Abstract
The aims of this study were to investigate whether hypertrophy of the small intestinal smooth muscle leads to alterations of myosin isoform composition and shortening velocity and whether possible changes correlate with a change in the sensitivity to ADP of shortening velocity in this tissue. A partial occlusion was introduced in the distal part of the ileum of guinea-pigs. After 2 weeks, the part of the small intestine just proximal of the stenosis was hypertrophied (indicated by a significantly increased cross-sectional area). The most proximal part of the small intestine was used as control, thus enabling comparisons between hypertrophic and normal tissue from the same animal. The outer longitudinal layer of the intestinal wall was gently peeled off and used for biochemistry, RT-PCR and mechanical experiments. The desmin/actin ratio was significantly increased following hypertrophy, although myosin and actin expression were similar in control and hypertrophic tissue. In hypertrophic tissue, the myosin heavy chain mRNA with a 21 base pair insert decreased significantly. The composition of the mRNA encoding the myosin essential light chains changed towards more of the basic type (LC17b). No change in the expression of non-muscle myosin heavy chains A and B was detected. The maximal shortening velocity (Vmax) of maximally activated skinned preparations was significantly lower in the hypertrophic tissue (≈50 % of control). The sensitivity of Vmax to ADP was increased in the hypertrophic smooth muscle tissue. We conclude that myosin expression is altered following intestinal hypertrophy and that these alterations affect reactions in the cross-bridge interaction, leading to a slower and more economical contractile function.
Smooth muscle is responsible for a wide variety of physiological tasks in the body. This functional diversity is reflected in a wide span of contractile properties between smooth muscle types (Somlyo & Somlyo, 1968; Malmqvist & Arner, 1991; Löfgren et al. 2001), from slow, economical phenotypes (e.g. large elastic arteries), to faster types (e.g. the intestine). Smooth muscle also has a large potential for structural and functional adaptation by hypertrophy of smooth muscle cells, for example the growth of the uterus during pregnancy or of the intestines and the urinary bladder following pathological distension (Gabella, 1979d; Malmqvist et al. 1991; Morano et al. 1997). Smooth muscle cell hypertrophy can involve an altered synthesis of different isoforms of structural and contractile elements. Studies of the mechanical function of hypertrophic smooth muscle may therefore reveal functions of the contractile and cytoskeletal elements, in addition to providing data of importance for the understanding of different pathophysiological conditions.
During hypertrophy of smooth muscle, both actin and myosin are synthesised in the growing smooth muscle cells. This has been demonstrated in the hypertrophying smooth muscle of blood vessels (Malmqvist & Arner, 1990) and urinary bladder (Malmqvist et al. 1991; Wang et al. 1995). One important issue is if this remodelling of the contractile apparatus leads to an altered expression of myosin isoforms. Both the isoforms of the myosin heavy chains (MHC, with and without a 7 amino acid insert in the head region) and of the essential light chains (acidic, LC17a, and basic, LC17b) have been proposed to be involved in modulation of shortening velocity in smooth muscle (Kelley et al. 1992; Fuglsang et al. 1993; Sjuve et al. 1996; Rovner et al. 1997; Lauzon et al. 1998; Babu et al. 2001). The composition of myosin isoforms is thought to directly influence the properties of enzyme kinetics of the actin-myosin interaction; slow muscles have higher ADP affinity and lower sensitivity to inorganic phosphate (Fuglsang et al. 1993; Löfgren et al. 2001). In a previous study, a decreased maximal shortening velocity (Vmax) was demonstrated in hypertrophic smooth muscle from the rat urinary bladder (Sjuve et al. 1996). This change towards a slower phenotype was associated with a decreased expression of the myosin heavy chain with the insert and an increase in LC17b. A study of the uterus of pregnant rats has shown a downregulation of the expression of LC17b and an increase in maximal shortening velocity (Morano et al. 1993). An increase in shortening velocity correlated with altered myosin isoform expression has also been demonstrated following thyroxine treatment (Löfgren et al. 2002).
The smooth muscle of the small intestine is functionally different from that of the urinary bladder in that it is almost constantly contracting to propagate the ingesta, whereas the urinary bladder is in a relaxed state most of the time. This might imply differences in the response to hypertrophic stimuli and/or in the mechanical and biochemical adaptations during hypertrophy between the intestine and the comparatively well-studied urinary bladder. In the present study, we have therefore examined the mechanical alterations in hypertrophic smooth muscle of the small intestine. We have used a model, modified from Gabella (1975), to induce hypertrophy of the small intestine in guinea-pigs. In principle, a partial stenosis is created in the most distal part of ileum which leads to a distension and hypertrophy, while the proximal part of the small intestine is not affected and can be used as control. This enables comparison between normal and hypertrophic tissue from the same animal, avoiding inter-individual differences. Using this model, we have addressed the following questions. (1) Is the expression of contractile and cytoskeletal proteins altered in smooth muscle tissue of hypertrophic small intestine? (2) Does the Vmax change during hypertrophy of the small intestinal smooth muscle? (3) Is the ADP sensitivity of maximal shortening velocity changed in the hypertrophic smooth muscle?
Methods
Animals
Female guinea-pigs (n = 9, ≈400 g) were anaesthetised using i.p. injections with Ketamine (100 mg (kg body weight)−1, KetalarR, Parke-Davis, Sweden) and Xylacin (25 mg (kg body weight)−1, Rompun-VetR, Bayer, Germany). An abdominal incision was performed and the distal part of ileum was dissected free. A 1 cm wide sterile cotton band was loosely placed around the distal part of ileum, close to the ileocaecal junction, and the ends of the band were glued together using cyanoacrylate glue. The animals were looked after daily and if an animal showed any sign of discomfort this animal was killed immediately (n = 1). The surgical procedure was carried out according to the Swedish national ethical guidelines for animal research and approved by the local ethics committee.
Ten to fourteen days after the operation the animals were killed by cervical dislocation and the small intestine was taken out. Two parts of the small intestine, the distal (hypertrophied in the operated animals) and the proximal (non-hypertrophied), were dissected. The dissection was performed in a buffer solution of the following composition (mm): NaCl, 118; KCl, 5; Na2HPO4, 1.2; MgCl2, 1.2; Hepes, 24; glucose, 10; CaCl2, 1.5. This solution was gassed with 100 % oxygen immediately before use. The outer, longitudinal smooth muscle layer of the small intestine was gently peeled off. The preparations were divided in two parts, one was chemically skinned and used for quick release experiments, the other frozen in liquid N2 for gel electrophoresis and RT-PCR and kept at -80°C. Permeabilisation to make chemically skinned preparations for the quick release experiments was performed using 1 % Triton X-100 (Arner & Hellstrand, 1985). Rings of the small intestinal wall from the hypertrophic and the normal part were also cut out to determine the extent of hypertrophy. The mucosa was removed and the circumference was measured using a microscope with an ocular scale. The cross-sectional area of the wall was calculated from the wet weight and circumference, using a density of 1.06 g ml−1.
Gel electrophoresis
Gel electrophoresis was performed in order to quantify the amounts of myosin, actin and desmin in the tissues (cf. Wede et al. 2002). The samples (smooth muscle from the outer, longitudinal layer of the small intestine) were thawed, weighed and thereafter homogenised (50 μl (mg wet weight)−1) in a buffer of the following composition: 25 mm Tris-HCl (pH 6.8), 10 % glycerol, 5 % mercaptoethanol, 1 mg ml−1 Bromophenol Blue and 2 % SDS. The homogenates were boiled for 2 min and quickly centrifuged. Four different amounts (1, 2, 3 and 5 μl) of each sample were loaded on 8 % SDS gels. On each gel different amounts of a standard of skeletal muscle actin with a known concentration were also loaded, to enable quantification of the amounts of the different proteins in the tissues. The gels were stained with Coomassie Blue overnight, destained, scanned in a GS-710 Calibrated Imaging Densitometer (BioRad, Richmond, CA, USA) and evaluated using Quantity One software (BioRad).
Western blot analysis
Semiquantitative Western blot analysis was performed to quantify the amounts of non-muscle myosins in the tissues. Three identical gels were run in parallel (as described above), one used for Coomassie staining and the two others for Western blot with antibodies against non-muscle myosin heavy chain A and non-muscle myosin heavy chain B, respectively. The antibody against non-muscle myosin heavy chain A was a gift from Dr Adelstein (NIH, Washington; Kelley et al. 1996) and the antibody against non-muscle myosin heavy chain B was a gift from Dr Morano (Berlin, Germany; Sjuve et al. 2001). Enhanced chemiluminescence (AmershamPharmaciaBiotech, UK) was used to detect antibody reactivity and the membranes were analysed using a Fluo-S Max and the Quantity One software. On each gel, a standard homogenate from urinary bladder tissue of a new born mouse, containing both non-muscle myosin heavy chain types, was used to enable comparisons between Western blots and gels.
Reverse transcriptase polymerase chain reaction (RT-PCR)
To determine the relative amounts of the different splice variants of the myosin heavy and light chains, we performed semi-quantitative RT-PCR. Since the different isoforms are formed by alternative splicing, we were able to detect both splice variants using two sets of primers, one set for the two heavy chains and one set for the two light chains (Fisher et al. 1997). For the heavy chains, the following primers were used (listed from the 5′ end to the 3′ end) ATGTACAAGGGCAAGAAGAGGC and GAGGAGT TGTCGTTCTTGAC. We used primers from the mammalian sequence (mouse, NCBI GI number: 7305294). It has been shown previously that the corresponding primers for gizzard generate PCR fragments of 351 and 330 bp (Fisher et al. 1997), which reflect the relative expression of mRNA for the myosin heavy chain with and without a 7 amino acid insert in the head region of the protein. The mRNA for the myosin light chains were detected using the following primers (listed from the 5′ end to the 3′ end): ATGTGTGACTTCACCGAGGAC and CATTCAGCACCATCCG GAC, generated using the mouse sequence (A. Arner, unpublished observation). PCR using these primers generates two fragments of 451 and 496 bp, reflecting the relative content of the two types of myosin essential light chain, LC17a and LC17b, respectively. The outer smooth muscle layer of the small intestine was homogenised and mRNA was extracted using the Micro Fast track kit (InVitrogen, Carlsbad, USA). cDNA was generated using Superscript First strand synthesis (Life Technologies, Carlsbad, USA). The PCR was executed with Taq polymerase (Qiagen, Washington, USA) in a Progene thermocycler (Techne, Cambridge, UK) using a denaturating temperature of 94 °C (1 min), and 35 cycles with annealing temperatures of 53.1 °C (heavy chains) or 51.7 °C (light chains) (1 min) and extension at 72 °C (2 min). The PCR products were separated on 3 % agarose gels stained with ethidium bromide and detected using Gel Doc 2000 and Quantity One software. The contents of the splice variants were expressed relative to the total PCR products.
Quick release experiments
The isotonic quick release method was used to determine the maximal shortening velocity of the tissues (Arner, 1982). Small preparations of the outer smooth muscle layer of the small intestine (approximate width and length: 1.5 mm and 3 mm) were attached between an isotonic lever arm and a force transducer (AE 801, SensoNor, Horten, Norway) using aluminium foil clips. In these experiments, solutions of the following basic composition were used: 30 mm Tes, 4 mm EGTA and 2 mm free Mg2+. The ionic strength was adjusted to 150 mm using KCl and the pH to 6.9 using KOH. Different free calcium concentrations were obtained by varying the ratio of CaEGTA/EGTA. The preparations were stretched to a very low passive tension (≈0.1 mN) and were allowed to relax in a solution with 3.2 mm MgATP, 12 mm phosphocreatine and 0.5 mg ml−1 creatine kinase. The degree of myosin light chain phosphorylation influences the shortening velocity of smooth muscle. In order to examine the cross-bridge kinetics in different tissues it is therefore important to keep the light chain phosphorylation constant. Repeated thiophosphorylation in a calcium containing (pCa 4.5) ATP-free solution (rigor solution) with 2 mm ATP-γ-S and 0.5 μm calmodulin was performed in order to keep the preparations maximally activated (Arheden et al. 1988). After thiophosphorylation, the preparations were rinsed in a calcium free (pCa 9) rigor solution. Contractions after each thiophosphorylation period were elicited by addition of the ATP-containing solution mentioned above. At the plateau of each contraction, a series of 15-25 releases to different afterloads was performed. A stable force level (afterload) was obtained within 5 ms and length was recorded during 1 s after the release. The maximal shortening velocity (Vmax) was calculated by fitting the Hill equation (Hill, 1938; eqn 1) to the force and velocity data and extrapolating the equation to zero afterload:
| (1) |
In this equation, a and b are constants, V is the velocity, P is the afterload and Po is the isometric force. The Vmax values presented in this study were measured at 100 ms after release in accordance with a previous report (Arner & Hellstrand, 1985). In the experiments with different concentrations of MgADP, ADP was introduced in the activating ATP-containing (6 mm MgATP, Löfgren et al. 2001) solution and the different concentrations were applied at random order. These experiments were performed in the absence of the ATP-regeneration system and with 0.2 mm of the myokinase inhibitor AP5A (Feldhaus et al. 1975) added. All quick-release experiments were performed at room temperature (22 °C).
The initial elastic recoil (ΔL) at each afterload (ΔP) was determined to evaluate the series elastic component. The series elastic component of smooth muscle is non-linear and can be described by an exponential spring. To evaluate the stiffness we fitted the data to a logarithmic function and determined the slope (K) of the (ΔP/Po/(ΔL/L) relation at Po (Arner, 1982).
Statistics
The values are given as mean values ± s.e.m. with n being the number of animals. Student's t test was used to evaluate statistical differences. Curve fitting was performed using a non-linear least squares method implemented in the Sigma plot software (SPSS Science, Chicago, USA).
Results
Structure and biochemical composition of the small intestinal wall
Partial obstruction of the ileum resulted in a marked increase in the circumference and cross-sectional area of the distal part of the small intestine (Table 1). The mean value of the cross-sectional area of the distal part of the small intestine, just above the stenosis was more than four times that of the normal, proximal part, suggesting hypertrophy of the intestine. Control experiments on normal (non-operated) guinea-pigs, slightly bigger than the ones used for surgery, showed that the cross-sectional areas of the proximal and the distal parts of the small intestine were similar (proximal part: 6 ± 1 mm2, n = 2, distal part: 7 ± 2 mm2, n = 2). Table 1 summarises data from the biochemical analysis of the distal, hypertrophic, and the proximal, normal, parts of the small intestine. The amount of the contractile proteins actin and myosin per tissue wet weight was similar in the two parts. Since the cross-sectional area of the small intestinal wall was significantly increased in the distal part, this suggests that synthesis of the contractile proteins had occurred in the hypertrophic tissue. The desmin/actin ratio was significantly increased in the tissue from the distal, hypertrophic part, showing a proportionally higher increase in the cytoskeletal protein desmin.
Table 1.
Dimensions and biochemical data of tissue from the normal and the hypertrophic parts of the small intestine of guinea-pig operated to induce intestinal obstruction
| Proximal control part | Distal hypertrophic part | |
|---|---|---|
| Circumference (mm) | 9.4 ± 0.8 | 19.2 ± 1.5 P <0.001 |
| Cross-sectional area (mm2) | 3.5 ± 0.3 | 16.2 ± 2.9 P <0.001 |
| [Actin] (μg actin (mg wet wt)−1) | 18.5 ± 2.5 | 20.4 ± 6.1 n.s. |
| [Myosin] (μg myosin (mg wet wt)−1) | 7.6 ± 1.6 | 7.8 ± 2.0 n.s. |
| Desmin/actin | 0.17 ± 0.02 | 0.39 ± 0.08 P <0.05 |
Circumference and cross sectional area were determined on rings of the intestinal wall with mucosa removed. The contents of actin and myosin were derived from the density of the respective bands on Coomassie stained SDS gels compared to the density of the bands from an actin standard of known concentration. The contents of these proteins are expressed relative to tissue wet weight. The desmin content was expressed as the desmin/actin ratio. n = 6-8.
Myosin isoform expression
In a previous study we have correlated changes in mRNA expression with changes in protein levels (Löfgren et al. 2002) for myosin heavy and light chain isoforms, and reported a correlation between mRNA and protein values when two muscle types (aorta and taenia coli) are compared and during thyroxine-induced remodelling. Although we cannot correlate the mRNA values with absolute protein levels, the changes in mRNA would correlate with changes in protein expression. In the present study we therefore examined the expression of myosin light and heavy chain isoforms at the mRNA level using RT-PCR. Figure 1 shows that the mRNA of the basic myosin essential light chain (LC17b) increased and that the mRNA of the myosin heavy chain with the insert (MHC+insert) decreased in the hypertrophic part of the small intestine compared to the control segment. The ratios between PCR products corresponding to the two myosin heavy and light chains isoforms were not influenced by the number of PCR cycles in the range 30-50 cycles. In control experiments, we compared expression of the myosin isoforms in the proximal and distal parts of the small intestine in normal (non-operated) guinea-pigs and found that the composition was similar in both parts for both myosin heavy and light chains (MHC+insert/total MHC: proximal 93 ± 0.2 %, distal 94 ± 2 %; LC17b/total LC17: proximal 26 ± 2 %, distal 23 ± 3 %; n = 2). These data suggest that the differences in myosin expression between the hypertrophic and control segments of operated animals are not due to differences along the length of the small intestine.
Figure 1. Results from RT-PCR experiments detecting the mRNA expression of myosin isoforms in smooth muscle of the distal/hypertrophic and the proximal/normal of the small intestine.
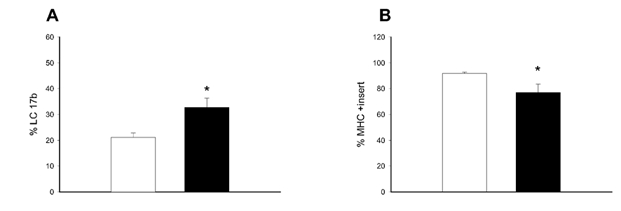
A shows the relative expression of the myosin essential light chain b (LC17b) expressed as percentage of the total expression of the myosin essential light chains. B shows the relative expression of the myosin heavy chain with the insert (MHC+insert) expressed as percentage of the total expression of the myosin heavy chains. *P < 0.05, n = 6 in each group.
Using semi-quantitative Western blot analysis we examined the expression of non-muscle myosin heavy chain A (NMMHC-A) and B (NMMHC-B) in hypertrophic and control tissue. The enhanced chemiluminescence signals of the Western blots for non-muscle myosins A and B were related to the Coomassie staining of the myosin band on the gels. To enable comparisons between gels, these values were normalised to the corresponding values of a standard from newborn mouse bladders containing both non-muscle myosin isoforms as described previously (Löfgren et al. 2002). No difference in the expression of these non-muscle myosins could be detected in the hypertrophic compared to the control part (NMMHC-A: control 0.48 ± 0.07, hypertrophic 0.49 ± 0.16; NMMHC-B: control 0.13 ± 0.03, hypertrophic 0.16 ± 0.06; n = 4-6).
Maximal shortening velocity and its sensitivity to ADP
Figure 2 shows force-velocity data of the preparations from the hypertrophic and the normal parts of the small intestine during a contraction at optimal MgATP concentration (3.2 mm). The maximal shortening velocity was decreased by almost 50 % in the hypertrophic compared to the normal part. We also determined the Vmax of proximal and distal parts of the small intestine from normal, unoperated guinea-pigs. The velocity was similar in the two parts (proximal 0.20 ± 0.01muscle lengths s−1; distal 0.21 ± 0.01 muscle lengths s−1; n = 8), showing that there is no difference in Vmax between the two parts of the intestine in the absence of hypertrophy. Stiffness was also calculated from the quick-release experiments. No difference in the stiffness constant K was observed between hypertrophic and control preparations (control 54 ± 8, hypertrophic 55 ± 9; n = 6).
Figure 2. Force-velocity relations.
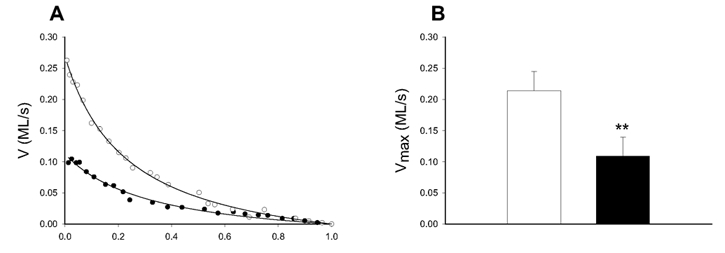
A shows original force-velocity relations of smooth muscle preparations from the normal (○) and the hypertrophic (•) part of the small intestine. The Hill equation (eqn (1), Methods) was fitted to the velocity (V in muscle lengths s−1) and relative afterload (P/Po) data and extrapolated to the maximal shortening velocity (Vmax). B shows mean values of Vmax of smooth muscle preparations from the normal (□) and the hypertrophic (▪) parts. ** P < 0.01, n = 6 in each group.
Figure 3 shows the dependence of Vmax on the MgADP concentration in the normal and the hypertrophic part of the small intestine. The Vmax in this solution was similar to that at 6 mm MgATP with ATP regeneration (Vmax at 6 mm MgATP without ATP regeneration/Vmax at 6 mm MgATP with ATP regeneration: control 1.06 ± 0.12, hypertrophic 1.16 ± 0.18; n = 4), suggesting that this [MgATP] was saturating for Vmax. In control preparations, MgADP inhibited velocity by about 20 % at the highest [MgADP] (5.2 mm) investigated. In hypertrophic tissue, the effect was more pronounced (about 40 % inhibition), suggesting an increased sensitivity to MgADP.
Figure 3. ADP effects on the maximal shortening velocity.
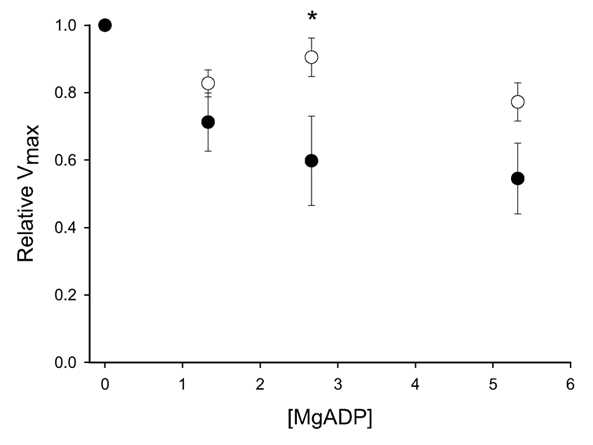
The figure shows the effects of addition of different concentrations of MgADP on maximal shortening velocity (Vmax) of the preparations from normal (○) and hypertrophic (•) tissues. * P < 0.05, n = 4 in each group.
Discussion
We have used a model for small intestinal hypertrophy of guinea-pigs introduced by Gabella (1975). The partial ligation results in a pronounced growth of the smooth muscle which has provided a useful model for extensive morphological studies (Gabella, 1975, 1979a-d, 1984; Ekblad et al. 1998). Mechanical investigations are, however, very limited (Gabella, 1979d). One advantage with this model is that control and hypertrophic smooth muscle tissue can be obtained from the same animal, thereby avoiding inter-individual differences and differences that are introduced as a consequence of the surgical trauma. This model involves a slowly increasing obstruction due to the developing fibrosis around the ligature which constricts the intestine. Thus the onset of the obstruction is gradual, which is in contrast to other commonly used models to induce smooth muscle hypertrophy, e.g. partial ligation of the urethra with subsequent hypertrophy of the urinary bladder, where the stenosis is acute. A gradual stenosis is probably more similar to that occurring in most situations of pathological obstruction.
In several previous studies it has been reported that hypertrophy of smooth muscle leads to upregulation of intermediate filaments (Gabella, 1979d; Berner et al. 1981; Malmqvist & Arner, 1990; Malmqvist et al. 1991). In studies by Gabella (1979d), increased numbers of desmin intermediate filaments were demonstrated using electron microscopy and we confirm these results, using biochemical determination of the desmin protein. It was suggested that a lower force of hypertrophic ileum muscle was due to the increased number of intermediate filaments and a concomitant relative decrease in myofilaments (Gabella, 1979d). We find that the actin and myosin concentrations are not influenced in the hypertrophic guinea-pig ileum, suggesting that a decreased expression of the contractile proteins does not occur in this model of hypertrophy, in contrast to the hypertrophying portal vein model (Malmqvist et al. 1991). This indicates that in some situations or stages of hypertrophic growth (e.g. in the portal vein), the myosin content does not increase in proportion to the increase in cell size. It is possible that the increased number of desmin filaments per se can reduce force. This is, however, unlikely since complete ablation of the desmin gene leads to a decrease in force development of smooth muscle from the urinary bladder and microarteries (Sjuve et al. 1998; Wede et al. 2002). In a study on smooth muscle from desmin-deficient mice it was shown that lack of desmin decreases maximal shortening velocity (Vmax) of skinned preparations from vas deferens and urinary bladder (Sjuve et al. 1998). The decrease in Vmax observed in the hypertrophic tissue in this study can thus not readily be explained by an increase in desmin expression. An increase in desmin content and intermediate filaments in hypertrophic smooth muscle is thus not a likely explanation for the observed decrease in force, nor for the decrease in Vmax reported previously (Gabella, 1979d; Arner et al. 1990; Sjuve et al. 1998). Rather these mechanical changes are due to other alterations in tissue structure or contractile protein expressions.
We report in this study that the composition of the myosin heavy and essential light chains is changed following hypertrophic remodelling of the small intestine. Both these constituents of the myosin hexamer have been implicated in determining the shortening velocity of the smooth muscle tissue (Kelley et al. 1992; Fuglsang et al. 1993; Sjuve et al. 1996; Rovner et al. 1997; Lauzon et al. 1998). We do not know the guinea-pig sequence for the myosin heavy chain with the insert. However, the insert is recognised in guinea-pig tissue by an antibody raised against the mammalian (rat) sequence (Löfgren et al. 2002). The changes observed in this study, i.e. increased expression of the LC17b and decreased expression of the myosin heavy chain with the insert are consistent with the previous findings on hypertrophied urinary bladder (Sjuve et al. 1996). In both models of hypertrophy, the light and heavy chains of myosin are altered, possibly reflecting a co-ordinated regulation. However, it will not be possible to determine, from these experiments, which myosin isoform is responsible for the mechanical change.
Non-muscle myosins can be expressed in smooth muscle and have recently been suggested to support contractions in some tissues and give a low shortening velocity (Morano et al. 2000). The non-muscle myosin heavy chain B has been reported to be upregulated in interstitial connective tissue cells of the bladder during hypertrophy (Sjuve et al. 2001). We report that the expression of these myosins is low in the outer muscle layer of the small intestine and is not influenced by hypertrophic remodelling. It is therefore unlikely that altered expression of non-muscle myosins is causing the lower Vmax of hypertrophic small intestine.
In a previous study, we have shown that the inhibitory influence of ADP on Vmax is much more pronounced in slow compared to fast smooth muscle types (Löfgren et al. 2001): the apparent inhibition constant (Ki) of MgADP of the aorta (slow) and the taenia coli (fast) differed 30-fold. We now extend the investigation to determine whether there is a change in ADP sensitivity and a change of myosin isoform composition in a smooth muscle tissue following hypertrophic remodelling.
It is possible that the lower shortening velocity in hypertrophic smooth muscle could involve a decrease in the number of series-coupled contractile units. In a simple model, a decrease in series coupling would increase the number of contractile units in parallel and lead to an increase in force, since the myosin concentration is unchanged. This is not consistent with the lower force reported in the hypertrophic guinea-pig small intestine (Gabella, 1979d). This suggests that the low Vmax of the hypertrophic small intestine rather reflects a change in the kinetics of the actin-myosin interaction. This explanation is further supported by the altered ADP sensitivity as discussed below.
One interesting question is whether ADP binding to the cross-bridge system and the shortening velocity are co-ordinated (through the expression of different myosin isoforms) or whether the correlation between the slow shortening velocity and high ADP sensitivity observed in comparisons between smooth muscles (Fuglsang et al. 1993; Löfgren et al. 2001) is just coincidental, reflecting different tissue properties. We have addressed this question using the hypertrophic small intestinal smooth muscle, where myosin isoforms and velocity are altered in the same muscle tissue. We find an increased ADP sensitivity (i.e. increased MgADP levels decreased Vmax at saturating MgATP to a higher extent in the hypertrophic tissue compared to the control). These results thus suggest that the ADP sensitivity as well as the maximal shortening velocity can be changed in one muscle as a result of physiological adaptation in vivo and that these two parameters are, at least under these conditions, regulated in a co-ordinated manner.
A change towards a slower/more economical smooth muscle phenotype with a lower Vmax, decreased relative expression of the myosin heavy chain with the insert and increased LC17b seems to be a common phenomenon in smooth muscle hypertrophy (Sjuve et al. 1998). A smooth muscle with a low Vmax would generally be more economical in maintaining tone (Rüegg, 1971) and the shift in myosin isoforms and mechanical properties could enable the muscle to maintain tension at low energy expenditure. In a distended hypertrophic organ, e.g. intestine or urinary bladder, the wall tension must be increased to maintain a given intraluminal pressure, according to the law of Laplace. The change in contractile properties might be beneficial in such situations where the contractions become more prolonged and where tension has to be maintained for longer times. Interestingly, the increased ADP sensitivity would slow the shortening velocity and promote economical tension maintenance in situations where the metabolic supply is limited.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (04x-8268), the Medical Faculty Lund University, the Crafoord foundation and the Swedish Heart Lung foundation. We want to acknowledge Dr Adelstein, NIH, Washington and Dr Morano, MDC, Berlin, Germany for the gifts of antibodies against the non-muscle myosin isoforms.
References
- Arheden H, Arner A. Cross-bridge behaviour in skinned smooth muscle of the guinea-pig taenia coli at altered ionic strength. Journal of Physiology. 1988;403:539–558. doi: 10.1113/jphysiol.1988.sp017263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A. Mechanical characteristics of chemically skinned guinea-pig taenia coli. Pflügers Archiv. 1982;395:277–284. doi: 10.1007/BF00580790. [DOI] [PubMed] [Google Scholar]
- Arner A, Hellstrand P. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. Journal of Physiology. 1985;360:347–365. doi: 10.1113/jphysiol.1985.sp015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner A, Malmqvist U. Metabolism and force in hypertrophic smooth muscle from rat urinary bladder. American Journal of Physiology. 1990;258:C923–932. doi: 10.1152/ajpcell.1990.258.5.C923. [DOI] [PubMed] [Google Scholar]
- Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nature Cell Biology. 2001;3:1025–1029. doi: 10.1038/ncb1101-1025. [DOI] [PubMed] [Google Scholar]
- Berner PF, Somlyo AV, Somlyo AP. Hypertrophy-induced increase of intermediate filaments in vascular smooth muscle. Journal of Cell Biology. 1981;88:96–100. doi: 10.1083/jcb.88.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Sjuve R, Arner A, Sundler F. Enteric neuronal plasticity and a reduced number of interstitial cells of Cajal in hypertrophic rat ileum. Gut. 1998;42:836–844. doi: 10.1136/gut.42.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus P, Frohlich T, Goody RS, Isakov M, Schirmer RH. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. European Journal of Biochemistry. 1975;57:197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Ikebe M, Brozovich F. Endothelin-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circulation Research. 1997;80:885–893. doi: 10.1161/01.res.80.6.885. [DOI] [PubMed] [Google Scholar]
- Fuglsang A, Khromov A, Torok K, Somlyo AV, Somlyo AP. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. Journal of Muscle Research and Cell Motility. 1993;14:666–677. doi: 10.1007/BF00141563. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophy of intestinal smooth muscle. Cell and Tissue Research. 1975;163:199–214. [PubMed] [Google Scholar]
- Gabella G. Hypertrophic smooth muscle. I. Size and shape of cells, occurrence of mitoses. Cell and Tissue Research. 1979a;201:63–78. doi: 10.1007/BF00238048. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophic smooth muscle. II. Sarcoplasmic reticulum, caveolae and mitochondria. Cell and Tissue Research. 1979b;201:79–92. doi: 10.1007/BF00238049. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophic smooth muscle. III. Increase in number and size of gap junctions. Cell and Tissue Research. 1979c;201:263–276. doi: 10.1007/BF00235062. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophic smooth muscle. IV. Myofilaments, intermediate filaments and some mechanical properties. Cell and Tissue Research. 1979d;201:277–288. doi: 10.1007/BF00235063. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophic smooth muscle. V. Collagen and other extracellular materials. Vascularization. Cell and Tissue Research. 1984;235:275–283. doi: 10.1007/BF00217851. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of shortening. Proceedings of the Royal Society. 1938;126:231–252. [Google Scholar]
- Kelley CA, Sellers JR, Gard DL, Bui D, Adelstein RS, Baines IC. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. Journal of Cell Biology. 1996;134:675–687. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Goldsmith PK, Adelstein RS. Smooth muscle myosin is composed of homodimeric heavy chains. Journal of Biological Chemistry. 1992;267:2127–2130. [PubMed] [Google Scholar]
- Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. Journal of Muscle Research and Cell Motility. 1998;19:825–837. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- Löfgren M, Fagher K, Woodard G, Arner A. Effects of thyroxine on myosin isoform expression and mechanical properties in guinea-pig smooth muscle. Journal of Physiology. 2002;543:757–766. doi: 10.1113/jphysiol.2002.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren M, Malmqvist U, Arner A. Substrate and product dependence of force and shortening in fast and slow smooth muscle. Journal of General Physiology. 2001;117:407–418. doi: 10.1085/jgp.117.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist U, Arner A. Isoform distribution and tissue contents of contractile and cytoskeletal proteins in hypertrophied smooth muscle from rat portal vein. Circulation Research. 1990;66:832–845. doi: 10.1161/01.res.66.3.832. [DOI] [PubMed] [Google Scholar]
- Malmqvist U, Arner A. Correlation between isoform composition of the 17 kDa myosin light chain and maximal shortening velocity in smooth muscle. Pflügers Archiv. 1991;418:523–530. doi: 10.1007/BF00370566. [DOI] [PubMed] [Google Scholar]
- Malmqvist U, Arner A. Contractile and cytoskeletal proteins in smooth muscle during hypertrophy and its reversal. American Journal of Physiology. 1991;260:C1085–1093. doi: 10.1152/ajpcell.1991.260.5.C1085. [DOI] [PubMed] [Google Scholar]
- Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nature Cell Biology. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- Morano I, Erb G. Expression of myosin heavy and light chains changes during pregnancy in the rat uterus. Pflügers Archiv. 1993;423:434–441. doi: 10.1007/BF00374938. [DOI] [PubMed] [Google Scholar]
- Morano I, Koehlen S, Haase H, Erb G, Baltas LG, Rimbach S, Wallwiener D, Bastert G. Alternative splicing and cycling kinetics of myosin change during hypertrophy of human smooth muscle cells. Journal of Cellular Biochemistry. 1997;64:171–181. doi: 10.1002/(sici)1097-4644(199702)64:2<171::aid-jcb1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Freyzon Y, Trybus KM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. Journal of Muscle Research and Cell Motility. 1997;18:103–110. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- Rüegg JC. Smooth muscle tone. Physiological Reviews. 1971;51:201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Arner A, Li Z, Mies B, Paulin D, Schmittner M, Small JV. Mechanical alterations in smooth muscle from mice lacking desmin. Journal of Muscle Research and Cell Motility. 1998;19:415–429. doi: 10.1023/a:1005353805699. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Haase H, Ekblad E, Malmqvist U, Morano I, Arner A. Increased expression of non-muscle myosin heavy chain-B in connective tissue cells of hypertrophic rat urinary bladder. Cell and Tissue Research. 2001;304:271–278. doi: 10.1007/s004410000262. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Haase H, Morano I, Uvelius B, Arner A. Contraction kinetics and myosin isoform composition in smooth muscle from hypertrophied rat urinary bladder. Journal of Cellular Biochemistry. 1996;63:86–93. doi: 10.1002/(SICI)1097-4644(199610)63:1%3C86::AID-JCB7%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacological Reviews. 1968;20:197–272. [PubMed] [Google Scholar]
- Wang ZE, Gopalakurup SK, Levin RM, Chacko S. Expression of smooth muscle myosin isoforms in urinary bladder smooth muscle during hypertrophy and regression. Laboratory Investigation. 1995;73:244–251. [PubMed] [Google Scholar]
- Wede OK, Löfgren M, Li Z, Paulin D, Arner A. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. Journal of Physiology. 2002;540:941–949. doi: 10.1113/jphysiol.2001.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]


