferents before (- tropisetron) and after (+tropisetron) tropisetron (300 μg kg−1, i.v.). C, neurograms displaying responses of an ischaemically sensitive cardiac C-fibre (CV = 0.45 m s−1) to PRP+collagen (1.5 ml, LA) before (Ca) and after (Cb) administration of tropisetron (300 μg kg−1, i.v.). This cardiac afferent innervated the posterior wall of the left ventricle. Data are presented as means ± s.e.m. * P < 0.05 compared with control; † P < 0.05, post-tropisetron vs. pre-tropisetron.
Table 3.
Locations of ischaemically sensitive cardiac sympathetic afferent nerve endings
| 5-HT agonist | Ischaemia/tirofiban | Repeated ischaemia | Ischaemia/Trop | Repeated 5-HT | 5-HT/Trop | 5-HT, BK ischaemia | Repeated PRP+Th | PRP+Th/Trop | Repeated PRP+Col | PRP ± col/Trop | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left ventricle | |||||||||||
| Anterior | 3 | 2 | 2 | 4 | 3 | 2 | 4 | 4 | 2 | 4 | 3 |
| Posterior | 5 | 6 | 4 | 4 | 4 | 4 | 2 | 4 | 5 | 4 | 4 |
| Right ventricle | |||||||||||
| Anterior | 1 | — | — | — | — | 1 | — | — | — | — | 1 |
| Posterior | — | — | 1 | 1 | 1 | — | 1 | — | 1 | — | — |
| Total | 9 | 8 | 7 | 9 | 8 | 7 | 7 | 8 | 8 | 8 | 8 |
PRP, platelet-rich plasma; Th, thrombin; Col, collagen; Trop, tropisetron.
Effects of tropisetron on the responses of afferents to platelets activated with thrombin
Figure 3B summarizes the effect of tropisetron on the responses of eight other ischaemically sensitive cardiac afferents (one Aδ-fibre, CV = 3.52 m s−1; seven C fibres, CV = 0.90 ± 0.27 m s−1) to platelets activated with thrombin. The activity of the afferents was increased from 0.42 ± 0.09 to 2.38 ± 0.46 impulses s−1 by brief myocardial ischaemia. Injection of 1.5 ml of PRP+thrombin into the LA stimulated all eight afferents, significantly increasing their discharge activity from 0.61 ± 0.14 to 1.73 ± 0.21 impulses s−1. Tropisetron (300 μg kg−1, i.v.) virtually eliminated the afferent responses to repeated injection of PRP+thrombin (Fig. 3B). Each of the eight afferents still responded to LA injection of 3 μg of BK (0.37 ± 0.11 vs. 2.01 ± 0.38 impulses s−1, before vs. after, P < 0.05) after administration of tropisetron. Conversely, we observed that eight other ischaemically sensitive cardiac afferents (two Aδ-fibres, CV = 4.12 and 2.97 m s−1; seven C-fibres, CV = 0.74 ± 0.38 m s−1) responded consistently to repeated injections of PRP+thrombin over the same time frame (Fig. 3A). Injection of PPP+thrombin into the LA did not stimulate any of the eight afferents (0.59 ± 0.10 vs. 0.62 ± 0.09 impulses s−1, before vs. after), an observation consistent with our previous study (Fu & Longhurst, 2002). Each afferent nerve ending was located in the left or right ventricle (Table 3).
Figure 3. Effect of tropisetron on responses of cardiac afferents to PRP+thrombin.
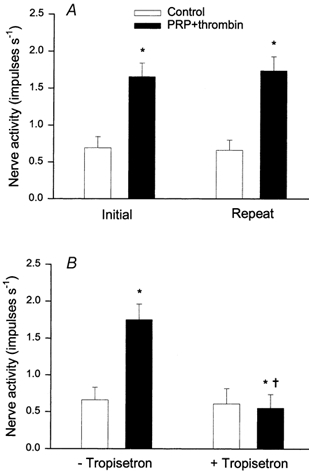
A, reproducibility of responses of nine cardiac sympathetic afferents to PRP+thrombin (1.5 ml, LA). B, responses of eight other cardiac sympathetic afferents to PRP+thrombin before (-tropisetron) and after (+ tropisetron) treatment with tropisetron (300 μg kg−1, i.v.). Columns and error bars represent means ± s.e.m. * P < 0.05 compared with control; † P < 0.05 post- tropisetron vs. pre- tropisetron
Cardiac venous blood flow and plasma 5-HT
During myocardial ischaemia cardiac venous blood flow significantly decreased from the pre-ischaemia level of 1.10 ± 0.09 to 0.24 ± 0.02 ml min−1, while during reperfusion blood flow significantly increased to 1.79 ± 0.12 ml min−1. We also observed a similar alteration of cardiac venous flow during pre-ischaemia, ischaemia and reperfusion in the tirofiban group. However, there was no alteration of cardiac venous flow (1.04 ± 0.09 ml min−1) during sham ischaemia and reperfusion compared with the pre-ischaemia level. The concentrations of 5-HT in plasma tended to increase (P = 0.053) during myocardial ischaemia and were significantly elevated immediately after release of the occlusion cuff (P < 0.01) compared to the pre-ischaemia levels in seven cats. In contrast, after administration of tirofiban, the cardiac 5-HT levels during ischaemia and reperfusion remained unchanged from the pre-ischaemia level (Table 2). The concentrations of 5-HT in cardiac venous plasma were at the same levels during pre-ischaemia, sham ischaemia and reperfusion in the sham control group. The concentration of 5-HT in cardiac venous plasma was significantly higher in the ischaemia group than the 5-HT levels in both the tirofiban and sham control groups during reperfusion.
Table 2.
Concentrations of 5-HT in cardiac venous plasma during myocardial ischaemia and reperfusion
| 5-HT (μm) | ||||
|---|---|---|---|---|
| n | Pre-ischaemia | Ischaemia | Reperfusion | |
| Sham control | 7 | 3.44 ± 0.41 | 3.40 ± 0.43 | 3.37 ± 0.44 |
| Ischaemia | 7 | 3.28 ± 0.39 | 3.81 ± 0.30 | 4.57 ± 0.27*† |
| Tirofiban | 7 | 3.37 ± 0.38 | 3.43 ± 0.32 | 3.39 ± 0.39 |
P < 0.05 compared with pre-ischaemia and ischaemia
P < 0.05 compared with sham control tirofiban group.
Effect of inhibition of platelet GP IIb-IIIa receptors on the response of cardiac afferents to myocardial ischaemia
Before application of tirofiban, a specific inhibitor of platelet GP IIb-IIIa receptor, we observed that the summed impulse activity of seven cardiac afferents (one Aδ-fibre, CV = 4.8 m s−1; six C-fibres, CV = 0.53 ± 0.21 m s−1) during the entire 5 min period of ischaemia was increased from 6.9 to 19.8 impulses s−1 (Fig. 4A). Inhibition of platelet GP IIb-IIIa receptors with tirofiban attenuated the response to ischaemia by 50 % (Fig. 4B). Similarly, brief myocardial ischaemia significantly increased the mean discharge activity of seven cardiac afferents from 0.74 ± 0.15 to 2.56 ± 0.41 impulses s−1. The responses of these cardiac afferents to ischaemia were attenuated significantly from 2.56 ± 0.41 to 1.28 ± 0.17 impulses s−1 after tirofiban treatment. Seven additional ischaemically sensitive C-fibre afferents (CV = 0.57 ± 0.14 m s−1) responded consistently to repeated 5 min periods of myocardial ischaemia (Fig. 9A). The neurograms in Fig. 4 show the response of a C-fibre to 5 min of ischaemia before and after tirofiban. The response of this afferent to ischaemia was attenuated 52 % by tirofiban. The response of one additional cardiac afferent to ischaemia was not attenuated by treatment with tirofiban (from 0.15 to 1.59 impulses s−1 before tirofiban vs. from 0.54 to 1.78 impulses s−1 after tirofiban treatment). The locations of all eight cardiac afferent nerve endings are shown in Table 3.
Figure 4. Influence of tirofiban on the response of cardiac afferents to myocardial ischaemia.
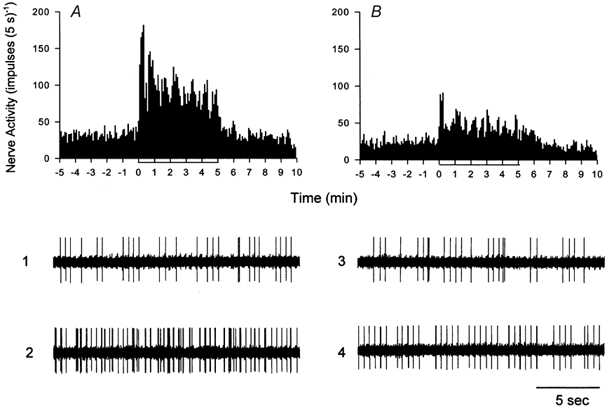
Neurohistograms showing summed 5 s discharge activity of seven cardiac sympathetic afferents during myocardial ischaemia before (A) and after (B) administration of tirofiban (100 μg kg−1, i.v. followed by infusion of 5 μg kg−1 min−1 for 25-30 min). Neurograms 1-4 are representative tracings of activity of a cardiac afferent innervating the anterior wall of the left ventricle (CV = 1.55 m s−1) during pre-ischaemia (1 and 3) and ischaemia (2 and 4) in the absence (1 and 2) and presence (3 and 4) of tirofiban.
Figure 9. Effect of tropisetron on group responses of cardiac afferents during myocardial ischaemia.
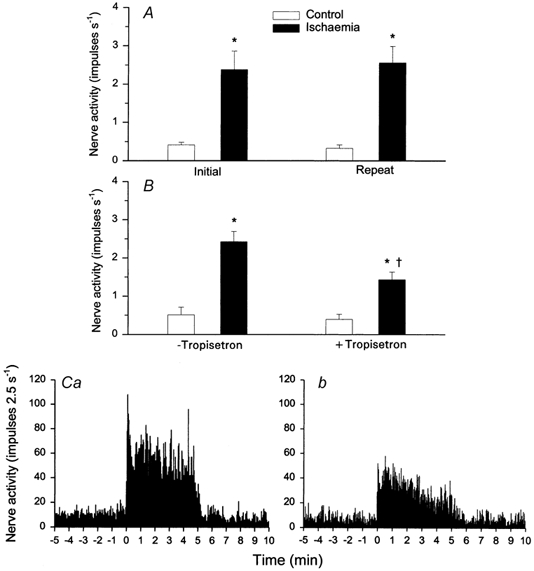
A, bar graph summarizing changes in activity of seven ischaemically sensitive cardiac afferents before (□) and during (▪) 5 min of repeated myocardial ischaemia. B, bar graph displaying discharge frequency of nine cardiac afferents before and during 5 min of ischaemia before (- tropisetron) and after (+ tropisetron) administration of tropisetron (300 μg kg−1, i.v.). C, neurohistograms provide summed 2.5 s discharge activity of all nine cardiac afferents during ischaemia before (Ca) and after (Cb) treatment with tropisetron. Columns and error bars represent means ± s.e.m. * P < 0.05vs. pre-ischaemia; † P < 0.05, post-tropisetron vs. pre-tropisetron.
Responses of ischaemically sensitive cardiac afferents to 5-HT and specific 5-HT receptor agonists
Figure 5 displays original tracings of an ischaemically sensitive C-fibre (CV = 0.53 m s−1) innervating the posterior wall of the left ventricle, during ischaemia and injection of 5-HT, 5-CT, α-M-5-HT, PBG and SC53116 (Fig. 5, panels A-F, respectively). The discharge activity of this cardiac C-fibre afferent increased from 0.67 to 2.73 impulses s−1 during ischaemia. After release of coronary arterial occlusion, the discharge activity of this fibre gradually returned to control levels. Injection of 5-HT and PBG (Fig. 5, panels B and E, respectively) resulted in an immediate burst of afferent activity. In contrast, injection of 5-CT, α-M-5-HT and SC53116 (Fig. 5, panels C, D and F, respectively) did not alter the impulse activity of this afferent.
Figure 5. Representative discharge activity of a cardiac sympathetic afferent during myocardial ischaemia and stimulation with 5-HT and specific 5-HT receptor agonists.
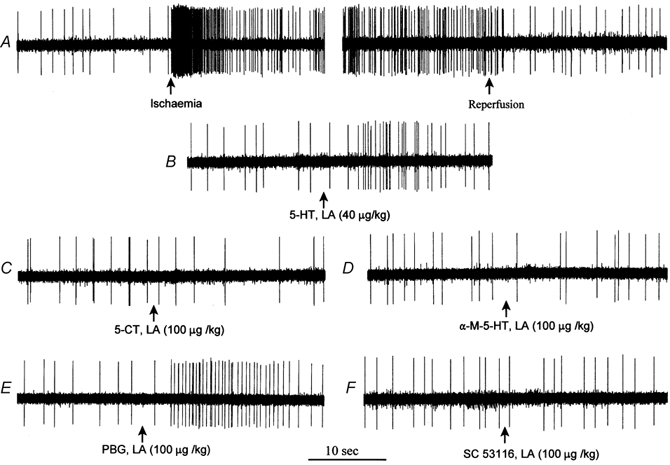
A, myocardial ischaemia increased average discharge activity of this afferent from 0.67 to 2.73 impulses s−1. B-F, responses of this afferent during injection of 5-HT (B), 5-CT (C), α-M-5-HT (D), PBG (E) or SC53116 (F) into the left atrium (LA). This C-fibre afferent (CV = 0.53 m s−1) innervated the posterior wall of the left ventricle.
The effects of 5-HT, 5-CT, α-M-5-HT, PBG and SC53116 on the entire group of nine ischaemically sensitive cardiac afferents (one Aδ-fibre, CV = 3.42 m s−1; eight C-fibres, CV = 0.61 ± 0.18 m s−1) are summarized in Fig. 6. The location of each of the nine afferent nerve endings is shown in Table 3. Injection of 5-HT (20-40 μg kg−1) into the left atrium stimulated all nine fibres, significantly increasing their discharge activity from 0.42 ± 0.14 to 1.81 ± 0.30 impulses s−1. Left atrial injection of PBG (100 μg kg−1) also stimulated eight of the nine C-fibres, significantly increasing their discharge activity from 0.45 ± 0.12 to 1.60 ± 0.39 impulses s−1. In contrast, application of α-M-5-HT (100 μg kg−1, LA) stimulated only two of the nine fibres, and thus did not significantly alter the impulse activity of this group of fibres (0.49 ± 0.13 to 0.81 ± 0.29 impulses s−1, P = 0.26). Injection of 5-CT (100 μg kg−1, LA) or SC53116 (100 μg kg−1, LA) did not stimulate any of the nine fibres tested (0.47 ± 0.14 to 0.44 ± 0.16, or 0.59 ± 0.19 to 0.50 ± 0.14 impulses s−1).
Figure 6. Responses of ischaemically sensitive cardiac afferents to 5-HT and specific 5-HT receptor agonists.
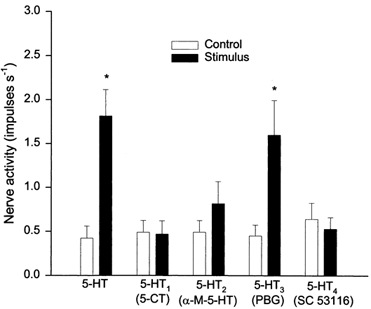
Bar graph showing peak activity of nine ischaemically sensitive cardiac afferents before (□) and after (▪) left atrial injection of 5-HT (20-40 μg kg−1), 5-CT (100 μg kg−1), α-M-5-HT (100 μg kg−1), PBG (100 μg kg−1) or SC53116 (100 μg kg−1). Data are presented as means ± s.e.m. * P < 0.05vs. control.
Responses of ischaemically insensitive afferents to 5-HT
Figure 7A summarizes the responses of seven ischaemically insensitive cardiac afferents (one Aδ-fibre, CV = 2.75 m s−1; six C-fibres, CV = 0.88 ± 0.19 m s−1) to 5-HT. Five minutes of myocardial ischaemia did not alter the discharge activity of this group of afferents (0.63 ± 0.20 vs. 0.62 ± 0.17 impulses s−1, P > 0.05). Also, injection of 5-HT (40 μg kg−1) into the LA did not stimulate any of the seven cardiac afferents (0.51 ± 0.14 vs. 0.50 ± 0.15 impulses s−1, P > 0.05). In contrast, this group of afferents did respond to LA injection of 3 μg of BK (0.59 ± 0.19 vs. 2.88 ± 0.69 impulses s−1, before vs. after, P < 0.05). The locations of all seven ischaemically insensitive afferent nerve endings are shown in Table 3.
Figure 7. Responses of ischaemically sensitive and insensitive cardiac afferents to 5-HT before and after tropisetron.
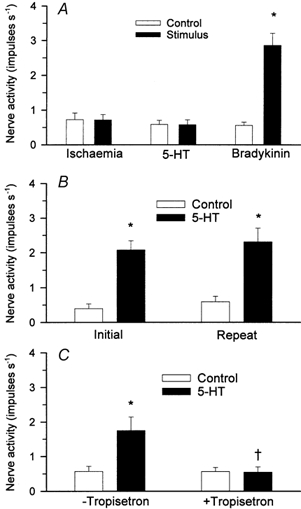
A, bar graph showing discharge activity of seven cardiac afferents during 5 min of myocardial ischaemia, stimulation with 5-HT (40 μg kg−1, LA) and bradykinin (3 μg, LA). B, bar graph showing reproducibility of responses of nine ischaemically sensitive cardiac afferents to repeated LA administration of 5-HT (40 μg kg−1). C, bar graph summarizing responses of six other ischaemically sensitive cardiac afferents to 5-HT (40 μg kg−1, LA) before (-tropisetron) and after (+ tropisetron) treatment with tropisetron (300 μg kg−1, i.v.). Data are presented as means ± s.e.m. * P < 0.05vs. respective control; † P < 0.05, post-tropisetron vs. pre-tropisetron.
Influence of tropisetron on responses of ischaemically sensitive cardiac afferents to 5-HT
The effects of 5-HT3 receptor blockade with tropisetron on the response of seven ischaemically sensitive afferents (one Aδ-fibre, CV = 3.64 m s−1; six C-fibres, CV = 0.84 ± 0.14 m s−1) to 5-HT are shown in Fig. 7C. 5-HT (40 μg kg−1, LA) significantly increased the discharge activity of all seven C-fibres from a baseline activity of 0.51 ± 0.13 impulses s−1 to 1.65 ± 0.35 impulses s−1. Tropisetron (300 μg kg−1, i.v.) virtually eliminated the afferent responses to 5-HT (Fig. 7C). Conversely, each of the seven afferents still responded to injection of 3 μg of BK (from 0.48 ± 0.18 to 2.69 ± 0.39 impulses s−1, P < 0.05) after tropisetron. This decrease in responsiveness of the afferent to 5-HT was not due to a general decrease in reactivity over time because eight other ischaemically sensitive afferents (one Aδ-fibre, CV = 3.24 m s−1; seven C-fibres, CV = 0.62 ± 0.11 m s−1) responded consistently to repeated administration of 5-HT (40 μg kg−1, LA) over the same time frame (Fig. 7B). The locations of the 15 cardiac afferent nerve endings are shown in Table 3.
Effects of tropisetron on responses of cardiac afferents to myocardial ischaemia
Figure 8 shows histograms and original recordings of an ischaemically sensitive C-fibre with a conduction velocity of 1.93 m s−1 that innervated the anterior wall of the left ventricle. Ischaemia increased the baseline activity of this afferent from 0.09 to 2.90 impulses s−1 (Fig. 8A). Antagonism of 5-HT3 receptors with tropisetron (300 μg kg−1, i.v.) attenuated the increase in discharge activity of this afferent (from 0.12 to 1.40 impulses s−1) by 52 % during repeat ischaemia (Fig. 8B).
Figure 8. Response of a cardiac afferent to myocardial ischaemia before and after the 5-HT3 receptor blockade.
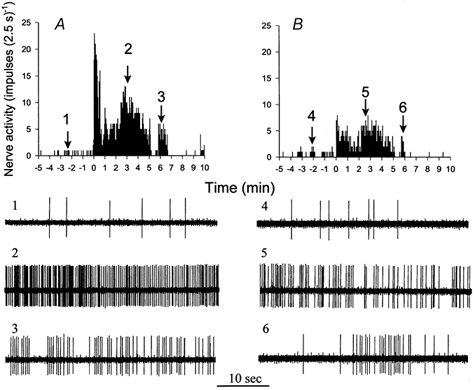
A, histograms showing increase in activity of a cardiac C-fibre afferent (CV = 1.93 m s−1) from 0.09 to 2.90 impulses s−1 during 5 min of myocardial ischaemia. B, tropisetron (300 μg kg−1, i.v.) attenuated the increase in discharge activity of this afferent (from 0.12 to 1.40 impulses s−1) during repeated ischaemia. This cardiac afferent innervated the anterior wall of the left ventricle. Neurograms 1-6 are representative tracings of the afferent at times indicated by arrows above histograms.
Ischaemia significantly increased the baseline discharge activity of nine C-fibre afferents (CV = 0.65 ± 0.17 m s−1) from 0.51 ± 0.21 to 2.42 ± 0.28 impulses s−1 (Fig. 9B). However, blockade of 5-HT3 receptors with tropisetron significantly attenuated the mean discharge activity of these cardiac afferents from 2.42 ± 0.28 to 1.43 ± 0.22 impulses s−1 during ischaemia (Fig. 9B). Seven additional afferents (CV = 0.57 ± 0.14 m s−1) responded consistently to repeated 5 min periods of myocardial ischaemia (Fig. 9A). The locations of the 16 afferent nerve endings are provided in Table 3.
Figure 9C shows summed 2.5 s discharge activity during 5 min of ischaemia in all nine C-fibre afferents before and after application of tropisetron. Similar to the changes in mean discharge activity, we observed that summed impulse activity during the entire 5 min period of ischaemia was attenuated by 41 % after application of tropisetron.
Discussion
This is the first study to demonstrate that activated platelets stimulate cardiac sympathetic afferents during ischaemia, at least in part, through a 5-HT3 receptor mechanism. Several pieces of data support this conclusion. First, we found that collagen and thrombin activate platelets to release large amounts of 5-HT. Second, suspensions of platelets activated with thrombin or collagen stimulated ischaemically sensitive cardiac sympathetic afferents and this stimulation is eliminated by tropisetron, a specific 5-HT3 receptor antagonist. Third, preventing activation of platelets with tirofiban, a specific inhibitor of platelet GP IIb-IIIa receptors, reduced the increase in concentration of 5-HT in cardiac venous plasma draining from the ischaemic region during ischaemia and reperfusion. Fourth, administration of tirofiban attenuated the responses of cardiac afferents to myocardial ischaemia. Fifth, application of 5-HT and PBG, a 5-HT3 receptor agonist, stimulated ischaemically sensitive cardiac sympathetic afferents, but 5-CT, a 5-HT1 receptor agonist, α-M-5-HT, a 5-HT2 receptor agonist, and SC53116, a 5-HT4 receptor agonist, did not. Finally, tropisetron abolished the response of these afferents to 5-HT and attenuated the response to myocardial ischaemia. Thus, through a 5-HT3 receptor mechanism, endogenous 5-HT, released from activated platelets during myocardial ischaemia, contributes to the ischaemia-mediated excitation of cardiac sympathetic afferents.
Cardiac sympathetic afferents compose a primary pathway for chest pain and the associated excitatory cardiovascular reflex responses during myocardial ischaemia. Based on the responses of cardiac sympathetic afferents to myocardial ischaemia, the afferents in this pathway can be classified as ischaemically sensitive afferents, which respond to myocardial ischaemia (Uchida & Murao, 1974; Huang et al. 1995a) or ischaemically insensitive afferents whose activity is not altered during myocardial ischaemia (Huang et al. 1995a). Ischaemically sensitive cardiac sympathetic afferents are thought to transmit nociceptive information to the central nervous system to elicit the perception of chest pain and initiate sympatho-excitatory cardiovascular reflexes that augment arterial blood pressure and heart rate and cause cardiac tachyarrhythmias (Uchida & Murao, 1974; Meller & Gebhart, 1992; Huang et al. 1995b). We recently demonstrated that activated platelets contribute to the increases in the responses of cardiac afferents during myocardial ischaemia (Fu & Longhurst, 2002). However, the mechanisms underlying the stimulating effect of activated platelets on ischaemically sensitive cardiac sympathetic afferents have not yet been identified.
Platelets are activated during myocardial ischaemic syndromes, particularly during acute myocardial infarction and unstable angina (Grande et al. 1990; Fitzgerald, 1991; Flores & Sheridan, 1994). Recent studies demonstrated that as the final common pathway, stimulation of platelet GP IIb-IIIa receptors leads to platelet activation irrespective of any of the agonists that stimulate platelets or of the stimulus-response-coupling pathway (Lefkovits et al. 1995; Coller, 1997). In this regard, numerous studies have documented that: (1) the GP IIb-IIIa receptors or ‘αIIbβ3′ (integrin nomenclature) receptors are expressed only in megakaryocytes and platelets and so are uniquely adapted to their role in platelet physiology. The density of GP IIb-IIIa receptors on the surface of platelets is extraordinary (≈80 000 copies per platelet), and there is an additional internal pool of GP IIb-IIIa receptors in α-granules; (2) vessel damage, adhesion and shear forces initiate signals that transform the GP IIb-IIIa receptors into a high affinity state that binds plasma-borne adhesive proteins such as fibrinogen and von Willebrand factor; (3) this binding reaction leads to platelet activation irrespective of the agonists; (4) highly specific competitive inhibitors of the platelet GP IIb-IIIa receptors include abciximab, eptifibatide, tirofiban, lamifibn and fradafiban. These inhibitors combine with the resting and active forms of platelet GP IIb-IIIa and therefore bind to non-stimulated and stimulated platelets, thereby completely inhibiting platelet activation during stimulation by the platelet agonists, including ADP, thrombin, collagen and TxA2 in several animal models (Lefkovits et al. 1995; Coller, 1997; Hiramatsu et al. 1997). Clinical trials of GP IIb-IIIa antagonists have shown a high degree of inhibition of platelets leading to a significant reduction in the number of ischaemic events in patients with myocardial infarction and unstable angina (Hiramatsu et al. 1997; Phillips & Scarborough, 1997; Lincoff et al. 2000). In the present study, we employed tirofiban, a specific inhibitor of platelet GP IIb-IIIa receptor, to prevent ischaemia-induced platelet activation. We observed that blockade of platelet GP IIb-IIIa receptors with tirofiban attenuated the response of cardiac afferents to myocardial ischaemia. These results, as well as our previous study (Fu & Longhurst, 2002), indicate that activated platelets during ischaemia stimulate cardiac sympathetic afferents.
During platelet activation many mediators including ATP, TxA2, histamine and 5-HT are released. Of the mediators, serotonin has been considered to be a potentially important chemical mediator that is responsible, at least in part, for the stimulation of cardiac sympathetic afferents during myocardial ischaemia when platelets are activated. In this respect, Meyers et al. (1982) demonstrated that as much as 0.9 μmol of 5-HT can be released from 100 million human platelets. Collagen induces the release of 5-HT from rabbit platelets in vitro (Packham et al. 1977; Fujimori et al. 1982; Packham et al. 1991) while thrombin stimulates the release of 5-HT from human and rat platelets (Belville et al. 1979; Verhoeven et al. 1984). The present study showed that both thrombin and collagen stimulate feline platelets to release 5-HT. Serotonin was very stable in PRP solutions since we measured it more than 24 h after the solutions were manipulated. This observation is in agreement with Douglas (1970) who demonstrated that degradation of 5-HT in tissue samples is slow (approximately 17 h).
Furthermore, there are data demonstrating that the concentration of 5-HT is increased during myocardial ischaemia. For example, Van den Berg et al. (1989) observed that the concentration of 5-HT in coronary sinus blood of patients with coronary disease is significantly higher than in patients without coronary disease. Brief coronary occlusion with angioplasty also causes an increase in the level of 5-HT in coronary sinus blood of patients with angina (Golino et al. 1994). Experimental data have shown that the concentration of 5-HT is increased at the site of coronary arterial stenosis and in coronary sinus blood during thrombosis-induced coronary artery occlusion (Ashton et al. 1986; Benedict et al. 1986). In the present model there was a tendency for an increase in the concentration of 5-HT in cardiac venous plasma during brief myocardial ischaemia and a significant increase during the immediate reperfusion period. These data suggest that 5-HT was released and accumulated in the ischaemic region during myocardial ischaemia and then was ‘washed’ out when coronary blood flow was restored. In contrast, treatment with tirofiban reduced the increase in the concentration of 5-HT during ischaemia and early reperfusion in this model. Thus, these data lead us to conclude that activated platelets during brief ischaemia are capable of releasing sufficient concentrations of 5-HT to contribute to ischaemia-mediated excitation of cardiac sympathetic afferents.
Previously, a number of studies have shown that 5-HT is capable of stimulating the visceral sensory nerve system. For instance, administration of 5-HT evokes hypertension in anaesthetized dogs (Zucker & Cornish, 1980). Our previous study demonstrated that 5-HT stimulates ischaemically sensitive abdominal sympathetic afferents (Fu & Longhurst, 1998). Others have demonstrated that exogenous 5-HT excites cardiac sympathetic Aδ afferents (Nishi et al. 1977). The physiological role of serotonin in peripheral tissues could be mediated by any of a large family of 5-HT receptors, which have been divided into at least seven types (Hoyer et al. 1994). Pharmacological data indicate that four major types of 5-HT receptors, 5-HT1, 5-HT2, 5-HT3 and 5-HT4, exist in the brain, spinal cord and cells of the peripheral nervous system (Hoyer et al. 1994). These receptors are coupled to a ligand-gated ion channel, intracellular adenyl cyclase or phospholipase C and inositol triphosphate (Hoyer et al. 1994). These four types of 5-HT receptors are involved in activation of the sensory nerve system. For instance, Taiwo & Levine (1992) observed that 5-HT produces hyperalgesia through direct stimulation of 5-HT1A receptors on the primary afferent nerves. Activation of 5-HT2 receptors potentiates pain produced by inflammatory mediators (Abbott et al. 1996) and increases transmission of nociception at the spinal level following stimulation of somatic C-fibre afferents (Peroutka, 1994). Rhodes et al. (1992) found that activation of 5-HT4 receptors depolarizes the cervical vagus nerve. Furthermore, Grundy et al. (1994) demonstrated that exogenous 5-HT stimulates vagal mucosal chemosensitive afferents through a 5-HT3 receptor mechanism. In this regard, activation of 5-HT3 receptors in the heart and lungs leads to a depressor reflex in conscious rabbits (Evans et al. 1990). However, there is no information on the relative importance of 5-HT and the 5-HT receptor subtypes in activation of cardiac sympathetic sensory nerves during ischaemia. Because we observed that 5-HT and PBG, a selective 5-HT3 receptor agonist, but not 5-HT1, 5-HT2 or 5-HT4 receptor agonists, stimulated ischaemically sensitive cardiac afferents in the present study, we suggest that 5-HT3 receptors are selectively responsible for the action of 5-HT on ischaemically sensitive cardiac sympathetic afferents. This conclusion was confirmed further by our observation that blockade of 5-HT3 receptors with tropisetron completely abolished the response of these afferents to 5-HT and attenuated the response of these afferents to myocardial ischaemia. As such, the present study provides consistent neurophysiological data indicating that endogenous 5-HT contributes to activation of cardiac sympathetic afferents during ischaemia through a 5-HT3 receptor mechanism.
An important goal of the present investigation was to determine whether 5-HT is the major mediator involved in the stimulation of activated platelets on ischaemically sensitive cardiac afferents since activated platelets release not only 5-HT but also other mediators including TxA2, ATP and histamine, which potentially could play a role in platelet-mediated excitation of cardiac afferents (Pelleg & Hurt, 1996; Kenagy et al. 1997; Fu et al. 1997b). We therefore assessed the influence of blockade of 5-HT3 receptors with tropisetron on the response of ischaemically sensitive cardiac afferents to the suspensions of activated platelets. Consistent with our previous observations (Fu & Longhurst, 2002), the present results confirm that platelets activated with thrombin and collagen excite ischaemically sensitive cardiac sympathetic afferents. However, tropisetron substantially reduced the responses of these afferents to thrombin-activated platelets as well as to collagen-activated platelets. Taking these data together with the in vivo data, we believe that during ischaemia platelets are activated and release serotonin, which, though a 5-HT3 receptor mechanism, stimulates cardiac sympathetic afferents during ischaemia.
Although our observation that blockade of 5-HT3 receptors virtually eliminated the response of cardiac afferents to administration of the suspensions of activated platelets suggests that 5-HT is an important mediator involved in the action of exogenously activated platelets on cardiac afferents, this observation does not exclude the contributions of other platelet-derived mediators such as TxA2, ATP, and histamine to the stimulation of activated platelets on cardiac sympathetic afferents. In this regard, previous studies have shown that these platelet-derived mediators are capable of stimulating somatic, vagal and sympathetic sensory nerves (Pelleg & Hurt, 1996; Kenagy et al. 1997; Fu et al. 1997b). Also, these mediators are released from platelets when they are activated (Hamberg et al. 1975; Meyers et al. 1982; Stormorken, 1986). However, a number of studies have shown that after release from activated platelets, TxA2 and ATP are unstable and their half-lives in suspensions of platelets are short (Hamberg et al. 1975; Trams, 1980; Ronca-Testoni & Borghini, 1982). For example, the half-lives of TxA2 and ATP are approximately 30 s and 10 min (Hamberg et al. 1975; Trams, 1980), respectively. In addition, the concentration of histamine in suspensions of activated platelets may be low because platelets release only small amounts of histamine (Masini et al. 1998). Conversely, data from the present study as well as from Douglas's study (Douglas, 1970) have shown that 5-HT is very stable in solutions of platelets, lasting for at least 17 h, much longer than other platelet-derived mediators. Taken together, we conclude that in suspensions of platelets activated exogenously, 5-HT is a major mediator released from platelets that can stimulate cardiac afferents. Other short lived platelet-derived mediators potentially may contribute to endogenously activated platelet-mediated stimulation of cardiac afferents during myocardial ischaemia although further investigation is necessary to confirm this possibility.
Since cardiac sympathetic afferents can be either ischaemically sensitive or ischaemically insensitive, we evaluated the relative responsiveness of these two groups of afferents to serotonin. We found that, although ischaemically sensitive ventricular afferents responded in a robust fashion to 5-HT, ischaemically insensitive ventricular afferents did not respond to this mediator. In contrast, ischaemically insensitive cardiac afferents responded consistently to BK. These data indicate that endogenous 5-HT is a more selective stimulus of ischaemically sensitive cardiac sympathetic afferents than BK. Such selectivity for ischaemically sensitive afferents also has been demonstrated with the action of hydroxyl radicals on visceral C fibre afferents (Stahl et al. 1993).
Differential responsiveness of ischaemically sensitive and insensitive afferent endings suggests that ischaemically sensitive C-fibre afferent endings in the heart and other visceral organs may be uniquely positioned to function as nociceptors that are particularly responsive to the noxious stimulus such as ischaemia (Pan & Longhurst, 1996). This observation lends weight to the argument that nociceptors can be distinguished by their unique properties rather than the possibility that all C-fibre afferents function as potential nociceptors based on the extent of stimulation. Future work will be required to determine the membrane properties of the endings and/or cell bodies that serve to distinguish these two class of receptors.
In conclusion, myocardial ischaemia activates feline platelets to release sufficient concentrations of serotonin to serve as a primary mechanism underlying stimulation of ischaemically sensitive cardiac sympathetic afferents during myocardial ischaemia. Inhibition of platelet GP IIb-IIIa receptors with tirofiban prevents ischaemia-induced activation of platelets and subsequently reduces the release of serotonin and activation of cardiac afferents during ischaemia. Importantly, serotonin originating from endogenously and exogenously activated platelets stimulates ischaemically sensitive but not ischaemically insensitive cardiac sympathetic afferents through 5-HT3 receptors, but not through 5-HT1, 5-HT2, or 5-HT4 receptors. These data extend our previous studies demonstrating that brief myocardial ischaemia leads to the activation of platelets which, in turn, stimulate ischaemically sensitive cardiac sympathetic afferents during ischaemia through release of serotonin. Activation of this cardiac sensory nerve system during even brief myocardial ischaemia may provoke the perception of cardiac pain and, through a neuronal reflex mechanism, could elicit reflex vasoconstriction that would help to maintain blood pressure in the presence of diminished pump capacity. Unfortunately, however, under some circumstances these reflex adjustments may become exaggerated and thus hinder survival during myocardial ischaemia (Longhurst et al. 2001).
Acknowledgments
We gratefully acknowledge the technical assistance of Ms Melissa Crisostomo and the secretarial assistance of Ms Sherry Ong and Ms Daniell McClellan. We thank Bethesda Y. Gee and Hovsep Babayan, the Student Fellows of the American Heart Association, Western States Affiliate, for their active participation in this project. We also thank Merck for the gift of tirofiban used in this study. This study was supported by grants HL-66217 from the National Institutes of Health and by a Beginning Grant-in-Aid 9960007Y from the American Heart Association Western States Affiliate.
References
- Abbott FV, Hong Y, Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35:99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Ashton JH, Benedict CR, Fitzgerald C, Raheja S, Taylor A, Campbell WB, Buja LM, Willerson JT. Serotonin as a mediator of cyclic flow variation in stenosed canine coronoary arteries. Circulation. 1986;73:572–578. doi: 10.1161/01.cir.73.3.572. [DOI] [PubMed] [Google Scholar]
- Belville JS, Bennett WF, Lynch G. A method for investigating the role of calcium in the shape change, aggregation and secretion of rat platelets. Journal of Physiology. 1979;297:289–297. doi: 10.1113/jphysiol.1979.sp013040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CR, Mathew B, Rex KA, Cartwright J, Sordahl LA. Correlation of plasma serotonin changes with platelet aggregation in an in vivo dog model of spontaneous occlusive coronary thrombus formation. Circulation Research. 1986;58:58–67. doi: 10.1161/01.res.58.1.58. [DOI] [PubMed] [Google Scholar]
- Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. Journal of Clinical Investigation. 1997;100(suppl. 11):S57–60. [PubMed] [Google Scholar]
- Douglas WW. Histamine and antihistamines; 5-hydroxytryptamine and antagonists. In: Goodman LS, Gilman A, editors. The Pharmacological Basis of Therapeutics. New York: Macmillan; 1970. pp. 621–662. [Google Scholar]
- Evans RG, Ludbrook J, Michalicek J. Characteristis of cardiovascular reflexes originating from 5-HT3 receptors in the heart and lungs of unanaethetized rabbits. Clinical and Experimental Pharmacology and Physiology. 1990;17:665–679. doi: 10.1111/j.1440-1681.1990.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DJ. Platelet activation in the pathogenesis of unstable angina: importance in determining the response to plasminogen activators. American Journal of Cardiology. 1991;68:51B–57B. doi: 10.1016/0002-9149(91)90384-w. [DOI] [PubMed] [Google Scholar]
- Flores NA, Sheridan DJ. The pathophysiological role of platelets during myocardial ischemia. Cardiovascular Research. 1994;28:295–302. doi: 10.1093/cvr/28.3.295. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Role of 5-HT3 receptors in activation of abdominal sympathetic C-fibre afferents during ischaemia in cats. Journal of Physiology. 1998;509:729–740. doi: 10.1111/j.1469-7793.1998.729bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Activated platelets stimulate cardiac afferents through a 5-HT receptor mechanism. FASEB Journal. 2000;14:A377. [Google Scholar]
- Fu L-W, Longhurst JC. Role of activated platelets in excitation of cardiac afferents during myocardial ischemia in cats. American Journal of Physiology. 2002;282:H100–H109. doi: 10.1152/ajpheart.2002.282.1.H100. [DOI] [PubMed] [Google Scholar]
- Fu L-W, O'Neill CA, Longhurst JC. Increased histamine and 5-HT in portal vein plasma and mesenteric lymph during brief ischemia and reperfusion. American Journal of Physiology. 1997a;273:H1135–1141. doi: 10.1152/ajpheart.1997.273.3.H1135. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Pan H -L, Longhurst JC. Endogenous histamine stimulates ischemically sensitive abdominal visceral afferents through H1 receptors. American Journal of Physiology. 1997b;273:H2726–2737. doi: 10.1152/ajpheart.1997.273.6.H2726. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Yamanishi Y, Yamatsu K, Tajima T. High performance liquid chromatography (HPLC) determination of endogenous serotonin released from aggregating platelets. Journal of Pharmacological Methods. 1982;7:105–113. doi: 10.1016/0160-5402(82)90022-5. [DOI] [PubMed] [Google Scholar]
- Golino P, Piscione F, Benedict C, Anderson H, Cappelli-Bigazzi M, Indolfi C, Condorelli M, Chiariello M, Willerson J. Local effect of serotonin released during coronary angioplasty. New England Journal of Medicine. 1994;330:523–528. doi: 10.1056/NEJM199402243300802. [DOI] [PubMed] [Google Scholar]
- Grande P, Grauholt A-M, Madsen JK. Unstable angina pectoris: Platelet behavior and prognosis in progressive angina and intermediate coronary syndrome. Circulation. 1990;81(suppl. I):I16–19. [PubMed] [Google Scholar]
- Grundy D, Blackshae LA, Hillsley K. Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Digestive Diseases Sciences. 1994;30(suppl.):44S–47S. doi: 10.1007/BF02300369. [DOI] [PubMed] [Google Scholar]
- Hamberg M, Svensson J. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proceedings of the National Academy of Sciences of the USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu Y, Gikakis N, Anderson HL, 3rd, Gorman JH, 3rd, Marcinkiewicz C, Gould RJ, Niewiarowski S, Edmunds LH., Jr Tirofiban provides “platelet anesthesia” during cardiopulmonary bypass in baboons. Journal of Thoracic Cardiovascular Surgery. 1997;113:182–193. doi: 10.1016/S0022-5223(97)70414-8. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ. International union of parmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacology Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Huang H-S, Pan H-L, Stahl GL, Longhurst JC. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. American Journal of Physiology. 1995a;269:H888–901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Huang H-S, Stahl GL, Longhurst JC. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. American Journal of Physiology. 1995b;268:H2114–2124. doi: 10.1152/ajpheart.1995.268.5.H2114. [DOI] [PubMed] [Google Scholar]
- Joseph R, Saroff DM, Delfs JR. Platelet secretory products have a damaging effect on neurons. Neuroscience Letters. 1992;135:153–158. doi: 10.1016/0304-3940(92)90425-7. [DOI] [PubMed] [Google Scholar]
- Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46, 619, on pulmonary vagal afferents in the cat. Respiration Physiology. 1992;87:383–396. doi: 10.1016/0034-5687(92)90019-s. [DOI] [PubMed] [Google Scholar]
- Kenagy J, Vancleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Research. 1997;744:175–178. doi: 10.1016/s0006-8993(96)01211-5. [DOI] [PubMed] [Google Scholar]
- Lee CR, Plosker GL, McTavish D. Tropisetron: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential as an antiemetic. Drugs. 1993;46:925–943. doi: 10.2165/00003495-199346050-00009. [DOI] [PubMed] [Google Scholar]
- Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. New England Journal of Medicine. 1995;332:1553–1559. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Califf RM, Topol EJ. Platelet glycoprotein IIb/IIIa receptor blockade in coronary artery disease. Journal of Am Coll Cardiol. 2000;35:1103–1115. doi: 10.1016/s0735-1097(00)00554-4. [DOI] [PubMed] [Google Scholar]
- Longhurst J, Tjen-A-Looi S, Fu L-W. Cardiac sympathetic afferent activation provocked by myocardial ischemia and reperfusion: Mechanism and reflexes. New York Academy of Sciences. 2001;940:74–95. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- Malliani A. Cardiocardiac excitatory reflexes during myocardial ischemia. Basic Research in Cardiology. 1990;85:243–252. doi: 10.1007/978-3-662-11038-6_20. [DOI] [PubMed] [Google Scholar]
- Masini E, Di Bello MG, Raspanti S, Fomusi Ndisang J, Baronti R, Cappugi P, Mannaioni PF. The role of histamine in platelet aggregation by physiological and immunological stimuli. Inflammation Research. 1998;47:211–220. doi: 10.1007/s000110050319. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- Meyers KM, Holsmen H, Seachord CL. Comparative study of platelet dense granule constituents. American Journal of Physiology. 1982;243:R454–461. doi: 10.1152/ajpregu.1982.243.3.R454. [DOI] [PubMed] [Google Scholar]
- Nishi K, Sakanashi M. Activation of afferent cardiac sympathetic nerve fibres of the cat by pain producing substances and by noxious heat. Pflüger's Archiv. 1977;372:53–61. doi: 10.1007/BF00582206. [DOI] [PubMed] [Google Scholar]
- Oei HHH, Hughes WE, Schaffer SW, Longenecker GL, Glenn TM. Platelet serotonin uptake during myocardial ishemia. American Heart Journal. 1983;106:1077–1081. doi: 10.1016/0002-8703(83)90655-5. [DOI] [PubMed] [Google Scholar]
- Packham MA, Guccione MA, Greenberg JP, Kinlough-Rathbone RL, Mustard JF. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977;50:915–926. [PubMed] [Google Scholar]
- Packham MA, Rand ML, Ruben DH, Kinlough-Rathbone RL. Effect of calcium concentration and inhibitors on the responses of platelets stimulated with collagen: contrast between human and rabbit platelets. Comparative Biochemistry and Physiology a: Comparative Physiology. 1991;99:551–557. doi: 10.1016/0300-9629(91)90130-5. [DOI] [PubMed] [Google Scholar]
- Pan H-L, Longhurst C. Ischaemia-sensitive sympathetic afferents innervating the gastrointestinal tract function as nociceptors in cats. Journal of Physiology. 1996;492:841–850. doi: 10.1113/jphysiol.1996.sp021350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. Journal of Physiology. 1996;490:265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM, Soler-Baillo JM, Polansky M. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in dogs. American Journal of Physiology. 1993;265:H681–690. doi: 10.1152/ajpheart.1993.265.2.H681. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. 5-Hydroxytryptamine receptors. Journal of Neurochemistry. 1994;60:408–416. doi: 10.1111/j.1471-4159.1993.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. American Journal of Cardiology. 1997;80(4A):11B–20B. doi: 10.1016/s0002-9149(97)00572-9. [DOI] [PubMed] [Google Scholar]
- Rhodes KF, Coleman J, Lattimer N. A component of 5-HT-evoked depolarization of the rat isolated vagus nerve is mediated by a putative 5-HT4 receptor. Naunyn-Schemiedeberg's Archives of Pharmacology. 1992;346:496–503. doi: 10.1007/BF00169003. [DOI] [PubMed] [Google Scholar]
- Ronca-Testoni S, Borghini F. Degradation of perfused adenine compounds up to uric acid in isolated rat heart. Journal of Molecular and Cellular Cardiology. 1982;14:177–180. doi: 10.1016/0022-2828(82)90116-x. [DOI] [PubMed] [Google Scholar]
- Stahl GL, Pan H-L, Longhurst JC. Activation of ischemia and reperfusion-sensitive abdominal visceral C fiber afferents: role of hydrogen peroxide and hydroxyl radicals. Circulation Research. 1993;72:1266–1275. doi: 10.1161/01.res.72.6.1266. [DOI] [PubMed] [Google Scholar]
- Stormorken H. Platelets in hemostasis and thrombosis. In: Holmsen H, editor. Platelet Responses and Metabolism. Vol. 1. Boca Raton, FL, USA: CRC Press; 1986. pp. 3–32. [Google Scholar]
- Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48:485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- Tjen-A-Looi S, Pan H-L, Longhurst JC. Endogenous bradykinin activates ischaemically sensitive cardiac visceral afferents through kinin B2 receptors in cats. Journal of Physiology. 1998;510:633–641. doi: 10.1111/j.1469-7793.1998.633bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol EJ, Byzova TV, Plow EF. Platelet GPIIb-IIIa blockers. Lancet. 1999;353:227–231. doi: 10.1016/S0140-6736(98)11086-3. [DOI] [PubMed] [Google Scholar]
- Trams EG. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. Journal of Theoretical Biology. 1980;87:609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Murao S. Excitation of afferent cardiac sympathetic nerve fibers during coronary occlusion. American Journal of Physiology. 1974;226:1094–1099. doi: 10.1152/ajplegacy.1974.226.5.1094. [DOI] [PubMed] [Google Scholar]
- Van Den Berg EK, Schmitz JM, Benedict CR, Malloy CR, Willerson JT, Dehmer GJ. Transcardiac serotonin concentration is increased in selected patients with limiting angina and complex coronary lesion morphology. Circulation. 1989;79:116–124. doi: 10.1161/01.cir.79.1.116. [DOI] [PubMed] [Google Scholar]
- Verhoeven AJM, Mommersteeg ME, Akkerman JWN. Quantification of energy consumption in platelets during thrombin-induced aggregation and secretion. Biochemical Journal. 1984;221:771–787. doi: 10.1042/bj2210777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC. Cardiac pain: anatomic pathway and physiologic mechanisms. Circulation. 1957;16:644–655. doi: 10.1161/01.cir.16.4.644. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Cornish KG. Reflex cardiovascular and respiratory effects of serotonin in conscious and anesthetized dogs. Circulation Research. 1980;47:509–515. doi: 10.1161/01.res.47.4.509. [DOI] [PubMed] [Google Scholar]


