Abstract
In the fetal sheep, parturition is triggered by an increase in the activity of the fetal hypothalamus- pituitary-adrenal (HPA) axis which, in turn, augments the biosynthesis of oestrogen by the placenta. Parturition can be prevented or delayed by destruction of the paraventricular nucleus (PVN), pituitary or adrenal, or stimulated by infusions of adrenocorticotropin (ACTH) or glucocorticoids. We have previously reported that physiological increases in fetal plasma concentrations of oestradiol have a neuroendocrine effect to increase both basal and hypotension-stimulated ACTH secretion. The present study was performed to test the effect of oestradiol on the central baroreceptor and chemoreceptor reflex pathways. We used immunohistological techniques to identify various neuroanatomical regions which are activated by hypotension and, subsequently, those areas modified by oestrogen's action and baroreceptor and chemoreceptor denervation. We assessed cellular activation in these brain regions by immunostaining for Fos, the protein product of c-fos, an immediate early response gene. We found that oestradiol increased Fos abundance in nucleus tractus solitarius (NTS), rostral ventrolateral medulla (RVLM), and PVN, and augmented the increase in Fos in these regions in response to a 10 min period of brachiocephalic arterial occlusion (BCO). Carotid sinus denervation blocked the Fos response to BCO, but not to oestrogen alone, in these regions. In contrast, the hippocampus responded to BCO with increase Fos in intact fetuses, but did not respond to oestrogen treatment. None of the treatments altered Fos expression in cerebral cortex or in cerebellum. We conclude that oestradiol augments the activity of the central baroreceptor and chemoreceptor reflex pathways, and that it may influence fetal ACTH secretion via this site of action.
In the fetal sheep, parturition is triggered by an increase in the activity of the fetal HPA axis (Liggins et al. 1973; Challis & Brooks, 1989). Parturition can be delayed by destruction of the paraventricular nucleus (McDonald & Nathanielsz, 1991; Gluckman et al. 1991), or pituitary (Liggins et al. 1966, 1967; Liggins & Kennedy, 1968) or stimulated by infusions of ACTH (Liggins, 1968), or glucocorticoids (Liggins, 1969). Cortisol acts at the placenta to increase the activity of cytochrome P450c17 (17-hydroxylase and 17,20 lyase activities) which increases the rate of oestrogen biosynthesis (Anderson et al. 1975; Pomerantz & Nalbandov, 1975; Steele et al. 1976; Yu et al. 1983). Oestrogen plays a central role in the initiation of parturition via a direct action on the uterus to increase myometrial contractility (Liggins, 1974). However, oestrogen also has a neuroendocrine action. 17β-oestradiol augments both basal and stimulated ACTH secretion (Saoud & Wood, 1997). The effect of oestrogen on ACTH secretion in fetal sheep is similar to but more pronounced than that in adult rats (Viau & Meaney, 1991).
The present study was designed to test whether oestrogen increases Fos expression in regions of the fetal brain which, in turn, are known to influence HPA axis function. More specifically, we tested the responsiveness of brain regions known to include baro- and chemoreflex pathways. We tested the effect of oestradiol on Fos expression in normotensive, hypotensive, intact, and baro- and chemoreceptor denervated fetuses. Cellular activity was assessed by measuring the level of Fos, the protein product of c-fos, the immediate early response gene, in brain areas important for HPA axis control. This method has been used in numerous studies to assess neuronal activation due to physiological stress (Hoffman et al. 1991; Kononen et al. 1992).
Methods
Surgical procedures
We studied 40 pregnant ewes and fetuses of mixed Western breeds. All of the experiments and animal use were performed according to the Guiding Principles for Animal Care and Use of the American Physiological Society and were approved by the University of Florida Animal Care and Use Committee. All ewes were between 114 and 125 days gestation at the time of surgery. Food and water were withheld from ewes 24 h prior to surgery. After shaving and cleaning with povidone iodine (Betadine, Purdue Fredrick Co., Norwalk, CT, USA), anaesthesia was induced via mask inhalation, the ewes were intubated and general anaesthesia was maintained (0.5-2.0 % halothane, balance O2). Heart rate, blood pressure, end-tidal O2 and CO2 tensions, electrocardiogram, and rectal temperature were all monitored during surgery. Animals were closely monitored from the time of intubation until recovery when the animal could stand on its own effort. Ewes were allowed free access to food and water throughout the post-operative period.
The uterus was exposed using a ventral midline incision. Once the hindlimbs were located, a small incision was made in the uterus. One hindlimb was delivered through the uterine incision and a polyvinyl chloride catheter (0.03 in i.d., 0.05 in o.d.) was placed into the tibial artery and advanced to the level of the diaphragm. An oestradiol implant (5 mg over 21 days or 238 μg day−1; Innovative Research of America, Toledo, OH, USA) or placebo implant was inserted subcutaneously into the area of the gluteous medius. Hindlimb and uterine incisions were closed and an amniotic catheter made of polyvinyl chloride (0.05 in i.d., 0.09 in o.d.) was sutured to the exterior of a hindlimb. All uterine incisions were closed in two layers.
Using a technique similar to the one described above, catheters (0.03 in i.d., 0.05 in o.d.) were placed in the lingual arteries and advanced approximately 1 cm into the carotid artery toward the heart. Upon closure of the neck incision, lingual catheters were anchored to the chin of the fetus. This surgical procedure has been described previously (Cudd & Wood, 1992).
Some of the fetuses were subjected to carotid sinus denervation as described previously (Wood, 1989; Tong et al. 1998). After exposing the common carotid artery but prior to catheterization of the lingual arteries, denervation was accomplished by bilaterally stripping all nerves and connective tissue between the carotid-occipital arterial junction and the carotid-lingual arterial junction. The occipital artery was ligated and the lingual artery was stripped rostrally of all nerves and connective tissue for approximately 1 cm from the carotid-lingual junction.
Before returning the head of the fetus to the uterus, an 8 mm occluder (In Vivo Metric, cat. no. OC8, Healdsburg, CA, USA) was placed around the brachiocephalic artery. The left forelimb of the fetus was delivered through the uterine incision. An incision was made in the second intercostal space, the brachiocephalic artery was exposed, and the occluder positioned. Once the occluder was sewn in place, the incision under the left forelimb was closed, the fetus was returned to the uterus, and the uterine incision was closed. Ampicillin (750 mg, Polyflex, Ft Dodge Laboratories, Ft Dodge, IA, USA) was administered into the amniotic cavity before closure of the maternal linea alba and skin in separate layers.
All catheters were filled with heparin (1000 units ml−1, Elkins-Sinn, Inc., Cherry Hill, NJ, USA) and closed with a sterile 17 gauge brass brad inserted into the end. Catheters and occluders were tunnelled subcutaneously to the flank and exteriorized. Maternal incisions were closed in layers and catheters were held in place with an elastic bandage (Spandage, Medi-Tech International, Brooklyn, NY, USA).
In ewes with twin pregnancies, both fetuses were surgically manipulated the same way. At the time of experimentation however, only one of the animals was made hypotensive while the other animal served as a control.
All ewes were treated with 750 mg ampicillin (i.m.) at the time of surgery and twice per day for 5 days thereafter. Rectal temperatures were monitored twice daily and food intake was monitored each day for signs of infection or distress.
In vivo experimental procedures
All ewes were allowed 5 days to recovery from surgery. On the day of experimentation, catheters were removed from the elastic bandage and the distal ends were cleaned with povidone iodine and alcohol. Each brad was removed and a sterile blunt adapter with a three-way stopcock was inserted. All catheters were flushed with heparinized saline (10 U ml−1). One femoral arterial, one lingual arterial, and the amniotic catheter were attached to transducers (Statham P23Id, Statham Instruments, Oxnard, CA, USA) for measurement of fetal arterial and amniotic fluid pressures. Arterial and amniotic fluid pressures were measured for the first 35 min of the experiment using a Grass Model 7 recorder. Lingual arterial pressure was measured as the arterial pressure downstream from the brachiocephalic occlusion. The data were digitized and stored using an IBM AT Microcomputer and a Keithley analog-to-digital converter on-line.
All experiments were performed between 117 and 140 days gestation. There were eight different experimental groups (n = 5 per group) in this study, represented in Table 1. Thirty-three of the 40 total experiments were in fetuses which were between 120 and 130 days and 38 of the 40 total experiments were in fetuses between 119 and 133 days gestation. All animals were studied in their home pens and care was taken to limit the amount of external stress placed upon the animals. Arterial blood samples (5 ml) were drawn at 0, 10, 20, and 60 min. In approximately half of the experiments, the brachiocephalic occluder was inflated with saline between the 0 and 10 min blood samples. Blood samples were placed in chilled tubes containing Na4EDTA (50 μg EDTA (ml blood)−1, Sigma Chemical Co., St Louis, MO, USA). An additional 1.5 ml of blood was drawn anaerobically into syringes coated with heparin for measurement of fetal blood gases using a Ciba-Corning 288 Blood Gas System. A small aliquot of this blood was used to measure haematocrit using an IEC microhaematocrit centrifuge. Blood gas and haematocrit data are shown as group means ± s.e.m. in Table 2. Blood samples were kept on ice until they were centrifuged at 3000 g for 30 min at 4 °C in a refrigerated centrifuge (Sorvall RT 6000B, DuPont, Newtown, CA, USA). After centrifugation, the plasma was divided into aliquots and transferred to polypropylene tubes and stored at -20 °C until hormones were assayed.
Table 1.
Description of the eight experimental groups
| Group | Oestradiol or placebo | Carotid sinus denervation (CSD) or intact | Brachiocephalic occlusion (BCO) or normotensive (-BCO) | Gestational ages (days) |
|---|---|---|---|---|
| 1 | Oestradiol | Intact | +BCO | 121, 124, 130, 132, 134 |
| 2 | Oestradiol | Intact | −BCO | 122, 126, 130, 130, 140 |
| 3 | Oestradiol | CSD | +BCO | 117, 123, 126, 127, 133 |
| 4 | Oestradiol | CSD | −BCO | 119, 121, 125, 126, 130 |
| 5 | Placebo | Intact | +BCO | 124, 125, 126, 126, 128, |
| 6 | Placebo | Intact | −BCO | 122, 124, 125, 125, 128 |
| 7 | Placebo | CSD | +BCO | 121, 125, 126, 127, 133 |
| 8 | Placebo | CSD | −BCO | 119, 123, 123, 127, 133 |
BCO = brachiocephalic occlusion; CSD = carotid sinus denervated.
Table 2.
Haematocrit, partial pressure of oxygen (Pa,O2) and carbon dioxide (Pa,CO2) in arterial blood and aretial H (pHa) measured in eight experimental groups
| Haematocrit | Pa,O2 (mmHg) | Pa,CO2 (mmHg) | pHa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | 0 min | 10 min | 20 min | 0 min | 10 min | 20 min | 0 min | 10 min | 20 min | 0 min | 10 min | 20 min |
| Control | 36.2 ± 2.0 | 36.0 ± 1.5 | 35.6 ± 1.3 | 16.8 ± 1.2 | 17.4 ± 2.2 | 17.5 ± 0.9 | 55.2 ± 3.2 | 53.9 ± 2.6 | 54.9 ± 3.1 | 7.362 ± 0.02 | 7.372 ± 0.04 | 7.370 ± 0.03 |
| Hypo | 33.8 ± 1.7 | 36.0 ± 2.0 | 33.6 ± 2.0 | 19.5 ± 1.1 | 22.0 ± 2.3 | 19.8 ± 1.5 | 53.9 ± 2.7 | 48.7 ± 2.4 | 51.0 ± 2.9 | 7.345 ± 0.03 | 7.355 ± 0.03 | 7.344 ± 0.02 |
| CSD | 32.4 ± 1.6 | 32.2 ± 1.5 | 32.4 ± 1.9 | 17.7 ± 1.6 | 17.7 ± 1.2 | 18.0 ± 2.0 | 53.4 ± 3.0 | 50.3 ± 2.5 | 50.6 ± 1.9 | 7.362 ± 0.02 | 7.364 ± 0.03 | 7.369 ± 0.03 |
| Hypo, CSD | 32.6 ± 2.2 | 33.0 ± 1.4 | 33.0 ± 1.5 | 18.4 ± 2.0 | 21.3 ± 1.8 | 19.3 ± 1.4 | 53.9 ± 1.9 | 49.4 ± 1.9 | 50.3 ± 2.3 | 7.364 ± 0.03 | 7.366 ± 0.02 | 7.354 ± 0.03 |
| E2 | 28.8 ± 1.3 | 27.8 ± 1.1 | 28.0 ± 1.1 | 18.0 ± 1.6 | 18.7 ± 1.9 | 18.2 ± 3.0 | 55.5 ± 1.7 | 52.7 ± 4.1 | 52.5 ± 2.7 | 7.343 ± 0.02 | 7.358 ± 0.02 | 7.354 ± 0.02 |
| E2, Hypo | 33.4 ± 1.8 | 35.0 ± 1.6 | 32.8 ± 1.3 | 15.4 ± 1.8 | 18.4 ± 2.2 | 17.9 ± 2.1 | 58.0 ± 2.3 | 55.6 ± 3.6 | 54.1 ± 2.6 | 7.335 ± 0.03 | 7.341 ± 0.03 | 7.378 ± 0.02 |
| E2, CSD | 33.0 ± 1.5 | 33.2 ± 1.4 | 32.6 ± 1.7 | 16.4 ± 1.9 | 17.7 ± 1.3 | 18.0 ± 1.6 | 55.3 ± 3.2 | 54.0 ± 2.5 | 54.6 ± 4.1 | 7.345 ± 0.03 | 7.358 ± 0.02 | 7.360 ± 0.03 |
| E2, Hypo, CSD | 34.4 ± 1.3 | 36.0 ± 1.2 | 34.2 ± 1.6 | 17.6 ± 0.9 | 23.0 ± 1.5 | 20.3 ± 1.7 | 52.8 ± 4.0 | 49.6 ± 2.4 | 50.0 ± 3.9 | 7.351 ± 0.02 | 7.353 ± 0.04 | 7.344 ± 0.02 |
All values are means ± s.e.m. (n = 5) of measurements taken at 0, 10 and 20 min (brachiocephalic occlusion occurs between 0 and 10 min). Hypo = hypotensive; CSD = carotid sinus denervated; E2 = oestradiol treated.
Immediately after drawing the 60 min blood sample, ewes were killed with an overdose of sodium pentobarbital via the jugular vein. Fetuses were immediately removed for perfusion of the brain. The chest of each fetus was opened and the brachiocephalic artery was located and cannulated. Either by means of a pump or by syringe, brains were perfused with 1 l of phosphate-buffered saline (pH 7.4, with 20 U ml−1 heparin) followed by 2 l of 4 % paraformaldehyde. Brains were removed and stored in 4 % paraformaldehyde until processing for immunohistochemistry. The time course of the experimental design is represented in Fig. 1.
Figure 1. Experimental design for in vivo studies.
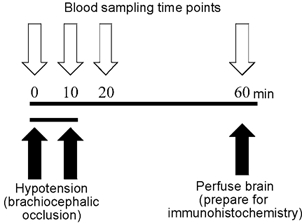
Blood samples (5 ml) were taken at 0, 10, 20 and 60 min (additional blood was taken at 0, 10 and 20 min for blood gas and haematocrit measurement). Hypotension via brachiocephalic occlusion occurred between 0 and 10 min. Blood pressure and heart rate were recorded for the first 35 min of the experiment. Animals were killed at 60 min and brains were prepared for immunohistochemistry.
Hormone assays
Plasma oestradiol concentrations were measured utilizing an enzyme immunoassay (EIA) kit from Oxford Biochemical Inc. (no. EA70) after extraction with 8 volumes of ethyl ether. The antiserum provided in this kit was highly specific for oestradiol. The cross-reactivity with oestrone was 0.10 %. Plasma ACTH, arginine vasopressin (AVP), and cortisol concentrations were measured by specific radioimmunoassays (RIA) which have been previously described (Wood et al. 1990, 1993; Bell et al. 1991).
Immunohistochemical techniques
There were a total of 24 fetal brains used for the histological experiments (n = 3 per group). Tissue was prepared for embedding by dehydration with progressively increasing concentrations of ethanol, followed by xylene. All tissue was embedded in paraffin and cut into 7 μm sections using a Zeiss microtome. Sections were mounted on poly-l-lysine slides, deparaffinized with xylene and rehydrated in decreasing concentrations of ethanol. Immunohistochemistry and visualization were made possible utilizing a Histostain-SP kit from Zymed (South San Francisco, CA, USA) and metal-enhanced 3,3′-diaminobenzidine (DAB; Pierce, Rockford, IL, USA). Sections were stained for Fos using a polyclonal anti-Fos antiserum from Oncogene Sciences (cat no. PC38, Ab5). The primary antibody was diluted 1:10 000 in diluent (1 % BSA in phosphate-buffered saline with 0.01 % Triton X-100). Specific staining was confirmed by dilution tests. Specific staining was absent upon replacing primary antibodies with 10 % normal goat serum. All slides were dehydrated prior to mounting coverslips with Permount (Fisher Scientific, Pittsburgh, PA, USA).
Fetal brains regions were analysed for Fos abundance using commercial densitometry software (Microcomputer Imaging Device from Imaging Research Inc., Ontario, Canada). The following brain regions were analysed: (1) paraventricular nucleus (PVN), (2) nucleus of the tractus solitarius (NTS), (3) rostral ventral lateral medulla (RVLM), (4) hippocampus, (5) cerebellum, (6) cortex, and (7) pituitary gland. As paraffin blocks were cut on the microtome, every fifth section was used once the region of interest was identified. This assured homogeneity among fetal brains.
Using densitometry, we quantified the proportion of positive stained cells in each brain section. Values were then analysed via three-way analysis of variance (ANOVA) (Winer, 1971). Pairwise comparisons were made using Student-Newman-Keuls multiple range test (Winer, 1971). Plasma ACTH, AVP, and cortisol concentrations were analysed using three-way ANOVA. Oestradiol-treated fetuses were analysed separately from placebo-treated fetuses by this means using ±BCO, ±CSD, and time as factors. Oestradiol-treated fetuses were compared to placebo-treated fetuses relative to time and treatment group by analysis of variance. Comparison of plasma oestradiol concentrations in oestradiol-treated fetuses and placebo-treated fetuses was made using Student's t test (Zar, 1984). The influence of initial arterial oxygen tension on regional Fos staining intensity was tested using forward stepwise multiple regression analysis (Winer, 1971).
Results
Haematocrit and blood gases
All group means ± s.e.m. are shown in Table 2. The arterial blood gases are somewhat variable, and values of arterial Pa,O2 are similar to, but in some groups tend to be lower than, those reported in previous studies on heavily instrumented fetal sheep (Wood, 1989; Saoud & Wood, 1997). Analysis of the initial values of Pa,O2 by three-way ANOVA revealed no statistically significant main effects of denervation status, oestradiol treatment, or assignment to brachiocephalic occlusion group, although there was a significant three-way interaction among these three main treatment groups (P < 0.05). Post hoc analysis using Tukey's multiple range test did not detect any statistically significant differences between individual pairs of groups. There were no statistically significant differences among groups in the values of haematocrit, Pa,CO2 or pHa.
Blood pressure
Mean arterial blood pressure (MAP) ± s.e.m. are shown in Table 3. All group means are shown at baseline (0 min) while blood pressure measurement during brachiochephalic occlusion is shown for hypotensive fetuses only. MAP was significantly elevated in oestradiol-treated fetuses compared to placebo-treated fetuses (n = 5 per group, P < 0.001). Also, MAP during occlusion was consistently significantly higher compared to each respective baseline value for each group (n = 5 per group, P < 0.001).
Table 3.
Fetal ovine mean arterial blood pressure (MAP) ±s.e.m. in eight experimental groups measured before and during brachiocephalic occlusion (n = 5)
| Experimental group | Baseline MAP (mmHg) | MAP during occlusion (mmHg) |
|---|---|---|
| Control | 50.0 ± 4.3 | — |
| Hypo | 54.2 ± 5.2 | 72.9 ± 8.1† |
| CSD | 52.9 ± 3.9 | — |
| Hypo, CSD | 53.3 ± 7.1 | 70.8 ± 5.3† |
| E2 | 68.8 ± 6.2* | — |
| E2, Hypo | 66.7 ± 5.2* | 85.4 ± 7.4† |
| E2, CSD | 65.6 ± 4.7* | — |
| E2, Hypo, CSD | 65.6 ± 3.2* | 83.3 ± 5.2† |
Oestradiol-treated fetuses revealed significantly higher MAP compared to placebo-treated fetuses
P < 0.001. MAP during occlusion was also found to be significantly elevated
P < 0.001. Hypo = hypotensive; CSD = carotid sinus denervated; E2 = oestradiol treated.
Hormonal variables
Plasma oestradiol concentrations levels were significantly higher in oestradiol-treated fetuses than in placebo-treated fetuses (n = 20 per group, P < 0.001; Fig. 2). Fetal plasma ACTH levels are shown in Fig. 3 and fetal plasma cortisol concentrations are shown in Fig. 4. In both placebo- and oestradiol-treated fetuses, BCO greatly increased fetal plasma ACTH secretion. The increase in fetal plasma ACTH concentration was significantly attenuated by carotid sinus denervation. Fetal plasma ACTH concentrations were not altered significantly by blood sampling alone in either placebo- or oestradiol-treated fetuses. Oestradiol treatment significantly increased fetal plasma ACTH concentration in all groups. Fetal plasma cortisol concentrations reflected the changes in fetal plasma ACTH concentration.
Figure 2. Oestradiol levels + s.e.m. for fetuses treated with 5 mg/21 day oestradiol implant and fetuses treated with a placebo implant.
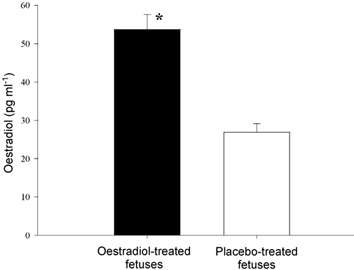
The group means are significantly different (n = 20 per group, P < 0.001).
Figure 3. ACTH plasma levels plotted as group means + s.e.m.
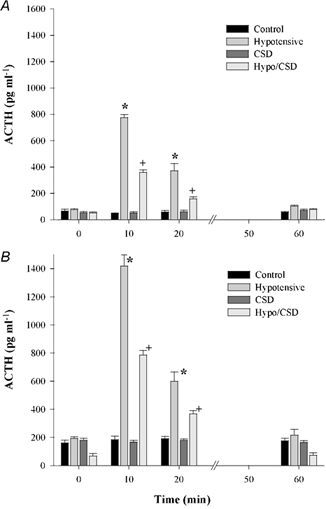
A, placebo-treated fetuses; B, oestradiol-treated fetuses. * and + denote statistically significant differences (n = 5 per group, P < 0.001). All oestradiol groups were significantly different from placebo groups relative to treatment and time (n = 5 per group, P < 0.01).
Figure 4. Cortisol plasma levels plotted as group means + s.e.m.
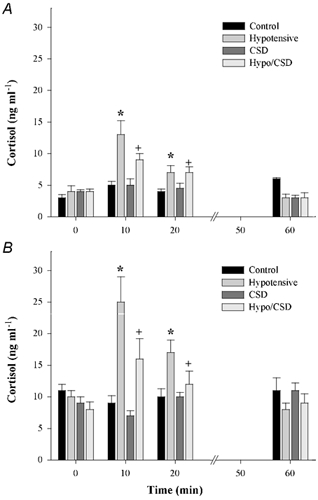
A, placebo-treated fetuses; B, oestradiol-treated fetuses. * and + denote statistically significant differences (n = 5 per group, P < 0.001). All oestradiol groups were significantly different from placebo groups relative to treatment and time (n = 5 per group, P < 0.01).
Fetal plasma AVP concentrations are shown in Fig. 5. The pattern of fetal plasma AVP responses was similar to that for the fetal ACTH responses. BCO increased AVP and carotid sinus denervation attenuated these responses in both placebo- and oestradiol-treated fetuses. Oestradiol treatment significantly increased fetal plasma AVP concentration in all experimental groups.
Figure 5. AVP plasma levels plotted as group means + s.e.m.
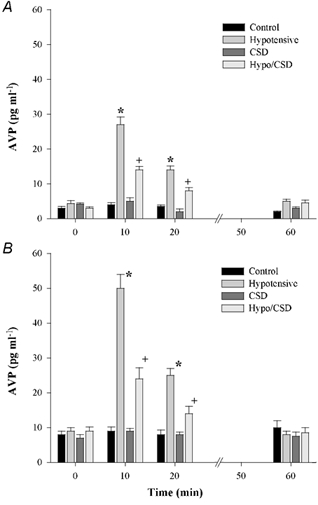
A, placebo-treated fetuses; B, oestradiol-treated fetuses. * and + denote statistically significant differences (n = 5 per group, P < 0.001). All oestradiol groups were significantly different from placebo groups relative to treatment and time (n = 5 per group, P < 0.01).
Immunohistochemistry
Fos staining in the PVN was significantly different among the eight treatment groups by three-way ANOVA (n = 3 per group, P < 0.001). The mean densitometry values + s.e.m. are plotted in Fig. 6 along with representative photomicrographs. Fos abundance was increased in response to BCO in both placebo- and oestradiol-treated fetuses. Carotid sinus denervation, by itself, did not alter Fos abundance, but did completely block the Fos response to BCO in both oestradiol- and placebo-treated fetuses. Oestradiol treatment significantly increased Fos abundance in all groups. Photomicrographs reveal that oestradiol-treated fetuses have more Fos in the PVN when compared to control fetuses. Also shown is significantly more positive staining in oestradiol-treated, hypotensive fetuses compared to placebo-treated, hypotensive fetuses.
Figure 6. Fos immunohistochemistry staining in the fetal ovine paraventricular nucleus.
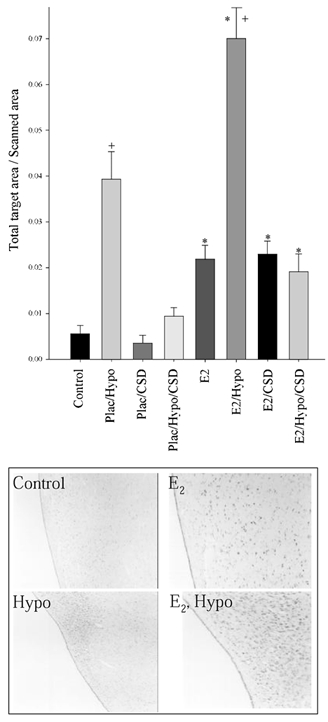
Plac = placebo implant, E2 = oestradiol implant, Hypo = hypotensive, CSD = carotid sinus denervated. There is a significant difference between Plac vs. E2 (* P < 0.001) and Control vs. Hypo (+P < 0.001). Representative photomicrographs are shown below the Fos densitometry.
The pattern of Fos staining in the NTS with regard to the experimental manipulations was remarkably similar to that in the PVN (Fig. 7). There was an overall significant effect of oestradiol treatment on Fos abundance. Within both placebo- and oestradiol-treated groups, BCO increased Fos abundance. In both groups, this effect on Fos abundance was eliminated by carotid sinus denervation.
Figure 7. Fos immunohistochemistry staining in the fetal ovine nucleus of the tractus solitarius.
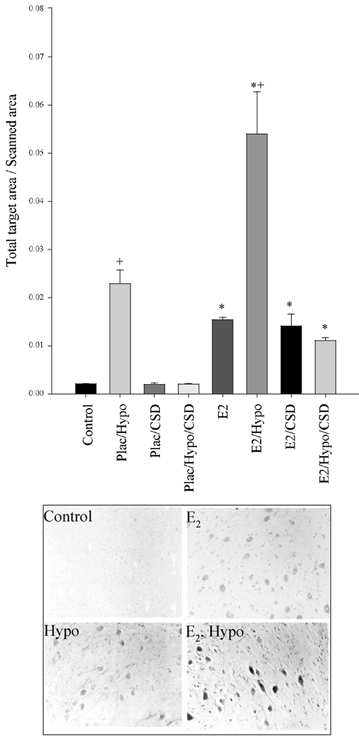
Plac = placebo implant, E2 = oestradiol implant, Hypo = hypotensive, CSD = carotid sinus denervated. There is a significant difference between Plac vs. E2 (* P < 0.001) and Control vs. Hypo (+P < 0.001). Representative photomicrographs are shown below the Fos densitometry.
The pattern of staining in the RVLM was similar to that in the PVN and in the NTS. The mean densitometry values + s.e.m. are plotted in Fig. 8 along with representative photomicrographs.
Figure 8. Fos immunohistochemistry staining in the fetal ovine rostral ventral lateral medulla.
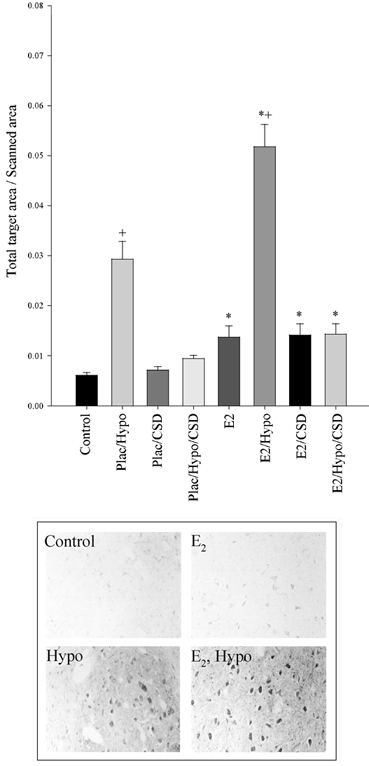
Plac = placebo implant, E2 = oestradiol implant, Hypo = hypotensive, CSD = carotid sinus denervated. There is a significant difference between Plac vs. E2 (* P < 0.001) and Control vs. Hypo (+P < 0.001). Representative photomicrographs are shown below the Fos densitometry.
Fos staining in the hippocampus was somewhat different than in the PVN, NTS, or RVLM. In this brain region, there was a statistically significant effect of BCO on Fos abundance (P < 0.001), an effect which was eliminated by carotid sinus denervation. However, in this region, there was no significant effect of oestradiol treatment (P = 0.407). The mean densitometry values + s.e.m. are plotted in Fig. 9 along with representative photomicrographs. Note that the y-axis is smaller than that of the PVN, etc. because the level of Fos staining is lower in the hippocampus. It can be seen visually that hypotensive fetuses have more Fos generation in the hippocampus when compared to normotensive fetuses.
Figure 9. Fos immunohistochemistry staining in the fetal ovine hippocampus.
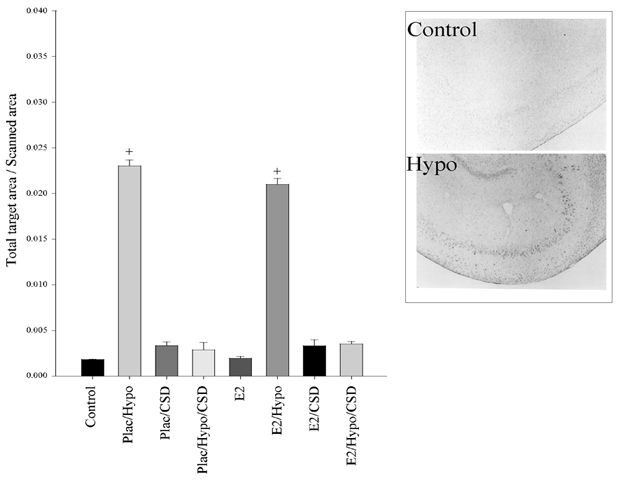
Plac = placebo implant, E2 = oestradiol implant, Hypo = hypotensive, CSD = carotid sinus denervated. There is a significant difference between Control vs. Hypo (+P < 0.001). Representative photomicrographs are shown to the right of the Fos densitometry.
Cerebellum did not respond to any of the treatments (n = 3 per group, P = 0.721). Similar to the cerebellum, none of the treatments altered Fos abundance in cerebral cortex (n = 3 per group, P = 0.399).
Discussion
In fetal sheep, the hypothalamus-pituitary-adrenal axis plays an important role in the generation of responses to stress which are integral for the survival of the fetus in utero. This endocrine axis also plays a role in the initiation of the chain of events which ultimately culminates in parturition. For these reasons, a fundamental understanding of the mechanisms controlling the activity of this axis is key to understanding fetal homeostasis as well as the events which lead to parturition. We have previously demonstrated that oestradiol, in concentrations which are within the endogenous range of plasma concentrations, has a potent effect on both basal and stimulated activity of the fetal hypothalamus-pituitary-adrenal axis (Saoud & Wood, 1997; Wood et al. 2001). We designed the present study to investigate the site of action of oestrogen on this endocrine axis.
The fetal HPA axis is influenced by both baroreceptors and chemoreceptors in the peripheral circulation. We have previously found that the ACTH responses to arterial hypotension, produced by vena caval obstruction (Wood, 1989; Tong et al. 1998), by carotid occlusion (Wood, 1995), or by brachiocephalic occlusion (see present results), are attenuated by denervation of the arterial baroreceptors and chemoreceptors. The baroreceptor- chemoreceptor reflex pathway is clearly an important controller of fetal HPA axis activity (Wood & Tong, 1999), and the activity of the reflex can be modulated by various paracrine or endocrine substances. For example, prostanoids augment reflex responsiveness to baroreceptor or chemoreceptor activity (Tong et al. 1998). It is theoretically possible than any hormone which influences HPA axis activity could exert its influence in the paraventricular nucleus (PVN) or in any brain region which is known to affect PVN function. Because oestrogen increases both basal and hypotension-stimulated fetal ACTH secretion, we designed the present study to investigate the cellular response to this hormone along the baroreceptor and chemoreceptor reflex pathways as well as in other brain regions which are important for HPA axis control. The results of this study reveal much about the action of oestrogen on the fetal brain, as well as the activity of the baroreceptor and chemoreceptor reflex pathways in response to hypotension in the fetus.
Immunohistochemical analysis of Fos abundance demonstrates that the PVN, nucleus tractus solitarius (NTS), and rostral ventrolateral medulla (RVLM) all respond to oestrogen treatment with increased neuronal activity, and that in all of these areas the cellular activity is increased in response to brachiocephalic occlusion. The influences of oestrogen and hypotension on these brain regions appeared to be additive (i.e. oestrogen does not appear to increase HPA axis function by altering the baro- or chemoreflex). All of these brain regions have oestrogen receptors (Simerly et al. 1990; Lehman et al. 1993), enabling them to respond directly to oestradiol treatment, and the response to oestradiol does not depend upon the presence of an intact reflex pathway (oestrogen still has an action in the carotid sinus-denervated fetuses).
In contrast to the PVN, NTS, and RVLM, neither oestrogen nor brachiocephalic occlusion altered Fos abundance in cerebral cortex or cerebellum. This demonstrated that the response to oestrogen treatment within the CNS was not the result of generalized and non-specific neuronal activation. The Fos immunostaining in the hippocampus revealed an intermediate pattern. Like the PVN, NTS, and RVLM, an increase in Fos staining was seen in hypotensive animals and this effect was eliminated with carotid sinus denervation. However, there was no apparent effect of oestradiol. This is interesting because there have been oestrogen receptors reported within the hippocampus (Simerly et al. 1990; Lehman et al. 1993). Perhaps the answer to this discrepancy lies in fact that the hippocampus is a site of cortisol negative feedback (Feldman et al. 1992; Feldman & Weidenfeld, 1999). The fetal HPA axis is inhibited by cortisol (Wood & Rudolph, 1983), and oestrogen increases the circulating concentrations of cortisol as well as ACTH (Wood & Saoud, 1997; Saoud & Wood, 1997). It is therefore possible that oestrogen had no net effect on Fos immunostaining in this region because of counterbalancing inhibitory effects of cortisol and stimulatory effects of oestrogen.
The results of these experiments do not reveal the site of action of oestrogen within the fetal brain that is relevant to fetal HPA axis activity. However, the pattern of Fos staining does demonstrate that: (1) oestrogen stimulates specific brain regions and does not have a general stimulatory action in the fetal brain; (2) among the regions stimulated by oestrogen is what might be referred to as the ‘core’ of the baroreflex-chemoreflex pathway (NTS, RVLM, PVN); (3) oestrogen augments the activity of these regions after hypotension; and (4) oestrogen actions on the baroreflex-chemoreflex pathway do not require intact baro- and chemoreceptors. The oestrogen treatment significantly increased Fos immunostaining in NTS, RVLM, and PVN, but had no measurable effect in hippocampus, cerebral cortex, and cerebellum. Along with the increased Fos staining in these regions was an increase in fetal mean arterial blood pressure. One could suggest that the increased staining in the oestrogen-treated animals was secondary to the increase in blood pressure. However, this seems very unlikely in light of the fact that oestrogen had the same effect in both intact and baro- and chemo-denervated fetuses. It seems more likely that the increased activity within the central pathways might have been the cause of the increased blood pressure.
We have previously demonstrated that baroreceptor and chemoreceptor denervation dramatically reduces the endocrine response to hypotension (Wood, 1989; Tong et al. 1998). The inhibition of the response is dramatic, but not complete. In light of our previous results, we were surprised to find that the baroreceptor and chemoreceptor denervation completely blocked the Fos response to hypotension in NTS, RVLM, PVN, and in hippocampus. The most straightforward interpretation of this result is that these brain regions were not activated in response to hypotension in the denervated fetuses. It is possible that there was a direct effect of the hypotension and resultant hypoperfusion on the pituitary, causing release of ACTH. We believe that this is not likely, however, because we have consistently observed a release of vasopressin in response to hypotension in sinoaortic denervated fetuses (Wood, 1989; Tong et al. 1998). The release of vasopressin from the posterior pituitary must be associated, at some time, with an increase in PVN activity. We therefore suggest that the sensitivity of the Fos immunostaining technique is not sufficient to detect the level of neuronal activation in the PVN after hypotension in the denervated fetal sheep. Alternatively, it is possible that the timing of the collection of the tissue relative to the onset of brachiocephalic occlusion was not appropriate to demonstrate the full extent of the Fos response to this stimulus. If this is correct, we would not be able to make any conclusions about whether the effect of the PVN was direct or secondary to input from medullary centres or other brain regions.
One potential confounding influence in the present experiments is the apparent variability in initial values of Pa,O2 among the various experimental groups. The variability was not great enough to allow us to identify individual group differences. Nevertheless, we considered the possibility that variations in Pa,O2 in the individual experiments might have biased the results of the Fos immunostaining. We analysed the data using a forward stepwise multiple regression analysis (Winer, 1971). This analysis revealed no statistically significant effect of Pa,O2 in the values of Fos immunostaining intensity. Although we believe that relative hypoxia was not a systematic confounding factor in our experiments, we do acknowledge that hypoxia per se is likely to alter the pattern of Fos immunostaining in the fetal brain. We propose, however, that the effect of the hypoxia was likely to influence the groups in a random, rather than systematic, distribution. Another potential confounding influence in these experiments is the possibility that some or all of the effects on Fos production might have resulted secondarily from an action of oestradiol outside the brain. For example, it is possible that oestradiol stimulated prostaglandin E2 production in placenta and that the PGE2, in turn, stimulated the Fos production that we report in this study. While this is possible, the extent to which oestradiol stimulates fetal placental PGE2 production is unknown. The present study differs from most previous studies in this laboratory in the method for reducing cerebral perfusion pressure. In several other studies (Wood et al. 1982; Wood, 1989; Tong et al. 1998), we used a technique of vena caval obstruction to reduce venous return and therefore combined ventricular output and, ultimately, fetal arterial blood pressure. More recently, we have used the technique of brachiocephalic occlusion as a method of reducing cerebral blood flow without systemic hypotension (Tong & Wood, 1999; Tong et al. 1999). We view this method as a more specific stimulus, allowing a direct manipulation of cerebral perfusion pressure and blood flow (Tong & Wood, 1999).
The effect of oestrogen on the fetal HPA axis response to hypotension provides, perhaps, some insight into the physiological changes which occur at the end of gestation and which ready the fetus for birth. There is an increase in fetal plasma oestradiol concentration at the end of gestation in the sheep which precedes normal parturition (Yu et al. 1983). We have proposed that this increase in plasma oestradiol and/or other oestrogens acts as one limb of a positive feedback loop which ultimately culminates in parturition (Saoud & Wood, 1997; Wood & Saoud, 1997). From the results of the present study, we would add to this that we propose that oestradiol augments cardiovascular reflex responsiveness. This increase in reflex responsiveness might improve survival of perinatal stress. Because of the fundamental importance of the fetal HPA axis in physiological control mechanisms in the perinatal period, it is possible that it ties together what might be thought of as disparate physiological systems. Specifically, it is possible that endocrine influences on the fetal HPA axis or neuronal maturation which ultimately initiate parturition might occur along the baroreflex and chemoreflex pathways. While these and other experiments have not directly addressed this idea, we propose that an influence of the baroreflex and chemoreflex pathways on the general activation of the fetal HPA axis at the end of gestation would be consistent with our experience so far.
Acknowledgments
This work was supported by a Grant-in-Aid (to C.E.W.) and by a predoctoral fellowship grant (to S.P.) from the Florida/Puerto Rico Affiliate of the American Heart Association.
References
- Anderson ABM, Flint AP, Turnbull AC. Mechanism of activation of glucocorticoids in induction of ovine parturition: Effect on placental steroid metabolism. Journal of Endocrinology. 1975;66:61–70. [PubMed] [Google Scholar]
- Bell ME, Wood CE, Keller-Wood M. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domestic Animal Endocrinology. 1991;8:245–254. doi: 10.1016/0739-7240(91)90060-w. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Brooks AN. Maturation and activation of hypothalamic-pituitary-adrenal function in fetal sheep. Endocrine Reviews. 1989;10:182–204. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- Cudd TA, Wood CE. Prostaglandin E2 releases ovine fetal ACTH from a site not perfused by the carotid vasculature. American Journal of Physiology. 1992;263:R136–140. doi: 10.1152/ajpregu.1992.263.1.R136. [DOI] [PubMed] [Google Scholar]
- Feldman S, Saphier D, Weidenfeld J. Corticosterone implants in the paraventricular nucleus inhibit ACTH and corticosterone responses and the release of corticotropin-releasing factor following neural stimuli. Brain Research. 1992;578:251–255. doi: 10.1016/0006-8993(92)90254-7. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Glucocorticoid receptor antagonists in the hippocampus modify the negative feedback following neural stimuli. Brain Research. 1999;821:33–37. doi: 10.1016/s0006-8993(99)01054-9. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Mallard C, Boshier DP. The effect of hypothalamic lesions on the length of gestation in fetal sheep. American Journal of Obstetrics and Gynecology. 1991;165:1464–1468. doi: 10.1016/0002-9378(91)90392-5. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, McDonald T, Shedwick R, Nathanielsz PW. Activation of cFos in ovine fetal corticotropin-releasing hormone neurons at the time of parturition. Endocrinology. 1991;129:3227–3233. doi: 10.1210/endo-129-6-3227. [DOI] [PubMed] [Google Scholar]
- Kononen J, Honkaniemi J, Alho H, Koistinaho J, Iadorola M, Pelto-Huikko M. Fos-like immunoreactivity in the rat hypothalamic-pituitary-axis after immobilization stress. Endocrinology. 1992;130:3041–3047. doi: 10.1210/endo.130.5.1315265. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Ebling FJP, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133:876–886. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. Journal of Endocrinology. 1968;42:323–329. doi: 10.1677/joe.0.0420323. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. Journal of Endocrinology. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Parturition in the sheep and the human. In: Coutinho EM, Fuchs F, editors. Physiology and Genetics of Reproduction. New York: Plenum Press; 1974. pp. 423–443. [Google Scholar]
- Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Recent Progress in Hormone Research. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Holm LW, Kennedy PC. Prolonged pregnancy following surgical lesions of the foetal lamb pituitary. Journal of Reproduction and Fertility. 1966;12(419) [Google Scholar]
- Liggins GC, Kennedy PC. Effects of electrocoagulation of the foetal lamb hypophysis on growth and development. Journal of Endocrinology. 1968;40:371–381. doi: 10.1677/joe.0.0400371. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Kennedy PC, Holm LW. Failure of initiation of parturition after electrocoagulation of the pituitary of the fetal lamb. American Journal of Obstetrics and Gynecology. 1967;98:1080–1086. doi: 10.1016/0002-9378(67)90031-2. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. American Journal of Obstetrics and Gynecology. 1991;165:764–770. doi: 10.1016/0002-9378(91)90325-l. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK, Nalbandov AV. Androgen level in the sheep fetus during gestation. Proceedings of the Society for Experimental Biology and Medicine. 1975;149:413–416. doi: 10.3181/00379727-149-38818. [DOI] [PubMed] [Google Scholar]
- Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. American Journal of Physiology. 1997;272:R1128–1134. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Steele PA, Flint APF, Turnbull AC. Activity of steroid C-17, 20 lyase in the ovine placenta: Effect of exposure to foetal glucocorticoid. Journal of Endocrinology. 1976;69:239–246. doi: 10.1677/joe.0.0690239. [DOI] [PubMed] [Google Scholar]
- Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. American Journal of Physiology. 1998;275:R735–741. doi: 10.1152/ajpregu.1998.275.3.R735. [DOI] [PubMed] [Google Scholar]
- Tong H, Richards E, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. Journal of the Society of Gynecological Investigation. 1999;6:127–135. doi: 10.1016/s1071-5576(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. American Journal of Physiology. 1999;277:R1268–1273. doi: 10.1152/ajpregu.1999.277.5.R1268. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2. New York: McGraw-Hill; 1971. pp. 514–603. [Google Scholar]
- Wood CE. Sinoaortic denervation attenuates the reflex responses to hypotension in fetal sheep. American Journal of Physiology. 1989;256:R1103–1110. doi: 10.1152/ajpregu.1989.256.5.R1103. [DOI] [PubMed] [Google Scholar]
- Wood CE. Fetal responses to carotid occlusion: immaturity of buffering systems. American Journal of Physiology. 1995;268:R343–348. doi: 10.1152/ajpregu.1995.268.2.R343. [DOI] [PubMed] [Google Scholar]
- Wood CE, Cudd TA, Kane C, Engelke K. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. American Journal of Physiology. 1993;265:R858–862. doi: 10.1152/ajpregu.1993.265.4.R858. [DOI] [PubMed] [Google Scholar]
- Wood CE, Kane C, Raff H. Peripheral chemoreceptor control of fetal renin responses to hypoxia and hypercapnia. Circulation Research. 1990;67:722–732. doi: 10.1161/01.res.67.3.722. [DOI] [PubMed] [Google Scholar]
- Wood CE, Keil LC, Rudolph AM. Hormonal and hemodynamic responses to vena caval obstruction in fetal sheep. American Journal of Physiology. 1982;243:E278–286. doi: 10.1152/ajpendo.1982.243.4.E278. [DOI] [PubMed] [Google Scholar]
- Wood CE, Rudolph AM. Negative feedback regulation of adrenocorticotropin secretion by cortisol. Endocrinology. 1983;112:1930–1936. doi: 10.1210/endo-112-6-1930. [DOI] [PubMed] [Google Scholar]
- Wood CE, Saoud CJ. Influence of estradiol and androstenedione on ACTH and cortisol secretion in the ovine fetus. Journal of the Society of Gynecological Investigation. 1997;4:279–283. [PubMed] [Google Scholar]
- Wood CE, Saoud CJ, Stoner TA, Keller-Wood M. Estrogen and androgen influence hypothalamic AVP and CRF concentrations in fetal and adult sheep. Regulatory Peptides. 2001;98:63–68. doi: 10.1016/s0167-0115(00)00231-7. [DOI] [PubMed] [Google Scholar]
- Wood CE, Tong H. Central nervous system regulation of reflex responses to hypotension during fetal life. American Journal of Physiology. 1999;277:R1541–1552. doi: 10.1152/ajpregu.1999.277.6.R1541. [DOI] [PubMed] [Google Scholar]
- Yu HK, Cabalum T, Jansen CA, Buster JE, Nathanielsz PW. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology. 1983;113:2216–2220. doi: 10.1210/endo-113-6-2216. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 2. Englewood Cliffs, NJ, USA: Prentice-Hall; 1984. [Google Scholar]


