Abstract
Neonatal rat motoneurones are electrically coupled via gap junctions and the incidence of this coupling declines during postnatal development. The mechanisms involved in this developmental regulation of gap junctional communication are largely unknown. Here we have studied the role of NMDA receptor-mediated glutamatergic synaptic activity in the regulation of motoneurone coupling. Gap junctional coupling was demonstrated by the presence of graded, short latency depolarising potentials following ventral root stimulation, and by the transfer of the low molecular weight tracer Neurobiotin to neighbouring motoneurones. Sites of close apposition between the somata and/or dendrites of the dye-coupled motoneurones were identified as potential sites of gap junctional coupling. Early postnatal blockade of the NMDA subtype of glutamate receptors using the non-competitive antagonist dizocilpine maleate (MK801) arrested the developmental decrease in electrotonic and dye coupling during the first postnatal week. These results suggest that the postnatal increase in glutamatergic synaptic activity associated with the onset of locomotion promote the loss of gap junctional connections between developing motoneurones.
Gap junctional coupling is widespread during early development of the nervous system and plays an important role in generating synchronous electrical and metabolic signals (Kiehn & Tresch, 2002). In newborn animals, motoneurones are extensively coupled and the extent of this coupling decreases markedly during the first two weeks after birth (Fulton et al. 1980; Becker & Navarrete, 1990; Walton & Navarrete, 1991), at the time when locomotor activity normally appears (Navarrete & Vrbová, 1983). The mechanisms involved in this developmental regulation are not well understood.
There is evidence that synaptic activity may be involved in regulating gap junctional communication. In Xenopus embryos, the developmental loss of dye coupling between muscle cells is prevented when neuromuscular transmission is blocked at the postsynaptic level (Armstrong et al. 1983). In addition, neurotransmitters such as dopamine and noradrenaline acutely modulate gap junctional communication in various parts of the central nervous system (see Rörig & Sutor, 1996). In the neonatal spinal cord, glutamate receptors are involved in the generation of rhythmic locomotor activity (Cazalets et al. 1992; Beato et al. 1997) and blockade of NMDA receptors, both in vivo and in vitro, has been shown to depress the locomotor rhythm (Fenaux et al. 1991; Beato et al. 1997). Spinal motoneurones are activated by glutamatergic inputs from primary afferents, interneurones and descending pathways and transmission at these sites is mediated by both NMDA and non-NMDA subclasses of glutamate receptors (Ziskind-Conhaim, 1990; Pinco & Lev-Tov, 1994). The earliest synaptic potentials recorded on motoneurones (at embryonic day 16) have slow rates of rise and long durations and are blocked by NMDA receptor antagonists, whereas EPSPs with fast rates of rise, mediated by AMPA receptors, appear at least a day later (Ziskind-Conhaim, 1990). These results emphasize the importance of NMDA receptors for spinal network development.
Here we have investigated the effects of early postnatal blockade of the NMDA type of glutamate receptors on gap junctional communication between identified ankle flexor motoneurones using MK801 (dizocilpine maleate). MK801, a non-competitive NMDA receptor antagonist, is known to cross the blood-brain barrier and has been reported to reduce polysynaptic reflexes when administered in vivo (Fenaux et al. 1991; Farkas & Ono, 1995). The extent of motoneuronal coupling was assessed by a combination of electrophysiological and morphological techniques.
Methods
All surgical procedures were carried out in compliance with the UK Animals (Scientific Procedures) Act 1986. Newborn rats (postnatal day (P) 0) were anaesthetised with ether and the right tibialis anterior (TA) muscle was injected with 0.5 μl of an aqueous suspension of 2.5 % Fast Blue (FB) (EMS-Polyloy, Groβ-Umstadt, Germany). This dye is retrogradely transported and labels the somata of motoneurones and was used in this study as a visual guide for intracellular recordings. Two days later (P2), allowing for retrograde transport of FB to take place, rats were injected daily with either: (i) the NMDA receptor antagonist MK801 (2 mg kg−1; i.p.) or (ii) vehicle (sterile saline), until the day of the experiment. Intracellular recordings from antidromically and visually identified (FB signal) TA motoneurones were performed using an in vitro spinal cord-hindlimb preparation (Walton & Navarrete, 1991). A total of 32 control TA motoneurones (24 preparations) and 15 MK801-treated TA motoneurones (8 preparations) from rats aged from P0-P9 are included in this study. Animals were divided into five groups: (i) P0-P2 normal (10 motoneurones, 7 preparations); (ii) P5-P7 saline-treated control (12 motoneurones, 10 preparations); (iii) P8-P9 saline-treated control (10 motoneurones, 7 preparations); (iv) P5-P7 MK801-treated animals (8 motoneurones, 5 preparations); and (v) P8-P9 MK801-treated animals (7 motoneurones, 3 preparations).
Electrophysiology
On the day of the experiment, the animal was anaesthetised with ether and decapitated. The spinal cord was dissected rapidly and hemisected mid-sagittally and isolated in continuity with the spinal roots, the sciatic nerve and the ankle flexor and extensor muscles. Details of the spinal cord-hindlimb preparation have been described previously (Walton & Navarrete, 1991). The preparation was perfused with oxygenated Krebs solution at room temperature and placed on the stage of a modified epifluorescence microscope to visualise the retrogradely labelled motoneurone pool. Krebs solution consisted of (mm): 113 NaCl, 4.5 KCl, 1 MgSO4, 2 CaCl2, 1 Na2HPO4, 25 NaHCO3 and 11 glucose (pH 7.4-7.6). Intracellular recordings were performed with sharp microelectrodes pulled to an average DC resistance of 20-35 MΩ. The electrodes were filled with 1.5 m potassium acetate and 2 % Neurobiotin (Vector Laboratories, Burlingame, CA, USA). Recordings were made with an Axoclamp 2B amplifier (Axon Instruments, Union City, USA) in bridge mode. Data were recorded on videotape using a DR-890 Neurocorder digitising unit (Neurodata Instruments Corporation, Delaware Water Gap, USA) for off-line analysis using a CED 1401 laboratory computer interface and Signal software (CED, Cambridge, UK). Stable intracellular recordings were carried out for a minimum of 30 min from motoneurones with a resting potential of at least -55 mV and overshooting action potentials. All cells reported in this study were prelabelled with Fast Blue. Fluorescently labelled TA motoneurones were further identified by the presence of an antidromic action potential following stimulation of the ventral root and the muscle nerve.
Morphological analysis
To investigate the presence of dye coupling, following electrophysiological characterisation, motoneurones were iontophoretically injected with the low molecular weight tracer Neurobiotin (Vector Laboratories) using depolarising current pulses (0.5-3 nA, 200 ms in duration at 3.3 Hz for 30 min). The tracer was further allowed to diffuse for at least 30 min before the preparation was fixed in 4 % paraformaldehyde. In cases where more than one motoneurone was impaled per preparation, care was taken to separate the second electrode track by at least 500 μm in the rostro-caudal axis in order to avoid accidental labelling of other cells. Transient impalement, where the cell was held hyperpolarised for less than 5 min, did not result in motoneurone labelling, since Neurobiotin has a positive charge and requires the application of depolarising current in order to inject the tracer. At the end of the recording period, the precise location of each impaled motoneurone was mapped with reference to the lumbar spinal roots L4 and L5, from images captured using a CCD camera. Serial parasagittal sections (100 μm) were cut using a Vibratome and the intracellularly labelled motoneurones were visualised with the ABC method (Vector Laboratories) according to the procedure described in detail by Horikawa & Armstrong (1988). Camera lucida reconstructions were carried out on a light microscope equipped with a drawing tube (BH2 Olympus) at ×750 final magnification (a ×100 oil-immersion objective was used).
Results are expressed as means ± standard errors of the mean (s.e.m.). Statistical differences were assessed by Student's t test and one-way ANOVA, using commercially available software (SigmaStat, Jandel-Scientific, Germany).
Results
Electrotonic coupling
Electrotonic coupling between motoneurones was determined by the presence of subthreshold short latency depolarising (SLD) potentials, evoked by graded stimulation of the ventral root and occurring within 1.5 ms of the onset of the antidromic action potential. Figure 1A illustrates antidromic action potentials evoked from the ventral root L4 and the TA muscle nerve, respectively, from an MK801-treated preparation. Graded stimulation of the ventral root at a subthreshold level for the antidromically evoked action potential resulted in four discrete SLD components, indicative of electrotonic coupling from four separate motoneurones (Fig. 1B). The SLDs occurred shortly after the onset of the antidromic action potential (saline-treated: 0.89 ± 0.09 ms and MK801-treated: 1.12 ± 0.17 ms). These potentials were resistant to: (i) high frequency (50 Hz) stimulation: (ii) changes of the membrane potential; (iii) collision with a somatofugal action potential; and (iv) removal of Ca2+ from the bathing solution, indicating that they were generated by electrotonic transmission (Walton & Navarrete, 1991).
Figure 1. Electrotonic and dye coupling between tibialis anterior motoneurones.
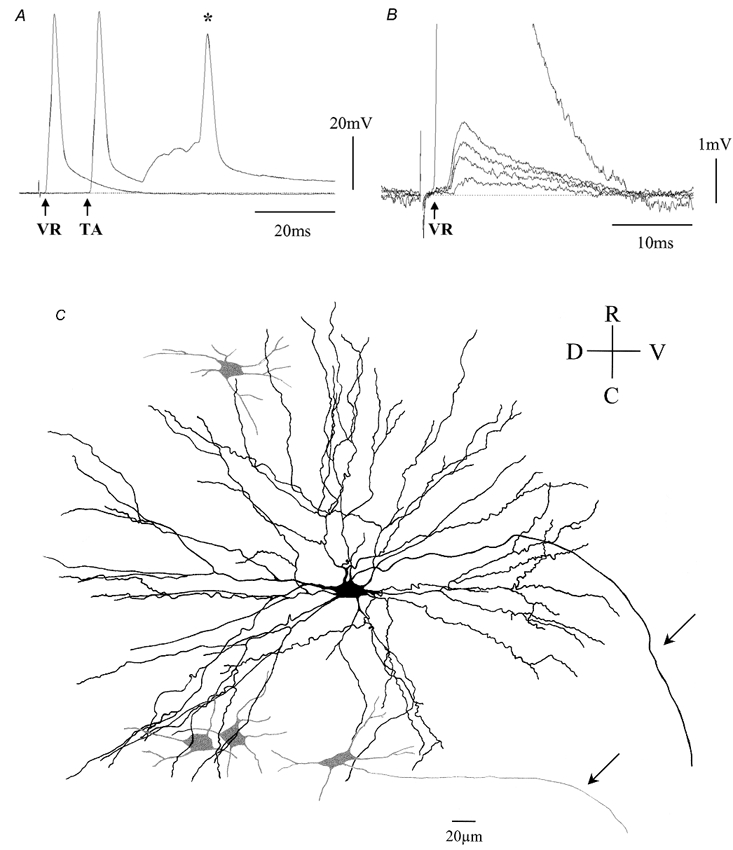
A, antidromic action potentials following ventral root L4 stimulation (VR) and tibialis anterior (TA) muscle nerve stimulation. Note that the TA antidromic action potential has a longer latency and is followed by a synaptically mediated depolarisation leading to an orthodromic action potential (*). B, graded short latency depolarisations elicited by VR stimulation. Note that four discrete short latency depolarising potentials can be discerned at stimulus intensities below the threshold for the antidromic spike (arrow), indicating electrical communication between the impaled motoneurone and four other motoneurones. C, camera lucida reconstruction of the Neurobiotin-filled TA motoneurone shown in A and B, revealing four dye-coupled cells (grey) within the boundaries of the dendritic tree of the impaled motoneurone. The arrows denote the axons of the impaled and of one of the dye-coupled motoneurones. R, rostral; C, caudal; D, dorsal; and V, ventral. P7, MK801-treated preparation.
Dye coupling
To establish whether electrotonic coupling is accompanied by the transfer of low molecular weight tracers known to cross gap junctions (Peinado et al. 1993), Neurobiotin was injected intracellularly following completion of the electrophysiological tests. Figure 1C shows a cluster of four motoneurones, which were dye coupled to the impaled TA motoneurone. The impaled motoneurone was usually darker than the other dye-coupled cells and in some cases the axon of some of the coupled cells were seen to exit the ventral root alongside that of the impaled motoneurone (arrows in Fig. 1C and Fig. 2A). Overall, there was a good correlation between the number of SLD components and the number of dye-coupled cells, although on average, the number of dye-coupled cells was slightly lower (see Table 1).
Figure 2. Putative sites of coupling between motoneurones.
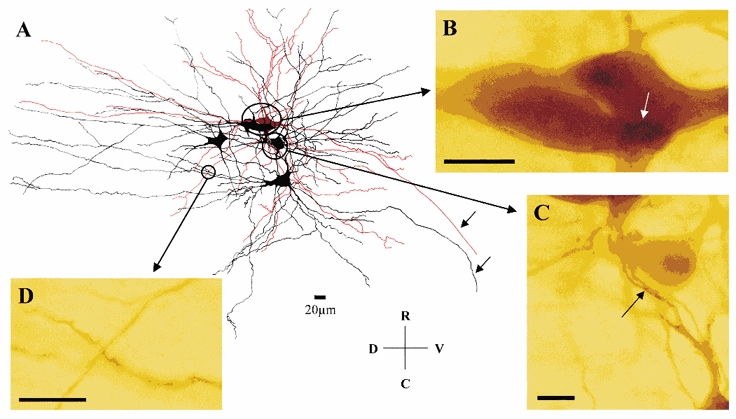
A, camera lucida reconstruction of dye-coupled motoneurones from a P8 MK801-treated preparation. The impaled motoneurone is shown in red and a cluster of four dye-coupled cells are shown in black. Note the extensive overlap of the dendritic trees of the motoneurones containing several sites of close apposition as illustrated at the light microscope level (B-D). B, somato-somatic and somato-dendritic (white arrow) sites of close apposition. C, proximal dendro-dendritic sites of close apposition (arrow). D, distal dendro-dendritic site of close apposition. Note that the axons of two of the motoneurones are seen leaving the spinal cord (arrows). Scale bars in B, C and D: 20 μm. R, rostral; C, caudal; D, dorsal; and V, ventral.
Table 1.
Increased incidence of electrotonic and dye coupling following NMDA receptor blockade
| Treatment | Rin | Electrical coupling | Dye coupling | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | n | RP (mV) | Mean (MΩ) | Range (MΩ) | Mean no. SLD components | Range | n | Mean no. components | Range | n | |
| Normal | P0-P2 | 10 | −68.2 ± 2.5 | 19.3 ± 5.7 | 5.2–36.1 | 5.30 ± 0.7 | 3–9 | 10 | 3.00 ± 0.8 | 1–5 | 4 |
| Saline | P5-P9 | 22 | −63.3 ± 1.3 | 6.3 ± 0.9* | 2.3–16.9 | 1.78 ± 0.4* | 0–8 | 22 | 1.06 ± 0.3 | 0–4 | 16 |
| Saline group 1 | P5-P7 | 12 | −62.8 ± 2.1 | 7.2 ± 1.4* | 3.4–16.9 | 1.83 ± 0.7* | 0–8 | 12 | 1.71 ± 0.3 | 0–4 | 10 |
| Saline group II | P8-P9 | 10 | −63.9 ± 1.3 | 5.0 ± 0.6* | 2.3–7.1 | 1.70 ± 0.5* | 0–4 | 10 | 0.83 ± 0.4 | 0–2 | 6 |
| MK801 | P5-P9 | 15 | −68.1 ± 1.4 | 5.7 ± 1.3* | 1.7–14.4 | 4.08 ± 0.7† | 1–11 | 14 | 3.90 ± 1.1† | 0–10 | 10 |
| MK801 group I | P5-P7 | 8 | −68.5 ± 1.8 | 5.9 ± 1.9* | 1.7–14.3 | 4.62 ± 1.0† | 1–11 | 8 | 3.67 ± 1.4 | 0–10 | 6 |
| MK801 group II | P8-P9 | 7 | −67.8 ± 2.4 | 5.5 ± 2.1* | 1.8–14.4 | 3.33 ± 0.7 | 2–6 | 6 | 4.00 ± 1.9 | 0–9 | 4 |
Means ± s.e.m. and ranges are shown. RP, resting potential; Rin input resistance; SLD, short latency depolarisation. Significance (one way ANOVA, Tukey's test) is indicated by
P < 0.05 compared to P0-P2 group
P < 0.05 compared to the corresponding saline treated age group.
Putative sites of coupling
To investigate further the putative sites of dye coupling, we looked for sites of close apposition between the somata and dendrites of the impaled motoneurone and those of the coupled cells. Figure 2A illustrates a camera lucida reconstruction of a cluster of dye-coupled cells, showing the extensive overlapping of the dendritic tree of the impaled motoneurone and that of the dye-coupled cells. However, careful mapping of potential sites of close apposition, using oil immersion at the light microscopy level, revealed the presence of only a small number of putative contacts at the somato-somatic (Fig. 2B), somato- dendritic (Fig. 2B, arrow) and dendro-dendritic (Fig. 2C, proximal; 2D, distal) levels.
Blockade of NMDA receptors in vivo halts the developmental decrease in motoneurone coupling
In order to determine whether activation of NMDA receptors regulates the extent of motoneurone coupling, we compared the incidence of electrotonic and dye coupling in TA motoneurones from newborn rats (P0-P2) with that of animals aged P5-P9, which had been treated daily with the NMDA receptor blocker MK801 or saline (control) for 3-7 days.
Table 1 shows a comparison of the basic electrophysiological properties (membrane potential and input resistance), and the incidence of electrotonic and dye coupling between the various groups. There was a large decrease of input resistance during normal development (compare P0-P2 normal and P5-P7 and P8-P9 saline treated groups). However, there was no significant difference in either the mean resting potential or input resistance between the age-matched saline and MK801-treated groups, suggesting that MK801 treatment does not significantly affect the passive membrane properties of the motoneurones.
In control motoneurones, the percentage incidence of electrotonic coupling decreased from 100 % in the normal P0-P2 group (100 % dye coupling) to 75 % in the P5-P7 saline treated control group (73 % dye coupling) and 70 % in the P8-P9 saline control group (66.7 % dye coupling). In MK801-treated animals however, 100 % of the studied cells were electrotonically coupled in the P5-P7 and P8-P9 age groups (dye coupling: 93.7 % at P5-P7 and 75 % at P8-P9). Comparison of the entire group of MK801-treated animals (P5-P9) against the age matched saline-treated control group showed that the percentage incidence of both electrotonic and dye coupling was statistically significant (Table 1). Similarly, the mean number of SLD components and dye-coupled cells was higher in MK801-treated rats compared to saline-treated controls (Table 1). In addition, the mean number of SLD components in the MK801-treated animals was not significantly different from that of normal motoneurones in the younger P0-P2 age group. These results therefore demonstrate that early postnatal blockade of NMDA receptors prevents the developmental decrease in gap junctional coupling between motoneurones, which takes place during the first two weeks of postnatal development.
Discussion
Our results show the simultaneous occurrence of electrotonic and dye coupling between identified neonatal TA motoneurones, and its decrease during early postnatal development, thus confirming and extending previous studies (Fulton et al. 1980; Becker & Navarrete, 1990; Walton & Navarrete, 1991; Chang et al. 1999). In the present study, we show further that early postnatal blockade of the NMDA subtype of glutamate receptors delays the normal developmental decrease in coupling during the first postnatal week. These results suggest that the increase in glutamatergic synaptic activity associated with the postnatal onset of locomotion promotes the loss of gap junctional connections between developing motoneurones.
Correlation between electrotonic and dye coupling
Although the results obtained by the two methods used in this study were in general agreement, it appears that the dye coupling method was less sensitive and showed a greater variability than the electrophysiological method. Neurobiotin is a low molecular weight tracer and has been reported to be a better marker for neuronal coupling than Lucifer Yellow (Peinado et al. 1993). This is consistent with the fact that the percentage of Neurobiotin-coupled motoneurones in normal neonatal (P0-P2) motoneurones observed here was higher than the percentage of Lucifer Yellow-coupled cells reported previously for a similar age group (Becker & Navarrete, 1990). In addition, the number and intensity of labelling of dye-coupled cells depends on several factors such as: (i) the number of gap junctional contacts; (ii) the location of the contacts between the impaled and the coupled cell (i.e. proximal or distal); and (iii) the time allowed for the tracer to diffuse throughout the dendritic tree. Furthermore, differences between the extent of electrotonic and dye coupling could be related to factors that affect gap junctional proteins by modulating their assembly or permeability (Chang et al. 1999).
We identified potential sites of gap junctional coupling as points of close apposition between the somata and/or dendrites of the dye-coupled motoneurones. Dendro- dendritic and dendro-somatic gap junctions have been identified in the rat spinal cord at the electron microscopic level (Matsumoto et al. 1988) and work in the locus coeruleus suggest that electrical interactions between dendrites outside the cell body region can account for synchronous activity within the nucleus (Ishimatsu & Williams, 1996). Recent studies also indicate that several types of connexin proteins are expressed by developing motoneurones and their expression is regulated during postnatal development (Chang et al. 1999). Thus, it is likely that the points of close apposition between somata and dendrites observed here may correspond to sites of functional gap junctional coupling.
The presence of electrical coupling between motoneurones might be expected to result in a relatively lower input resistance in the recorded neuron compared to that in non-coupled cells. However, we did not find a significant difference in input resistance between coupled and non-coupled cells either in normal or MK801-treated animals at any of the ages studied (data not shown). Indeed, contrary to expectation, we found that the mean value of input resistance was highest in neonatal animals (P0-P2), at a time when electrotonic and dye coupling was most pronounced. There are several reasons why a simple correlation between these parameters might not exist. In order for electrotonic coupling to have a significant effect on the input resistance it is necessary to assume that most sites of coupling are located close to the cell body, since dendro-dendritic gap junctions located at distal sites might not significantly affect the input resistance measured at the cell body. In addition, the input resistance depends not only on the gap junctional conductance between a coupled pair but also on the non-junctional conductance due to ion channels present in the membrane as well as shunt conductances due to active synaptic inputs on the recorded neuron.
The fact that electrotonic and dye coupling between motoneurones is functionally specific and developmentally regulated (Walton & Navarrete, 1991; Chang et al. 1999) suggests that gap junctional communication may play an important role in synchronising electrical activity as well as metabolic signals during early development (Kiehn & Tresch, 2002). Indeed, the presence of synchronised action potentials has been directly demonstrated by dual intracellular recordings from electrically coupled sympathetic preganglionic neurons in neonatal spinal cord slices (Logan et al. 1996). Furthermore, synchronous membrane potential oscillations have been recorded intracellularly and extracellularly from neonatal spinal motoneurones after abolishing all chemical synaptic neurotransmission (Tresch & Kiehn, 2000). This is also consistent with our previous electromyographic finding that motor unit activity in rat hindlimb muscles shows a greater tendency to synchronization during the first compared to the second postnatal week (Navarrete & Vrbová, 1983). These results therefore suggest that gap junctional coupling influences the pattern of motoneurone activity prior to the time when spinal neuronal circuits begin to display adult-like locomotor activity and thus plays an important role in motor control during embryonic and early postnatal development (Navarrete & Vrbová, 1993).
Role of afferent synaptic activity in the regulation of motoneurone coupling
The mechanisms involved in developmental downregulation of gap junctional coupling are unknown. Synaptic activity has previously been shown to regulate electrical coupling, as blockade of cholinergic neuromuscular transmission prevents the developmental loss of electrical coupling between muscle cells (Armstrong et al. 1983). Furthermore, in visual cortical neurons, the postnatal reduction in dye coupling is associated with a developmental increase in the amount of spontaneous synaptic activity (Kandler & Katz, 1998).
The present results demonstrate that blockade of the NMDA receptors during a critical neonatal period arrests the normal developmental decrease in motoneurone coupling. The simplest explanation of our findings is that it results from a selective reduction of the NMDA receptor-mediated component of the glutamatergic synaptic drive to motoneurones. However, it could be argued that since MK-801 was administered systemically its effects could have resulted from blockade of NMDA-mediated synaptic transmission upstream of the motoneurones. That MK801 affects the general growth of the animals is evident from their retarded weight gain, as has been previously reported (Facchinetti et al. 1993; Maier et al. 1995). However, it has been reported that these effects cannot be attributed to a general developmental retardation of the motor system since motor reflexes (e.g. righting reflex) and locomotor skills were not delayed in MK-801-treated animals (Maier et al. 1995). This is also reflected at the level of functional maturation of the motoneurone itself. In the present study we found that the developmental decrease in input resistance that occurs during normal postnatal development was not significantly affected by MK801 treatment (see Table 1). Thus, while systemic MK801 administration can be expected to block NMDA receptors in different parts of the nervous system, it does not appear to have generalised effects in all aspects of spinal motor development.
Several lines of evidence suggest that the effects of MK-801 are, at least in part, due to direct effects on synaptic transmission to motoneurones. Previous electrophysiological studies indicate that MK801 selectively reduces by about 10-30 % the mono- and polysynaptic spinal reflex input to motoneurones leaving the larger, non-NMDA component of glutamatergic transmission intact (Farkas & Ono, 1995). Similarly, NMDA receptor blockade in vitro depresses, but does not abolish, the activity of the spinal pattern generators involved in locomotion (Fenaux et al. 1991; Beato et al. 1997). Behavioural studies indicate that in vivo systemic administration of high doses (0.5 mg kg−1) of MK-801 to neonatal animals decreases the general level of hindlimb motor activity (Mc Ewen et al. 1999).
Other evidence in favour of a direct effect on motoneurones is the finding that systemic MK801 treatment using a similar protocol to that employed in the present study rescues motoneurones from axotomy-induced cell death (Mentis et al. 1993; Casanovas et al. 1996) and disrupts the maturation of the motoneurone dendritic tree (Kalb, 1994). Chronic neonatal blockade of NMDA receptors has also been shown to prolong the period of plasticity of spinal cord circuitry in postnatal rats (Maier et al. 1995), highlighting the critical role of NMDA-mediated synaptic activity in the postnatal refinement of spinal synaptic circuits, as has also been demonstrated in the visual system (Kleinschmidt et al. 1987). It is possible that, during normal development, the increase in glutamatergic synaptic activity associated with the maturation of locomotion may lead to the downregulation of motoneurone gap junctional coupling. This would provide an activity-dependent mechanism for the refinement of motor control, since the loss of motoneurone coupling would reduce the extent of synchronised motoneurone activity and allow for independent recruitment of motoneurones.
The mechanism by which glutamatergic synaptic activity downregulates gap junctional coupling during development is unclear. Previous studies have shown that neurotransmitters acutely modulate gap junctional coupling in both neurons and glial cells (Rörig & Sutor, 1996; Roerig & Feller, 2000). In the rat somatosensory cortex, dye coupling is significantly reduced by noradrenaline and 5-HT (Roerig & Feller, 2000). In addition, activation of calcium-permeable ionotropic glutamate receptors has been shown to result in a decrease in gap junctional conductance in cerebellar Bergman glia (Müller et al. 1996), an effect that could be blocked in calcium-free medium. Furthermore, directly increasing the levels of cytosolic calcium in hippocampal neurons leads to occlusion of dye coupling (Rao et al. 1987). Such effects may be mediated directly or via calcium-activated phosphorylation of the connexin subunits by protein kinases (Rörig & Sutor, 1996). Thus, it is possible that sustained activation of NMDA receptors may lead in the short term to occlusion, and in the longer term to degradation or downregulation of gap junctional proteins located in the vicinity of glutamatergic synapses.
Finally, it is possible that other subtypes of glutamate receptors (e.g. calcium-permeable AMPA receptors) could also be involved in regulating gap junctional coupling and further studies investigating blockade of these receptors should be carried out. In addition, it would be interesting to investigate the effects of increased afferent synaptic activity upon this process to confirm the influence of synaptic activity in the regulation of neuronal coupling.
Acknowledgments
We are grateful to Wellcome Trust and the European Union BIO4-96-0649 for financial support.
References
- Armstrong DL, Turin L, Warner AE. Muscle activity and the loss of electrical coupling between striated muscle cells in Xenopus embryos. Journal of Neuroscience. 1983;3:1414–1421. doi: 10.1523/JNEUROSCI.03-07-01414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Bracci E. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proceedings of the Royal Society B. 1997;264:877–884. doi: 10.1098/rspb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DL, Navarrete R. Dye coupling between lumbar motoneurones in the embryonic and neonatal rat spinal cord: an in vitro study. Journal of Physiology. 1990;423:98P. [Google Scholar]
- Casanovas A, Ribera J, Hukkanen M, Riveros-Moreno V, Esquerda JE. Prevention by lamotrigine, MK-801 and Nomega-nitro-L-arginine methyl ester of motoneuron cell death after neonatal axotomy. Neuroscience. 1996;71:313–325. doi: 10.1016/0306-4522(95)00461-0. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Squalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. Journal of Physiology. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. Journal of Neuroscience. 1999;19:10813–10828. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas S, Ono H. Participation of NMDA and non-NMDA excitatory amino acid receptors in the mediation of spinal reflex potentials in rats: an in vivo study. British Journal of Pharmacology. 1995;114:1193–1205. doi: 10.1111/j.1476-5381.1995.tb13333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux F, Corio M, Palisses R, Viala D. Effects of an NMDA-receptor antagonist, MK-801, on central locomotor programming in the rabbit. Experimental Brain Research. 1991;86:393–401. doi: 10.1007/BF00228963. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Miledi R, Takahasji T. Electrical synapses between motoneurones in the spinal cord of the newborn rat. Proceedings of the Royal Society B. 1980;208:115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. Journal of Neuroscience Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. Journal of Neuroscience. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Relationship between dye coupling and spontaneous activity in developing ferret visual cortex. Developmental Neuroscience. 1998;20:59–64. doi: 10.1159/000017299. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Tresch MC. Gap junctions and motor behavior. Trends in Neurosciences. 2002;25:108–115. doi: 10.1016/s0166-2236(02)02038-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. Journal of Physiology. 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen ML, Van Hartesveldt C, Stehouwer DJ. The NMDA antagonist, MK-801, alters l-DOPA-induced air-stepping in neonatal rats. Developmental Brain Research. 1999;115:33–40. doi: 10.1016/s0165-3806(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Maier DL, Kalb RG, Stelzner DJ. NMDA antagonism during development extends sparing of hindlimb function to older spinally transected rats. Developmental Brain Research. 1995;87:135–144. doi: 10.1016/0165-3806(95)00065-l. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. Journal of Neuroscience. 1988;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Greensmith L, Vrbová G. Motoneurons destined to die are rescued by blocking N-methyl-d-aspartate receptors by MK-801. Neuroscience. 1993;54:283–285. doi: 10.1016/0306-4522(93)90253-c. [DOI] [PubMed] [Google Scholar]
- Müller T, Möller T, Neuhaus J, Kettenmann H. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia. 1996;17:274–284. doi: 10.1002/(SICI)1098-1136(199608)17:4<274::AID-GLIA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Navarrete R, Vrbová G. Changes of activity patterns in slow and fast muscles during postnatal development. Developmental Brain Research. 1983;8:11–19. [Google Scholar]
- Navarrete R, Vrbová G. Activity-dependent interactions between motoneurones and muscles: their role in the development of the motor unit. Progress in Neurobiology. 1993;41:93–124. doi: 10.1016/0301-0082(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Synaptic transmission between ventrolateral funiculus axons and lumbar motoneurons in the isolated spinal cord of the neonatal rat. Journal of Neurophysiology. 1994;72:2406–2419. doi: 10.1152/jn.1994.72.5.2406. [DOI] [PubMed] [Google Scholar]
- Rao G, Barnes CA, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. Brain Research. 1987;408:267–270. doi: 10.1002/cne.902590405. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Research Reviews. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Rörig B, Sutor B. Regulation of gap junction coupling in the developing neocortex. Molecular Neurobiology. 1996;12:225–249. doi: 10.1007/BF02755590. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potential in the mammalian spinal cord. Nature Neuroscience. 2000;3:593–599. doi: 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Walton KD, Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat spinal cord. Journal of Physiology. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. Journal of Neuroscience. 1990;10:125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


