Abstract
Paired transcranial magnetic stimulation has greatly advanced our understanding of the mechanisms which control excitability in human motor cortex. While it is clear that paired-pulse excitability depends on the exact interstimulus interval (ISI) between the first (S1) and second stimulus (S2), relatively little is known about the effects of the intensities of S1 and S2, and the effects of manipulating neurotransmission through the GABAA receptor. When recording the motor evoked potential (MEP) from the resting abductor digiti minimi (ADM) muscle, using a fixed ISI of 1.5 ms, and expressing the interaction between S1 and S2 as MEPS1+S2/(MEPS1 + MEPS2), then a systematic variation of the intensities of S1 and S2 revealed short-interval intracortical facilitation (SICF) if S1 and S2 were approximately equal to MEP threshold (RMT), or if S1 > RMT and S2 < RMT. In contrast, short-interval intracortical inhibition (SICI) occurred if S1 < RMT and S2 > RMT. Contraction of the ADM left SICI unchanged but reduced SICF. The GABAA receptor agonist diazepam increased SICI and reduced SICF in the resting ADM while diazepam had no effect during ADM contraction. Surface EMG and single motor unit recordings revealed that during ADM contraction SICI onset was at the I3-wave latency of S2, whereas SICF typically ‘jumped up’ by one I-wave and started with the I2-wave latency of S2. Findings suggest that SICI is mediated through a low-threshold GABAA receptor-dependent inhibitory pathway and summation of IPSP from S1 and EPSP from S2 at the corticospinal neurone. In contrast, SICF originates through non-synaptic facilitation at the initial axon segment of interneurones along a high-threshold excitatory pathway.
Short-interval paired-pulse transcranial magnetic stimulation (TMS) in humans has been used to explore the excitability of various inhibitory (Kujirai et al. 1993; Di Lazzaro et al. 1998b; Hanajima et al. 1998; Fisher et al. 2002) and excitatory (Tokimura et al. 1996; Ziemann et al. 1996c, 1998; Di Lazzaro et al. 1999b; Hanajima et al. 2002) neuronal circuits at the motor cortical level. Most previous studies employed fixed intensities of the first (S1) and second stimulus (S2) within a given protocol. If S1 is below threshold for a motor evoked potential (MEP) in the target muscle and S2 is clearly above the MEP threshold, then the interaction between S1 and S2 is inhibitory at very short interstimulus intervals (ISI) of 1–5 ms (Kujirai et al. 1993; Fisher et al. 2002). However, if S1 and S2 are close to the MEP threshold (Tokimura et al. 1996) or S1 is clearly above the MEP threshold and S2 is below or around the MEP threshold (Ziemann et al. 1998; Hanajima et al. 2002), then MEP facilitation occurs at discrete ISIs of about 1-1.5, 2.5-3.0 and 4.0-4.5 ms. Cervical epidural recordings of the descending corticospinal volley provided strong evidence that all these interactions occur at the level of the motor cortex (Di Lazzaro et al. 1998b, 1999b). The exact mechanisms, however, are not fully understood. It is thought that short-interval intracortical inhibition (SICI) reflects inhibition mediated by GABAA receptors (Kujirai et al. 1993). Most probably, the sub-threshold S1 produces IPSP at the cortico-spinal neurones that lead to a reduced number of action potentials by the subsequent suprathreshold S2. In contrast, it is thought that short-interval intracortical facilitation (SICF) reflects direct excitation of axon initial segments of excitatory intracortical interneurons by S2, which had been depolarised and therefore made hyperexcitable by the preceding S1 (Hanajima et al. 2002). This suggests that the physiology underlying the interaction between S1 and S2 may be rather complex. Previous studies demonstrated that the exact ISI between S1 and S2 determines what kind of interaction occurs (Kujirai et al. 1993; Tokimura et al. 1996; Ziemann et al. 1998; Fisher et al. 2002). In contrast, the effects of S1 and S2 intensity have not yet been systematically explored. It was noted in preliminary experiments, for instance, that SICI at an ISI of 3 ms is maximal if S1 was approximately 80 % of resting motor threshold (RMT) and S2 clearly above RMT (Kujirai et al. 1993). If S1 was increased above RMT then SICI turned into SICF (Kujirai et al. 1993). Another paired-pulse TMS study showed, by using an ISI of 1.2 ms and a threshold-hunting protocol, that the interaction between S1 and S2 was inhibitory if S1 was < 65 % RMT, but facilitatory if S1 exceeded 65 % RMT (Awiszus et al. 1999). The aim of this study was to test in greater detail the effects of S1 and S2 intensity on the interaction between S1 and S2 at short ISIs of less than or equal to 5 ms. Most experiments were performed at an ISI of 1.5 ms and with the ADM at rest. In order to explore the physiology of the interaction between S1 and S2, experiments were also conducted during voluntary isometric contraction of the ADM, and single motor units (SMU) were recorded in addition. This allows the exact determination of the onset of the interaction between S1 and S2 relative to the D-wave elicited by direct activation of the proximal axon of the corticospinal neurone by anodal transcranial electrical stimulation (TES) (Hanajima et al. 2002). Finally, the effects of a single oral dose of the GABAA receptor agonist diazepam (DZP) were tested in the resting and active ADM in order to see to what extent the inhibitory and facilitatory interactions between S1 and S2 are affected by changes in inhibitory neurotransmission.
Methods
Subjects
Twelve healthy volunteers (mean age, 30.2 ± 4.4 years, range, 23–36 years; 2 women, 10 men) participated in the experiments. Nine subjects were right-handed and three left-handed when tested with the Edinburgh Inventory (Oldfield, 1971). Informed written consent was obtained from all subjects. The study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the J. W. Goethe University of Frankfurt, Germany.
Recording and stimulation procedures
Subjects were seated comfortably in a reclining chair. Surface EMG was recorded from the abductor digiti minimi (ADM) muscle of the dominant hand, using surface electrodes in a belly-tendon montage, with the active electrode placed over the motor point and the reference electrode on the proximal interphalangeal joint of the small finger. After amplification and 10 Hz to 2 kHz bandpass filtering (Counterpoint Electromyograph, Dantec Electronics, Skovlunde, Denmark) the EMG signal was passed through a CED micro 1401 laboratory interface (Cambridge Electronic Design, Cambridge, UK) and fed into a personal computer (sampling rate 4 kHz), using customised data collection and conditional averaging software (Spike 2 for Windows, Version 3.05, Cambridge Electronic Design, Cambridge, UK) for off-line analysis.
Transcranial magnetic stimulation (TMS) was applied over the hand area of the dominant motor cortex through a figure-of-eight coil (outer diameter of each loop, 9 cm; peak magnetic field ≈1.5 T) using two Magstim 200 magnetic stimulators (Magstim, Whitland, Carmarthenshire, UK) connected to the BiStim module (Magstim) throughout all measurements. The stimulating coil was placed flat on the skull with the handle pointing backwards and rotated 45 ° away from the mid-line. Thus, the current induced in the brain was directed approximately perpendicular towards the assumed line of the central sulcus. This is the optimal orientation for a predominantly trans-synaptic activation of the corticospinal neurone (e.g. Kaneko et al. 1996; Di Lazzaro et al. 2001). The optimal coil position for activating the contralateral ADM was determined as the site where stimulation at a slightly suprathreshold stimulus intensity consistently produced the largest MEP. This site was marked with a pen in order to assure a constant placement of the coil throughout the experiment. Resting motor threshold (RMT) was determined in the resting ADM to the nearest 1 % of maximum stimulator output using single-pulse TMS. RMT was defined as the lowest stimulus intensity which elicited MEPs > 50 μV in at least five of ten consecutive trials (Rossini et al. 1994). Active motor threshold (AMT) was obtained during a slight isometric contraction (5-10 % of maximum voluntary contraction) and defined as the lowest stimulus intensity which elicited a mean MEP > 100 μV from five single-trial rectified sweeps. RMT and AMT are reported as a percentage of the maximum stimulator output. The EMG was displayed continuously at a high gain (50 μV per division) of the recording device on the computer screen and played through a loudspeaker for acoustic feedback. In those experiments with the ADM at rest, trials contaminated by EMG activity were discarded from analysis.
Effects of S1 and S2 intensity at different interstimulus intervals
These experiments were conducted in six subjects with the ADM at rest. In each subject, four different ISIs of 1.5, 2.1, 3.3 and 5.0 ms were tested in pseudorandomised order and in separate sessions. These particular ISIs were selected because they had revealed different interactions between S1 and S2 in previous paired-pulse TMS experiments. The interval of 1.5 ms showed marked SICF if S1 > RMT and S2 < RMT (Ziemann et al. 1998), or if both stimuli were approximately equal to RMT (Tokimura et al. 1996), but no SICI if S1 < RMT and S2 > RMT (Fisher et al. 2002). In contrast, the interval of 2.1 ms showed no or much less SICF if S1 > RMT and S2 < RMT (Ziemann et al. 1998), or if both stimuli were approximately equal to RMT (Tokimura et al. 1996), while clear SICI was observed if S1 < RMT and S2 > RMT (Fisher et al. 2002). While these were robust between-subject effects in the previous studies, the interaction between S1 and S2 was more variable for the interval of 3.3 ms. Some subjects showed SICF while others showed no interaction (Tokimura et al. 1996; Ziemann et al. 1998). If S1 < RMT and S2 > RMT, there was clearly less SICI than for the interval of 2.1 ms (Fisher et al. 2002). Finally, the interval of 5.0 ms was selected because it is usually the turning point between inhibition and facilitation in the paired-pulse protocols where S1 < RMT and S2 > RMT (Kujirai et al. 1993; Ziemann et al. 1996c).
In all experiments, S1 and S2 were varied in steps of 10 % RMT between 60 and 140 % RMT (i.e. nine intensity steps). The paired-pulse conditions consisted of all possible combinations of S1 and S2 intensities (i.e. 9 × 9 = 81 conditions). In addition, nine single-pulse conditions were tested at the nine different intensities. Five trials were performed for each condition (i.e. 5 × 90 = 450 trials per session). The different conditions were applied in pseudorandomised order. The correct intensities of the two magnetic stimulators were set automatically by customised software (Spike 2) via the CED 1401 laboratory interface and the remote port of the Magstim stimulators. The intertrial interval varied randomly between 8 ± 2 s. Conditional averages of the peak-to-peak MEP amplitudes were calculated, and the interaction between S1 and S2 was expressed as the ratio of the MEP amplitude elicited by paired TMS (MEPS1+S2) over the arithmetic sum of the MEP amplitudes produced by the corresponding single stimuli (MEPS1 and MEPS2):
Interaction = MEPS1+S2/(MEPS1 + MEPS2).
Effects of DZP on the interactions between S1 and S2 at an ISI of 1.5 ms in the resting ADM
This experiment was conducted in 11 subjects. The paired-pulse TMS protocol was applied as above (nine intensity steps) at an ISI of 1.5 ms immediately prior to and 2 h after intake of a single oral dose of 20 mg DZP. The ISI of 1.5 ms was selected because it has been extensively explored in previous studies (Ziemann et al. 1998; Fisher et al. 2002; Hanajima et al. 2002). In particular, it was shown by replacement of one magnetic stimulus by anodal TES (Tokimura et al. 1996; Ziemann et al. 1998) and by epidural recordings of the descending corticospinal volley at the cervical spinal cord (Di Lazzaro et al. 1998b, 1999b) that the interactions between S1 and S2 most likely occur in the motor cortex.
Three subjects were excluded from the analysis of the DZP effects because they had already shown a significant depression of MEP amplitude to single-pulse TMS (MEP intensity curve), similar to previous findings (Boroojerdi et al. 2001). This, by itself, might affect the interaction between S1 and S2 (Kujirai et al. 1993). For the remaining eight subjects with stable MEP intensity curves, the interaction between S1 and S2 was calculated as above, separately for the pre- and post-DZP measurements. Furthermore, in order to provide a condition-by-condition comparison pre- versus post-DZP, each condition of S1 and S2 intensity was expressed for each subject as a weighted difference of the MEPS1+S2/(MEPS1 + MEPS2) data:
with possible values between −1 and +1. Negative values would indicate either more SICI or less SICF, depending on the value of MEPS1+S2/(MEPS1 + MEPS2) prior to DZP intake.
As a control for the DZP experiment, four subjects were tested in an identical manner (‘pre’ and ‘post’ measurements, separated by 2 h of waiting), but without taking DZP.
Effects of DZP on the interactions between S1 and S2 at an ISI of 1.5 ms during contraction of the ADM and SMU recordings
The DZP experiment in the resting ADM was repeated in seven subjects during slight isometric contraction of the ADM (5-10 % of maximum contraction). Pauses were allowed whenever needed to avoid fatigue. Stimulus intensity was related to AMT. Otherwise, the experiment was conducted the same way as during muscle rest.
In addition to the analyses described above, the interaction between S1 and S2 was further tested by plotting the function MEPS1+S2 - (MEPS1 + MEPS2) against time. To this end, the individual D-wave latency was determined by anodal TES using a Digitimer D185 electrical stimulator with a time constant of 50 μs. In each subject, MEPS1+S2 - (MEPS1 + MEPS2) was then related to the individual D-wave latency of S2 which was assigned a time of zero. Finally, curves were averaged across subjects for each of the 81 conditions of S1 and S2 (Fig. 5). This approach allows the precise determination of the time course of the interactions between S1 and S2 relative to the D-wave. The onset of interaction was determined as the first consistent deviation of the MEPS1+S2 - (MEPS1 + MEPS2) function away from zero. It is important to note that a valid interpretation of this analysis is possible in the active muscle only, and only at interaction onset. At this point voluntary muscle activation eliminates the requirement for temporal summation at the neuronal elements along the motor pathway to reach action potential threshold. However, only the fastest conducting corticospinal neurones are tested.
Figure 5. Time course of the interaction MEPS1+S2 - (MEPS1 + MEPS2) during slight isometric contraction of the ADM.
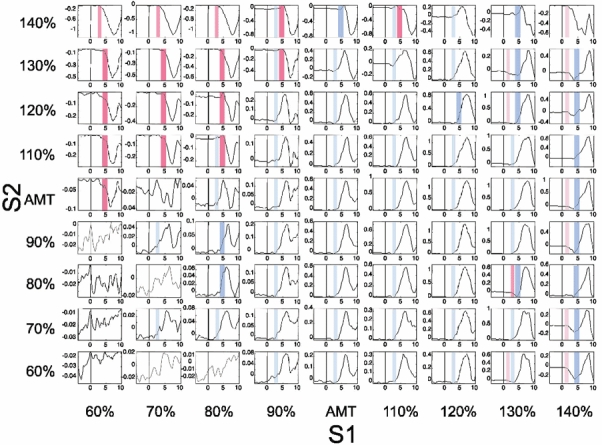
The intensities of S1 relative to AMT are given on the x-axis, those of S2 on the y-axis. One diagram was constructed for each of the 81 conditions of S1 and S2 intensity. Each diagram displays the time course of the mean MEPS1+S2 - (MEPS1 + MEPS2) (in mV) from seven subjects. Before averaging, each individual curve was related to the individual anodal D wave latency of S2 in the active ADM which was assigned a time of zero. The onset of a clear deviation of the MEPS1+S2 - (MEPS1 + MEPS2) curve away from zero was marked by a box. Three boxes with fixed different timings 1.0-2.0, 2.5-3.5 and 4-5.5 ms after the D-wave latency of S2 were used, in order to indicate an onset of the interaction between S1 and S2 falling into the range of the I1, I2 or I3 latency of S2. Note, that the onset of SICI (indicated by red boxes) was mainly during the I3-wave of S2. In contrast, the onset of SICF (blue boxes) was mainly during the I2-wave of S2.
In order to verify the correctness of this analysis and its validity for a broader sample of neurones, recordings were made in three subjects from 12 SMU altogether. The anodal D-wave latency was determined for each SMU. In addition, three different paired-pulse TMS conditions were compared with the corresponding single-pulse conditions. The paired-pulse conditions were selected from the previous surface EMG data (see Fig. 3A). One condition tested SICI (S1 = 60 % AMT; S2 = 120 % AMT), the other two conditions tested SICF (S1 = 120 % AMT; S2 = 60 % AMT or S1 = 90 % AMT; S2 = 90 % AMT). The 60 % AMT single-pulse condition was usually not tested because it never resulted in any evoked SMU response. Conditional post-stimulus time histograms (PSTHs) were constructed from 100 trials. Conditions were applied in pseudorandom order. Trials with multiple-unit evoked responses were discarded on-line from analysis. The mean voluntary SMU firing rate during the period of 100 ms prior to stimulation was 7 s−1. The timing of bins (bin width = 0.25 ms) in the PSTH was related to the anodal D-wave of S2 of the individual SMU which was assigned a value of zero. Bins were counted when they fell into an I-wave latency window. According to previous work (cf. Fig. 2 in Day et al. 1989), the windows of the I1-, I2- and I3-wave were set to 1.0-2.0, 2.5-3.5 and 4.0-5.5 ms, respectively, after the anodal D-wave. The interactions between S1 and S2 were analysed for each unit and SICI or SICF condition and separately for the I1-, I2- and I3-wave window by the weighted difference:
Therefore, values between +1 and −1 are possible, with positive values indicating a facilitatory interaction.
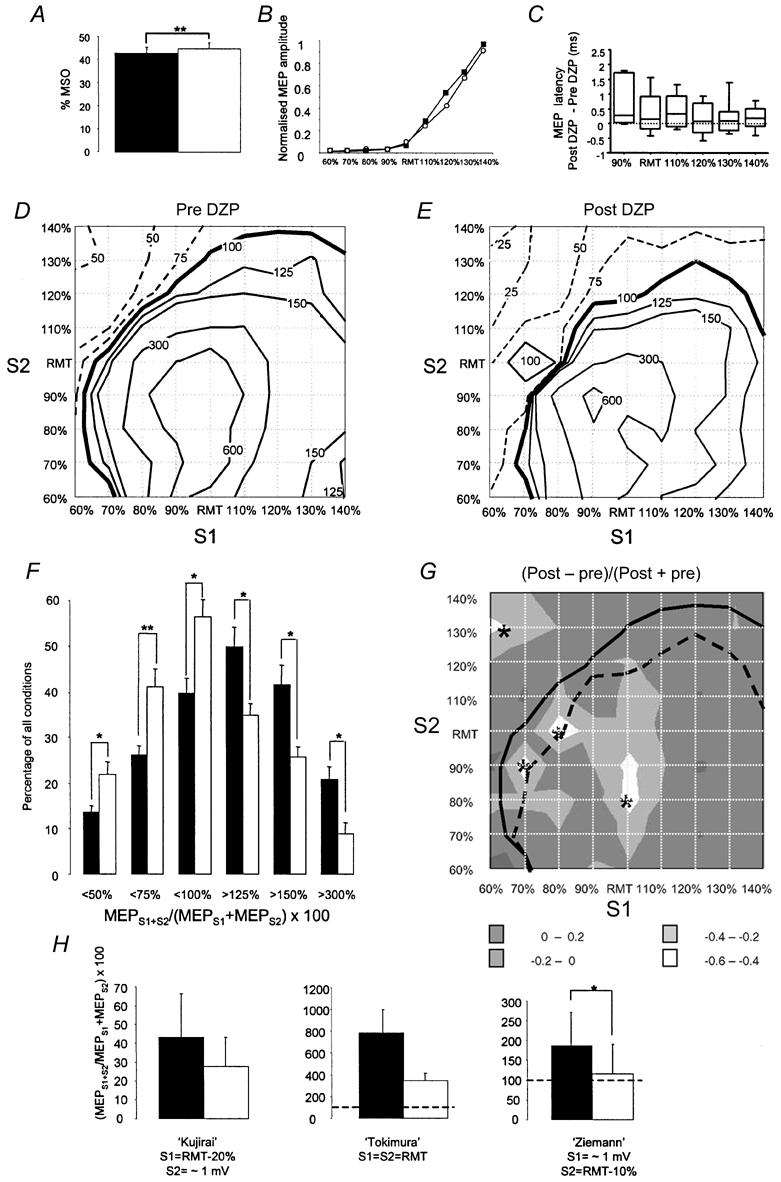
Figure 3. Effects of diazepam (DZP) on single-pulse and paired-pulse TMS measures of motor excitability in the active ADM.
Conventions and arrangement are the same as in Fig. 2. Note that DZP no longer produced significant effects during slight isometric contraction of the ADM.
Statistical procedures
The main measure of the present experiments was the interaction of S1 and S2 expressed as MEPS1+S2/(MEPS1 + MEPS2). The effect of ISI was evaluated by counting those intensity conditions of S1 and S2 resulting in MEPS1+S2/(MEPS1 + MEPS2) values below or above a given limit. These counts were compared across the four ISIs using Student's paired t test. The effects of DZP on RMT and AMT were analysed using Student's paired t test. The effects on the MEP intensity curve were tested with a repeated-measures two-way ANOVA with time (‘pre’ and ‘post’) and stimulus intensity (nine levels) as the within-subject factors. The effects of DZP on MEPS1+S2/(MEPS1 + MEPS2) were analysed by counting conditions resulting in values below or above a given limit and subjecting these data to multiple t tests. Furthermore, for each of the 81 single conditions of S1 and S2 the weighted difference (post - pre)/(post + pre) was calculated for each subject and these data were tested against zero, using one-sample multiple t tests. The weighted difference SMU data were analysed according to paired-pulse condition and I-wave latency window by testing against zero, using one-sample multiple t tests. All multiple comparisons were corrected using Bonferroni's method. Statistical significance was assumed whenever P < 0.05.
Results
Effects of S1 and S2 intensity at different interstimulus intervals
All four ISIs resulted in an inhibitory interaction of S1 and S2, when S1 < RMT and S2 > RMT. This is in accord with previous paired-pulse experiments where S1 was typically set to around 80 % RMT and S2 to produce an unconditioned MEP of ≈1 mV peak-to-peak amplitude (S21mV) (Kujirai et al. 1993). The comparison between the different ISI shows that the ‘area’ of SICI (i.e. the number of conditions with MEPS1+S2/(MEPS1 + MEPS2) × 100 < 100 %) was larger for ISI = 2.1 ms (Fig. 1B) compared to any of the other ISIs (Fig. 1A and C and D). This difference was statistically significant between the ISIs of 1.5 and 2.1 ms for levels of SICI < 100 and < 75 % (Fig. 1E, paired t test, corrected for multiple comparisons, P < 0.0083).
Figure 1. Short-interval paired-pulse inhibition and facilitation as a function of stimulus intensity and interstimulus interval in the resting ADM.
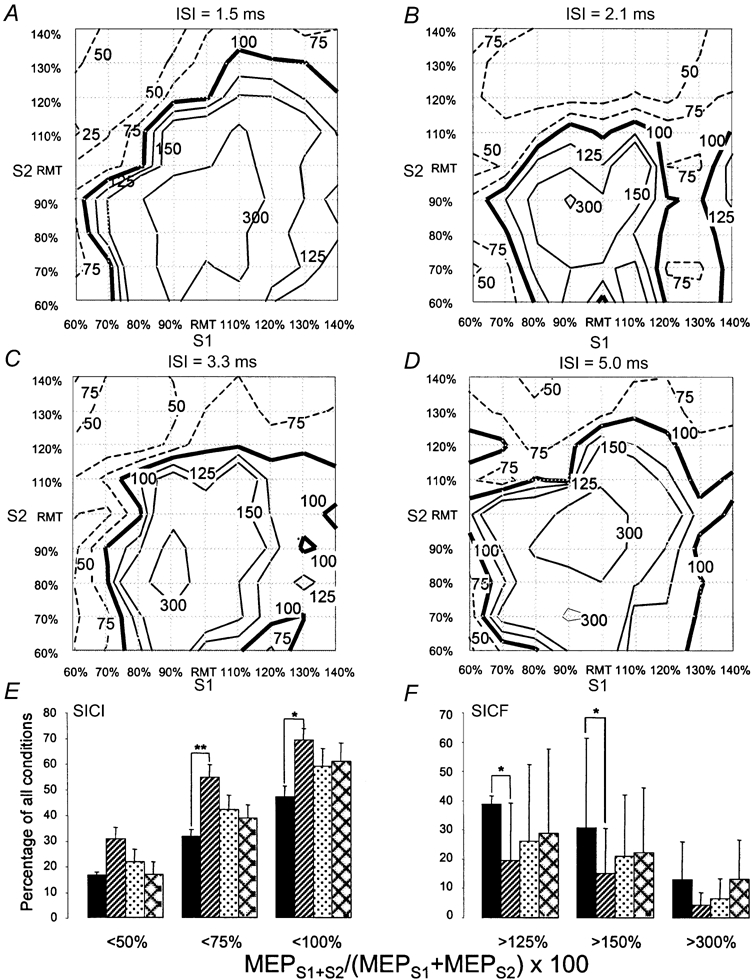
A-D refer to interstimulus intervals (ISIs) of 1.5 (A), 2.1 (B), 3.3 (C) and 5.0 ms (D). In each diagram, stimulus intensity of the first stimulus (S1, x-axis) and the second stimulus (S2, y-axis) is related to resting motor threshold (RMT) of the abductor digiti minimi (ADM) muscle. As there are nine different stimulus intensities for S1 and S2, each diagram consists of 81 conditions. For each condition, the interaction between S1 and S2 was expressed as the percentage of ADM motor evoked potential (MEP) amplitudes produced by paired TMS (MEPS1+S2) over the arithmetic sum of the MEP produced by the single stimuli (MEPS1 + MEPS2). All data are means of six subjects and are given as contour plots. The thick continuous line in each diagram represents no interaction (100 %), dashed lines show inhibitory (< 100 %) and thin continuous lines facilitatory (> 100 %) interaction, the numbers indicate the contour line values. Note that the area of facilitation is much more extensive with the ISI of 1.5 ms compared with 2.1 ms. E, number of conditions (given as percentage of all 81 conditions, y-axis) below or above discrete interaction levels of (MEPS1+S2/MEPS1 + MEPS2) × 100 as indicated on the x-axis. The different bars refer to ISIs of 1.5 (black), 2.1 (hatched), 3.3 (stippled) and 5.0 ms (cross-hatched). Error bars are s.e.m. Asterisks indicate significant differences (*P < 0.05, **P < 0.01; t tests corrected for multiple comparisons). Note that the ISI of 1.5 ms resulted in less short-interval intracortical inhibition (SICI) and more facilitation (SICF) compared with the ISI of 2.1 ms.
All ISIs resulted in a facilitatory interaction of S1 and S2, if S1 and S2 were approximately equal to RMT, in accord with previous findings (Tokimura et al. 1996). However, the ‘area’ of SICF (i.e. the number of conditions with MEPS1+S2/(MEPS1 + MEPS2) × 100 > 100 %) was larger for ISI = 1.5 ms (Fig. 1A) than for the other ISIs (Fig. 1B-D), ‘expanding’ to conditions with S1 > RMT and S2 < RMT. This is in line with previous reports (Ziemann et al. 1998; Hanajima et al. 2002). This difference was statistically significant between the ISI of 1.5 ms and 2.1 ms for levels of SICF > 125 and > 150 % (Fig. 1E, paired t tests, corrected for multiple comparisons, P < 0.0083).
RMT was not different across the different ISIs (1.5 ms, 45.0 ± 5.0 %; 2.1 ms, 45.2 ± 5.6 %; 3.3 ms, 44.0 ± 6.3 %; 5.0 ms, 44.5 ± 5.9 %; repeated-measures ANOVA, P = 0.84). Similarly, there was no difference of single-pulse MEP intensity curves (repeated-measures ANOVA, P = 0.96 (ISI), P = 0.99 (ISI × stimulus intensity)). These are important negative results because single-pulse MEP amplitude or TMS intensity may affect the interaction between S1 and S2 (Kujirai et al. 1993; Ziemann et al. 1996c).
Effects of DZP on the interactions between S1 and S2 at an ISI of 1.5 ms in the resting ADM
DZP resulted in a very slight but significant increase in RMT of, on average, 1.9 % of the maximum stimulator output (paired t test, P = 0.001; Fig. 2A). The MEP intensity curve with stimulus intensity adjusted to RMT remained unaffected (repeated-measures ANOVA, F = 1.71, P = 0.232 (drug); F = 1.05, P = 0.411 (drug × stimulus intensity); Fig. 2B). Furthermore, MEP onset latency was not affected by DZP (Fig. 2C). Therefore, alterations in single-pulse MEP amplitude and MEP onset latency cannot account for the effects of DZP on the interaction between S1 and S2 in the paired-pulse experiment (see below). The single-pulse results are not at variance with the depressive effects of the GABAA receptor agonist lorazepam on MEP amplitude in one previous report (Boroojerdi et al. 2001) because those subjects who showed a MEP depression (n = 3) were excluded from analysis (see Methods).
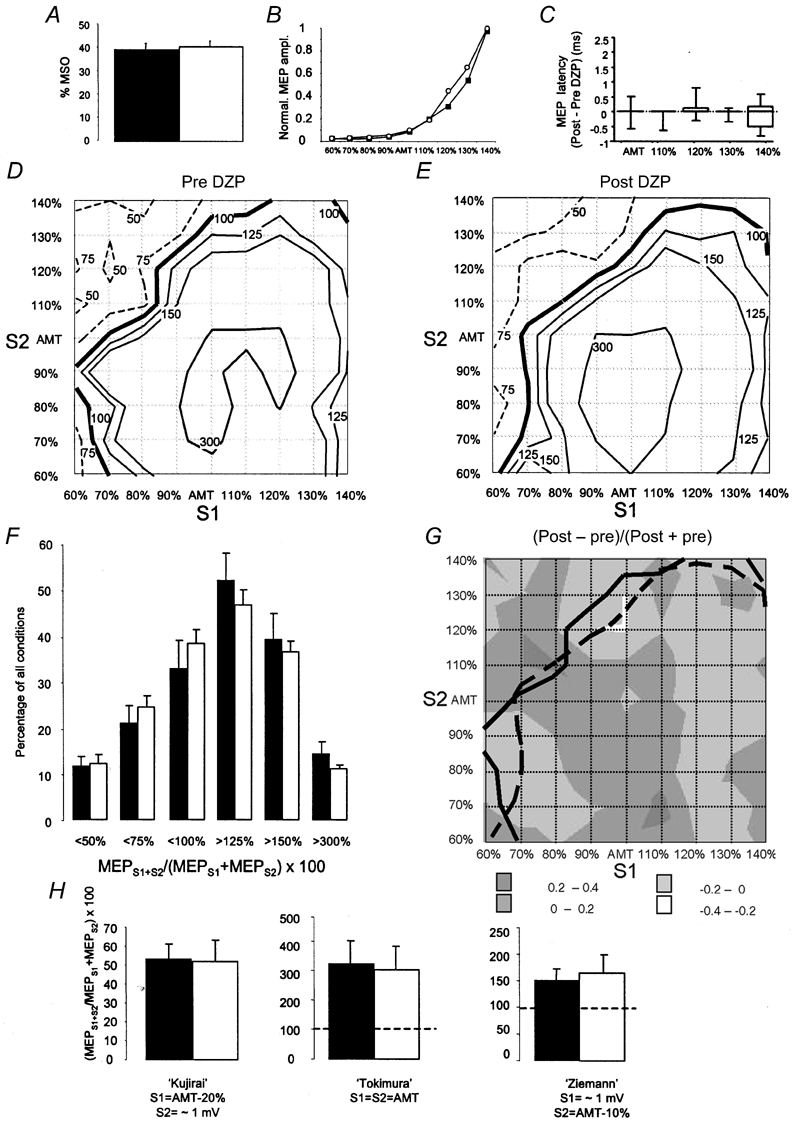
Figure 2. Effects of diazepam (DZP) on single-pulse and paired-pulse TMS measures of motor excitability in the resting ADM.
All measures were taken immediately before and 2 h after a single oral dose of 20 mg of DZP and are means from eight subjects. A, resting motor threshold expressed as percentage of the maximum stimulator output (%MSO, y-axis) before (▪) and after DZP (□). ** P < 0.01 (paired t test). B, motor evoked potential (MEP) intensity curves before (▪) and after DZP (○). Stimulus intensity of the single TMS pulse was related to resting motor threshold (RMT, x-axis). MEP amplitudes are normalised for each subject to the maximum MEP before DZP, which was assigned a value of 1. C, changes of MEP onset latency, calculated as latency differences ‘Post - pre’ DZP (in ms). For the intensity of RMT - 10 %, the data are based on only five subjects because the other subjects had no visible MEP at this subthreshold intensity. D and E, contour plots (average from 8 subjects) of the interaction between the first (S1) and second stimulus (S2) expressed as MEPS1+S2/MEPS1 + MEPS2 × 100 pre- (D) and post-DZP (E). The interstimulus interval was 1.5 ms. Conventions for the contour plots are the same as in Fig. 1A. F, number of conditions (given as percentage of all 81 conditions, y-axis) below or above discrete interaction levels of MEPS1+S2/MEPS1 + MEPS2 × 100 as indicated on the x-axis. ▪ and □, pre- and post-DZP, respectively. Error bars are s.e.m. *P < 0.0083, **P < 0.001; t tests corrected for multiple comparisons. G, the data in the contour plots in D and E are shown as weighted differences (post-DZP - pre-DZP)/(post-DZP + pre-DZP). Accordingly, values between −1 and +1 are possible. Negative values indicate either an increase of short-interval intracortical inhibition (SICI), or a decrease of facilitation (SICF), depending on the value of (MEPS1+S2/MEPS1 + MEPS2 × 100 pre-DZP. The grey-shaded areas refer to the different ranges of weighted difference values as given in the inset. The asterisks indicate conditions that were statistically significantly different from zero (P < 0.0006, one-sample t tests, corrected for multiple comparisons). The continuous and dashed lines are the (MEPS1+S2/MEPS1 + MEPS2) × 100 = 100 % contour lines pre- and post-DZP from D and E, respectively, to delineate SICI and SICF ‘areas’. Note that DZP led to increase of SICI and reduction of SICF. H, short-interval (1.5 ms) paired-pulse intracortical inhibition (SICI) according to the ‘Kujirai protocol’ (left diagram) and short-interval paired-pulse intracortical facilitation (SICF) according to the ‘Tokimura protocol’ (middle diagram) and the ‘Ziemann protocol’ (right diagram) pre- (▪) and post DZP (□). The particular conditions of the first (S1) and second stimulus (S2) in the different protocols are given below each diagram. Error bars indicate s.e.m. * P < 0.05 (paired t test).
The main effects of DZP on the MEPS1+S2/ (MEPS1 + MEPS2) interaction were an increase of the ‘area’ of SICI and a decrease of the ‘area’ of SICF (Fig. 2D-E). This difference was significant for the levels of MEPS1+S2/(MEPS1 + MEPS2) × 100 < 75 %, < 100 % and > 125 % (Fig. 2F, paired t tests, corrected for multiple comparisons, P < 0.0083).
DZP resulted in weighted differences < −0.4 in two ‘regions’ of conditions of S1 and S2 (Fig. 2G). One such effect occurred with S1 = 60 % RMT and S2 = 130 % RMT, indicating a marked increase in SICI (Fig. 2G). The other occurred with S1 = RMT and S2 = 80–90 % RMT, or S1 = 70–80 % RMT and S2 = RMT (Fig. 2G), indicating a marked decrease of SICF (Fig. 2G). The asterisks in Fig. 2G point to interactions between S1 and S2 that were significantly different from zero (one-sample t tests corrected for multiple comparisons, P < 0.0006).
If those conditions of S1 and S2 were selected as defined in previously published paired-pulse TMS protocols (Kujirai et al. 1993; Tokimura et al. 1996; Ziemann et al. 1996c; see also Introduction), only the ‘Ziemann’ protocol revealed the depressive effect of DZP (Fig. 2H; paired t test, t = 3.398, P = 0.011), while the ‘Kujirai’ and ‘Tokimura’ protocols were suggestive of more SICI and less SICF, respectively, but the differences did not reach statistical significance (Fig. 2H, P > 0.05).
The control experiment (2 h waiting without DZP) showed no differences in the single-pulse and paired-pulse measures between baseline and after 2 h of waiting (data not shown). This suggests that the alterations of SICI and SICF under DZP were due to a specific drug effect.
Effects of DZP on the interactions between S1 and S2 at an ISI of 1.5 ms in the active ADM
During slight isometric contraction, SICI and SICF were expressed very similarly as during muscle rest (Figs 2D, 3D, 4A and 4B). Only the highest level of SICF > 300 % was reduced during muscle contraction, suggesting a saturation effect during muscle contraction. The MEPS1+S2/(MEPS1 + MEPS2) × 100 = 100 % contour line, which marks the border between SICI and SICF was not shifted by contraction (Fig. 4B).
Figure 4. Effects of voluntary ADM contraction compared to rest on paired-pulse TMS measures of motor excitability.
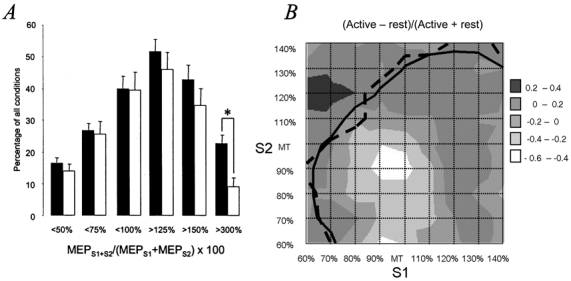
A, number of conditions (given as percentage of all 81 conditions, y-axis) below or above discrete interaction levels of (MEPS1+S2/MEPS1 + MEPS2) × 100 as indicated on the x-axis. ▪ and □, resting and active ADM before DZP intake, respectively. Data are from the seven subjects who participated in both experiments. Error bars indicate s.e.m. Note that voluntary contraction did not affect SICI but resulted in a significant decrease in the highest SICF level. *P < 0.01. B, mean weighted differences (n = 7 subjects) of each condition of S1 and S2 comparing the paired-pulse interaction (MEPS1+S2/MEPS1 + MEPS2) in the active and resting ADM. The continuous and dashed lines are the (MEPS1+S2/MEPS1 + MEPS2) × 100 = 100 % contour lines at rest and during contraction, respectively, to delineate SICI and SICF ‘areas’. Note that contraction decreased the highest values of SICF but did not result in a shift of the 100 % contour line.
DZP had no significant effect either on the single-pulse measures of motor excitability (AMT, MEP intensity curve, MEP onset latency, Fig. 3A-C), or on SICI and SICF (Fig. 3D-G). Neither were the selected paired-pulse conditions as defined in the previous protocols (Kujirai et al. 1993; Tokimura et al. 1996; Ziemann et al. 1996c) affected by DZP (Fig. 3H).
Time course of the interactions between S1 and S2 at an ISI of 1.5 ms in the active ADM
The time course of the function MEPS1+S2 - (MEPS1 + MEPS2) was plotted relative to the anodal D-wave latency of S2 for each of the 81 conditions of S1 and S2 (Fig. 5). Consistently, the onset of SICI coincided with the I3-wave of S2 (Fig. 5, dark red bands). Only in a few exceptions, at high intensities of S2, the onset of SICI occurred at the I2-wave latency of S2 (Fig. 5, light red bands). In contrast, the onset of SICF occurred consistently at the I2-wave latency of S2 (Fig. 5, dark blue bands), and in a few conditions even at the I1-wave latency of S2 (Fig. 5, light blue bands).
The SMU recordings confirmed these findings. The representative SMU in Fig. 6A-C showed a suppression of the I3-wave of S2 in a condition testing SICI (S1 = 60 % AMT; S2 = 120 % AMT, Fig. 6A). In contrast, the same SMU displayed facilitation coincident with the I2-wave of S2 in conditions testing SICF (S1 = 120 % AMT; S2 = 60 % AMT; Fig. 6B; S1 = 90 % AMT; S2 = 90 % AMT; Fig. 6C). Accordingly, the analysis of all 12 SMUs demonstrated a significant inhibition of the I3-wave of S2 in the ‘Kujirai’ SICI condition, and significant facilitation coincident with the I2-wave of S2 in the ‘Ziemann’ and ‘Tokimura’ SICF conditions (Fig. 6D). Furthermore, there was a trend for a facilitation coincident with the I1-wave of S2 (P = 0.03, not significant after correction for multiple comparisons) in the Ziemann condition. Finally, the I1-wave of S2 remained completely unaffected in the Kujirai and Tokimura conditions (Fig. 6D).
Figure 6. Interaction between S1 and S2 in SMU recordings.
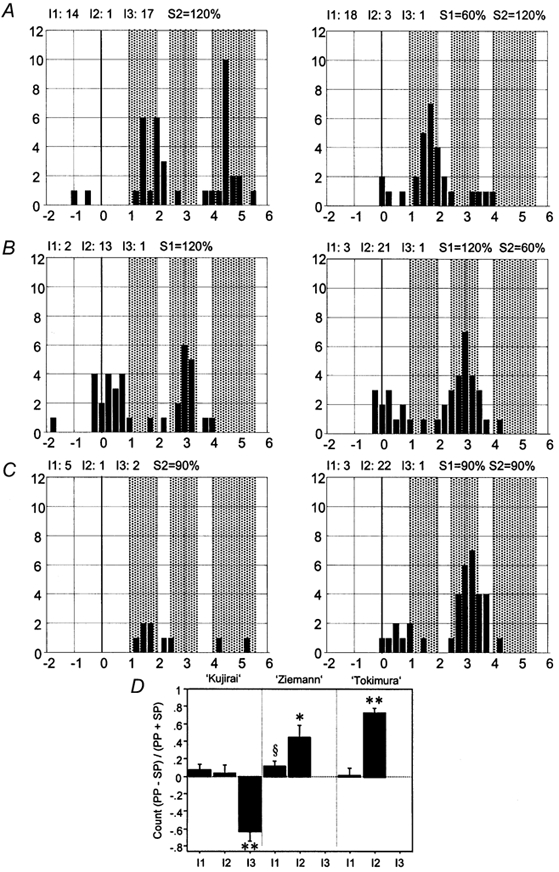
A-C, one representative SMU tested for SICI (A, S1 = 60 % AMT; S2 = 120 % AMT), and SICF (B, S1 = 120 % AMT; S2 = 60 % AMT; C, S1 = 90 % AMT; S2 = 90 % AMT). Each PSTH (bin width = 0.25 ms) was constructed from 100 trials. Time zero refers to the anodal D-wave latency of S2 of this unit. The left PSTHs refer to single-pulse stimulation by the higher intensity pulse, the right PSTHs to the corresponding paired-pulse stimulation. The dotted boxes indicate intervals of 1.0-2.0, 2.5-3.5 and 4-5.5 ms after the D-wave which refer to the range of I1-, I2- and I3-wave latencies of S2, respectively. Counts of SMU discharge in the range of the three I-wave latencies are given above each diagram. Note that SICI (A) was produced by a complete inhibition of the I3-peak of S2, whereas SICF (B and C) resulted from a facilitation coincident with the I2 latency of S2. D, summary display of the data from all 12 SMU. The interaction of S1 and S2 is expressed as the weighted difference between the paired-pulse (PP) and single-pulse (SP) conditions on the y-axis. Note that SICI (Kujrai protocol) resulted consistently from a depression coincident with the I3-wave of S2. In contrast, SICF (Ziemann and Tokimura protocols) resulted consistently from a facilitation coincident with the I2-wave of S2. §P = 0.03 (non-significant after correction for multiple comparisons); *P < 0.015; **P < 0.001.
Discussion
Effects of S1 and S2 intensity at different interstimulus intervals
Systematic variation of the intensities of S1 and S2 led to consistent patterns of paired-pulse interactions. If the intensities of S1 and S2 were approximately equal to RMT, SICF occurred, in line with one previous paired-pulse TMS study (Tokimura et al. 1996). In addition, SICF was obtained if S1 > RMT and S2 < RMT, but only with an ISI of 1.5 ms. This facilitation was absent or expressed to a much lesser extent with ISIs of 2.1, 3.3 and 5.0 ms. This is in accordance with one previous report which demonstrated SICF between a suprathreshold S1 and a subthreshold S2 only at discrete ISIs of 1.1-1.5, 2.3-2.9 and 4.1-4.4 ms (Ziemann et al. 1998). SICI was obtained if S1 < RMT and S2 > RMT, in agreement with previous findings (Kujirai et al. 1993; Ziemann et al. 1996c). SICI was most pronounced with an ISI of 2.1 ms. At this interval, SICI ‘expanded’ to conditions with S1 > RMT and S2 > RMT. The stronger SICI with an ISI of 2.1 ms compared with the other intervals may have been predicted from one recent study which showed, by using a threshold tracking technique, that intervals of 1.0 and 2.2-2.6 ms were particularly effective in producing SICI while intervals of 1.5 and 3.0-4.5 ms were much less effective (Fisher et al. 2002).
Site of the interaction between S1 and S2
The site of the paired-pulse interactions was not specifically tested in the present experiments because there exists already a wealth of consistent evidence from previous studies for placing the site of this interaction into the motor cortex. When either the magnetic S1 or magnetic S2, or both stimuli were substituted by anodal TES, SICI (Kujirai et al. 1993) and SICF (Tokimura et al. 1996; Ziemann et al. 1998) disappeared. The implication is that anodal TES activates the corticospinal system preferentially directly at the proximal corticospinal axon, some nodes of Ranvier distant to the cell body, and therefore is resistant to changes in excitability of the corticospinal neurone (Di Lazzaro et al. 1999a). Even stronger evidence in favour of a cortical site of the paired-pulse interactions came from epidural cervical spinal cord recordings of the descending corticospinal volley elicited by TMS of the hand area of motor cortex. These recordings showed that SICI and SICF were associated with a marked decrease or increase, respectively, of the I2-wave and later I-waves, while the I1-wave remained unaffected (Di Lazzaro et al. 1998b, 1999b). Still, it cannot be fully excluded that some interaction between S1 and S2 in the present experiments took place at a subcortical or even spinal level, in particular when both stimuli were above motor threshold.
Effects of DZP and voluntary contraction on the interactions between S1 and S2 at an ISI of 1.5 ms
SICI and SICF remained largely unaffected by voluntary contraction, with the exception of the highest SICF values which were reduced by contraction (Figs 2D, 3D and 4). At first sight, the SICI data are at variance with one previous study which showed a reduction of SICI during contraction (Ridding et al. 1995). However, a close look reveals that there was a slight (non-significant) trend toward less SICI during contraction also in the present experiments (e.g. 42 vs. 52 % SICI in the ‘Kujirai’ condition during rest vs. contraction, Fig. 2H and Fig. 3H). Reduction of the highest SICF values during contraction (S1 and S2 approximately equal to motor threshold) is consistent with previous findings (Tokimura et al. 1996).
The small effects of voluntary contraction on SICI and SICF suggest that the intracortical pathways mediating the voluntary drive and the paired-pulse interaction are largely independent and converge only at a distal point such as the corticospinal neurone (Fig. 7). Epidural recordings of the corticospinal volley showed that voluntary contraction increased the size and number of I-waves (Di Lazzaro et al. 1998a). This suggested an increased excitability of the corticospinal neurone during contraction. If one assumes a fixed range of excitabilities of the corticospinal neurone, voluntary contraction would shift the excitability toward the maximum excitability. SICI could then still be transmitted without much alteration while the highest values of SICF would saturate.
Figure 7. Connectivity model to explain SICI and SICF.
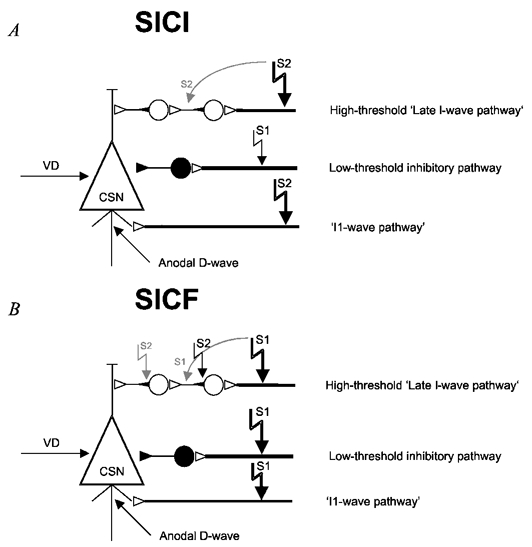
The connectivity model is derived from Fig. 4 in Amassian et al. (1987). The model is a gross simplification of nature but it is sufficient to explain all experimental data. It assumes that there exists one low-threshold inhibitory pathway, and high-threshold excitatory ‘I1- and late I-wave pathways’. CSN, corticospinal neurone; VD, voluntary drive. • denotes a GABAergic inhibitory interneurone, the ○s are excitatory interneurones. A, for SICI, a low-intensity S1 (indicated by the small filled arrow) and a high-intensity S2 (indicated by the large filled arrow) are used. S1 only activates the low-threshold inhibitory pathway. S2 given 1.5 ms after S1 only activates the I1- and late I-wave pathways, while the low-intensity pathway is refractory. The IPSP and EPSP from the inhibitory pathway and the ‘late I-wave pathway’ summate at the CSN at a delay of three I-wave intervals relative to the anodal D-wave latency. In some instances, S2 may activate the axon of the second-order interneurone, in particular if high intensity is used (indicated by the grey curved arrow). In this case, the EPSP from S2 would interact with the IPSP from the inhibitory pathway at the SCN two I-wave intervals later than the anodal D-wave latency. B, for SICF, a high-intensity S1 and a low-intensity S2 are used. S1 activates all pathways. S2 cannot activate any axon due to refractoriness. However, the initial axon segment of the second-order interneurone in the ‘late I-wave pathway’ (indicated by the small filled triangle adjacent to the cell soma) is hyperexcitable due to the EPSP from S1 and can be excited directly by S2. Therefore, the site of excitation by S2 ‘jumps up’ by one I-wave latency, and the facilitatory interaction between S1 and S2 lags the anodal D-wave latency by only two I-wave intervals. In some instances, S1 may activate in addition the axon of some second-order interneurones (indicated by the grey curved arrow). In this case, the initial axon segment of first-order interneurones is hyperexcitable due to the EPSP from S1 and can be excited by S2. The facilitatory interaction between S1 and S2 would then lag the anodal D-wave latency by only one I-wave interval.
Systematic variation of S1 and S2 intensity showed that the system underlying SICI can be activated by very low-intensity S1 at ≤ 60 % AMT (Figs 1, 2D, 3D and 5). In contrast, there was no indication that S1 at intensities of 60–70 % RMT or AMT was capable of producing any facilitatory interaction. This confirms that the lowest threshold system activated by TMS in the hand area of human motor cortex is inhibitory (Davey et al. 1994; Ziemann et al. 1996c; Awiszus et al. 1999). In the resting ADM, DZP significantly increased SICI, in particular with conditions of very low-intensity S1 (60-70 % RMT, Fig. 2D, E and G). These are non-optimal for producing SICI (Kujirai et al. 1993; Fisher et al. 2002), and therefore escape a possible ‘floor effect’ which may have been the reason for the lack of effects of benzodiazepines on SICI in previous experiments (Inghilleri et al. 1996; Ziemann et al. 1996a). The increase of SICI by DZP in the present experiments confirms the early notion that SICI is mediated by the GABAA receptor (Kujirai et al. 1993; Ziemann et al. 1996b). During isometric contraction, DZP completely lost its effect on SICI (Fig. 3D-H) although contraction by itself prior to DZP intake did not significantly affect SICI (see above). The most parsimonious explanation for this dissociation is that voluntary contraction is capable of modifying the GABAA receptor of corticospinal neurones by reducing its sensitivity to regulation by benzodiazepines while its sensitivity to GABA is maintained. A similar rapidly developing dissociation was described in rat hippocampal dentate granule cell GABAA receptors during epileptic seizures (Kapur & Macdonald, 1997).
Systematic variation of S1 and S2 also showed that the excitatory system mediating SICF comes into play only if the intensity of S1 is ≥ 80 % RMT or AMT (Figs 1, 2D, 3D and 5), similar to previous observations (Awiszus et al. 1999). This suggests the existence of, at least, two largely independent systems which may converge at the corticospinal neurone, the low-threshold inhibitory system and a high-threshold excitatory system (Fig. 7). Facilitation in the excitatory system must have the potential to ‘overrule’ the inhibitory system. Otherwise, SICF would not be possible. SICF was strongly reduced by DZP in the resting ADM (Fig. 2D-H). This was an expected finding because S1 always activates the inhibitory system (which is enhanced by DZP) in parallel with the excitatory system. The complete lack of effect of DZP during isometric ADM contraction (Fig. 3D-H) confirms previous recordings of the descending corticospinal volley from the cervical spinal cord (Di Lazzaro et al. 2000) and is consistent with the idea (see above) that voluntary contraction decreases the sensitivity of the GABAA receptor to benzodiazepine regulation.
Time course of the interactions between S1 and S2 at an ISI of 1.5 ms in the active ADM
We have proposed here a novel way to analyse the time course of paired-pulse interactions during isometric contraction of the target muscle by using the surface EMG derived MEPS1+S2 - (MEPS1 + MEPS2) curve related to the anodal D-wave. The results of this analysis were fully compatible with the SMU data (Fig. 5 and Fig. 6). The analysis based on surface EMG data has, however, the advantage that a larger range of stimulus intensities can be tested which would result in multiple-unit responses in SMU recordings.
The onset of SICI coincided with the I3-wave of S2, while the I1-wave was never affected (Fig. 5 and Fig. 6). Sometimes, SICI started already at the I2-wave of S2, but this effect was small and inconsistent (Fig. 5). These findings are compatible with previous epidural recordings of the descending corticospinal volley which showed that the I1-wave was never inhibited and the I2-wave was only inhibited at an ISI of 1 ms, but not at ISI ≥ 2 ms. The sparing of the I1-wave is consistent with several other observations which also showed that this wave is much less sensitive to modulation than later I-waves (Nakamura et al. 1997; Hanajima et al. 1998; Tokimura et al. 2000). These dissociated effects are best explained by segregated pathways for the I1-wave and the later I-waves, with very direct access of the ‘I1-wave pathway’ to proximal parts of the corticospinal neurone (Amassian et al. 1987; Sakai et al. 1997; Fig. 7). In contrast to SICI, the onset of SICF typically coincided with the I2-latency of S2, and in some instances even with the I1-latency of S2 (Fig. 5 and Fig. 6). This is entirely consistent with recent SMU data (Hanajima et al. 2002). Those authors managed, by variation of the coil orientation, to elicit selectively an I1- or I3-wave in a given SMU. Paired-pulse TMS using a suprathreshold S1 and a subthreshold S2 at an ISI of 1.5 ms resulted in a facilitation of the I3-wave of S1 (coincident with the I2-wave of S2), and no effect on the I1-wave of S1 but the appearance of an I2-peak (coincident with the I1-wave of S2).
Mechanisms of SICI and SICF
The major question is by which mechanisms SICI and SICF occur. A crucial argument can be based on the observation of the ‘jumping up’ of SICF by one or even two I-wave latencies compared to SICI, even if a fixed intensity of S2 was used (e.g. S2 = 120 % in Fig. 5).
One elegant way to explain this jumping up would be to assume that: (1) SICI occurs through an interaction at the corticospinal neurone by summation of IPSP mediated by the low-threshold inhibitory pathway and EPSP mediated by the higher-threshold excitatory ‘late I-wave pathway’ (Fig. 7A). Low-intensity S1 will activate the low-threshold inhibitory pathway only, and higher-intensity S2 will activate the high-threshold ‘late I-wave pathway’ only. S2 will not activate the low-threshold inhibitory pathway because, according to the low intensity of S1 required to obtain optimal SICI (Kujirai et al. 1993; Ziemann et al. 1996c; Fisher et al. 2002), S1 had most probably already activated most or all parts of this pathway which is then refractory for subsequent S2 activation at an interval of 1.5 ms (Amassian et al. 1998). The onset of SICI coincides with the conduction time in the excitatory late I-wave pathway, which is approximately three I-wave latencies later than the anodal D-wave of S2; (2) SICF occurs due to an interaction of S1 and S2 along the high-threshold excitatory late I-wave pathway (Fig. 7B). In contrast to the pure SICI situation, the higher-intensity S1 will now activate at least some axons of the late I-wave pathway. If S2 is given 1.5 ms after S1, and the intensity of S1 ≥ S2, then S2 cannot excite any axon, due to the refractory period of corticocortical axons (Amassian et al. 1998). However, S2 may directly excite the initial axon segment of those excitatory interneurones which had received an EPSP from S1, and therefore, may be hyperexcitable at the time of S2 (Amassian et al. 1990; Deletis et al. 2001; Fig. 7B). This model explains why the onset of SICF jumps up by one I-wave latency compared with SICI because S2 activates hyperexcitable interneurones one I-wave latency ahead of the site of excitation when S2 is given alone.
Several properties of SICF can be predicted from this model. Evidence from previous studies suggested that the neuronal time constant of the initial axon segment is probably very short. Chronaxies of about 300 μs were reported for interneurones in rat visual cortex (Nowak & Bullier, 1998) and of 60–130 μs in intrinsic collaterals of pyramidal tract cells in cat motor cortex (Asanuma et al. 1976). If values of approximately 300 μs were correct for the axons of interneurones in the late I-wave pathway, then shifting the ISI between S1 and S2 away from the I-wave interval (≈1.5 ms) should result in a significant reduction or even lack of SICF. S2 would hit initial axon segments which were no longer hyperexcitable due to the rapid decay of the EPSP at the initial axon segment. Indeed, previous studies showed that SICF occurred only at discrete ISI which are approximately multiples of the I-wave interval (Tokimura et al. 1996; Ziemann et al. 1998). ISI intermediate between I-wave intervals should even result in SICI for many conditions of S1 and S2 because of the lack of facilitatory interaction along the late I-wave pathway. This was demonstrated here for the ISI of 2.1 ms (Fig. 1B).
In conclusion, the present experiments showed that the interactions between the effects of the first and second stimulus of paired TMS at an ISI of 1.5 ms may be inhibitory or facilitatory, depending on the intensities of the two stimuli. Examining the exact onset of the interactions between the first and second pulse relative to the anodal D-wave latency by using SMU recordings and a novel analysis of the surface EMG supported the view that the inhibition most likely occurred through summation at the corticospinal neurone of IPSP elicited by the first pulse mediated through a low-threshold GABAA receptor dependent inhibitory pathway, and EPSP elicited by the second pulse mediated through a high-threshold excitatory late I-wave pathway. In contrast, facilitation originated mainly non-synaptically through direct excitation of the axon initial segment of interneurones along the late I-wave pathway by the second pulse which were made hyperexcitable through EPSP by the first pulse. Using the dimension of stimulus intensity in paired-pulse TMS may help to advance our understanding of disordered paired-pulse cortical excitability in neurological diseases such as epilepsy or movement disorders which had exhibited a rather unspecific deficiency of SICI in previous conventional paired-pulse TMS protocols (Ziemann, 1999).
Acknowledgments
T.V.I. was a research fellow of the Alexander v. Humboldt Foundation.
References
- Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalography Clinical Neurophysiology. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Rothwell JC, Cracco RQ, Maccabee PJ, Vergara M, Hassan N, Eberle L. What is excited by near-threshold twin magnetic stimuli over human cerebral cortex. Journal of Physiology. 1998;506.P:122–123P. [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Experimental Brain Research. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H, Urbach D, Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding inhibition or I-wave facilitation using a threshold-hunting paradigm. Experimental Brain Research. 1999;129:317–324. doi: 10.1007/s002210050901. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clinical Neurophysiology. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. Journal of Physiology. 1994;477:223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deletis VV, Isgum VV, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 1. Recovery time of corticospinal tract direct waves elicited by pairs of transcranial electrical stimuli. Clinical Neurophysiology. 2001;112:438–444. doi: 10.1016/s1388-2457(01)00461-8. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clinical Neurophysiology. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial magnetic stimulation over the motor cortex hand area in conscious humans. Experimental Brain Research. 1999a;124:525–528. doi: 10.1007/s002210050649. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Experimental Brain Research. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1998a;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Experimental Brain Research. 1999b;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Experimental Brain Research. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired- pulse magnetic stimulation in humans. Journal of Physiology. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. Journal of Physiology. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Experimental Brain Research. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalography Clinical Neurophysiology. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. Journal of Neuroscience. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Experimental Brain Research. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. Journal of Physiology. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, LüCKING CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furabayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of -eight shaped coil. Experimental Brain Research. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. Journal of Physiology. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalography and Clinical Neurophysiology. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalography and Clinical Neurophysiology. 1999;51(suppl.):127–136. [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals of Neurology. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. Journal of Physiology. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I-wave interaction in the human motor cortex by paired transcranial magnetic stimulation. Journal of Physiology. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


