Abstract
It remains unclear in pre-eclampsia whether or not a functional change occurs in the role played by prostacyclin in endothelium-dependent relaxation in resistance arteries. We examined this using human omental resistance arteries (obtained from pre-eclamptic or normotensive pregnant women) in the presence of NG-nitro-l-arginine (l-NNA, an inhibitor of nitric oxide synthase). In endothelium-intact strips from both groups, 9,11-epithio-11,12-methano-thromboxane A2 (STA2, a thromboxane A2 mimetic) produced a contraction. Diclofenac (an inhibitor of cyclooxygenase) enhanced the STA2 contraction only in the normotensive pregnant group (1.4 times control, P < 0.01). In the presence of STA2, bradykinin (0.1 μm) produced an endothelium-dependent relaxation in both groups, the relaxation being significantly smaller for the pre-eclamptic group (P < 0.002). Diclofenac significantly attenuated the bradykinin-induced relaxation only for the normotensive pregnant group (31 % inhibition, P < 0.001). The bradykinin-induced membrane hyperpolarization consisted of diclofenac-sensitive and -insensitive components. The former, but not the latter, was significantly smaller in pre-eclampsia (-4.3 vs. −2.6 mV, P < 0.05). The concentrations of 6-keto-PGF1α (a stable metabolite of prostacyclin) in these arteries were significantly lower in pre-eclampsia in both the absence and presence of bradykinin (about 0.2-0.4 times the normotensive pregnant value in each case, P < 0.01). By contrast, both the relaxation and the membrane hyperpolarization in response to beraprost (10 nm, a stable analogue of prostacyclin) were similar between the two groups. We conclude that, in pre-eclampsia, a reduced part is played by prostaglandins in the endothelium-dependent relaxation seen in resistance arteries and that this may be due to a reduced production of prostacyclin by the endothelium.
Vascular endothelial cells release vasorelaxing factors, such as nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarizing factor (EDHF), and these play an important role in the regulation of vascular tone, vascular permeability and blood coagulation, thus helping to maintain circulatory homeostasis (Vallance et al. 1989; Moncada et al. 1991; Kuriyama et al. 1998). Pre-eclampsia is a pregnancy-specific syndrome characterized by marked increases in peripheral vascular resistance and vascular permeability together with a disturbance of blood coagulation (Lenfant et al. 1990; Cines et al. 1998; Cunningham, 2001). Among the endothelium-derived relaxing factors, an abnormality in the role played by endothelium-derived NO has been suggested in resistance arteries in pre-eclampsia (Poston et al. 1995; Sladek et al. 1997; Suzuki et al. 2000), while the function of EDHF seems to be preserved in some human resistance arteries in this condition (Pascoal et al. 1998; Suzuki et al. 2000).
Prostacyclin is synthesized in vascular endothelium and is known to be a potent vasodilator as well as a powerful inhibitor of platelet aggregation (Moncada et al. 1976). During pregnancy, the synthesis of 6-keto-PGF1α (a stable metabolite of prostacyclin) has been found to be increased in fetoplacental and umbilical tissues and is thought to play an important role in the regulation of not only the maternal but also the fetal blood circulation in pregnancy (Remuzzi et al. 1979). In pre-eclampsia, the urinary and blood concentrations of 6-keto-PGF1α are significantly decreased by comparison with those in normotensive pregnant women (Goodman et al. 1982; Fitzgerald et al. 1987). Furthermore, the production of placental 6-keto-PGF1α in pre-eclampsia is less than one-half that seen in normal pregnant women (Walsh, 1985), suggesting that in pre-eclampsia, a reduced function of endothelium-derived prostacyclin occurs in vascular beds. However, contrary evidence has also been reported in that, in pre-eclampsia, the endothelium-dependent relaxation mediated by prostaglandins (PGs) is not modified in the arteries of subcutaneous fat, although the endothelium-dependent relaxation mediated by NO is reduced (compared with that in normotensive pregnant women; Knock & Poston, 1996). Thus, it remains to be clarified whether or not the role played by endothelium-derived prostacyclin is changed in resistance arteries in pre-eclampsia. Furthermore, if this happens, the underlying mechanism has not yet been clarified.
To examine this issue, we first investigated the effect of bradykinin (to stimulate endothelial release of PGs and EDHF) on the contraction induced by the stable thromboxane A2 analogue, 9,11-epithio-11,12-methano-thromboxane A2 (STA2; Kanmura et al. 1987), in the presence of the NO synthase inhibitor, NG-nitro-l-arginine (l-NNA, to prevent production of endothelial NO), in endothelium-intact strips from omental resistance arteries from normotensive pregnant and pre-eclamptic women. Next, we examined the effect of bradykinin on the smooth muscle cell membrane potential (to look for a change in the effect of PGs on the membrane potential) in endothelium-intact strips treated with l-NNA. Finally, we measured the production of 6-keto-PGF1α in the absence and presence of bradykinin in l-NNA-treated, endothelium-intact strips from both groups of women.
Methods
Preparations
The procedures used in this study conformed to the standards set by the Declaration of Helsinki and were approved by the institutional review boards of Nagoya City University Medical School. Written informed consent was obtained from all patients. Muscle strips with intact endothelium were cut from omental resistance arteries (outer diameter, 0.1-0.3 mm) obtained from 25 normotensive pregnant and 13 pre-eclamptic women at Caesarean section. Pre-eclampsia was diagnosed according to the criteria laid down by the National High Blood Pressure Education Program (Lenfant et al. 1990; Table 1). Women with a history of smoking or a history complicated by certain diseases (diabetes mellitus, renal disease, collagen disorders or infections) were excluded from both groups since the function of the endothelium is known to be changed by smoking and by these diseases. Two out of 13 pre-eclamptic patients took oral hydralazine (to decrease their blood pressure) after the onset of pre-eclampsia. The other 11 pre-eclamptic women received no medication except anaesthetic agents (for the surgical operation). No medication (except anaesthetic) was received by the normotensive pregnant women. Among the normotensive pregnant women, two received 2.5 ml of 0.5 % bupivacaine to induce spinal anaesthesia while the other 23 received ketamine (5 mg kg−1, given intramuscularly) for general anaesthesia. In pre-eclamptic patients, thiopentone (5 mg kg−1, given intravenously) was used for general anaesthesia (2 patients) and 2.5 ml of 0.5 % bupivacaine for spinal anaesthesia (11 patients).
Table 1.
Patient details
| Normotensive pregnant | Pre-eclampsia | |
|---|---|---|
| n | 25 | 13 |
| Age (years) | 30 ± 1 | 30 ± 2 |
| Parity (primiparous/multiparous) | 15/10 | 12/3 |
| Gestational age (weeks) | 39 ± 1 | 37 ± 1 |
| Blood pressure | ||
| Systolic (mmHg) | 110 ± 3 | 157 ± 4* |
| Diastolic (mmHg) | 72 ± 9 | 101 ± 4* |
| Proteinuria (g l−1) | — | 3.0 ± 0.5 |
| Generalized oedema | 0 | 5 |
Data are expressed as mean ± s.e.m.
Significant vs. normotensive pregnant at P < 0.05 by Student's unpaired t test with F test.
The tissue specimens (≈4 cm2) were immediately placed in Krebs solution and transported to the laboratory. Omental artery segments (3 cm in length) were excised and the tissues were prepared as follows in Krebs solution at room temperature. The connective tissue was carefully removed under a binocular microscope and the artery was cut along its long axis with small scissors so as not to damage the endothelium. Small circularly cut muscle strips with intact endothelium (0.1-0.3 mm in width, 0.05-0.08 mm in thickness, 0.3-0.4 mm in length) were prepared, as described previously (Itoh et al. 1992; Yamakawa et al. 1997; Suzuki et al. 2000). The usefulness of this preparation in investigations of endothelium-dependent responses in small resistance arteries has been reported previously in studies involving membrane potential changes (Yamakawa et al. 1997), the production of prostanoids (Suzuki et al. 1991) or measurement of isometric tension (Suzuki et al. 2000). In some experiments, the endothelium was removed using a small razor blade, as described previously (Itoh et al. 1992; Suzuki et al. 2000) and the lack of endothelial function was pharmacologically verified by the absence of a bradykinin-induced relaxation on the noradrenaline (3 μm)-induced contraction. When an endothelium-intact strip was used, the strip was pretreated with the NO synthase inhibitor, l-NNA, for 90 min before the start of the experiment and l-NNA was then present throughout. The concentration of l-NNA used was 0.3 mm, as in a previous report (Kakuyama et al. 1998). Guanethidine (5 μm, to prevent effects due to release of sympathetic transmitters; Mishima et al. 1984) was present in all the solutions used in the present experiments.
Recording of mechanical responses
An endothelium-intact muscle strip was placed in a chamber with a capacity of 0.3 ml and superfused with Krebs solution at a flow rate of about 2 ml min−1. Both ends of the preparation were fixed and isometric tension was recorded using a strain-gauge transducer (AE801; SensoNor a.s., Horten, Norway), as described previously (Suzuki et al. 2000). A resting tension of 2–3 mg was applied so as to obtain a maximum contraction in 80 mm K+. Each preparation was allowed to equilibrate for 2 h before the start of the experiments. The length, width, thickness and the cross-sectional area of the preparation were measured with the aid of an inverted microscope at ×250 magnification (using a calibrated scale). The transverse cross-sectional area was calculated assuming a rectangular cross-section, as described previously (Itoh et al. 1992; Suzuki et al. 2000). Tension measurements were then expressed in kilonewtons per metre squared (kN m−2).
In previous studies, we found that STA2 (a stable analogue of thromboxane A2, 0.03-10 nm) produced a concentration-dependent contraction in both groups of women, the sensitivity to STA2 of endothelium-intact strips being higher for pre-eclamptic women than for normotensive pregnant women (Suzuki et al. 2000). For this reason, unless otherwise stated, the concentration of STA2 used was 3 nm in strips from normotensive pregnant women and 1 nm in strips from pre-eclamptic women; these concentrations were used to obtain contractions of approximately equal amplitude (Table 2). When oscillations in tension occurred in the presence of STA2, the level was taken to be at the mid-point of the amplitude of the oscillations. When the effect of the cyclooxygenase inhibitor, diclofenac, was to be observed, the concentration used was 3 μm so as to obtain its selective action, as described previously (Yamashita et al. 1999). Strips were pretreated with diclofenac for 60 min after recording a reproducible control response and diclofenac was then present throughout the experiment.
Table 2.
Effect of diclofenac on mechanical responses
| STA2-induced tension | BK-induced relaxation | ||||||
|---|---|---|---|---|---|---|---|
| Maximum (kN m−2) | Maximum (%) | Duration (s) | |||||
| n | STA2 | STA2+ DF | BK | BK + DF | BK | BK + DF | |
| Normotensive pregnant | 7 | 61.5 ± 3.4 | 85.0 ± 7.5* | 81.5 ± 2.9 | 55.9 ± 3.4* | 348 ± 12 | 221 ± 26* |
| Pre-eclampsia | 4 | 57.5 ± 5.4 | 66.8 ± 9.1 | 64.5 ± 2.0† | 57.7 ± 3.5 | 189 ± 10† | 174 ± 30 |
Data are expressed as means ± s.e.m. STA2 was applied to endothelium-intact strips treated with the NO synthase inhibitor, l-NNA. The concentration of the thromboxane A2 mimetic, STA2, was 2 nm for normotensive pregnant women and 1 nm for pre-eclamptic women. Bradykinin (BK, 0.1 μm) was applied for 2 min during the STA2-induced contraction. After recording the control response, the strip was pretreated with cyclooxygenase inhibitor, diclofenac (DF, 3 μm), for 60 min, which was then present throughout the experiment.
Significant at P < 0.05 vs. before application of diclofenac.
P < 0.05 vs. normotensive pregnant women.
Endothelium-dependent relaxation was induced by an application of bradykinin (0.1 μm) during the contraction induced by STA2. For this set of experiments, the preparations were first contracted with STA2 and, after a steady-state contraction had been attained, bradykinin was then applied for 2 min during the on-going STA2-induced contraction. The concentration of bradykinin used was 0.1 μm so as to obtain a reproducible endothelium-dependent relaxation, as reported previously (Suzuki et al. 2000).
To examine the concentration-dependent effects of the stable prostacyclin analogue, beraprost, on the STA2-induced contraction, endothelium-intact strips from both groups of women were treated with l-NNA (0.3 mm) plus diclofenac (3 μm). The preparations were first contracted with STA2 and, after a steady-state contraction had been attained, beraprost (0.1-10 nm) was then cumulatively applied for 2 min at each concentration from low to high concentration during the on-going STA2 -induced contraction.
Recording of membrane potential changes
The membrane potential was measured using a conventional microelectrode technique, as described previously (Yamashita et al. 1999; Ohashi et al. 2000). To enable recording of membrane potentials, an endothelium-intact strip was placed in a chamber of 0.7 ml volume, pinned down to the bottom of the chamber and superfused with warmed (36-37 °C) Krebs solution at a flow rate of about 2 ml min−1. Glass microelectrodes were made from borosilicate glass tubing (o.d., 1.2 mm with a glass filament inside; Hilgenberg, Malsfeld, Germany), then filled with 1 m KCl. The resistance of the electrodes was 80–180 MΩ. The electrode was inserted into smooth muscle cells from the luminal side using a micromanipulator (model MHW-3; Narishige International, Tokyo, Japan). Membrane potentials recorded using an Axoclamp-2B amplifier (Axon Instruments, Foster City, CA, USA) were displayed on a cathode-ray oscilloscope (model VC-6020; Hitachi, Tokyo, Japan) and data were stored at an acquisition rate of 100 Hz using an Axoscope 7.0/Digidata 1200 data acquisition system (Axon Instruments) on an IBM/AT-compatible PC.
To examine its effect on the smooth muscle cell membrane potential, bradykinin (0.1 μm) was applied for 1.5 min to endothelium-intact strips treated with l-NNA (both groups of women). The effects of diclofenac with or without charybdotoxin (CTX, a blocker of intermediate- and large-conductance Ca2+-activated K+ channels) plus apamin (a blocker of small-conductance Ca2+-activated K+ channels; Garland et al. 1995; Marchenko & Sage, 1996) on the bradykinin-induced hyperpolarization were examined as follows. In endothelium-intact, l-NNA-treated strips, bradykinin was applied for 1.5 min (to record the control response) followed by a 30 min washout (to allow the response to recover). Diclofenac (3 μm) was then applied for 60 min and bradykinin was again applied in the presence of diclofenac. Finally, after a 30 min washout with a solution containing diclofenac, CTX (0.1 μm) plus apamin (0.1 μm) were applied for 15 min and bradykinin was then applied in the presence of CTX plus apamin. The concentration of CTX and apamin was 0.1 μm, so as to obtain their selective actions (Garland et al. 1995; Marchenko & Sage, 1996). This series was performed in one and the same strip.
To observe its effect on the smooth muscle cell membrane potential, beraprost (10 nm) was applied for 1.5 min in endothelium-intact strips treated with diclofenac plus l-NNA (both groups of women).
Assay for 6-keto-PGF1α
After equilibration for 2 h in Krebs solution, endothelium-intact strips were transferred to 0.4 ml Krebs solution containing l-NNA (0.3 mm) plus guanethidine (5 μm) and equilibrated for 90 min at 36 °C. Bradykinin (final concentration, 0.1 μm) was then added to the tube for 5 min. For the assay, a 50 μl sample of the solution was taken from the tube, and the concentration of 6-keto-PGF1α-like immunoreactivity was measured using an enzyme immunoassay kit purchased from Amersham Pharmacia Biotech (Tokyo, Japan). The assay protocol for this followed a manual supplied by Amersham Pharmacia Biotech (Yamashita et al. 1999). The strips were homogenized, then centrifuged and the pellets were entered into a protein assay performed using a modified Lowry method (DC protein Assay kit; Bio-Rad, Hercules, CA, USA). The content of 6-keto-PGF1α-like immunoreactivity is expressed per milligram protein for measurements made over a 5 min period with or without bradykinin.
Solutions
The ionic composition of the Krebs solution was as follows (mm): Na+, 137.4; K+, 5.9; Mg2+, 1.2; Ca2+, 2.6; HCO3−, 15.5; H2PO4−, 1.2; Cl−, 134; and glucose, 11.5. The Krebs solution contained guanethidine (5 μm, to prevent noradrenaline outflow from sympathetic nerves) and l-NNA (to prevent the release of NO from the endothelium). High-K+ solution (80 mm) was prepared by replacing sodium chloride with potassium chloride isosmotically. The solutions were bubbled with 95 % oxygen and 5 % carbon dioxide and the pH values were adjusted to 7.3-7.4 using NaOH and HCl.
Drugs
The drugs used were as follows: bradykinin (Peptide Institute Inc., Osaka, Japan), l-NNA and diclofenac sodium (Sigma Chemical Co., St Louis, MO, USA) and guanethidine (Tokyo Kasei, Tokyo, Japan). Beraprost sodium was kindly provided by Yamanouchi Pharmaceutical (Tokyo, Japan) and STA2 by Ono Pharmaceutical (Osaka, Japan). l-NNA was directly diluted in Krebs solution (0.3 mm). STA2 was diluted in absolute ethanol to make a 10 mm stock solution and stored at −80 °C until use. The other agents were dissolved in ultra-pure MilliQ water (Milli-Q SP/Milli RX system; Japan Millipore Corp., Tokyo, Japan).
Statistical analysis
All data are expressed as means ± s.e.m., with the n values representing the number of subjects. The concentration-response curve for the relaxation induced by beraprost on the STA2-induced contraction was obtained by fitting the data points for each strip by a nonlinear least-squares method using commercial software (Kaleida graph; Synergy Software, PA, USA). A two-way repeated-measures ANOVA followed by Scheffé‘s post hoc F test were used for the statistical analysis, or Student's paired and unpaired t tests with an F test were used. Probabilities less than 5 % (P < 0.05) were considered significant.
Results
Effects of diclofenac on STA2-induced contraction
STA2 produced a contraction with oscillations in endothelium-intact strips from both groups of women (Fig. 1A). The magnitude of the force induced by 3 nm STA2 for normotensive pregnant women was not significantly different from that induced by 1 nm STA2 for pre-eclamptic women (P = 0.7349; Table 2). Diclofenac attenuated the oscillations and enhanced the contraction induced by STA2 in strips from normotensive pregnant women (P = 0.015; Table 2 and Fig. 1A). By contrast, in strips from pre-eclamptic women, this agent attenuated the oscillations but did not modify the contraction induced by STA2 (P = 0.349; Table 2 and Fig. 1B).
Figure 1. Effect of diclofenac on bradykinin-induced relaxation.
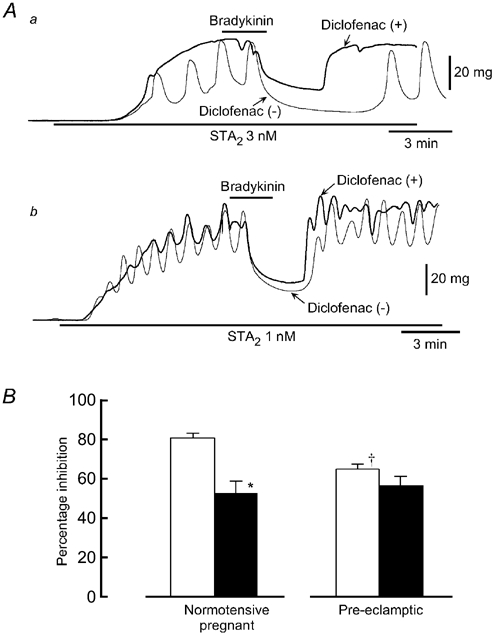
A, original traces of the relaxation induced by bradykinin (0.1 μm) of the contraction induced by the stable thromboxane A2 mimetic, STA2, in endothelium-intact strips from a normotensive pregnant woman (a) and from a pre-eclamptic woman (b). Bradykinin was applied for 2 min as indicated by the bars. After recording the control response (Diclofenac(-)),the strip was pretreated for 60 min with the cyclooxygenase inhibitor, diclofenac. STA2 was then applied in the presence of diclofenac (Diclofenac (+)) and bradykinin was applied. B, summary of the effect of diclofenac on the bradykinin-induced relaxation before (□) and after an application of diclofenac (▪). Results are shown as means ± s.e.m. * P < 0.05 vs. before application of diclofenac (Student's paired t test with an F test). † P < 0.05 vs. normotensive pregnant women (Student's unpaired t test with F test).
In endothelium-denuded strips, STA2 (1 nm) induced a contraction with no oscillations for both groups of women (not shown). In absolute terms, the magnitude of the STA2-induced tension in endothelium-denuded strips was almost the same as that seen in endothelium-intact strips treated with l-NNA plus diclofenac. In endothelium-denuded strips, the value obtained in the presence of 3 nm STA2 for normotensive pregnant women was 100.6 ± 10.6 kN m−2 (n = 4, P = 0.215 vs. endothelium-intact strips treated with l-NNA + diclofenac) and that for pre-eclamptic women in the presence of 1 nm STA2 was 90.5 ± 15.3 kN m−2 (n = 4, P = 0.175). Under our conditions, diclofenac had no effect on the STA2-induced contraction in endothelium-denuded strips from either group of women (P > 0.15 in each case, n = 4).
Effects of diclofenac on bradykinin-induced relaxation
Bradykinin (0.1 μm) produced a relaxation of the contraction induced by STA2 in strips from both normotensive pregnant (Fig. 1Aa) and pre-eclamptic women (Fig. 1Ab). Bradykinin did not produce a relaxation (rather, it produced a contraction) in endothelium-denuded strips from either group (n = 4, data not shown). Both the magnitude and the duration of the bradykinin-induced relaxation were significantly smaller for pre-eclamptic women than for normotensive pregnant women (for the magnitude, P = 0.002 and for the duration, P < 0.001; Table 2). Diclofenac (3 μm) significantly attenuated both the magnitude and the duration of the relaxation induced by bradykinin in normotensive pregnant women (for the magnitude, P < 0.001 and for the duration, P = 0.001). By contrast, in strips from pre-eclamptic women, diclofenac modified neither the magnitude nor the duration of the relaxation induced by bradykinin (for the magnitude, P = 0.098 and for the duration, P = 0.604). The bradykinin-induced relaxation observed in the presence of diclofenac was not significantly different between the two groups (for the magnitude, P = 0.717 and for the duration, P = 0.243; Fig. 1B and Table 2).
Effect of bradykinin on membrane potential
The resting membrane potential of smooth muscle cells in endothelium-intact strips was −59.9 ± 1.3 mV for normotensive pregnant women (n = 9) and −56.0 ± 1.1 mV for pre-eclamptic women (n = 6), and these values were not significantly different from each other (P = 0.053). Under our conditions, bradykinin (0.1 μm) produced an initial, followed by a second phase of membrane hyperpolarization in both groups of women. The initial phase was not significantly different between the two groups (P = 0.5936), but the second phase was significantly smaller for pre-eclamptic women than that for normotensive pregnant women (P = 0.041; Fig. 2 and Table 3).
Figure 2. Bradykinin-induced hyperpolarization.
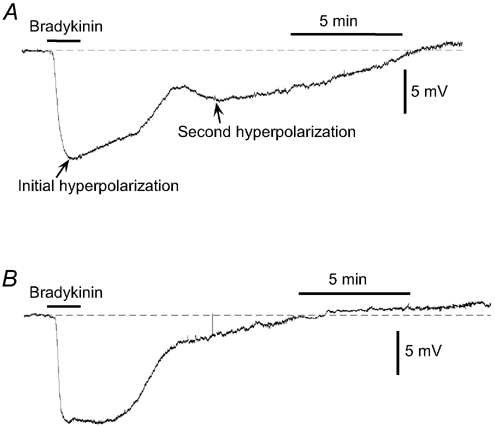
Original traces of the effect of bradykinin on membrane potential in smooth muscle cells of endothelium-intact omental resistance arteries from a normotensive pregnant woman (A) and a pre-eclamptic woman (B). Bradykinin (0.1 μm) was applied for 1.5 min as indicated by the bar. All experiments were carried out in the presence of the NO synthase inhibitor, l-NNA (0.3 mm), and guanethidine (5 μm, to prevent noradrenaline outflow from sympathetic nerves), both of which were applied as pretreatments for 90 min before the start of the experiment. Dashed lines indicate the resting membrane potential level in the presence of l-NNA + guanethidine.
Table 3.
Effect of diclofenac with and without CTX + apamin on bradykinin-induced hyperpolarization
| Normotensive pregnant (mV) | Pre-eclampsia (mV) | |
|---|---|---|
| I. Resting membrane potential | ||
| Control | −59.9 ± 1.3 (9) | −56.0 ± 1.1 (6) |
| +DF | −55.1 ± 0.8†(9) | −53.2 ± 1.2 (6) |
| +DF + CTX + apamin | −51.8 ± 0.4‡(4) | −49.4 ± 0.7‡(4) |
| II. Bradykinin-induced hyperpolarization | ||
| Control | ||
| initial hyperpolarization | −12.4 ± 1.4 (6) | −11.3 ± 1.2 (6) |
| second hyperpolarization | −4.3 ± 0.7 (6) | −2.6 ± 0.4* (6) |
| +DF | ||
| initial hyperpolarization | −11.5 ± 1.8 (6) | −16.4 ± 1.0 (4) |
| second hyperpolarization | −0.4 ± 0.6†(6) | −0.4 ± 1.6 (4) |
| +DF + CTX + apamin | ||
| initial hyperpolarization | −0.6 ± 0.6‡(4) | −1.2 ± 1.2‡ (3) |
Data are expressed as mean ± s.e.m. The number of women is given in parentheses. The initial hyperpolarization was calculated as the maximum hyperpolarization obtained within a 3 min period after application of bradykinin, and the second hyperpolarization was calculated as the maximum hyperpolarization obtained within a 5–17 min period after washout of bradykinin. The cyclooxygenase inhibitor, diclofenac (DF, 3 μm), was given as pretreatment for 60 min and was present throughout the experiment. Charybdotoxin (CTX) + apamin (Ca2+-activated K+ channel inhibitors) were given as pretreatment for 15 min in the presence of diclofenac and were present thereafter.
P < 0.05 vs. normotensive pregnant women.
P < 0.05 vs. before application of diclofenac.
P < 0.05 vs. before application of CTX + apamin.
Diclofenac slightly depolarized the membrane for normotensive pregnant women (P = 0.035) but had no effect for pre-eclamptic women (P = 0.097; Table 3). Figure 3 shows the effect of diclofenac on the bradykinin-induced hyperpolarization in endothelium-intact strips from normotensive pregnant women. Diclofenac had no effect on the bradykinin-induced initial phase of hyperpolarization for either group of women (for normotensive pregnant women, P = 0.5936 and for pre-eclamptic women, P = 0.054) and it significantly inhibited the second phase of the hyperpolarization for normotensive pregnant women only (P < 0.01; Table 3). In the presence of diclofenac, charybdotoxin (CTX, 0.1 μm) plus apamin (0.1 μm) depolarized the membrane (for normotensive pregnant women, P = 0.003 and for pre-eclamptic women, P = 0.003) and greatly attenuated the bradykinin-induced initial hyperpolarization (P < 0.001 in each case; Table 3).
Figure 3. Effect of diclofenac without and with charybdotoxin + apamin on bradykinin-induced hyperpolarization in an endothelium-intact strip from a normotensive pregnant woman.
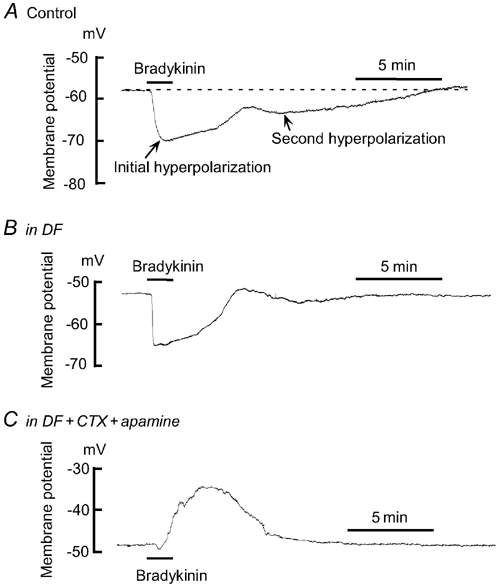
A, original trace of the effect of bradykinin (0.1 μm) on membrane potential in smooth muscle cells of an endothelium-intact strip from a normotensive pregnant woman (Control). B, effect of the cyclooxygenase inhibitor, diclofenac (DF). After recording the control response (shown in A), the strip was pretreated with DF (3 μm) and bradykinin was applied in the presence of DF. C, effect of the Ca2+-activated K+ channel inhibitors, charybdotoxin (CTX) + apamin, in the presence of diclofenac. In the presence of diclofenac, the strip was pre-treated for 15 min with CTX (0.1 μm) + apamin (0.1 μm) and bradykinin was applied in the presence of DF + CTX + apamin.
Effect of beraprost on electrical and mechanical activities
In endothelium-intact strips treated with l-NNA (0.3 mm) plus diclofenac (3 μm), beraprost (0.1-10 nm) produced a concentration-dependent relaxation of the contraction induced by STA2 in both groups of women. The relaxation response to beraprost was not significantly different between the two groups (by a two-way repeated-measures ANOVA, P = 0.996; Fig. 4).
Figure 4. Effect of beraprost on STA2-induced contraction.
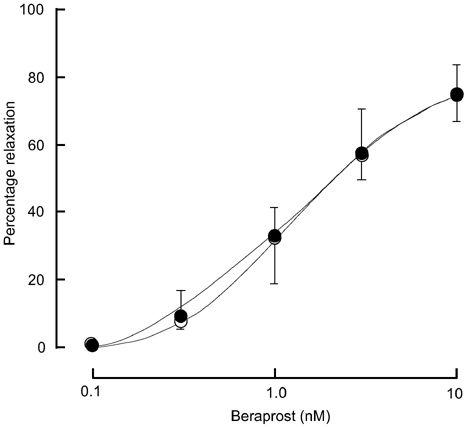
A concentration-dependent relaxation in response to a stable analogue of prostacyclin, beraprost (0.1-10 nm), was obtained during the on-going STA2-induced contraction in endothelium-intact strips treated with l-NNA + diclofenac from normotensive pregnant women (○, n = 6) and from pre-eclamptic women (•, n = 5). Results are shown as means ± s.e.m.
In endothelium-intact strips treated with l-NNA (0.3 mm) plus diclofenac (3 μm), beraprost (10 nm) produced a hyperpolarization in both groups of women. The hyperpolarization was by −12.8 ± 2.2 mV for normotensive pregnant women (n = 5) and by −11.7 ± 1.3 mV for pre-eclamptic women (n = 4), and these values were not significantly different (P = 0.645).
Effect of bradykinin on prostacyclin synthesis
In endothelium-intact strips treated with l-NNA (0.3 mm), the basal concentration of 6-keto-PGF1α was significantly lower for pre-eclamptic women (0.45 ± 0.15 ng (mg protein)−1 (5 min)−1, n = 8) than for normotensive pregnant women (1.39 ± 0.27 ng (mg protein)−1 (5 min)−1, n = 12, P = 0.007; Fig. 5). Bradykinin (0.1 μm) significantly increased the concentration of 6-keto-PGF1α in both groups of women but the concentration recorded in the presence of bradykinin was significantly lower for pre-eclamptic women (3.54 ± 0.92 ng (mg protein)−1 (5 min)−1, n = 5) than for normotensive pregnant women (19.78 ± 1.81 ng (mg protein)−1 (5 min)−1, n = 4, P < 0.001; Fig. 5).
Figure 5. Effect of bradykinin on the synthesis of prostacyclin.
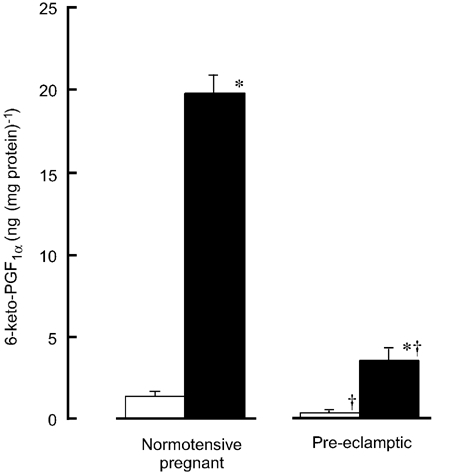
Bradykinin (0.1 μm) was applied for 5 min in endothelium-intact strips treated with l-NNA from normotensive pregnant and pre-eclamptic women. Data show the concentration of a stable metabolite of prostacyclin, 6-keto-PGF1α, in the absence (□) and presence of bradykinin (▪). Results are shown as means ± s.e.m. * P < 0.05 vs. in the absence of bradykinin (Student's unpaired t test with an F test). † P < 0.05 vs. normotensive pregnant women (Student's unpaired t test with an F test).
Discussion
Pregnancy is associated with profound physiological alterations in vascular behaviour, including a marked vasodilatation and a refractoriness to vasopressor responses (Friedman, 1988). The vascular adaptations to pregnancy are thought to depend to a large extent on an increase in the function of endothelium-derived vasorelaxing substances, such as prostacyclin, NO and EDHF (Friedman, 1988; Knock & Poston, 1996; Sladek et al. 1997; Pascoal et al. 1998; Suzuki et al. 2000). Pre-eclampsia is a human pregnancy-specific syndrome that is characterized by a marked increase in peripheral vascular resistance with an abnormality in the role played by endothelium-derived vasorelaxing substances, especially NO (Lenfant et al. 1990; Poston et al. 1995; Sladek et al. 1997; Suzuki et al. 2000).
Change in the role played by spontaneously released PGs in pre-eclampsia
It has been found that, in the blood of pre-eclamptic women, the lipid peroxides that inhibit prostacyclin synthase are abnormally increased, while the concentration of 6-keto-PGF1α (a stable metabolite of prostacyclin) is significantly reduced (Whorton et al. 1985; Wang et al. 1992). In the present experiments, in endothelium-intact omental resistance arteries treated with the NO synthase inhibitor, l-NNA, cyclooxygenase inhibition by diclofenac significantly enhanced the contraction induced by a stable analogue of thromboxane A2 (STA2) for normotensive pregnant women but not for pre-eclamptic women. This action of diclofenac was not seen in endothelium-denuded strips. Furthermore, diclofenac depolarized the membrane of smooth muscle cells in endothelium-intact strips for normotensive pregnant women but not for pre-eclamptic women. Moreover, in endothelium-intact strips, the basal concentration of 6-keto-PGF1α was significantly lower for pre-eclamptic women than for normotensive pregnant women although the stable prostacyclin analogue, beraprost, at a low concentration (10 nm), produced a hyperpolarization of the same magnitude in the smooth muscle cells from both groups of women. These results suggest that the role played by spontaneously released prostacyclin from the endothelium may be reduced in resistance arteries in pre-eclampsia (by comparison with the situation in normotensive pregnant women).
Change in the role played by PGs in the presence of bradykinin in pre-eclampsia
It has been found that, in addition to EDHF, endothelium-derived PGs also hyperpolarize the membrane in vascular preparations from some animals and humans (Nishiyama et al. 1998; Yamashita et al. 1999; Coleman et al. 2001). In the present experiments, we found that, in endothelium-intact human omental resistance arteries, bradykinin produced an initial, followed by a second phase of membrane hyperpolarization in smooth muscle cells from both normotensive pregnant and pre-eclamptic women. Since these strips were treated with l-NNA, the role of endothelium-derived NO in these hyperpolarizations can be ruled out. Diclofenac inhibited the second phase (but not the initial phase) of the hyperpolarization, and the initial phase remaining in the presence of diclofenac was blocked by a combined application of CTX (a blocker of intermediate- and large-conductance Ca2+-activated K+ channels) plus apamin (a blocker of small-conductance Ca2+-activated K+ channels; Garland et al. 1995; Marchenko & Sage, 1996). These results suggest that, in human omental resistance arteries, the bradykinin-induced initial hyperpolarization is due to an action of EDHF while the second phase is mediated by the action of PGs. Most importantly, we found that, in endothelium-intact strips treated with l-NNA, the bradykinin-induced PGs-mediated hyperpolarization (but not the EDHF-mediated one) was significantly smaller for pre-eclamptic women than for normotensive pregnant women. Furthermore, under our conditions, diclofenac inhibited the bradykinin-induced relaxation only for normotensive pregnant women. Taken together, these results suggest that the functional role played by endothelium-derived PGs is reduced in omental resistance arteries in pre-eclampsia.
Although bradykinin significantly increased the concentration of 6-keto-PGF1α in endothelium-intact omental arteries treated with l-NNA from both groups of women, the concentrations of this metabolite recorded in the absence and presence of bradykinin were significantly smaller for pre-eclamptic women than for normotensive pregnant women. However, in endothelium-intact strips treated with l-NNA plus diclofenac, both the relaxation of the STA2-induced contraction and the hyperpolarization in response to beraprost (a stable analogue of prostacyclin, 10 nm) were identical between the two groups, indicating that the action of prostacyclin on smooth muscle is not modified in pre-eclampsia. Although, so far, there is no available supporting evidence in pregnant human resistance arteries, these results suggest that a reduction in the synthesis of prostacyclin in the endothelium could be responsible for the reduced endothelium-dependent, PGs-mediated relaxation seen in omental resistance arteries in pre-eclampsia. However, since vessel function may vary among vascular beds, the overall significance of the present findings will remain uncertain until vessels from other resistance arteries (such as uterine arteries) have been evaluated. On this point, it should be noted that in arteries of subcutaneous fat, the bradykinin-induced prostanoids-dependent relaxation is apparently not modified in pre-eclampsia (compared with the response in normotensive pregnant women; Knock & Poston, 1996). We do not know the reason for the discrepancy but it may, in part, be due to the use of different resistance arteries (omental arteries vs. subcutaneous fat arteries) and/or different vasospasmogenic agents (STA2vs. noradrenaline). This remains to be clarified in future work.
Role played by EDHF in pre-eclampsia
The accumulated evidence supports the hypothesis that EDHF may play an important role in agonist-stimulated, endothelium-dependent relaxation in pregnant human resistance arteries (Knock & Poston, 1996; Pascoal et al. 1998; Suzuki et al. 2000).
It was recently found that, in human omental resistance arteries, bradykinin produces an endothelium-dependent relaxation through an action of NO- and PGs-independent factors (Pascoal et al. 1998). Since the action of these factors was blocked by CTX plus apamin, it was suggested that this endothelium-dependent relaxation is mediated by an action of EDHF (Suzuki et al. 2000), although there was no direct proof for bradykinin-induced membrane hyperpolarization in human pregnant resistance arteries. Furthermore, and more importantly, these authors suggested that the function of EDHF may not be modified in pre-eclampsia (as compared with the response in normotensive pregnant women; Pascoal et al. 1998; Suzuki et al. 2000). In the present experiments, we demonstrated that, in pregnant human omental resistance arteries, bradykinin does indeed produce a smooth muscle cell membrane hyperpolarization which is sensitive to CTX plus apamin, and is thus a supposed action of EDHF. Most importantly, the magnitude of the hyperpolarization mediated by EDHF in omental resistance arteries in pre-eclampsia is similar to the response seen in normotensive pregnant women. Thus, in accord with the previous notion, it is suggested that the function of EDHF remains normal in these human resistance arteries in pre-eclampsia. It is known that an increase in the intracellular concentration of Ca2+ ([Ca2+]i) in endothelial cells plays a crucial role in mediating agonist-induced responses, such as those induced not only by EDHF but also by PGs in smooth muscle cells (Kuriyama et al. 1998). We found that the synthesis of PGs induced by bradykinin (but not the EDHF response) is reduced in omental resistance arteries in pre-eclampsia (compared with that in normotensive pregnant women). Taken together, these pieces of evidence suggest that the reduced synthesis of PGs seen in pre-eclamptic women may not be due simply to a reduction in the bradykinin-induced increase in [Ca2+]i in endothelial cells. The underlying mechanism still needs to be clarified in future work. Furthermore, the present results suggest that, in pre-eclamptic women, caution should be used in the employment of a cyclooxygenase inhibitor (especially at high concentrations) under conditions in which visceral blood flow needs to be kept constant.
In conclusion, in human omental resistance arteries, the synthesis of prostacyclin in endothelial cells is reduced in pre-eclampsia and thus the relaxation mediated by endothelium-derived PGs in the presence of bradykinin is down-regulated. When taken together with our previous findings (Suzuki et al. 2000), this suggests that in pre-eclampsia, the function not only of NO but also of PGs in endothelium-dependent relaxation is down-regulated, while the function of EDHF remains normal.
Acknowledgments
We thank Dr Robert J. Timms for a critical reading of the manuscript. We also thank Drs K. Teshigawara, S. Kajiura, K. Kojima and T. Wada and the staff of the delivery suites at Nagoya City University Hospital for their cooperation in this study. This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, by a grant from the JAOG Ogyaa Donation Foundation, Tokyo, Japan and by a Grant-in-Aid for Research in Nagoya City University.
References
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. Journal of Physiology. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FG. Hypertensive disorders in pregnancy. In: Cunningham FG, Gant NF, Gilstrap LC III, Hauth JC, Wenstrom KD, editors. Williams Obstetrics. New York: McGraw-Hill; 2001. pp. 567–618. [Google Scholar]
- Fitzgerald DJ, Entman SS, Mulloy K, Fitzgerald GA. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation. 1987;75:956–963. doi: 10.1161/01.cir.75.5.956. [DOI] [PubMed] [Google Scholar]
- Friedman SA. Preeclampsia: a review of the prostaglandins. Obstetrics and Gynecology. 1988;71:122–137. [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends in Pharmacological Sciences. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Goodman RP, Killam AP, Brash AR, Branch RA. Prostacyclin production during pregnancy: comparison of production during normal pregnancy and pregnancy complicated by hypertension. American Journal of Obstetrics and Gynecology. 1982;142:817–822. doi: 10.1016/s0002-9378(16)32525-x. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kajikuri J, Kuriyama H. Characteristic features of noradrenaline-induced Ca2+ mobilization and tension in arterial smooth muscle of the rabbit. Journal of Physiology. 1992;457:297–314. doi: 10.1113/jphysiol.1992.sp019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuyama M, Vallance P, Ahluwalia A. Endothelium-dependent sensory NANC vasodilatation: involvement of ATP, CGRP and a possible NO store. British Journal of Pharmacology. 1998;123:310–316. doi: 10.1038/sj.bjp.0701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y, Itoh T, Kuriyama H. Mechanisms of vasoconstriction induced by 9,11,epithio-11,12-methano-thromboxane A2 in the rabbit coronary artery. Circulation Research. 1987;60:402–409. doi: 10.1161/01.res.60.3.402. [DOI] [PubMed] [Google Scholar]
- Knock GA, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. American Journal of Obstetrics and Gynecology. 1996;175:1668–1674. doi: 10.1016/s0002-9378(96)70123-0. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiological Reviews. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- Lenfant C, Gifford RW, Jr, Zuspan FP. National high blood pressure education program working group report on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology. 1990;163:1691–1712. doi: 10.1016/0002-9378(90)90653-o. [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Sage SO. Calcium-activated potassium channels in the endothelium of intact rat aorta. Journal of Physiology. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Miyahara H, Suzuki H. Transmitter release modulated by α-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. British Journal of Pharmacology. 1984;83:537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- Nishiyama M, Hashitani H, Fukuta H, Yamamoto Y, Suzuki H. Potassium channels activated in the endothelium-dependent hyperpolarization in guinea-pig coronary artery. Journal of Physiology. 1998;510:455–465. doi: 10.1111/j.1469-7793.1998.455bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M, Dohi Y, Itoh T. Possible mechanisms underlying the vasodilatation induced by olprinone, phosphodiesterase III inhibitor, in rabbit coronary artery. British Journal of Pharmacology. 2000;129:1000–1006. doi: 10.1038/sj.bjp.0703125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoal IF, Lindheimer MD, Nalbantian-Brandt C, Umans JG. Preeclampsia selectively impairs endothelium-dependent relaxation and leads to oscillatory activity in small omental arteries. Journal of Clinical Investigation. 1998;101:464–470. doi: 10.1172/JCI557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacology and Therapeutics. 1995;65:215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Misiani R, Muratore D, Marchesi D, Livio M, Schieppati A, Mecca G, De Gaetano G, Donati MB. Prostacyclin and human fetal circulation. Prostaglandins. 1979;18:341–347. doi: 10.1016/s0090-6980(79)80052-0. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. American Journal of Physiology. 1997;272:R441–463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kajikuri J, Itoh T. Effects of endothelin-1 on endothelial cells in the porcine coronary artery. Circulation Research. 1991;69:1361–1368. doi: 10.1161/01.res.69.5.1361. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kajikuri J, Suzumori K, Itoh T. Mechanisms underlying the reduced endothelium-dependent relaxation in human omental resistance arteries in pre-eclampsia. Journal of Physiology. 2000;527:163–174. doi: 10.1111/j.1469-7793.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;336:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. American Journal of Obstetrics and Gynecology. 1985;152:235–240. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Walsh SW, Kay HH. Placental lipid peroxidase and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. American Journal of Obstetrics and Gynecology. 1992;167:946–949. doi: 10.1016/s0002-9378(12)80017-2. [DOI] [PubMed] [Google Scholar]
- Whorton AR, Montogomery ME, Kent RS. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. Journal of Clinical Investigation. 1985;76:295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa N, Ohhashi M, Waga S, Itoh T. Role of endothelium in regulation of smooth muscle membrane potential and tone in the rabbit middle cerebral artery. British Journal of Pharmacology. 1997;121:1315–1322. doi: 10.1038/sj.bjp.0701285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Kajikuri J, Ohashi M, Kanmura Y, Itoh T. Inhibitory effects of propofol on acetylcholine-induced, endothelium-dependent relaxation and prostacyclin synthesis in rabbit mesenteric resistance arteries. Anesthesiology. 1999;91:1080–1089. doi: 10.1097/00000542-199910000-00029. [DOI] [PubMed] [Google Scholar]


