Abstract
Studies in cultured cells show that activation of endothelial nitric oxide (NO) synthase (eNOS) requires the dissociation of this enzyme from its inhibitory association with caveolin-1 (Cav-1), and perhaps its translocation from plasma membrane caveolae to other cellular compartments. We investigated the hypothesis that in vivo NO-dependent vasodilatation is associated with the translocation of eNOS from the cell membrane. To this end, we applied ACh topically (10-100 μm for 10 min) to the hamster cheek pouch microcirculation and measured NO production, blood flow and vessel diameter, and assessed subcellular eNOS distribution by Western blotting. Baseline NO production was 54.4 ± 5.2 pmol min−1 (n = 16). ACh increased NO release, caused arteriolar and venular dilatation and elevated microvascular flow. These responses were inhibited by NG-nitro-L-arginine (30 μm). The maximal increase in NO production induced by 10 μm and 100 μm ACh was 45 ± 20 % and 111 ± 33 %, respectively; the corresponding blood flow increases were 50 ± 10 % and 130 ± 24 %, respectively (n = 4-6). Both responses followed a similar time course, although increases in NO preceded flow changes. In non-stimulated tissues, eNOS was distributed mainly in the microsomal fraction. ACh-induced vasodilatation was associated with eNOS translocation to the cytosolic and Golgi-enriched fractions. After 1.5, 3.0 or 6.0 min of application, 10 μm ACh decreased the level of membrane-bound eNOS by -13 ± 4 %, -60 ± 4 % and -19 ± 17 %, respectively; at the same time points, 100 μm ACh reduced microsomal eNOS content by -38 ± 9 %, -61 ± 16 % and -40 ± 18 %, respectively (n = 4-5). In all cases, microsomal Cav-1 content did not change. The close ACh concentration dependence and the concomitance between eNOS subcellular redistribution and NO release support the concept that eNOS translocation from the plasma membrane is part of an activation mechanism that induces NO-dependent vasodilatation in vivo.
Endothelial cells control vascular tone by releasing vasoactive substances in response to physical and chemical signals. Nitric oxide (NO) has emerged as the most notable endothelium-derived vasodilator. Elegant pharmacological studies, in isolated or in situ arteries and arterioles, have documented the pivotal role of NO in the endothelium-dependent vasodilatation induced by several humoral agents and shear stress (Ishii et al. 1990; Koller & Kaley, 1991; Kuo et al. 1991; Moncada et al. 1991). On the other hand, the mechanisms triggering NO release by the activation of endothelial NO synthase (eNOS) have been studied mainly in cultured endothelial cells derived from large vessels, or in transfected non-endothelial cells (Michel et al. 1993, 1997a; Venema et al. 1996; Garcia-Cardeña et al. 1997; Feron et al. 1998). While a few recent reports address this subject in vitro in endothelial cells of microvascular origin (Thuringer et al. 2000; Kawanaka et al. 2002), much less is known about the mechanisms of eNOS activation in vivo.
In cultured cells, eNOS is targeted primarily to and anchored to the plasma membrane by dual acylation with myristate and palmitate (Busconi & Michel, 1993; Robinson & Michel, 1995; Sowa et al. 1999). In the membrane, the enzyme is found mainly in an inhibitory association with caveolin-1 (Cav-1), a structural protein of caveolae (Feron et al. 1996; Garcia-Cardeña et al. 1997; Ju et al. 1997). Furthermore, it has been proposed that following a rise in intracellular calcium, eNOS dissociates from Cav-1, allowing activation of the enzyme. This hypothesis is supported by reports that an increase in intracellular calcium is associated with a rise in NO production (Blatter et al. 1995; Kanai et al. 1995), as the interaction of eNOS with calcium- calmodulin dissociates the eNOS-Cav-1 complex (Garcia-Cardeña et al. 1997; Michel et al. 1997a,b; Feron et al. 1998). A role for tonic Cav-1 inhibition of eNOS activity in vivo is supported by the recent observation that the aortic rings of Cav-1-null mice present enhanced NOS-dependent relaxation in response to ACh (Drab et al. 2001; Razani et al. 2001). In addition, eNOS translocation from the membrane to the cytosol and/or perinuclear compartments has been observed following stimulation with calcium-mobilising agonists (Michel et al. 1993; Prabhakar et al. 1998; Goetz et al. 1999), possibly via enzyme de-palmitoylation (Robinson et al. 1995; Yeh et al. 1999). Translocation was presumed to be an inactivation mechanism of eNOS because it was a relatively slow process compared to the rapid transient NO signal reported in aortic endothelial cells (Malinski & Taha, 1992). This interpretation is questionable because the time course of NO production was not analysed together with eNOS translocation.
To what extent the mechanisms determined separately in cultured cells occur and are relevant in vivo has not been thoroughly investigated. It is conceivable that the dynamic environment in the intact circulation resulting in tonic eNOS activation may influence the kinetics and functional relevance of processes determined in tissue culture. To resolve whether eNOS translocation corresponds to activation or inactivation of the enzyme, this process should be analysed along with the time course of NO production. Furthermore, the functional relevance of such an event should be assessed in intact preparations or in vivo.
Therefore, in the work presented here, we tested the hypothesis that agonist-induced NO production and NO-dependent vasodilatation is associated with eNOS translocation from the cell membrane in microvascular endothelium in vivo. We decided to investigate this hypothesis in the hamster cheek pouch because this experimental model allows the direct visualisation and quantification of blood flow in the microcirculatory bed with minimal surgical trauma, as well as detection of NO production and eNOS subcellular distribution in a single preparation (Durán et al. 2000; Figueroa et al. 2001a). We chose ACh as the test agonist because it is a recognised calcium-mobilising endothelium-dependent vasodilator. We compared eNOS fractional distribution at rest and at several times during ACh-induced vasodilatation.
We report data on the correspondence between the time course of the rise in blood flow and microvessel diameter with NO production and the reduction of microsomal eNOS content. Our data support the hypothesis that translocation from the membrane represents eNOS activation in vivo.
Methods
Animal and drug sources
Adult male 100-120 g golden Syrian hamsters (Mesocricetus aureatus) were obtained from the Research Animal Facility of the Pontificia Universidad Católica de Chile. All studies were approved by the Institutional Bioethics Committee in compliance with the Guiding Principles in the Care and Use of Laboratory Animals endorsed by the American Physiological Society. We report the data of successfully completed experiments from a total of 142 hamsters.
Unless specified otherwise, all biochemical reagents and inhibitors were purchased from Sigma Chemical Co. (St Louis, MO, USA) and chemicals of analytical grade were from Merck (Darmstadt, Germany). Monoclonal anti-human eNOS and polyclonal anti-caveolin-1 primary antibodies were purchased from Transduction Laboratories (Lexington, KY, USA), and monoclonal anti-coat protein-beta (β-COP) was from Sigma. Monoclonal anti-Na+,K+-ATPase developed by Douglas M. Fambrough was obtained from the Developmental Studies Hybridoma Bank, under the auspices of NICHD, maintained by the University of Iowa (Iowa City, IA, USA). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were obtained from Pierce (Rockford, IL, USA).
Intravital microscopy of the cheek pouch
Hamsters were anaesthetised with sodium pentobarbital (60 mg kg−1, i.p.) and the right cheek pouch was prepared for intravital microscopy as described previously (Boric et al. 1990). Briefly, the trachea, left carotid artery and left jugular vein were cannulated. The right pouch was immobilised with a Lucite plate introduced through the mouth and exposed through a skin incision. The non-vascular layer of connective tissue was cleared and the observation chamber was placed on top of the pouch and secured to the skin. The observation chamber was placed on the middle section of the pouch, comprising mostly epithelial tissue and fine strands of skeletal muscle fibres. All observations and biochemical analyses were performed on this section of the tissue. The hamster was placed on the stage of a Nikon Optiphot microscope and the pouch was transilluminated with a fibre-optic bundle. The cheek pouch was superfused at 1 ml min−1 by a peristaltic pump with a bicarbonate buffer (mm: 125 NaCl, 1.17 MgSO4, 2 CaCl2, 20 NaHCO3) equilibrated with 95 % N2-5 % CO2, pH 7.4 and kept at 37 °C. The observation chamber was isolated from room air by a glass coverslip. All drugs were applied topically without interrupting the superfusate flow using either a sideline near the input to the observation chamber, or dissolved in the superfusion medium. During the experimental procedures and throughout the experiment, arterial carotid pressure was recorded continually on a Grass polygraph. Supplementary doses of anaesthetic were given whenever arterial pressure started to rise (usually 10-15 mg kg−1i.v., every 25-30 min). At the end of the experiment the animals were killed by an anaesthetic overdose (≈150 mg kg−1i.v.).
Microvascular flow and conductance determinations
After a 45 min stabilisation period, a 0.2 ml saline bolus containing 2 × 106 counts min−1 of 22Na radioisotope (22NaCl, NEZ-081, New England Nuclear, Boston, MA, USA) was injected i.v. The experiment was started 20 min later, to allow equilibration of the radioactive tracer between the plasma and extracellular compartment. The superfusion solution was collected over 2.5 min periods with a fraction collector. Duplicate 20 μl arterial blood samples were taken at hourly intervals. Total exchangeable plasma flow was determined by calculating the clearance of 22Na in the superfused tissue with a Wallac-Turku gamma counter, as detailed previously (Boric et al. 1990, 1995). The relative vascular conductance index (RVC) was calculated by dividing the clearance of 22Na by the mean arterial pressure at every collection interval (Boric et al. 1990, 1995).
Vessel diameters
The microcirculatory network was examined with a × 10 LWD Leitz objective and a few selected fields were recorded on videotape with a TV camera prior to, during and after the exposure to the different drugs. Vessel diameters were measured with a video calliper (Texas A&M, College Station, TX, USA) during playback at a magnification of × 900, with an accuracy of ± 0.5 μm. Arterioles (A) and venules (V) were grouped according to their branching order as described. The diameters of the observed vessels were: A4, 5-15 μm; A3, 16-30 μm; A2, 31-45 μm; A1, 46-70 μm; V3, 26-45 μm; V2, 46-65 μm.
Determination of NO production
The production of NO by the cheek pouch was quantified by measuring the superfusate content of authentic NO plus nitrite by chemiluminescence, as published previously (Figueroa et al. 2001a). To prevent contamination of the NO analyser with radioactive material, NO and RVC measurements were not performed together in the same preparation. Chemiluminescence is a highly sensitive and accurate method for NO measurements (Archer, 1993). However, due to the short half-life of NO in biological fluids, it is necessary to measure NO oxidised products, i.e. nitrites or nitrates. We used mild reducing conditions to reduce only nitrites, the first oxidised product of NO, because we have established that this procedure minimises background noise and prevents interference from chemicals containing amino groups (Boric et al. 1999). In addition, it has been reported that nitrates contribute less than 10 % to total NO metabolites recovered in the interstitial side of human microcirculation (Clough, 1999), and it has also been established that nitrite levels reflect NOS activity in living tissues (Lauer et al. 2001). A 50 μl superfusate sample was injected into the reduction chamber of a NO analyser (Sievers 280), filled with 8 ml glacial acetic acid containing 100 mg potassium iodide (75 mm), to rapidly reduce nitrites to NO. A nitrogen stream carried the resulting NO gas to a cell in which the specific chemiluminescence generated by the NO vs. ozone reaction was detected by a photomultiplier. The threshold sensitivity of this device is ≈0.5 pmol. Calibration of the equipment was performed daily using standards of 10-1000 nm sodium nitrite. In each experiment, background buffer readings were obtained every 10 min from a T-connector placed in the inlet line immediately before the observation chamber. The net NO plus nitrite output released by the exposed tissue was calculated after subtracting background readings and is expressed as picomoles per minute.
Determination of eNOS translocation
Subcellular fractionation
The exposed cheek pouch area (80-100 mg of wet tissue) was quickly excised (≈15 s) at defined times and homogenised for 15 s with an Ultraturrax in 500 μl cold antiprotease-lysis buffer (1 μg ml−1 aprotinin, 1 mm benzamidine, 10 μg ml−1 leupeptin, 1 mm phenylmethylsulfonyl fluoride, 200 μg ml−1 soybean trypsin inhibitor, 5 mm EGTA, 100 mm Tris, pH 7.4). For a detailed analysis of eNOS subcellular distribution, the crude homogenate was separated into three fractions, corresponding to a heavy membrane/organelles fraction, a light membrane (microsomal) fraction and a cytosolic fraction. The crude homogenate was first cleared of keratinised epithelium debris by a 5 min centrifugation at 150 g. The pellet was discarded and the supernatant was considered as the starting material to assess the whole tissue sample. A 50 μl sample of this total homogenate was saved for protein analysis, and the rest was centrifuged at 10 000 g for 30 min at 4 °C. The resulting pellet corresponded to the heavy membrane/organelles fraction. The second supernatant was ultracentrifuged at 100 000 g for 90 min (4 °C) to obtain the microsomal (pellet) and cytosolic (supernatant) fractions. To perform a quantitative time course analysis of microsomal eNOS translocation, the tissue homogenate was fractionated in two steps, to recover just the microsomal fraction. In this case, the crude homogenate was centrifuged at 10 000 g for 30 min at 4 °C. The pellet, containing debris, heavy membranes and organelles, was discarded and the supernatant was ultracentrifuged at 100 000 g for 90 min to produce the microsomal (pellet) and cytosolic (supernatant) fractions, as described previously (Figueroa et al. 2001a). The nucleic acid content was determined spectrophotometrically by absorbance at 260 nm (A260).
Western blotting
Each pellet was resuspended in 100 μl of 100 mm Tris pH 7.4 containing 1 % SDS. Protein content was determined by the Bradford method. Samples from each resuspended or supernatant fraction were mixed 1:1 with Laemmli's buffer (0.5 m Tris-HCl, pH 6.8, 10 % v/v glycerol, 10 % w/v SDS, 5 % v/v 2-mercaptoethanol, 1 % w/v bromophenol blue) and separated by 7.5 % SDS-PAGE. Human endothelial cell lysate, from an aortic endothelium cultured cell line (Transduction Laboratories, Lexington, KY, USA), was used as positive control for both eNOS and Cav-1 (data not shown). Molecular mass (mol. mass) was estimated with pre-stained markers (Bio-Rad, Hercules, CA, USA). Proteins were electro-transferred onto a nitrocellulose membrane. The membrane was blocked overnight with 5 % non-fat milk in Tris pH 7.4 at 4 °C, and cut horizontally at a mol. mass of 70-80 kDa. Each section was incubated separately with anti-eNOS (high mol. mass) or anti-Cav-1 (low mol. mass) primary antibodies (1:2500), for 3 h at room temperature. This was followed by 1 h incubation with the appropriate conjugated secondary antibody and developed by a 15 min incubation with 0.01 % 3,3′-diaminobenzidine, 0.5 % H2O2 in the dark, or by chemiluminescence (SuperSignal, WestFemto, Pierce, Rockford, IL, USA). Additional Western blots were performed with similar procedures, using primary antibodies for β-COP and Na+,K+-ATPase (1:1000), to determine the presence of these protein markers of Golgi and plasma membranes, respectively. Western blots were scanned and analysed densitometrically using the NIH-image software.
Experimental protocols
The experimental protocols were designed to measure three factors: (1) ACh-induced vasodilatation and its inhibition by a NOS antagonist, (2) ACh-induced microvascular NO production and (3) ACh-induced translocation of microsomal eNOS. All experiments were initiated with a 30 min baseline collection period. All concentrations reported correspond to the final concentrations attained in the observation chamber.
NOS-dependent vasodilatation induced by ACh
We assessed the extent and time course of microvascular dilatation in response to 10 min applications of ACh (1-100 μm) or sodium nitroprusside (SNP, 0.1-10 μm). Local inhibition of NOS was accomplished by superfusion with 10 or 30 μmNG-nitro-l-arginine (l-NNA) for a period of 60-120 min. These l-NNA concentrations and the duration of treatment were chosen based on our previous work on baseline NO production in the hamster cheek pouch (Figueroa et al. 2001a).
In a first series of experiments, we assessed the magnitude and time course of changes in arteriolar and venular diameters and microvascular flow in response to 100 μm ACh. These measurements were repeated after 60 min of superfusion with 30 μml-NNA. Subsequently, we tested the reactivity of the tissue after prolonged l-NNA treatment with 10 μm SNP.
To test for the development of tachyphylaxis, we assessed the magnitude and time course of vasodilatation induced by three successive 100 μm ACh applications. Each stimulus was separated by a 60 min drug washout period. Vasodilatation was measured as the change in RVC.
The inhibitory efficacy of 10 μml-NNA, administered for different periods, was tested with 100 μm ACh challenges applied 60 or 120 min after administration of the NOS inhibitor. In addition, we verified the reactivity of the tissue following prolonged l-NNA treatment by applying 10 μm SNP after 120 min in the presence of the NOS inhibitor.
The concentration dependence of agonist-induced vasodilatation was tested in additional hamsters stimulated with lower concentrations of ACh (1-10 μm) and SNP (0.1-1 μm).
ACh-induced microvascular NO production
Based on the results of the experiments described in the preceding sections, four groups of hamsters were used to measure ACh-induced NO release under control conditions and during NOS inhibition. The basic protocol consisted of a 30 min baseline measurement, followed by a 10 min sideline infusion with 10 μm ACh (n = 5), 100 μm ACh (n = 6) or buffer (n = 5), and collection of the superfusate outflow for an additional 30 min. The influence of NOS blockade on NO production was tested by a 10 min challenge with 100 μm ACh during the continuous superfusion with 30 μml-NNA (n = 4). The application of NOS inhibitor was begun 60 min prior to ACh.
ACh-induced subcellular eNOS translocation
Sixty-three hamsters were prepared as for intravital microscopy, as pairs or in groups of three. After a 30 min equilibration period, the volume in the chamber (1.5 ml) was replaced with fresh buffer (control), or buffer containing 10 or 100 μm ACh (stimulated). Four groups of three animals each were processed after 3 min incubation for a complete three-compartment subcellular eNOS distribution analysis by chemiluminescence. Three separate groups of three hamsters each were used to test the effects of ACh on the distribution of β-COP and Na+,K+-ATPase, which may be used as markers for Golgi and plasma membrane, respectively. Cheek pouches from the remaining 42 hamsters were processed after 1.5, 3 or 6 min for quantitative Western blot analysis of eNOS and Cav-1 present in the microsomal fraction, developed by diaminobenzidine. Matched control and ACh-treated tissues were processed simultaneously. These stimulation periods were chosen based on the results of the experiments in the previous sections, to study eNOS distribution during development and maximal arteriolar dilatation, encompassing the up-slope and maximum NO production. The microsomal fraction was chosen because membrane-bound eNOS represents the activatable pool of the enzyme (Robinson et al. 1995; Garcia-Cardeña et al. 1997; Ju et al. 1997; Michel et al. 1997a,b; Rizzo et al. 1998).
Statistical analysis
Results are presented as means ± s.e.m. Two-way ANOVA, or Student's paired t test, with Dunnett's tables for multiple comparisons against a control (Dunnett, 1964), was used to assess significance of differences as a function of time within groups. Comparisons between groups were made using Student's unpaired t test or one-way ANOVA plus Newman-Keuls post hoc test. The level of statistical significance was set at P < 0.05.
Results
Microvascular flow and conductance
ACh-induced vasodilatation
In control conditions, microvascular conductance, as assessed by the RVC index, was constant (Fig. 1 and Fig. 2). Topical application of ACh induced a rapid and reversible vasodilator response. Typically, RVC rose steeply during the 10 min stimulation period, reached a peak around 2.5-5 min after the end of the drug application, and returned to baseline in an exponential-like fashion within 20-25 min (Fig. 1A and Fig. 2A).
Figure 1. Changes in microvessel diameter and relative vascular conductance (RVC) induced by acetylcholine (ACh), NG-nitro-l-arginine (l-NNA) and sodium nitroprusside (SNP).
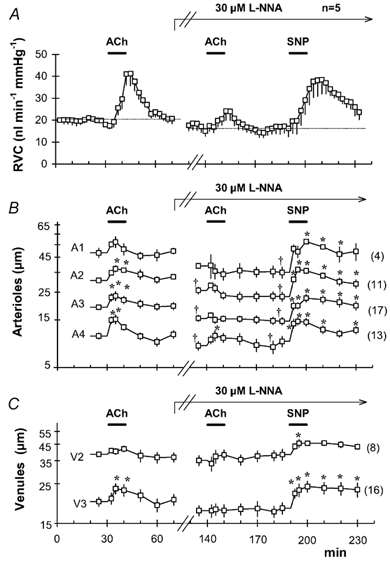
Time course of RVC (A), arteriolar (B) and venular (C) diameters measured simultaneously in 5 animals (mean ± s.e.m.). Microvessels from all 5 animals were grouped according to their branching order (arterioles A1-A4, venules V2-V3). The number of vessels in each category is shown in parentheses. After a 30 min baseline period, 100 μm ACh was applied topically for 10 min. Following 30 min of drug washout; superfusion with 30 μml-NNA was started and maintained for the remainder of the experiment (arrow). A second ACh application was administered after 60 min of nitric oxide synthase (NOS) inhibition with l-NNA. Finally, 10 μm SNP was applied topically after 120 min of NOS inhibition. Horizontal bars indicate the periods of superfusion with ACh or SNP. * Significant differences in vessel diameter as compared to the diameter immediately prior to each ACh or SNP application. † Significant difference between diameters at the moment just before the second ACh and SNP challenges, as compared to the initial control value (P < 0.05, paired t test, with Dunnett's tables for multiple comparisons against a single control).
Figure 2. Reproducibility of vasodilatation by successive applications of ACh and its inhibition by NOS blockade.

Variations in relative vascular conductance (RVC; mean ± s.e.m.) as a function of time in three experimental series. A, control response to successive ACh applications. ACh (100 μm) was applied topically for 10 min periods in the superfusion medium at indicated times (bars). B, time course of NOS inhibition and effect of SNP. After a 30 min control period, the cheek pouch was superfused with 10 μml-NNA for the remainder of the experiment (arrow). After 2 h of NOS inhibition, 10 μm SNP was applied topically (bar). The dotted line depicts the initial basal RVC level. C, time dependence of l-NNA treatment on ACh-induced vasodilatation. After 30 min in control conditions, the tissue was superfused continuously with 10 μml-NNA for 210 min. ACh (100 μm) was applied at 60 and 120 min after starting NOS inhibition.
In every case, arteriolar dilatation preceded the increase in microvascular flow. As shown in Fig. 1B, stimulation with 100 μm ACh caused significant diameter increase in A4, A3 and A2 arterioles. This vasodilatation lasted for the entire drug application period, attaining a maximum at 2.5-5 min. The magnitude of the vasodilator response was proportionally higher in smaller vessels: A4 > A3 > A2 > A1. Third-order venules also presented significant dilatation, albeit slightly delayed compared to arterioles (Fig. 1C). No significant changes were seen in larger, V2 venules. The diameter of all microvessels returned to baseline within 10 min of ACh washout (Fig. 1B and C).
The vasodilator response to successive 10 min applications of ACh was similar in magnitude and duration during a 3 h period (Fig. 2A), demonstrating the stability of the preparation and the absence of tachyphylaxis. A two-way ANOVA indicated that there was no significant change in baseline RVC before each stimulation (21.9 ± 6.2, 24.4 ± 5.1 and 27.2 ± 4.8 nl min−1 mmHg−1), or in the maximal RVC attained in the first, second and third ACh stimulation (50.2 ± 7.6, 51.6 ± 7.1 and 50.0 ± 10.7 nl min−1 mmHg−1, respectively). The corresponding net RVC increment above baseline, calculated as the area under the curve, was 185 ± 52, 153 ± 39 and 160 ± 47 nl mmHg−1, respectively; not significant).
Application of ACh at concentrations of 1-10 μm caused vasodilator responses with a similar time course but smaller amplitude than 100 μm ACh. Likewise, SNP caused concentration-related vasodilator responses with a similar time course. Figure 3A summarises the vasodilator action of three concentrations of ACh (1-100 μm) and SNP (0.1-10 μm). The maximal RVC increment was directly proportional to concentration for both agonists, but SNP was approximately one order of magnitude more effective than ACh in inducing vasodilatation.
Figure 3. Changes in microvascular flow as a function of agonist concentration.

A, vasodilator effect of ACh and SNP, as indicated by relative vascular conductance (RVC). Symbols denote mean ± s.e.m. of the maximal increase in RVC induced by a 10 min topical application of ACh or SNP in the hamster cheek pouch. The number of experiments is shown in parentheses. With each agonist, the maximal RVC increase changed significantly with concentration (P < 0.05, ANOVA). B, inhibition of vasodilatation as a function of the concentration of NOS inhibitor. Symbols denote the mean ± s.e.m. of maximal RVC increase induced by a 10 min application of 100 μm ACh or 10 μm SNP as a function of the concentration of NG -nitro-l-arginine (l-NNA). ACh was tested after 60 min superfusion with the NOS inhibitor. SNP was tested after 120 min of continuous application of the NOS inhibitor. The number of experiments is shown in parentheses. * Significant differences relative to agonist alone (P < 0.05, unpaired t test, with Dunnett's tables for multiple comparisons with a control).
NOS inhibition
Superfusion with l-NNA caused significant reductions in microvessel diameter, microvascular flow and the vasodilator capacity of ACh, yet the degree of the inhibition depended upon the concentration and duration of the application period (Figs 1-3).
After 60 min of superfusion with 30 μml-NNA, there was a significant reduction in A2, A3, and A4 arteriolar diameters (Fig. 1B). Application of 100 μm ACh, under these conditions, produced negligible changes in A1, A2, A3, V3 and V2 microvessels. However, A4 arterioles presented a moderate but significant vasodilator response (Fig. 1B), which preceded a correspondingly small RVC peak (Fig. 1A). After 100 min of treatment with 30 μml-NNA, all arteriolar diameters were significantly reduced compared to their initial control value. Administration of 10 μm SNP at this time caused significant dilatation of all microvessels and also increased RVC significantly (Fig. 1A-C).
Superfusion with 10 μml-NNA produced a progressive reduction in RVC, which levelled off at approximately 50 % of the baseline RVC after ≈75 min (Fig. 2B). Again, NOS inhibition did not impair microvascular responses to NO, as demonstrated by the robust vasodilatation induced by the administration of 10 μm SNP after 120 min of l-NNA treatment.
In order to draw a parallel between the reduction in baseline conductance and the ability of ACh to induce vasodilatation, we tested ACh responses at different times after starting superfusion with 10 μml-NNA. The vasodilator peak elicited by 100 μm ACh was reduced by 75-80 % after 60 min, and was abolished after 120 min of treatment with 10 μml-NNA (Fig. 2C).
The inhibitory efficacy of the two concentrations of l-NNA on ACh-induced vasodilatation is summarised in Fig. 3B. After 60 min of NOS inhibition, the residual ACh-induced vasodilatation was smaller with 30 μml-NNA than with 10 μml-NNA. In contrast, neither concentration of l-NNA affected the vasodilatation induced by an equipotent concentration of SNP.
ACh-induced NO production
Basal cheek pouch NO release ranged between 30 and 70 pmol min−1, with an average of 54.4 ± 5.2 pmol min−1 (n = 16; Fig. 4). Application of buffer vehicle did not change NO production. In contrast, superfusion with 10 or 100 μm ACh for 10 min produced a concentration-related rapid rise in NO release (Fig. 4). The ACh-induced NO peak reached a maximum at 2.5-5 min during the drug application period, and returned to control levels 10 min after the end of the stimulus. This time course of NO release resembles the time course of arteriolar vasodilatation (compare Fig. 1B and Fig. 4). The total NO release above baseline, calculated as the area under the curve, induced by 100 μm ACh (350 ± 124 pmol) was significantly larger than that induced by 10 μm ACh (154 ± 54 pmol, P < 0.05, unpaired t test).
Figure 4. ACh-induced microvascular nitric oxide (NO) production.

Time course of microvascular NO release as a function of ACh concentration (mean ± s.e.m.). After 30 min of baseline data collection, ACh or buffer (vehicle) was applied topically for 10 min (bar). NO release showed significant differences as a function of time in tissues treated with 10 μm ACh (F(15,60) = 2.08, P < 0.024) and 100 μm ACh (F(15,75) = 4.05, P < 0.0001), but not in controls (F(15,60) = 1.53, P < 0.123). Differences assessed by two-way ANOVA.
The administration of 30 μml-NNA abolished basal NO release with a time course entirely consistent with reductions in RVC and vessel diameters, confirming previous reports (Figueroa et al. 2001a). After 30 min of l-NNA treatment, NO release was 19.0 ± 6.6 pmol min−1; it dropped to 8.3 ± 3.4 pmol min−1 after 45 min of treatment with l-NNA, and fell bellow the lower detection limit for the assay thereafter (F(15,45) = 2.69, P < 0.006). In these conditions, application of 100 μm ACh did not cause any detectable increment in NO production (data not shown).
Translocation of eNOS
According to our homogenisation and fractionation procedure, cell nuclei precipitated in the first 5 min centrifugation at 150 g, along with tissue debris. This observation was corroborated by spectrophotometric analysis at A260, which demonstrated the presence of ≈85 % of total nucleic acid content in this fraction, while the remaining ≈15 % was found in the cytosolic fraction. Therefore, nuclei were not included in our subcellular fractionation analysis. The 10 000 g precipitate was enriched in β-COP, and was devoid of Na+,K+-ATPase, indicating that it corresponded to heavy membranes, including those of the Golgi apparatus (Fig. 5B). Mitochondria and peroxisomes may have been included in this fraction (not determined). The microsomal fraction was enriched in Na+,K+-ATPase, indicating that it corresponded mostly to plasma membrane (Fig. 5B). The microsomal fraction presented a weak signal for β-COP. Finally, the cytosolic fraction was devoid of Na+,K+-ATPase or β-COP, attesting to the purity of this fraction (data not shown).
Figure 5. Effect of ACh on subcellular endothelial NOS (eNOS) distribution.

Hamster cheek pouches were superfused with buffer (control) or with 10 μm or 100 μm ACh for 3 min, homogenised and submitted to subcellular fractionation. Western blots were revealed by chemiluminescence to detect eNOS, Na+,K+-ATPase, or anti-coat protein beta (β-COP) content in the microsomal fraction (M), cytosolic fraction (C), heavy membrane/Golgi-enriched fraction (G) and total homogenate (T). A, distribution of eNOS in a representative series of blots obtained from one out of four similarly treated groups. Protein load per lane: M, 30 μg; C, 140 μg; G, 30 μg; T, 80 μg. ACh-treated tissues showed a marked decrease in microsomal eNOS content and an equivalent increase in the Golgi-enriched fraction. B, distribution of Na+,K+-ATPase and β-COP in one out of three similarly treated hamster cheek pouches. Protein load per lane: M, 40 μg; G, 40 μg. Application of ACh failed to affect the distribution of these proteins, which are markers for plasma (Na+,K+-ATPase) and Golgi (β-COP) membranes.
In control tissues, eNOS was found mainly in the microsomal compartment, but it was also detectable in the cytosolic and heavy membrane/Golgi-enriched fractions (Fig. 5A). A clear redistribution of eNOS among different subcellular fractions was observed after 3 min of treatment with ACh. This sampling time was chosen because it corresponded with the moment of maximal vasodilatation. After a 3 min application of 10 μm or 100 μm ACh, eNOS content in the heavy membrane/Golgi-enriched fraction increased, while the amount of the enzyme in the microsomal fraction was reduced to a similar extent (Fig. 5A). With 100 μm, but not with 10 μm ACh, a distinguishable increase in the cytosolic eNOS content was also seen. Total eNOS content in the cheek pouch homogenate was similar between control and ACh-treated hamsters (n = 4 each). ACh treatment did not affect the distribution of Na+,K+-ATPase or β-COP, the protein markers for plasma and Golgi membranes, respectively (Fig. 5B).
To further characterise the time course of ACh-induced eNOS translocation, we performed a quantitative analysis of microsomal enzyme content at 1.5, 3 and 6 min of treatment. Because eNOS is bound to caveolae, we also measured Cav-1 as a control for this membrane domain. Figure 6 shows representative Western blots and the corresponding densitometric intensity for eNOS and Cav-1 at the time points studied. Treatment with 10 μm and 100 μm ACh reduced microsomal eNOS content in a rapid and dose-related way (Fig. 6, top). As compared to buffer-treated controls, at 1.5, 3 and 6 min, 100 μm ACh reduced membrane-bound eNOS by -37.6 ± 8.9 % (P < 0.01), -60.8 ± 16.4 % (P < 0.01) and -39.6 ± 18.2 % (P < 0.05), respectively. With 10 μm ACh, microsomal eNOS content was reduced at 1.5 min by -13.1 ± 3.7 % (P < 0.05) and at 3 min by -60.0 ± 3.7 % (P < 0.01), whereas at 6 min the reduction was not significant (-19.1 ± 16.8 %). In contrast, application of either 10 μm or 100 μm ACh did not change microsomal Cav-1 content at any of the time points studied (Fig. 6, bottom), despite the evident eNOS translocation.
Figure 6. Effect of acetylcholine (ACh) on microsomal eNOS and caveolin-1 (Cav-1) content.

Hamster cheek pouches were treated with buffer (Control) or 10 μm or 100 μm ACh, during a period of 1.5, 3 or 6 min, and then homogenised and prepared for Western blot analysis of the microsomal fraction. Top, time course of ACh-induced microsomal eNOS translocation. The boxes show Western blots of representative pools of each experimental group (80 μg total protein per lane). Pools were prepared using equal-protein aliquots of every individual tissue in each group. The underlying bar graph shows the corresponding densitometric image analysis of band intensities of individual hamsters (mean ± s.e.m.). Bottom, the same pools and individual tissue samples were analysed by Western blot and densitometric analysis for Cav-1. The number of animals in each group is shown inside the bars in the lower panel. Western blots were revealed with diaminobenzidine. * P < 0.05vs. control; † P < 0.01vs. control and vs. 10 μm ACh (ANOVA, Newman-Keuls test).
Discussion
Our work demonstrates a good correlation in the in vivo microcirculation between vasodilatation, blood flow, NO production, and the translocation of eNOS in response to ACh. The temporal changes in subcellular eNOS redistribution, NO production, arteriolar dilatation and blood flow are consistent with a cause and effect relationship, in which eNOS translocation from the cell membrane facilitates NO production. Our work constitutes the first direct evidence of dynamic regulation of eNOS activity associated with enzyme translocation from the membrane in the context of functional regulation of microvascular flow in vivo.
NO measurements
We have demonstrated the feasibility of continuously measuring microvascular NO production in the hamster cheek pouch by analysing superfusate chemiluminescence (Durán et al. 2000; Figueroa et al. 2001a). At baseline, the tissue locally produces more than 90 % of the NO recovered, exceeding by far the amount brought to the exchange area by the blood supply. In addition, the abolishment of measurable NO by l-NNA confirms the enzymatic origin of the NO signal, ruling out the possibility of artefact readings (Figueroa et al. 2001a). Our measurements reflect global cheek pouch NO production; detection of the exact regional or cellular origin of NO was beyond the scope of our experimental design. Based on studies in endothelium-denuded microvessels (Boric et al. 1999; Figueroa et al. 2001b), it is reasonable to assume that most microvascular NO comes from endothelial cells as a product of eNOS activity. In addition, in the hamster cheek pouch, eNOS is detected only in the endothelial lining of arterioles and venules (Durán et al. 2000; Figueroa et al. 2001a); other NOS isoforms are not detected in the endothelium (Segal et al. 1999). However, we cannot reject a possible contribution from other sources, such as the neuronal NOS (nNOS) that has been detected in microvascular smooth muscle cells of the same microvessels in which eNOS was localised to endothelium in the hamster cheek pouch (Segal et al. 1999).
Our results confirm that the resting hamster cheek pouch microcirculation is endowed with an important baseline NO dilatory tone, as evidenced by the comparison between arteriolar diameters and RVC before and after l-NNA treatment (Fig. 1 and Fig. 2). A pharmacokinetic barrier to l-NNA action is also confirmed by the delay in attaining a new steady-state blood flow after application of the NOS inhibitor (Figueroa et al. 2001a).
ACh-induced, eNOS-mediated vasodilatation
Arteriolar and venular dilatation was clearly seen after 2.5 min of ACh treatment, attaining a maximum at ≈5 min and fading in the next subsequent 5-10 min (Fig. 1B and C). The RVC increase was delayed and prolonged in time compared to changes in vessel diameter (Fig. 1A). This delay is due mainly to the accumulation and diffusion of the tracer in the interstitium and observation chamber compartments, which retards its appearance in the superfusate output by approximately one sampling period (Figueroa et al. 2001a). Blockade of eNOS with l-NNA reduced the vasodilator response to ACh and the RVC peak in a dose- and time-dependent manner (Figs 1-3). The slow reduction in basal RVC induced by 10 μml-NNA was associated with a parallel decrease in ACh responsiveness (Fig. 2B and C). These results agree with the well-established NO dependence of ACh-induced vasodilatation.
It is worth noting that our data also demonstrate a dose-response relationship between ACh and NO release in vivo (Fig. 4). By plotting the changes in RVC as a function of the amount of NO generated by known concentrations of ACh, in control and l-NNA-treated tissues, one obtains an increase in microvascular vasodilatation that is directly related to the amount of NO released (Fig. 7). Furthermore, a temporal sequence of NO production, vasodilatation and an increase in flow can be deduced by comparing their respective time course responses (Fig. 1 and Fig. 4). NO release rose within 2.5 min of the application of ACh, reached a peak by 5 min and remained above baseline for an additional ≈10 min (Fig. 4). The NO response clearly precedes the RVC peak, which attained a maximum during the first washout sample (Fig. 1 and Fig. 2). This finding rules out the possibility that NO was produced in the vessel wall secondary to increments in shear stress (Kuo et al. 1991; Ungvari et al. 2001). NO and RVC responses are directly comparable because NO-nitrite and the sodium tracer share the interstitial and superfusate pathways and reach the superfusate with similar transit times (Figueroa et al. 2001a). The idea of a cause and effect relationship between NO release and vasodilatation is reinforced by the observation that the rise in NO production coincides with instantly measured vessel dilatation (Figs 1B and C, and 4). The decay in NO release persisted longer, due to NO-nitrite diffusion, as discussed earlier. Altogether, these data constitute the first evidence that elevations in endogenous NO dilate the microvessels of the hamster cheek pouch in a dose-related manner, and extend previous data showing a temporal and concentration dependence between ACh-induced NO release and relaxation in isolated small arteries (Simonsen et al. 1999).
Figure 7. Correlation between ACh-induced changes in relative vascular conductance (RVC) and ACh-generated NO.

▪, 10 μm ACh (n = 5 for NO, n = 7 for RVC); •, 100 μm ACh (n = 6 for NO, n = 15 for RVC); □, 100 μm ACh plus 30 μml-NNA (n = 4 for NO, n = 5 for RVC). Mean ± s.e.m.
Because ACh does not induce relaxation in endothelium-denuded arteries (Furchgott & Zawadzki, 1980; Simonsen et al. 1999) and microvessels (Sun et al. 1994; Dornyei et al. 1997), and, to the best of our knowledge, it lacks the ability to produce NO directly in vascular smooth muscle, it is reasonable to interpret ACh-induced NO production as the result of eNOS stimulation. In control conditions, we detected significant dilatation of A4, A3 and A2 arterioles, and V3 venules, in response to 100 μm ACh. Venular dilatation is likely to correspond to an active phenomenon, since we have determined the presence of eNOS in arteriolar and venular endothelium in the cheek pouch (Durán et al. 2000; Figueroa et al. 2001a), and it has been reported that both arterioles and venules have the capacity to generate NO in response to physiological stimuli (Bohlen, 1998).
After 60 min of NOS blockade with 30 μml-NNA, ACh induced a small rise in RVC that was preceded by a significant dilatation of A4 arterioles (Fig. 1). However, in these conditions, NO release is practically abrogated. Therefore, this remnant vasodilatation observed in small terminal arterioles could be produced by another endothelium-derived factor. There is growing evidence that the dilatory effect of ACh is mediated not only by NO release, but also by endothelium-derived hyperpolarising signals (EDHF) (Feletou & Vanhoutte, 1999). Heterogeneous vasodilator mechanisms that are activated in response to ACh have been described in large arterioles (150-250 μm) isolated from different tissues of the hamster (Clark & Fuchs, 1997). In this context, our results indicate that the predominant mediator of relaxation in response to ACh in the cheek pouch is NO.
Translocation of eNOS
To study the occurrence and functional meaning of eNOS translocation, we performed a detailed subcellular analysis of eNOS distribution after 3 min of ACh application. We chose this sampling time because it was the moment when the majority of vessels attained maximal vasodilatation with either 100 μm ACh (Fig. 1B and C) or 10 μm ACh (not shown). Our results indicate that ACh stimulation causes eNOS translocation from the microsomal compartment to the Golgi and to the cytosolic compartments in vivo (Fig. 5). Total eNOS content did not change, attesting to the consistency of tissue homogenisation and sample handling.
We have previously shown that in the resting hamster cheek pouch in vivo, eNOS is distributed between the microsomal and cytosolic compartments in a roughly 2:1 proportion (Figueroa et al. 2001a). In this work, we also detected a sizeable amount of eNOS in the 10 000 g precipitate. According to the distribution of conventional protein markers, this membranous fraction does not contain detectable amounts of plasma membrane (Na+,K+-ATPase), whereas it is enriched in Golgi membrane (β-COP; Fig. 5B). Our present results agree with reports of eNOS distribution in both the plasma membrane and Golgi apparatus in cultured endothelial cells (Liu et al. 1997; Sowa et al. 1999). Moreover, confocal and electron microscope examination of vascular endothelial cells fixed in situ, has revealed that in vivo, eNOS is distributed mainly between a peripheral band-like structure and a perinuclear domain, identified as the Golgi complex (O'Brien et al. 1995; Andries et al. 1998).
The most conspicuous change induced by ACh was a reduction in the microsomal eNOS content; however, there was no equivalent increase in cytosolic eNOS that could account for the changes in membrane-bound enzyme. A minor but consistent increase in cytosolic eNOS was detected with 100 μm ACh. Interestingly, an increment of eNOS content in the Golgi-enriched fraction was observed with both ACh concentrations. Because total protein content in the microsomal and this heavy membrane fraction was similar, the opposite and symmetrical changes in eNOS concentration observed in these two compartments mainly account for the ACh-induced redistribution of this enzyme.
These results support and expand previous reports assessing eNOS translocation in cultured endothelial cells after agonist stimulation. When using fractionation or extraction techniques, increases in eNOS have been detected in the nuclear fraction or in a detergent-insoluble (cytoskeletal) fraction (Venema et al. 1996; Wang et al. 1997; Feng et al. 1999). By confocal microscopy, eNOS has been observed to translocate from the plasma membrane to a perinuclear domain (Prabhakar et al. 1998; Goetz et al. 1999). At present we cannot assess the exact destination of eNOS in the heavy membrane fraction in the hamster microcirculation. While we did not find cell nuclei in the 10 000 g precipitate (A260 readings), it is possible that mitochondria and other small organelles, such as peroxisomes, are also present in this fraction. However, there are no indications in the literature that eNOS is located in these organelles. Therefore, based on our results with markers for Golgi apparatus and plasma membrane, we favour the perinuclear Golgi complex domain as the most probable target for eNOS translocation in our study, in agreement with earlier studies (O'Brien et al. 1995; Liu et al. 1997; Andries et al. 1998; Sowa et al. 1999).
To understand further the significance of eNOS translocation in relation to the enzyme activation induced by ACh, we analysed three time points corresponding to the up-slope and established NO production (Fig. 4 and Fig. 6). We focused our quantitative analysis just on the microsomal fraction because membrane-bound eNOS represents the activatable pool of the enzyme (Robinson et al. 1995; Garcia-Cardeña et al. 1997; Ju et al. 1997; Michel et al. 1997a,b; Venema et al. 1997; Rizzo et al. 1998) and, as shown in Fig. 5A, changes in this compartment summarise variations in the other compartments. The absence of changes in microsomal Cav-1 content attests to a similar load of plasma membrane proteins in control and ACh-treated tissue samples. We found a significant reduction in microsomal eNOS content as early as 1.5 min after ACh application, and maximal enzyme translocation after 3 min of treatment (Fig. 6). This rapid eNOS translocation was observed when NO release was rising with either of the ACh concentrations used (Fig. 4). In addition, the magnitude of eNOS translocation was proportional to the concentration of ACh applied, because at 1.5 min, 100 μm ACh caused a larger degree of translocation than 10 μm ACh and, at 6 min, only 100 μm ACh was effective (Fig. 6). It is interesting to note that at 6 min of 100 μm ACh application, NO release was still rising, whereas with 10 μm ACh, NO production peaked earlier (Fig. 4). The current results strongly suggest that ACh-induced reduction in microsomal eNOS is a transient effect, although we cannot define exactly the time at which the enzyme fully returns to its baseline subcellular location after the 100 μm ACh challenge, because we limited the duration of our study to 6 min. The apparent difference between transient eNOS translocation (Fig. 6) with a more prolonged NO release (Fig. 4) can be explained by the delay between NO production and NO-nitrite collection in the superfusate, as discussed earlier. The time correspondence between the magnitude of eNOS translocation and arteriolar vasodilatation (Fig. 6 and Fig. 1B, respectively) reinforces the concept of enzyme activation, because both variables were measured instantly and are directly comparable. In general, the ACh-stimulated reduction in microsomal eNOS content, which is time-related to arteriolar dilatation, and is closely associated with an increase in NO production and blood flow (Figs 1, 4 and 6), is consistent with the idea that the translocation of eNOS is part of a mechanism that activates this enzyme.
A transient ACh-induced eNOS activation and translocation could be expected given previous reports that agonist-induced NO production is essentially transitory (Malinski & Taha, 1992; Blatter et al. 1995; Cohen et al. 1997). Our study neither rejects nor contradicts the existence of other eNOS activation mechanisms, such as Akt-mediated eNOS phosphorylation (Dimmeler et al. 1999; Fulton et al. 1999), which may possibly be acting either in parallel, or in a sequential way after agonist stimulation.
In general, our in vivo data agree with results obtained by others in cultured endothelial cells describing a reversible transit cycle for eNOS during prolonged bradykinin or oestradiol stimulation. It has been observed that eNOS translocation started at 1 min, attained a maximum at ≈5 min, remained at that maximum between 5 and 20 min, and receded thereafter (Michel et al. 1993; Wang et al. 1997; Prabhakar et al. 1998; Goetz et al. 1999). We may assume that differences in the source, stimulus, and physiological state of cultured endothelial cells as compared to our in vivo conditions may account for the slight discrepancies in the time course of the eNOS translocation response. The previously reported data have often been interpreted as evidence that translocation corresponds to an inactivation mechanism for eNOS, based on comparisons with NO measurements obtained in aortic endothelial cells, showing that NO synthesis starts to decrease after 10 min exposure to a bradykinin pulse (Malinski & Taha, 1992). However, in these previous studies, the time course of eNOS translocation was not determined in concert with NO production in the same preparation. On the other hand, our combined translocation and NO production data obtained in vivo are consistent with the participation of eNOS traffic in the activation mechanism of the enzyme. Interestingly, by using a specific intracellular NO-reporting dye in endothelial cells stimulated with vascular endothelial growth factor, it has been shown recently that eNOS, localised either in the perinuclear Golgi region or plasma membrane, is able to synthesise NO (Fulton et al. 2002).
The reduction in microsomal eNOS content in the absence of changes in Cav-1 content is consistent with the idea that activation of the enzyme implies eNOS translocation after its dissociation from Cav-1. This notion considers that eNOS is mainly associated with Cav-1 in the membrane and that this protein exerts an inhibitory role on eNOS activity (Garcia-Cardeña et al. 1997; Ju et al. 1997; Michel et al. 1997a,b). In support of this interpretation, carbachol-induced eNOS release from Cav-1 has been observed in cultured cells (Feron et al. 1998). The association of both proteins in the microcirculation is supported by the report that eNOS and Cav-1 are co-localised in the endothelium of hamster microvessels (Segal et al. 1999).
In conclusion, our in vivo data support the idea that eNOS translocation away from the cell membrane, and most likely its dissociation from Cav-1, participates in the ‘on’ signal for NO release and vasodilatation in vivo. What cellular pathways are involved in eNOS redistribution, and how this process connects with other activation signals for NO production, such as eNOS interaction with calcium- calmodulin (Garcia-Cardeña et al. 1997; Michel et al. 1997b; Rizzo et al. 1998) and/or phosphorylation (Michel et al. 1993; Dimmeler et al. 1999; Fulton et al. 1999, 2002; Durán et al. 2000), remain as interesting open questions for future studies.
Acknowledgments
The authors wish to give special thanks to Ms Sara Ayala for her dedicated technical assistance. This research was supported by grants No. 1971222 and No. 2990079, and Líneas Complementarias No. 8990008 from FONDECYT and USPHS NHLBI Grant 1RO1 HL 70634-01.
References
- Andries LJ, Brutsaert DL, Sys SU. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circulation Research. 1998;82:195–203. doi: 10.1161/01.res.82.2.195. [DOI] [PubMed] [Google Scholar]
- Archer S. Measurement of nitric oxide in biological models. FASEB Journal. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- Blatter LA, Taha Z, Mesaros S, Shacklock PS, Wier WG, Malinski T. Simultaneous measurements of Ca2+ and nitric oxide in bradykinin-stimulated vascular endothelial cells. Circulation Research. 1995;76:922–924. doi: 10.1161/01.res.76.5.922. [DOI] [PubMed] [Google Scholar]
- Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. American Journal of Physiology. 1998;275:H542–550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- Boric MP, Donoso V, Fournier A, St Pierre S, Huidobro-Toro JP. Endothelin reduces microvascular blood flow by acting on arterioles and venules of the hamster cheek pouch. European Journal of Pharmacology. 1990;190:123–133. doi: 10.1016/0014-2999(90)94119-i. [DOI] [PubMed] [Google Scholar]
- Boric MP, Figueroa XF, Donoso MV, Paredes A, Poblete I, Huidobro-Toro JP. Rise in endothelium-derived NO after stimulation of rat perivascular sympathetic mesenteric nerves. American Journal of Physiology. 1999;277:H1027–1035. doi: 10.1152/ajpheart.1999.277.3.H1027. [DOI] [PubMed] [Google Scholar]
- Boric MP, Martinez A, Donoso MV, Huidobro-Toro JP. Neuropeptide Y is a vasoconstrictor and adrenergic modulator in the hamster microcirculation by acting on neuropeptide Y1 and Y2 receptors. European Journal of Pharmacology. 1995;294:391–401. doi: 10.1016/0014-2999(95)00556-0. [DOI] [PubMed] [Google Scholar]
- Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. Journal of Biological Chemistry. 1993;268:8410–8413. [PubMed] [Google Scholar]
- Clark SG, Fuchs LC. Role of nitric oxide and Ca++-dependent K+ channels in mediating heterogeneous microvascular responses to acetylcholine in different vascular beds. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1473–1479. [PubMed] [Google Scholar]
- Clough GF. Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. Journal of Physiology. 1999;516:549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Plane F, Najibi S, Huk I, Malinski T, Garland CJ. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proceedings of the National Academy of Sciences of the USA. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dornyei G, Kaley G. Release of nitric oxide and prostaglandin H2 to acetylcholine in skeletal muscle venules. American Journal of Physiology. 1997;272:H2541–2546. doi: 10.1152/ajpheart.1997.272.6.H2541. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Dunnett C. New tables for multiple comparisons with a control. Biometrics. 1964;22:482–491. [Google Scholar]
- Durán WN, Seyama A, Yoshimura K, Gonzalez DR, Jara PI, Figueroa XF, Boric MP. Stimulation of NO production and of eNOS phosphorylation in the microcirculation in vivo. Microvascular Research. 2000;60:104–111. doi: 10.1006/mvre.2000.2250. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. The alternative: EDHF. Journal of Molecular and Cellular Cardiology. 1999;31:15–22. doi: 10.1006/jmcc.1998.0840. [DOI] [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochemical and Biophysical Research Communications. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. Journal of Biological Chemistry. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. Journal of Biological Chemistry. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Martinez AD, Gonzalez DR, Jara PI, Ayala S, Boric MP. In vivo assessment of microvascular nitric oxide production and its relation with blood flow. American Journal of Physiology - Heart and Circulatory Physiology. 2001a;280:H1222–1231. doi: 10.1152/ajpheart.2001.280.3.H1222. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Poblete MI, Boric MP, Mendizabal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. British Journal of Pharmacology. 2001b;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, Michell B, Kemp BE, Rodman D, Sessa WC. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. Journal of Biological Chemistry. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS). and caveolin. Functional significance of the NOS caveolin binding domain in vivo. Journal of Biological Chemistry. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the USA. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Chang B, Kerwin JF, Jr, Huang ZJ, Murad F. N omega-nitro-l-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. European Journal of Pharmacology. 1990;176:219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. Journal of Biological Chemistry. 1997;272:18522–18225. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kanai AJ, Strauss HC, Truskey GA, Crews AL, Grunfeld S, Malinski T. Shear stress induces ATP-independent transient nitric oxide release from vascular endothelial cells, measured directly with a porphyrinic microsensor. Circulation Research. 1995;77:284–293. doi: 10.1161/01.res.77.2.284. [DOI] [PubMed] [Google Scholar]
- Kawanaka H, Jones MK, Szabo IL, Baatar D, Pai R, Tsugawa K, Sugimachi K, Sarfeh IJ, Tarnawski AS. Activation of eNOS in rat portal hypertensive gastric mucosa is mediated by TNF-alpha via the PI 3-kinase-Akt signaling pathway. Hepatology. 2002;35:393–402. doi: 10.1053/jhep.2002.30958. [DOI] [PubMed] [Google Scholar]
- Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. American Journal of Physiology. 1991;260:H862–868. doi: 10.1152/ajpheart.1991.260.3.H862. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. American Journal of Physiology. 1991;261:H1706–1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proceedings of the National Academy of Sciences of the USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. Journal of Cell Biology. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. Journal of Biological Chemistry. 1997a;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. Journal of Biological Chemistry. 1997b;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the USA. 1993;90:6252–6256. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- O'brien AJ, Young HM, Povey JM, Furness JB. Nitric oxide synthase is localized predominantly in the Golgi apparatus and cytoplasmic vesicles of vascular endothelial cells. Histochemistry and Cell Biology. 1995;103:221–225. doi: 10.1007/BF01454027. [DOI] [PubMed] [Google Scholar]
- Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. Journal of Biological Chemistry. 1998;273:27383–27388. doi: 10.1074/jbc.273.42.27383. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. Journal of Biological Chemistry. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. Journal of Biological Chemistry. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Busconi L, Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. Journal of Biological Chemistry. 1995;270:995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Michel T. Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proceedings of the National Academy of Sciences of the USA. 1995;92:11776–11780. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. American Journal of Physiology. 1999;277:H1167–1177. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. Journal of Physiology. 1999;516:271–282. doi: 10.1111/j.1469-7793.1999.271aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa G, Liu J, Papapetropoulos A, Rex-Haffner M, Hughes TE, Sessa WC. Trafficking of endothelial nitric-oxide synthase in living cells. Quantitative evidence supporting the role of palmitoylation as a kinetic trapping mechanism limiting membrane diffusion. Journal of Biological Chemistry. 1999;274:22524–22531. doi: 10.1074/jbc.274.32.22524. [DOI] [PubMed] [Google Scholar]
- Sun D, Kaley G. Characteristics and origin of myogenic response in isolated gracilis muscle arterioles. American Journal of Physiology. 1994;266:H1177–1183. doi: 10.1152/ajpheart.1994.266.3.H1177. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Rucker-Martin C, Frelin C. Cardiac capillary cells release biologically active nitric oxide at an early stage of in vitro development. Cardiovascular Research. 2000;47:726–737. doi: 10.1016/s0008-6363(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Sun D, Huang A, Kaley G, Koller A. Role of endothelial [Ca2+]i in activation of eNOS in pressurized arterioles by agonists and wall shear stress. American Journal of Physiology - Heart and Circulatory Physiology. 2001;281:H606–612. doi: 10.1152/ajpheart.2001.281.2.H606. [DOI] [PubMed] [Google Scholar]
- Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochemical and Biophysical Research Communications. 1996;226:703–710. doi: 10.1006/bbrc.1996.1417. [DOI] [PubMed] [Google Scholar]
- Venema VJ, Zou R, Ju H, Marrero MB, Venema RC. Caveolin-1 detergent solubility and association with endothelial nitric oxide synthase is modulated by tyrosine phosphorylation. Biochemical and Biophysical Research Communications. 1997;236:155–161. doi: 10.1006/bbrc.1997.6921. [DOI] [PubMed] [Google Scholar]
- Wang Q, Patton WF, Hechtman HB, Shepro D. A novel anti-inflammatory peptide inhibits endothelial cell cytoskeletal rearrangement, nitric oxide synthase translocation, and paracellular permeability increases. Journal of Cellular Physiology. 1997;172:171–182. doi: 10.1002/(SICI)1097-4652(199708)172:2<171::AID-JCP4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yeh DC, Duncan JA, Yamashita S, Michel T. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca(2+)-calmodulin. Journal of Biological Chemistry. 1999;274:33148–33154. doi: 10.1074/jbc.274.46.33148. [DOI] [PubMed] [Google Scholar]


