Abstract
It is still not known whether leptin, an adipocyte-derived hormone, acts directly within the hypothalamus to stimulate the gonadotropin-releasing hormone (GnRH)-luteinizing hormone (LH) system. In order to address this question, the present study examined the effects of direct intrahypothalamic perfusions with leptin on the in vivo release of GnRH in ovarian steroid-primed ovariectomized rats utilizing the push-pull perfusion technique. Both α-melanocyte-stimulating hormone (α-MSH) and neuropeptide Y were also measured in the hypothalamic perfusates. In normally fed animals, the leptin infusion was without effect on the release of these three hypothalamic peptides and also without effect on plasma LH and prolactin (PRL), whether leptin was infused into the medial preoptic area (where the majority of GnRH neuronal cell bodies exist) or the median eminence-arcuate nucleus complex (where axon terminals of GnRH neurons are located). In contrast, in 3-day fasted rats leptin was effective in stimulating the secretion of GnRH, α-MSH, and LH, regardless of the site of perfusion. These three hormones were increased in a temporal order of α-MSH, GnRH and LH. Irrespective of the site of perfusion, leptin was without effect on the release of neuropeptide Y. Only when leptin was infused into the median eminence-arcuate nucleus complex was PRL secretion also stimulated, although its onset was 1 h behind that of LH. The leptin-induced elevations of GnRH, α-MSH, LH and PRL were all dose-dependently stimulated by subnormal (1.0 ng ml−1) and normal (3.0 ng ml−1) concentrations of leptin, but at higher concentrations (10 ng ml−1) it did not produce additional effects. Leptin infusion into the anterior hypothalamic area, a control site equidistant from both the medial preoptic area and the median eminence-arcuate nucleus complex, did not produce a significant change in any of the hormones in either the fed or fasted rats. These results demonstrate for the first time that leptin can act at both the cell bodies and axon terminals of GnRH neurons to stimulate the release of the neurohormone in vivo, and they also suggest that α-MSH may play a significant intermediary role in linking leptin and GnRH secretion.
Leptin, a polypeptide hormone secreted by adipose tissue, plays an important role in body weight homeostasis through reducing food intake and increasing thermogenesis. The adipose hormone is transported via the circulatory system into the CNS where it acts on leptin receptors (Fruhbeck et al. 2001). Increasing evidence suggests that leptin may also play a significant role in the regulation of a variety of neuroendocrine functions (Smith et al. 2002). Amongst the neuroendocrine actions of leptin, its stimulating effect on the hypothalamo-pituitary-gonadal axis seems to be of particular physiological significance in consideration of the well-established causal link between nutritional status and reproduction (Wade et al. 1996; Ahima et al. 2000). It is thus proposed that leptin may serve as a metabolic regulator of reproductive capability by signalling to the brain the amount of energy stored in the body (Smith et al. 2002).
Previous studies in vitro reported that leptin acts directly in both the hypothalamus and the pituitary to stimulate the release of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH), respectively (Yu et al. 1997a, b). However, the well-known reduction of LH levels in food-restricted animals, a phenomenon supposed to be mediated, at least in part, by fasting-induced hypoleptinaemia, is believed ultimately, to be a consequence of the decreased release of GnRH from the hypothalamus (Smith et al. 2002). It is well established that the hypothalamus is a site in the brain where leptin receptors are abundantly expressed (Mercer et al. 1996b; Schwartz et al. 1996; Fei et al. 1997; Elmquist et al. 1998; Friedman & Halaas, 1998). Although several isoforms of leptin receptor identified to date were all reported to mediate signal transduction (Bjorbaek et al. 1997), the long-form receptor is considered to play a major role in intracellular signalling (Uotani et al. 1999). The long isoform of the leptin receptor is especially abundant in the arcuate nucleus (ARC), the dorsomedial, paraventricular and ventromedial nuclei and the lateral hypothalamus (Mercer et al. 1996b; Fei et al. 1997; Elmquist et al. 1998).
Although a few reports have provided indirect evidence for the facilitatory action of leptin on GnRH secretion in vivo (Carro et al. 1997; Smith et al. 2002), no previous study has demonstrated it directly. In order to clarify this unresolved but important issue, the author has employed the technique of push-pull perfusion (PPP) of the rat hypothalamus as in previous studies (Watanobe & Takebe, 1993a, b, 1994). Utilizing sex steroid-primed ovariectomized rats that have dioestrous levels of oestradiol and progesterone in general circulation, in vivo influences of leptin infused directly into the medial preoptic area (MPOA) and the median eminence (ME)-ARC complex on the release of GnRH at these sites of the hypothalamus have been studied. The MPOA is the site where the majority of the GnRH neuronal cell bodies exist, and the ME-ARC is the anatomical structure where the axonal fibres of GnRH neurons are terminated before the neurohormone is released into the portal circulation (Lantos et al. 1995). In the hypothalamic perfusates, both α-melanocyte-stimulating hormone (α-MSH) and neuropeptide Y (NPY) were also measured. This is because both neurohormones have been implicated in mediating the actions of leptin on feeding and reproduction (Kalra et al. 1999). With respect to hormones in general circulation, alterations not only in LH but also prolactin (PRL) were determined before and during the leptin infusion. This was done in consideration of several previous studies including those of the author and others reporting a stimulating action of leptin on PRL secretion (Yu et al. 1997a; Gonzalez et al. 1999; Kohsaka et al. 1999a; Watanobe et al.1999a,b,2000).
Furthermore, in the present study, the author compares the hormonal effects of leptin infusion between fed and fasted rats. This attempt was made based on previous reports that the hypothalamo-pituitary-gonadal axis showed differential responses to exogenous leptin in fed vs. food-restricted animals (Cheung et al. 1997b; Henry et al. 1999, 2001; Nagatani et al. 2001).
Methods
Animals and PPP protocol
All the following procedures were approved by the Ethical Committee for Animal Experimentation of the International University of Health and Welfare. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Female rats (240-250 g) of the Wistar strain were used. They were housed in an air-conditioned room with controlled lighting (light on from 08:00 to 20:00 h) and were given free access to laboratory chow and tap water. Two weeks before PPP, the animals were bilaterally ovariectomized under anaesthesia with sodium pentobarbitone (pentobarbital; 40 mg (kg body wt)−1, i.p.). At the same time, a guide cannula with a removable inner stylet was stereotaxically implanted in the MPOA, the ME-ARC, or the anterior hypothalamic area (AHA), consistently on the right side. The AHA was chosen as a control site of perfusion, which is equidistant from both the MPOA and the ME-ARC. Stereotaxic co-ordinates for cannula placement were taken from the atlas of Pellegrino et al. (1979). Co-ordinates for the MPOA were 1.8 mm anterior to and 1.0 mm lateral to the bregma, and 7.8 mm ventral from the dura. Co-ordinates for the ME-ARC were 0.0 mm anterior to and 0.5 mm lateral to the bregma, and 9.8 mm ventral from the dura. Co-ordinates for the AHA were 0.8 mm anterior to and 1.2 mm lateral to the bregma, and 8.8 mm ventral from the dura. According to these stereotaxic co-ordinates, the tip of the guide cannula in the AHA was expected to be about 1.5 mm away from those in both the MPOA and the ME-ARC. The PPP cannulae used were the same as described in previous studies (Watanobe & Takebe, 1993a, b, 1994). The device was fixed on to the skull with anchor screws and dental cement. By 7 days after ovariectomy and PPP cannula placement, the body weight of each animal had returned to the presurgical level. On this postoperative day, all animals started receiving hormone replacement with both oestradiol and progesterone. The oestradiol treatment was administered using a s.c. implantation of a single silastic capsule (inner diameter, 1.5 mm; outer diameter, 3.0 mm; length, 25 mm; Dow Corning, Midland, MI, USA) containing oestradiol-17β (Sigma Chemical Company, St Louis, MO, USA) dissolved in peanut oil at 20 μg ml−1. This treatment has previously been reported to produce physiological levels of plasma oestradiol (about 30 pg ml−1) in ovariectomized rats for a period of at least 2 weeks (Cagampang et al. 1991). This concentration of oestradiol corresponds to that found on dioestrus day 2 of the oestrous cycle, and is too low to elicit surge-like secretions of LH and PRL (Freeman, 1988). A daily s.c. injection of progesterone (Sigma) dissolved in peanut oil was given at a dose of 5 mg (kg body wt)−1, which is the physiological dose used in previous studies (Watanobe & Takebe, 1987; Watanobe & Suda, 1999). The last injection of progesterone was performed 24 h before starting the PPP experiment. The MPOA, ME-ARC, and AHA groups were each divided into two subsets. One subset was allowed to feed ad libitum (fed rats), and the other subset was deprived of food for 3 days (fasted rats) until the day of PPP. Two days prior to PPP and under light ether anaesthesia, all animals were implanted with a jugular vein catheter filled with heparin solution.
At about 08:00 h on the day of PPP, an extension of the jugular vein catheter was installed for frequent blood sampling and the inner stylet within the guide cannula was replaced with the inner cannula perfusion assembly. Thereafter, artificial cerebrospinal fluid (ACSF) that had the same composition as in previous reports (Watanobe & Takebe, 1993a, b, 1994) was infused through the push cannula and collected from the pull cannula at a flow rate of 15 μl min−1. The dead space of the pull system (from the tip of the guide cannula to the distal end of the pull tubing) was adjusted to 150 μl (corresponding to a 10 min period of perfusion) so that each blood sample could be drawn in the middle of each time period for perfusate collection. Until the experiment was over, not only the fasted but also the fed groups were deprived of food, although they were given free access to drinking water. After a 3 h equilibration period, blood samples (200 μl) to measure LH and PRL were collected from the freely moving animals every 20 min between 11:00 and 17:00 h. At 11:00 h only, an additional 250 μl of blood was drawn to measure leptin, oestradiol and progesterone also. An equivalent volume of red blood cells taken from donor rats was suspended in 0.9 % NaCl and replaced through the jugular vein catheter after each blood sampling. Twenty minute perfusion fractions (300 μl) were collected into tubes placed on ice over a total period of 380 min (11:00-17:20 h). The reason for collecting a further perfusate between 17:00 and 17:20 h is the existence of the above-mentioned dead space within the pull system. All the MPOA, ME-ARC and AHA groups were perfused with 1.0, 3.0 or 10 ng ml−1 of the recombinant rat leptin (R & D Systems, Inc., Minneapolis, MN, USA) during the period of 14:00-17:20 h. The rat leptin was dissolved in the ACSF immediately before use. Control groups were perfused with the pure ACSF only throughout the period of 11:00-17:20 h. The actual time of day during which leptin was infused was between 13:50 and 17:10 h because the dead space of the push system (from the tip of the push cannula to the distal end of the push tubing) was adjusted to 150 μl (corresponding to a 10 min period of perfusion). The collected perfusates were immediately frozen on dry ice, lyophilized, and stored at −70 °C until assayed for GnRH, α-MSH, and NPY. The blood was collected into tubes containing disodium EDTA (2.5 mg (ml blood)−1), centrifuged and the plasma was stored at −70 °C until assayed for LH, PRL, leptin, oestradiol and progesterone. Within 30 min after completion of the experiment, the animals were killed by decapitation and their brains were removed and stored at −70 °C for histological examination.
Hormone assays
The lyophilized perfusates were reconstituted with 300 μl of an assay buffer (0.1 % bovine serum albumin, 100 mm phosphate-buffered saline, 0.1 % sodium azide, 0.1 % Triton X-100, pH 7.4) and subjected to radioimmunoassays (RIAs) for GnRH, α-MSH and NPY. A 50 μl aliquot was applied to the GnRH RIA and 100 μl aliquots to each of the α-MSH and NPY assays. Iodinated GnRH was purchased from New England Nuclear (Boston, MA, USA). GnRH antibody (Ab) and GnRH peptide as standards were both from the Peptide Institute, Inc. (Osaka, Japan). The GnRH Ab was used at a final dilution of 1:480 000. α-MSH was measured utilizing an α-MSH RIA kit purchased from Peninsula Laboratories Inc. (San Carlos, CA, USA). NPY RIA was carried out using iodinated porcine NPY (New England Nuclear), Ab against the rat, human NPY (Peninsula Laboratories Inc.), and the rat, human NPY (Peninsula Laboratories Inc.) as the standard. The NPY Ab was used at the final dilution of 1:160 000. The sensitivities of these assays (expressed per perfusate) were 0.2 pg for GnRH, 0.5 pg for α-MSH and 10 pg for NPY. These three peptides were also measured in reconstituted lyophilizates from blank perfusates (five samples per rat) containing 300 μl of the pure ACSF, and their mean values were subtracted from the levels in all the actual perfusates from every animal. In the perfusates from the MPOA and the ME-ARC, both GnRH and NPY were detectable in all samples from every animal, although α-MSH was sometimes undetectable (in fewer than three samples in one rat). By convention, such samples that contained undetectable levels of α-MSH were allotted the sensitivity threshold of the assay for calculation. In contrast, in the perfusates from the AHA, both GnRH and α-MSH were essentially undetectable, even though only NPY was detectable in all samples from every animal. GnRH, α-MSH and NPY did not cross-react with each other, or with their respective related compounds. LH and PRL levels were determined by RIA using reagents kindly donated by Dr A. F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, USA). Rat LH-RP-3 and PRL-RP-3 were used as the standards. The sensitivity of the LH assay was 0.2 ng ml−1, and that of PRL assay was 0.8 ng ml−1. For GnRH, α-MSH, NPY, LH and PRL, samples from individual rats were analysed within the same assay. Plasma concentrations of leptin, oestradiol and progesterone were determined by a rat leptin enzyme-linked immunosorbent assay kit (Morinaga Institute of Biological Science, Yokohama, Japan), oestradiol RIA kit (DPC Corp., Los Angeles, CA, USA), and progesterone RIA kit (DPC Corp.), respectively. The sensitivities of these assays were 0.2 ng ml−1, 8.0 pg ml−1, and 0.1 ng ml−1, respectively. In these eight hormone assays, both intra- and interassay coefficients of variation were less than 10 %.
Histology
Histological examination of the PPP cannula placement was carried out in the same manner as previously reported (Watanobe & Takebe, 1993a). Only animals that had the tip of the cannula within the respective target regions contributed to the data given in the Results.
Statistical analyses
To determine whether observed temporal fluctuations in hypothalamic and plasma hormones constituted endogenous pulses, results were analysed by the cluster analysis method (Veldhuis & Johnson, 1986). A t statistic of 2.0 was selected to maintain a maximal false-positive rate of 2.5 % or less, by using cluster sizes of one or two in the nadir and peak.
Results were expressed as means ± s.d. For the purpose of detecting significant differences from 11:00 h values, data of individual experimental groups were analysed by two-way ANOVA with repeated measures. One-way ANOVA was used to compare data among different groups. When significant F values were obtained, a Bonferroni multiple comparisons test was performed. The data for GnRH, α-MSH, NPY, LH, and PRL were also expressed as the area under the curve, which was calculated using the trapezoidal rule. Differences were considered significant if P was smaller than 0.05.
Results
Table 1 shows the plasma concentrations of leptin, oestradiol and progesterone in the six experimental groups examined in this study. The leptin levels in the three fed groups were similar to those in fed female rats with regular oestrous cycles as reported in a previous study (Watanobe & Suda, 1999). The plasma leptin concentrations in the three fasted groups were also consistent with previous data (Watanobe et al. 1999b). The plasma levels of oestradiol and progesterone indicated that all six groups had hormone levels that correspond to those found during the dioestrous stage of the oestrous cycle (Freeman, 1988).
Table 1.
Plasma concentrations of leptin, oestradiol and progesterone in the six experimental groups examined in this study
| Group | No. of rats a | Leptin (ng ml−1) | Oestradiol (pg ml−1) | Progesterone (ng ml−1) |
|---|---|---|---|---|
| MPOA (fed) | 38 | 3.13 ± 1.43 | 28 ± 12 | 5.3 ± 1.9 |
| MPOA (fasted) | 40 | 0.29 ± 0.13 | 30 ± 13 | 54 ± 2.5 |
| ME-ARC (fed) | 37 | 3.32 ± 1.46 | 29 ± 12 | 5.1 ± 1.8 |
| ME-ARC (fasted) | 35 | 0.28 ± 0.06 | 31 ± 18 | 5.3 ± 1.8 |
| AHA (fed) | 35 | 3.18 ± 0.06 | 30 ± 12 | 5.0 ± 1.2 |
| AHA (fasted) | 38 | 0.31 ± 0.06 | 32 ± 12 | 5.2 ± 1.9 |
The sum total of rats constituting the respective groups. MPOA, medial preoptic area; ME–ARC, median eminence-arcuate nucleus complex; AHA, anterior hypothalamic area.
Cluster analysis of results from any individual animal from any group did not reveal a significant pulsatile release of any hypothalamic or plasma hormone throughout the sampling period. It may be that the 20 min sampling period was too long to detect the pulses of GnRH and LH especially. Figure 1 shows the results of leptin perfusion in the fed MPOA group. For clarity, the figure shows the data from only the ACSF and leptin (10 ng ml−1) groups. In the rats perfused with ACSF (vehicle) alone, concentrations of plasma or hypothalamic hormones examined showed no significant changes over the entire period of observation. This was also the case with the three different concentrations of leptin infused. Data on the integrated release of the hormones are not shown.
Figure 1. Effects of MPOA perfusion with leptin or vehicle on local release of α-MSH, NPY and GnRH, as well as on plasma levels of LH and PRL, in fed female rats.
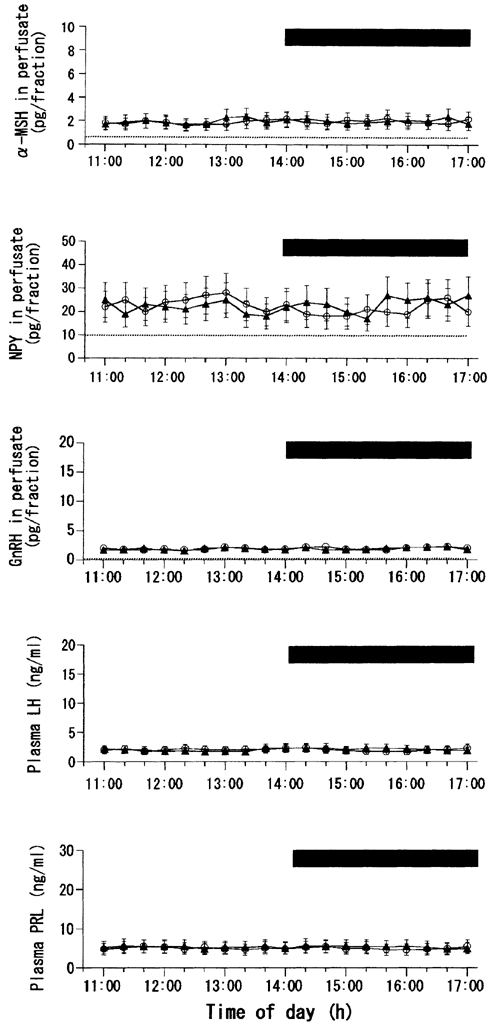
In this and subsequent figures (2, 4 and 5): (1) data from only the highest concentration of leptin (10 ng ml−1) are shown; (2) the horizontal bar indicates the period during which leptin (▴) or vehicle (ACSF, ○) was infused; (3) the time of perfusate collection in the upper three graphs is shifted 10 min ahead of the actual time of perfusion because the dead space of the pull system (150 μl) corresponds to a 10 min period of perfusion (flow rate, 15 μl min−1); (4) measurements of α-MSH, NPY and GnRH in the perfusates are expressed as point values at the centre of their collection periods; and (5) dotted lines in the graphs for α-MSH, NPY and GnRH indicate the limits of detection for each peptide. Number of rats in each subgroup = 8-10.
Figure 2 and Figure 3 show the results of leptin perfusion in the fasted MPOA group. For clarity, Fig. 2 shows the data from only the ACSF and leptin (10 ng ml−1) groups. As in the fed MPOA group, the vehicle was without effect on any of the five hormones measured. Differing from the data in the fed MPOA group, however, all the three different concentrations of leptin given to the fasted MPOA group led to significant elevations in the concentrations of α-MSH, GnRH and LH. The levels of these three hormones were increased dose dependently by 1.0 and 3.0 ng ml−1 concentrations of leptin. However, the effects of 10 ng ml−1 leptin were statistically indistinguishable from those of 3.0 ng ml−1 leptin (Fig. 3), which suggests that excitatory actions of leptin on α-MSH, GnRH and LH may already be maximal at its physiological concentration (3.0 ng ml−1). The time courses of these three hormones during the infusion of 10 ng ml−1 leptin were such that the significant rise in α-MSH concentration occurred first, and this was tracked by significant elevations in GnRH and LH levels with delays of 20 and 40 min, respectively (Fig. 2). Similar temporal patterns for these three hormones were also observed during the infusion of the other two concentrations of leptin (data not shown). The levels of NPY and PRL were not significantly affected by the leptin infusion.
Figure 2. Effects of MPOA perfusion with leptin or vehicle on local release of α-MSH, NPY and GnRH, as well as on plasma levels of LH and PRL,in fasted female rats.
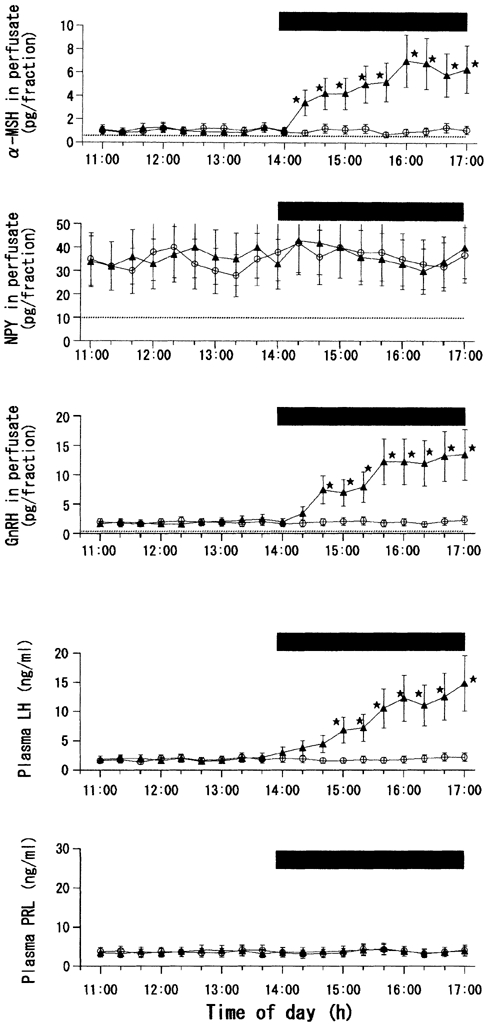
Number of rats in each subgroup = 8-11. * Statistically significant vs. the control group.
Figure 3. Integrated release of α-MSH, NPY, GnRH, LH and PRL before (11:00-14:00 h) and during (14:00-17:00 h) leptin or vehicle infusion into the MPOA in fasted female rats.
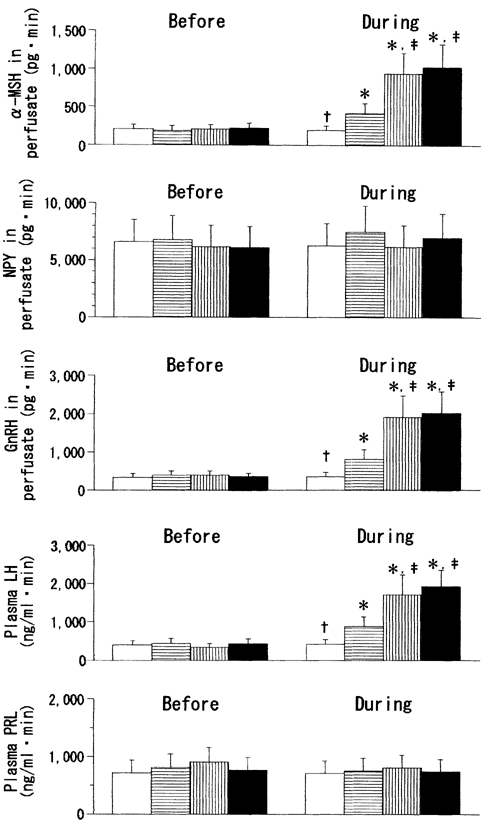
Open bars, ACSF (control); bars with horizontal lines, leptin (1.0 ng ml−1); bars with vertical lines, leptin (3.0 ng ml−1); filled bars, leptin (10 ng ml−1).* Statistically significant vs. the ‘before’ values of the respective groups. † Statistically significant vs. the other three groups. ‡ Statistically significant vs. the leptin (1.0 ng ml−1) group.
Figure 4 shows the data from the leptin perfusion in the fed ME-ARC group. For clarity, the figure shows the data from the ACSF and leptin (10 ng ml−1) groups only. Analogous with that observed in the fed MPOA group, none of the leptin concentrations exerted any significant influence on the plasma or hypothalamic hormones examined. Data on the integrated release of the hormones are not shown.
Figure 4. Effects of ME-ARC perfusion with leptin or vehicle on local release of α-MSH, NPY and GnRH, as well as on plasma levels of LH and PRL, in fed female rats.
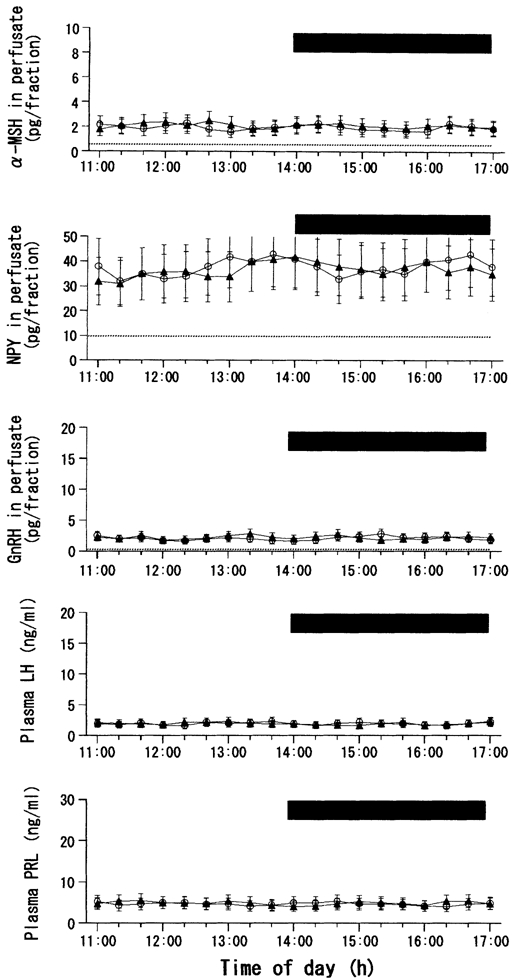
Number of rats in each subgroup = 9 or 10.
Figure 5 and Figure 6 show the results of leptin perfusion in the fasted ME-ARC group. For clarity, Fig. 5 shows the data from the ACSF and leptin (10 ng ml−1) groups only. Similar to the data for the fasted MPOA group, all three different concentrations of leptin given to the fasted ME-ARC group resulted in significant elevations of α-MSH, GnRH and LH levels. As with MPOA perfusion, the levels of these three hormones were dose-dependently increased by 1.0 and 3.0 ng ml−1 concentrations of leptin, and the influence of 10 ng ml−1 leptin was statistically similar to that of 3.0 ng ml−1 leptin (Fig. 6). During the infusion of 10 ng ml−1 leptin, a significant increase in α-MSH occurred 20 min earlier than that in GnRH, and this elevation of GnRH was followed by that of LH with a delay of 20 min (Fig. 5). Similar temporal profiles for these three hormones were also observed during the infusion of the other two concentrations of leptin (data not shown). In addition, as a finding observed only in the fasted ME-ARC group, leptin was also stimulatory to PRL secretion. Prolactin levels were significantly increased by both the 3.0 and 10 ng ml−1 concentrations of leptin to a similar degree, although 1.0 ng ml−1 leptin was without effect (Fig. 6). During the infusion of 10 ng ml−1 leptin, the first significant elevation of PRL levels occurred 1 h later than that of LH (Fig. 5). A similar time lag between these two hormones was also observed during the infusion of 3.0 ng ml−1 leptin (data not shown).
Figure 5. Effects of ME-ARC perfusion with leptin or vehicle on local release of α-MSH, NPY and GnRH, as well as on plasma levels of LH and PRL, in fasted female rats.
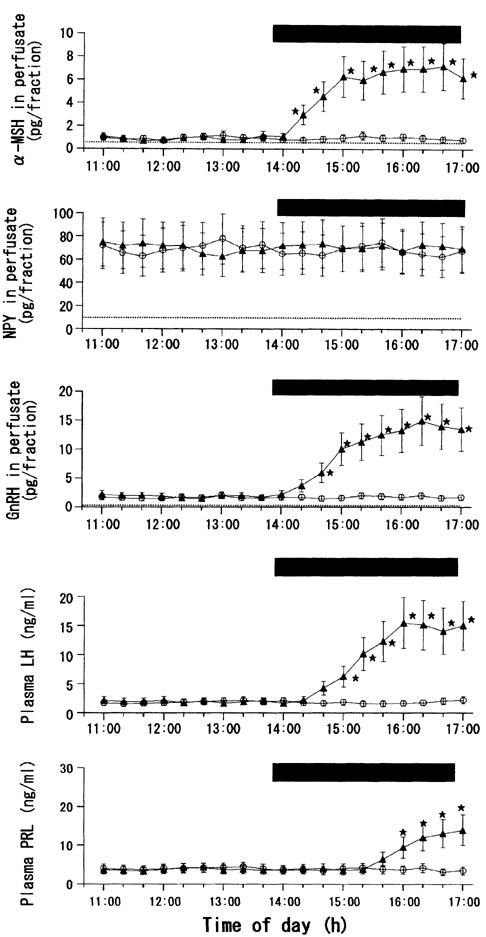
Number of rats in each subgroup = 7-9. * Statistically significant vs. the control group.
Figure 6. Integrated release of α-MSH, NPY, GnRH, LH and PRL before (11:00-14:00 h) and during (14:00-17:00 h) leptin or vehicle infusion into the ME-ARC in fasted female rats.
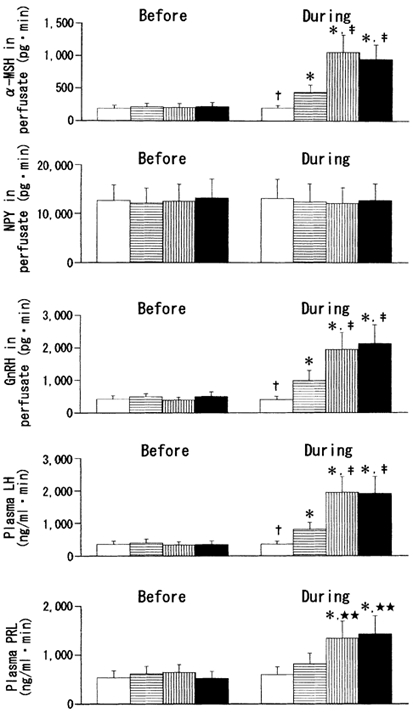
Open bars, ACSF (control); bars with horizontal lines, leptin (1.0 ng ml−1); bars with vertical lines, leptin (3.0 ng ml−1); filled bars, leptin (10 ng ml−1). *Statistically significant vs. the ‘before’ values of the respective groups. † Statistically significant vs. the other three groups. ‡ Statistically significant vs. the leptin (1.0 ng ml−1) group. ** Statistically significant vs. the ACSF and leptin (1.0 ng ml−1) groups.
Although data are not shown as figures, the leptin infusion into the AHA was without effect on any of the hypothalamic and pituitary hormones examined, irrespective of the leptin concentration and the nutritional state of the animals. In both the fed and fasted groups, NPY levels in the AHA perfusates were measurable, but those of α-MSH and GnRH were almost undetectable.
Figure 7 compares the integrated basal release of α-MSH and NPY in the MPOA, ME-ARC, and AHA (no data for α-MSH in the AHA) between the fed and fasted rats. The release of α-MSH in both the MPOA and the ME-ARC was significantly decreased after fast by 33 % (P < 0.05) or 38 % (P < 0.05), respectively. In contrast, the basal outputs of NPY in both the MPOA and the ME-ARC were significantly higher in the fasted than in the fed rats by 39 % (P < 0.05) or 95 % (P < 0.02), respectively. In the AHA, however, NPY levels were statistically indistinguishable between the two groups. Under both the fed and fasted conditions, the basal levels of NPY release in the three hypothalamic areas were significantly (P < 0.01-0.05) different from each other (ME-ARC > MPOA > AHA).
Figure 7. Comparison between fed and fasted female rats of the integrated basal release of α-MSH (A) and NPY (B) in MPOA, ME-ARC, and AHA.
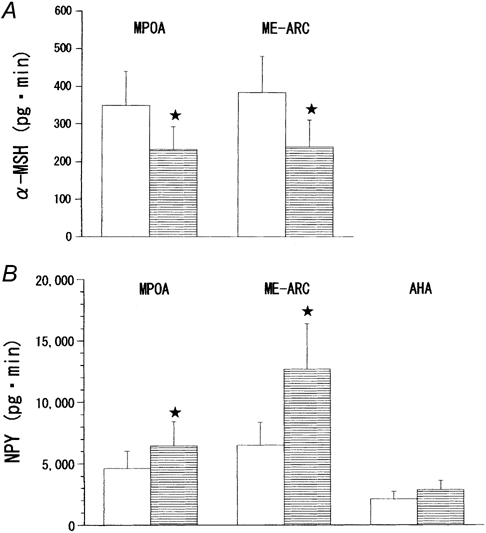
In order to give a single group of data for either hormone, the four different ‘before’ values from the respective four subgroups were combined. The measurements of α-MSH are not shown for the AHA because this hormone was undetectable in this site. Open bars, fed rats; bars with horizontal lines, fasted rats. The number of rats in each group is the same as shown in Table 1. Under both the fed and fasted conditions, NPY outputs in the three sites of perfusion were significantly different from each other. * Statistically significant vs. its fed counterpart.
Discussion
Concentrations of leptin infused
In this study, the hypothalamus was perfused with three different concentrations of leptin (1.0, 3.0 and 10 ng ml−1). The middle concentration was chosen as the one similar to that found in normally fed dioestrous female rats (Watanobe & Suda, 1999). The lowest concentration was set between this normal level and the value seen in 3-day fasted female rats (about 0.3 ng ml−1; Watanobe et al. 1999b). The highest concentration was chosen as the one comparable to that found in mildly obese humans and rats. Women with mild obesity are reported to have 3–4 times higher levels of circulating leptin than subjects of normal weight (Oppert et al. 1997; Rissanen et al. 1999). Normally fed female Otsuka-Long-Evans-Tokushima Fatty rats, a genetically obese rat strain with non-insulin-dependent diabetes mellitus and mild obesity, have about 10 ng ml−1 of leptin in the general circulation (Watanobe et al. 2001b). These concentrations of leptin are, however, higher than those in cerebrospinal fluid (CSF) of rats. It was reported that leptin levels in the CSF of normally fed female rats (0.2-0.3 ng ml−1) are about 10 times lower than in the plasma (Grueso et al. 2001; Rocha et al. 2001). This implies that the three concentrations of leptin infused in the present study (1.0-10 ng ml−1) are 3–50 times higher than the adipose hormone levels normally existing in the CSF and perhaps also the brain parenchyma of female rats.
Nutritional state and leptin actions on the GnRH-LH system
Regardless of whether MPOA or ME-ARC was perfused, there was a clear difference in hormonal responses to leptin between fed and fasted rats. In the fasted rats, 1.0 and 3.0 ng ml−1 of leptin produced a dose-dependent stimulation of GnRH release from either site of the hypothalamus, and this elevation of GnRH was followed by enhanced release of LH from the pituitary. Regardless of the site of perfusion, 10 ng ml−1 of leptin did not further elevate the GnRH or LH responses above the values seen after infusing 3.0 ng ml−1 of leptin. In the fed rats, both the 3.0 and 10 ng ml−1 concentrations of leptin, regardless of the 1.0 ng ml−1 concentration, were without effect on the GnRH or LH release, whether MPOA or ME-ARC was perfused. Overall, these results may suggest that the stimulatory influences of leptin on the reproductive hormones are already maximal at the concentration that corresponds to the physiological plasma levels in normally fed female rats. In contrast, however, Yu et al. (1997a) and Lebrethon et al. (2000) reported that leptin was able to stimulate static and pulsatile release of GnRH, respectively, from hypothalamic explants of normally fed rats. Even so, a close inspection of these reports reveals that the level of significance of the leptin effects that they observed was relatively small (only a 15–20 % increase over control values) when tested at the leptin concentrations similar to those employed in this study (1.0-10 ng ml−1 = 6.25 × 10−11 to 6.25 × 10−10m). It is possible that such marginal effects of leptin were statistically indiscernible in this study, in which the data variation was relatively large. Alternatively, this discrepancy might have been due to the in vivo vs. in vitro experimental conditions. It should be remembered that hypothalamic explants are deprived of humoral and neuroanatomical connections with neighbouring extrahypothalamic tissues. Therefore, if some, as yet unidentified, mediators originating outside the hypothalamus tonically inhibit the interplay between leptin and GnRH, leptin would not stimulate GnRH release from the in vivo hypothalamus.
It is very probable that the levels of leptin existing in the brain of normally fed rats are physiologically important to upregulate the GnRH-LH system under this nutritional state, because central administration of leptin Ab suppresses the pulsatile and surge-like secretion of LH in well-fed female rats (Carro et al. 1997; Kohsaka et al. 1999b). However, it should also be noted that all the concentrations of leptin employed in this study and previous ones in vitro (Yu et al. 1997a; Lebrethon et al. 2000) are supraphysiological given the normal leptin concentrations in the rat CSF (see above ‘Concentrations of leptin infused’). In agreement with present data, most of the previous studies in vivo reported that leptin was effective in stimulating the GnRH-LH system in fasted animals only, and excess leptin was without effect on the reproductive axis under well-fed conditions (Cheung et al. 1997b; Henry et al. 1999, 2001; Watanobe et al. 1999b; Nagatani et al. 2001). All these in vivo data, including the present ones, are consistent with Flier's view that an appropriate energy balance is sufficient to restore and maintain reproductive competence in sexually mature females (Flier, 1998).
Sites of leptin actions
The present data strongly suggest that leptin may directly act at both the cell bodies (MPOA) and axon terminals (ME-ARC) of GnRH neurons. The possibility of leptin diffusing from one site to another must always be considered when interpreting data from studies such as this one. However, this author considers it unlikely that such diffusion of leptin from MPOA to ME-ARC, or vice versa, significantly affects the data obtained. This is because the leptin infusion into the AHA, a control site, was totally ineffective in affecting the secretion of either hormone measured. The perfused site within the AHA is equidistant by about 1.5 mm from those within the MPOA and ME-ARC and this distance is shorter than that between the latter two sites (about 2.7 mm; Pellegrino et al. 1979).
The finding that leptin can act at the ME-ARC may be in agreement with previous studies reporting the existence in this area of high concentrations of leptin receptors demonstrated at both gene and protein levels (Mercer et al. 1996b; Schwartz et al. 1996; Fei et al. 1997; Elmquist et al. 1998; Friedman & Halaas, 1998). This suggests that leptin directly binds to its receptors in the ME-ARC and stimulates the release of GnRH stored in the ME. The observed higher sensitivity of ME-ARC to leptin in the fasted than in the fed rats may be similar to the fasting-induced increase in leptin receptors in the ARC that was shown at both its mRNA and protein levels (Baskin et al. 1998, 1999a). In contrast, little or no co-expression of the leptin receptor was demonstrated in the GnRH cell bodies in the MPOA, whether it was examined at its gene or protein level (Finn et al. 1998; Friedman & Halaas, 1998). These findings suggest that leptin may influence GnRH secretion indirectly through interneurons. However, the possibility cannot be excluded that a small population of GnRH neurons expressing the leptin receptor mediates the stimulatory influence of leptin on the neurohormone. Indeed, Magni et al. (1999) reported that leptin receptors are expressed in mouse immortalized GnRH neurons (GT1-7 cells) at both gene and protein levels. They also found that these leptin receptors are functional in modulating GnRH secretion. As the GT1-7 and similar GT1-1 cells are clonal cell lines which retain many of the properties of GnRH neurons (Mellon et al. 1990), the study of Magni et al. (1999) suggests that leptin may act directly on GnRH neurons to stimulate the release of neurohormone. Data in this paper obtained from the MPOA perfusion in fasted rats agree with this possibility.
Inasmuch as the ME is one of the structures known as circumventricular organs that lack the blood-brain barrier (Broadwell & Brightman, 1976), it is not surprising that the GnRH output in the ME-ARC of the fasted rats increased in response to the three concentrations of leptin that are within the physiological range in the plasma. In contrast, however, MPOA is not a circumventricular organ and therefore circulating leptin may not reach deeper brain structures including the MPOA to a significant degree (Banks et al. 1996, 1999). In this context, intriguing data were reported by several authors that the brain is also the site of leptin production (Wilkinson et al. 2000; Knerr et al. 2001; Ehrhardt et al. 2002) and leptin mRNA levels in the hypothalamus are subject to change under altered nutritional states (Wilkinson et al. 2000). It is thus possible that this brain-derived leptin acts directly on GnRH neuronal cell bodies in the MPOA to regulate the synthesis and/or secretion of GnRH under physiological circumstances. In addition, it is also interesting that leptin similarly stimulated the GnRH-LH system whether it was infused into the MPOA or ME-ARC despite the fact that the MPOA contains a small population of GnRH-containing nerve terminals (Lantos et al. 1995). However, it appears that neuroendocrine cells are capable of releasing neurosecretory granules by exocytosis at virtually any part of their plasmalemma, such as dendrites, cell bodies and axon collaterals, not merely the well-known perivascular nerve endings (Pow & Morris, 1989; Landgraf & Ludwig, 1991). Indeed, this author and collaborators have previously reported that in female rats infusion of a nitric oxide donor into MPOA or ME-ARC stimulated the local output of GnRH to a similar extent (Kohsaka et al. 1999b).
Leptin can also act directly on the anterior pituitary to stimulate LH secretion (Yu et al. 1997a, 1997b), and the expression of leptin receptors in this endocrine gland has also been demonstrated (Zamorano et al. 1997; Shimon et al. 1998; Jin et al. 1999, 2000; Iqbal et al. 2000; Lin et al. 2000; Sone et al. 2001). However, in the present study the lack of hormonal effects of leptin infusion into the AHA, in contrast to its positive actions when infused into the MPOA and ME-ARC, makes it unlikely that intrapituitary actions of leptin diffusing from the hypothalamus formed a significant proportion of the stimulated LH release. Furthermore, it was reported that within the rat anterior pituitary gland the leptin receptor is most abundantly expressed in somatotrophs, but is expressed in less than 1 % of gonadotrophs (Sone et al. 2001).
Roles of NPY and α-MSH
Several lines of evidence suggest that the ARC-NPY neurons are important targets of leptin. This may be in keeping with the morphological evidence that some populations of ARC-NPY neurons abundantly co-express leptin receptors (Mercer et al. 1996a; Finn et al. 1998; Baskin et al. 1999b). It has been reported that NPY mediates at least part of leptin actions on neuroendocrine functions (Kalra et al. 1999; Ahima et al. 2000). In addition, it is well established that NPY plays a crucial role in regulating the GnRH-LH system (Kalra et al. 1999). This author thus hypothesizes that leptin infusion into the hypothalamus would alter the local release of NPY, if leptin were able to stimulate the release of GnRH and LH. However, leptin did not affect the NPY release in either the MPOA or ME-ARC in fasted rats, which are the group that showed stimulation of the GnRH-LH system during leptin infusion. Here, it is shown that basal outputs of NPY in both the ME-ARC and MPOA were significantly increased by fasting, which may parallel reports that hypoleptinaemia upregulates the NPY synthesis in the ARC (Stephens et al. 1995; Schwartz et al. 1996, 1998) and also with the report that ARC-NPY neurons send projections to the MPOA (Li et al. 1999). This significant difference in the basal NPY release between these two nutritional states may endorse the effectiveness of the PPP method used here, which thus lends credence to the negative NPY data during leptin infusion. Collectively, the present results suggest that NPY does not mediate the acute effects of leptin on the GnRH-LH system, although it has been repeatedly shown that leptin modulates NPY neuronal activity and gene expression in a more chronic manner (Stephens et al. 1995; Schwartz et al. 1996, 1998; Baskin et al. 1999b). In agreement with these current in vivo data, recent in vitro studies in normal mice and rats reported that leptin does not acutely affect NPY release from the hypothalamus (Jang et al. 2000; King et al. 2000). In addition, the finding from NPY-deficient mice that these animals normally respond to exogenous leptin indicates that the ARC-NPY neurons are not the sole target of leptin (Erickson et al. 1996, 1997).
In contrast, the leptin infusion caused a significant stimulation of α-MSH in both the MPOA and ME-ARC in fasted, but not fed, rats. This elevation of α-MSH was sequentially tracked by that of GnRH and then of LH. These results seem to be consistent with the recent in vitro study of Kim et al. (2000) showing that leptin increased α-MSH release from hypothalamic explants from fasted, but not fed, rats. The present data that fasted animals had a significantly lower basal release of α-MSH in both the ME-ARC and MPOA than the fed animals, may be consistent with the previous reports that a decreased leptin signal lowers pro-opiomelanocortin (POMC) mRNA levels in ARC (Schwartz et al. 1997; Thornton et al. 1997; Mizuno et al. 1998), and also that ARC-POMC neurons send α-MSH-immunoreactive fibres to the MPOA (Leranth et al. 1988; Thind & Goldsmith, 1988; Chen et al. 1989). As it is known that the ARC-POMC neurons abundantly co-express leptin receptors (Cheung et al. 1997a; Finn et al. 1998), and the α-MSH-containing axon terminals make direct synaptic contacts with GnRH neurons (Leranth et al. 1988; Thind & Goldsmith, 1988; Chen et al. 1989), the temporal relationship between α-MSH and GnRH release during leptin infusion may suggest an intermediary role of α-MSH in linking leptin and the activation of GnRH neurons. This view seems to be in keeping with several previous studies in vivo and in vitro conducted in both rats (Alde & Celis, 1980; Durando et al. 1989; Caballero & Celis, 1993) and humans (Reid et al. 1981; Limone et al. 1997) that were in favour of α-MSH as a stimulator to the GnRH-LH system, although a few conflicting reports also exist (Khorram et al. 1984; Scimonelli & Celis, 1990). In addition, it is also possible that the leptin-induced release of α-MSH, an anorectic peptide, is associated with ingestive behaviour. It is generally accepted that the ARC plays a pivotal role in the central regulation of food intake and energy balance through synthesizing and integrating a number of appetite-regulating factors (Kalra et al. 1999).
It is known that among the five melanocortin (MC) receptors cloned to date, the MC4-R serves a crucial role in the central regulation of body weight homeostasis (Schiöth et al. 2001). Collaborators and I have previously reported that the MC4-R also plays a significant role in mediating the leptin-induced stimulation of LH surge in female rats (Watanobe et al. 1999a; Schiöth et al. 2001). As α-MSH is a potent endogenous agonist of the MC4-R (Schiöth, 2001), the present data suggest the existence of a functional communication of leptin → α-MSH → MC4-R → GnRH, in addition to the above-mentioned mechanism that leptin may directly activate GnRH neurons (Magni et al. 1999). To date, there are no published data demonstrating the existence of MC4-Rs in GnRH neurons in vivo, but a very recent report of Khong et al. (2001) supports this possibility. They reported that mouse immortalized GnRH neurons (GT1-1 and GT1-7 cells) express functional MC4-Rs that respond to α-MSH with the production of cAMP and GnRH. Although Murray et al. (2000a,b) also reported, as I have, a significant contribution of MC4-R to leptin-stimulated LH release, two other reports are in obvious contrast to our conclusion. Hohmann et al. (2000) and Raposinho et al. (2000) reported a lack of involvement of MC4-R signalling in the regulation of reproductive function in mice and rats. Although the author has no clear explanation for this discrepancy, it is possible that these conflicting results may reflect a sexually dimorphic manner by which MC4-R regulates the reproductive system. It may be important that the present and previous (Watanobe et al. 1999a; Schiöth et al. 2001) data of colleagues and myself and those of Murray et al. (2000a, 2000b) were all obtained from female rats, whereas the results of Hohmann et al. (2000) and Raposinho et al. (2000) were from male rodents. In this context, it may be informative to note that Parent et al. (2000) found leptin stimulatory to the pulsatile GnRH release from rat hypothalami in vitro only in females, and not in males.
Leptin and PRL secretion
Of note, the leptin infusion into the ME-ARC of fasted rats led to the stimulation of not only LH but also PRL. As observed for LH, leptin exerted its maximal effects on PRL release at 3.0 ng ml−1 concentration. This excitatory action of leptin on PRL secretion agrees with recent studies (Yu et al. 1997; Gonzalez et al. 1999; Kohsaka et al. 1999; Watanobe et al. 1999a,b, 2000), and also with a recent molecular study reporting that leptin upregulates PRL mRNA levels in mouse pituitary gland (Renz et al. 2000).
This author has found that leptin stimulated PRL secretion only when it was infused into the ME-ARC, but not into the MPOA. These results strongly suggest that the functional substrate mediating the leptin-induced PRL secretion may exist within the ME-ARC or its vicinity. It is well established that ARC plays a crucial role in the neuroendocrine regulation of PRL secretion (Freeman et al. 2000). My previous data suggest that the central MC4-R mediates not only the LH but also PRL surges (Watanobe et al. 1999a, 2001a; Schiöth et al. 2001). It is known that α-MSH exerts a stimulatory effect on PRL secretion (Freeman et al. 2000), and that MC4-R is the only MC-R subtype that exists in ME (Harrold et al. 1999). On the basis of these reports, my present data suggest that the stimulatory effects of leptin on PRL secretion, in analogy with its actions on the GnRH-LH system, may be mediated by the α-MSH → MC4-R line of communication, although additional roles for other PRL-regulating molecules cannot be excluded.
It deserves attention that in this study the leptin-induced release of PRL did not occur as early as that of LH. This finding is not surprising, if α-MSH triggers the PRL secretion. As α-MSH is considered to facilitate PRL release through enhancing the responsiveness of mammotrophs to physiologically relevant PRL secretagogues (Hill et al. 1991; Nunez & Frawley, 1998), it may be reasonable that mammotrophs need to be exposed to α-MSH for a sustained, not brief, period of time until α-MSH manifests its PRL-releasing action. In support of this view, my recent study demonstrates that chronic, but not acute, administration of leptin is able to stimulate PRL secretion in rats (Watanobe et al. 2000). It is also possible that in this study the leptin infused to the ME-ARC diffused into and acted on the pituitary gland to stimulate PRL release. However, the immunohistochemical evidence that leptin receptors are expressed in less than 1 % of rat mammotrophs makes this possibility unlikely (Sone et al. 2001).
Summary and conclusion
This study examines for the first time whether leptin acts directly within the hypothalamus to modulate the secretion of GnRH and LH in conscious, freely moving female rats. The data obtained suggest that leptin may act at both the cell bodies and axon terminals of GnRH neurons to stimulate the release of the neurohormone. Leptin is also stimulatory to PRL secretion when infused into ME-ARC. It is suggested that α-MSH may be an intermediary molecule linking leptin and these hormonal responses. Inasmuch as these effects of leptin were observed only in fasted rats and were maximal at its physiological concentrations, it appears that an appropriate energy balance is sufficient to restore and maintain reproductive capability in female rats.
Acknowledgments
This author thanks the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases, and Dr A. F. Parlow for the generous donation of reagents for rat LH and PRL RIAs. This study was supported in part by grants-in-aid from the Japan Society for the Promotion of Science (nos 12671072 and 14571071) to H.W.
References
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Alde S, Celis ME. Influence of α-melanotropin on LH release in the rat. Neuroendocrinology. 1980;31:116–120. doi: 10.1159/000123061. [DOI] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Breininger JF, Bonigut S, Miller MA. Leptin binding in the arcuate nucleus is increased during fasting. Brain Research. 1999a;828:154–158. doi: 10.1016/s0006-8993(99)01252-4. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999b;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 1998;47:538–543. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, Da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. Journal of Biological Chemistry. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. Journal of Comparative Neurology. 1976;166:257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- Caballero C, Celis ME. The effect of the blockade of α-melanocyte-stimulating hormone on LH release in the rat. Journal of Endocrinology. 1993;137:197–202. doi: 10.1677/joe.0.1370197. [DOI] [PubMed] [Google Scholar]
- Cagampang FRA, Maeda KI, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. Journal of Endocrinology. 1991;129:321–328. doi: 10.1677/joe.0.1290321. [DOI] [PubMed] [Google Scholar]
- Carro E, Pinilla L, Seoane LM, Considine RV, Aguilar E, Casanueva F, Dieguez C. Influence of endogenous leptin tone on the estrous cycle and luteinizing hormone pulsatility in female rats. Neuroendocrinology. 1997;66:375–377. doi: 10.1159/000127262. [DOI] [PubMed] [Google Scholar]
- Chen WP, Witkin JW, Silverman AJ. β-Endorphin and gonadotropin-releasing hormone synaptic input to gonadotropin-releasing hormone neurosecretory cells in the male rat. Journal of Comparative Neurology. 1989;286:85–95. doi: 10.1002/cne.902860106. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Pro-opiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997a;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997b;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Durando P, Ferreira A, Celis ME. Acute administration of α-melanotropin exerts a stimulatory control on puberty. Acta Endocrinologica. 1989;120:661–666. doi: 10.1530/acta.0.1200661. [DOI] [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW, Boisclair YR. Spatial and developmental regulation of leptin in fetal sheep. American Journal of Physiology–Regulatory, Integrative and Comparative Physiology. 2002;282:R1628–1635. doi: 10.1152/ajpregu.00750.2001. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distribution of leptin receptor mRNA isoforms in the rat brain. Journal of Comparative Neurology. 1998;395:535–547. [PubMed] [Google Scholar]
- Erickson JC, Ahima RS, Hollopeter G, Flier JS, Palmiter RD. Endocrine function of neuropeptide Y knockout mice. Regulatory Peptides. 1997;70:199–202. doi: 10.1016/s0167-0115(97)01007-0. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proceedings of the National Academy of Sciences of the USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- Flier JS. What's in a name ? In search of leptin's physiologic role. Journal of Clinical Endocrinology and Metabolism. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 1893–1928. [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiological Reviews. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology, Endocrinology and Metabolism. 2001;280:E827–847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin (116-130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology. 1999;70:213–220. doi: 10.1159/000054479. [DOI] [PubMed] [Google Scholar]
- Grueso E, Rocha M, Puerta M. Plasma and cerebrospinal fluid leptin levels are maintained despite enhanced food intake in progesterone-treated rats. European Journal of Endocrinology. 2001;144:659–665. doi: 10.1530/eje.0.1440659. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Widdowson PS, Williams G. Altered energy balance causes selective changes in melanocortin-4 (MC4-R), but not melanocortin-3 (MC3-R), receptors in specific hypothalamic regions. Diabetes. 1999;48:267–271. doi: 10.2337/diabetes.48.2.267. [DOI] [PubMed] [Google Scholar]
- Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A, Clarke IJ. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology. 1999;140:1175–1182. doi: 10.1210/endo.140.3.6604. [DOI] [PubMed] [Google Scholar]
- Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinising hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of body weight. Journal of Endocrinology. 2001;168:67–77. doi: 10.1677/joe.0.1680067. [DOI] [PubMed] [Google Scholar]
- Hill JB, Nagy GM, Frawley LS. Suckling unmasks stimulatory effect of dopamine on prolactin release: possible role for α-melanocyte-stimulating hormone as a mammotrope responsiveness factor. Endocrinology. 1991;129:843–847. doi: 10.1210/endo-129-2-843. [DOI] [PubMed] [Google Scholar]
- Hohmann JG, Teal TH, Clifton DK, Davis J, Hruby VJ, Han G, Steiner RA. Differential role of melanocortins in mediating leptin's central effects on feeding and reproduction. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;278:R50–59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000;141:1515–1520. doi: 10.1210/endo.141.4.7433. [DOI] [PubMed] [Google Scholar]
- Jang M, Mistry A, Swick A, Romsos DR. Leptin rapidly inhibits hypothalamic neuropeptide Y secretion and stimulates corticotropin-releasing hormone secretion in adrenalectomized mice. Journal of Nutrition. 2000;130:2813–2820. doi: 10.1093/jn/130.11.2813. [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan L, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. Journal of Clinical Endocrinology and Metabolism. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine Reviews. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Khong K, Kurtz SE, Sykes RL, Cone RD. Expression of functional melanocortin-4 receptor in the hypothalamic GT1-1 cell line. Neuroendocrinology. 2001;74:193–201. doi: 10.1159/000054686. [DOI] [PubMed] [Google Scholar]
- Khorram O, de Castro B, McCann SM. Physiological role of α-melanocyte-stimulating hormone in modulating the secretion of prolactin and luteinizing hormone in the female rat. Proceedings of the National Academy of Sciences of the USA. 1984;81:8004–8008. doi: 10.1073/pnas.81.24.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary-thyroid axis and may mediate the effects of leptin. Journal of Clinical Investigation. 2000;105:1005–1011. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PJ, Widdowson PS, Doods H, Williams G. Regulation of neuropeptide Y release from hypothalamic slices by melanocortin-4 agonists and leptin. Peptides. 2000;21:45–48. doi: 10.1016/s0196-9781(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Knerr I, Schuster S, Nomikos P, Buchfelder M, DöTSCH J, Schoof E, Fahlbusch R, Rascher W. Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone-secreting and non-functioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropathology and Applied Neurobiology. 2001;27:215–222. doi: 10.1046/j.0305-1846.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Watanobe H, Kakizaki Y, Habu S, Suda T. A significant role of leptin in the generation of steroid-induced luteinizing hormone and prolactin surges in female rats. Biochemical and Biophysical Research Communications. 1999a;254:578–581. doi: 10.1006/bbrc.1998.0112. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Watanobe H, Kakizaki Y, Suda T. A comparative study of the effects of nitric oxide and carbon monoxide on the in vivo release of gonadotropin-releasing hormone and neuropeptide Y from rat hypothalamus during the estradiol-induced luteinizing hormone surge: Estimation by push-pull perfusion. Neuroendocrinology. 1999b;69:245–253. doi: 10.1159/000054425. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Ludwig M. Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: Osmotic stimulation via microdialysis. Brain Research. 1991;558:191–196. doi: 10.1016/0006-8993(91)90768-q. [DOI] [PubMed] [Google Scholar]
- Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Research Reviews. 1995;20:209–249. doi: 10.1016/0165-0173(94)00013-f. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, Gerard A, Parent AS, Junien JL, Bourguignon JP. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology. 2000;141:1464–1469. doi: 10.1210/endo.141.4.7432. [DOI] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Research. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology. 1999;140:5382–5390. doi: 10.1210/endo.140.11.7093. [DOI] [PubMed] [Google Scholar]
- Limone P, Calvelli P, Altare F, Ajmone-Catt P, Lima T, Molinatti GM. Evidence for an interation between alpha-MSH and opioids in the regulation of gonadotropin secretion in man. Journal of Endocrinological Investigation. 1997;20:207–210. doi: 10.1007/BF03346904. [DOI] [PubMed] [Google Scholar]
- Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB. Long form leptin receptor mRNA expression in the brain, pituitary, and other tissues in the pig. Domestic Animal Endocrinology. 2000;19:53–61. doi: 10.1016/s0739-7240(00)00064-3. [DOI] [PubMed] [Google Scholar]
- Magni P, Vettor R, Pagano C, Calcagno A, Beretta E, Messi E, Zanisi M, Martini L, Motta M. Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology. 1999;140:1581–1585. doi: 10.1210/endo.140.4.6622. [DOI] [PubMed] [Google Scholar]
- Mellon P, Windle J, Goldsmith P, Padula C, Roberts J, Weiner R. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PT, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. Journal of Neuroendocrinology. 1996a;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Letters. 1996b;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and in ob/ob and db/db mice, but is stimulated by leptin (published erratum appears in Diabetes 1998; 47: 696) Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Murray JF, Adan RAH, Walker R, Baker BI, Thody AJ, Nijenhuis WA, Yukitake J, Wilson CA. Melanin-concentrating hormone, melanocortin receptors and regulation of luteinizing hormone release. Journal of Neuroendocrinology. 2000a;12:217–223. doi: 10.1046/j.1365-2826.2000.00440.x. [DOI] [PubMed] [Google Scholar]
- Murray JF, Mercer JG, Adan RAH, Datta J, Aldairy C, Moar KM, Baker BI, Stock MJ, Wilson CA. The effect of leptin on luteinizing hormone release is exerted in the zona incerta and mediated by melanin-concentrating hormone (published erratum appears in Journal of Neuroendocrinology 2001; 13: 109) Journal of Neuroendocrinology. 2000b;12:1133–1139. doi: 10.1046/j.1365-2826.2000.00577.x. [DOI] [PubMed] [Google Scholar]
- Nagatani S, Thompson RC, Foster DL. Prevention of glucoprivic stimulation of corticosterone secretion by leptin does not restore high frequency luteinizing hormone pulses in rats. Journal of Neuroendocrinology. 2001;13:371–377. doi: 10.1046/j.1365-2826.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- Nunez L, Frawley LS. Alpha-MSH potentiates the responsiveness of mammotropes by increasing Ca2+ entry. American Journal of Physiology. 1998;274:E971–977. doi: 10.1152/ajpendo.1998.274.6.E971. [DOI] [PubMed] [Google Scholar]
- Oppert JM, Lahlou N, LaferrÈRE B, Roger M, Basdevant A, Guy-Grand B. Plasma leptin and acute serotoninergic stimulation of the corticotropic axis in women who are normal weight or obese. Obesity Research. 1997;5:410–416. doi: 10.1002/j.1550-8528.1997.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Parent AS, Lebrethon MC, Gerard A, Vandersmissen E, Bourguignon JP. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regulatory Peptides. 2000;92:17–24. doi: 10.1016/s0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. 2. New York: Plenum Publishing Co.; 1979. [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Castillo E, D'Alleves V, Broqua P, Pralong FP, Aubert ML. Chronic blockade of the melanocortin 4 receptor subtype leads to obesity independently of neuropeptide Y action, with no adverse effects on the gonadotropic and somatotropic axes. Endocrinology. 2000;141:4419–4427. doi: 10.1210/endo.141.12.7842. [DOI] [PubMed] [Google Scholar]
- Reid R, Ling N, Yen SSC. α-Melanocyte stimulating hormone induces gonadotropin release. Journal of Clinical Endocrinology and Metabolism. 1981;52:159–161. doi: 10.1210/jcem-52-1-159. [DOI] [PubMed] [Google Scholar]
- Renz M, Tomlinson E, Hultgren B, Levin N, Gu Q, Shimkets RA, Lewin DA, Stewart TA. Quantitative expression analysis of genes regulated by both obesity and leptin reveals a regulatory loop between leptin and pituitary-derived ACTH. Journal of Biological Chemistry. 2000;275:10429–10436. doi: 10.1074/jbc.275.14.10429. [DOI] [PubMed] [Google Scholar]
- Rissanen P, MäKINATTILA S, Vehmas T, Taavitsainen M, Rissanen A. Effect of weight loss and regional fat distibution on plasma leptin concentration in obese women. International Journal of Obesity. 1999;23:645–649. doi: 10.1038/sj.ijo.0800896. [DOI] [PubMed] [Google Scholar]
- Rocha M, Grueso E, Puerta M. The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. Journal of Endocrinology. 2001;171:349–354. doi: 10.1677/joe.0.1710349. [DOI] [PubMed] [Google Scholar]
- SchiÖTH HB. The physiological role of melanocortin receptors. Vitamins and Hormones. 2001;63:195–232. doi: 10.1016/s0083-6729(01)63007-3. [DOI] [PubMed] [Google Scholar]
- SchiÖTH HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. NeuroReport. 2001;12:687–690. doi: 10.1097/00001756-200103260-00014. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology. 1998;139:2629–2635. doi: 10.1210/endo.139.5.6000. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. Journal of Clinical Investigation. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Scimonelli T, Celis ME. A central action of α-melanocyte-stimulating hormone on serum levels of LH and prolactin in rats. Journal of Endocrinology. 1990;124:127–132. doi: 10.1677/joe.0.1240127. [DOI] [PubMed] [Google Scholar]
- Shimon I, Yan X, Magoff DA, Friedman TC, Melmed S. Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. Journal of Clinical Endocrinology and Metabolism. 1998;83:4059–4064. doi: 10.1210/jcem.83.11.5273. [DOI] [PubMed] [Google Scholar]
- Smith GD, Jackson LM, Foster DL. Leptin regulation of reproductive function and fertility. Theriogenology. 2002;57:73–86. doi: 10.1016/s0093-691x(01)00658-6. [DOI] [PubMed] [Google Scholar]
- Sone K, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell and Tissue Research. 2001;305:351–356. doi: 10.1007/s004410100407. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, Mackellar W, Rosteck PR, Jr, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Thind KK, Goldsmith PC. Infundibular gonadotropin-releasing hormone neurons are inhibited by direct opioid and autoregulatory synapses in juvenile monkeys. Neuroendocrinology. 1988;47:203–216. doi: 10.1159/000124914. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- Uotani S, Bjorbaek C, Tornoe J, Flier JS. Functional properties of leptin receptor isoforms: Internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48:279–286. doi: 10.2337/diabetes.48.2.279. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: A simple, versatile, and robust algorithm for endocrine pulse detection. American Journal of Physiology. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. American Journal of Physiology. 1996;270:E1–19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- Watanobe H, SchiÖTH HB, Suda T. Stimulation of prolactin secretion by chronic, but not acute, administration of leptin in the rat. Brain Research. 2000;887:426–431. doi: 10.1016/s0006-8993(00)03019-5. [DOI] [PubMed] [Google Scholar]
- Watanobe H, SchiÖTH HB, Wikberg JES, Suda T. The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochemical and Biophysical Research Communications. 1999a;257:860–864. doi: 10.1006/bbrc.1999.0547. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Suda T. A detailed study on the role of sex steroid milieu in determining plasma leptin concentrations in adult male and female rats. Biochemical and Biophysical Research Communications. 1999;259:56–59. doi: 10.1006/bbrc.1999.0718. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Suda T, Wikberg JES, SchiÖTH HB. Evidence that physiological levels of circulating leptin exert a stimulatory effect on luteinizing hormone and prolactin surges in rats. Biochemical and Biophysical Research Communications. 1999b;263:162–165. doi: 10.1006/bbrc.1999.1331. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Role of postnatal gonadal function in the determination of thyrotropin (TSH) releasing hormone-induced TSH response in adult male and female rats. Endocrinology. 1987;120:1711–1718. doi: 10.1210/endo-120-5-1711. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Intrahypothalamic perfusion with interleukin-1-beta stimulates the local release of corticotropin-releasing hormone and arginine vasopressin and the plasma adrenocorticotropin in freely moving rats: a comparative perfusion of the paraventricular nucleus and the median eminence. Neuroendocrinology. 1993a;57:593–599. doi: 10.1159/000126412. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. In vivo release of neurotensin from the median eminence of ovariectomized estrogen-primed rats as estimated by push-pull perfusion: correlation with luteinizing hormone and prolactin surges. Neuroendocrinology. 1993b;57:760–764. doi: 10.1159/000126434. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Effects of intravenous administration of interleukin-1-beta on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: estimation by push-pull perfusion. Neuroendocrinology. 1994;60:8–15. doi: 10.1159/000126714. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Yoneda M, Kakizaki Y, Kohsaka A, Suda T, SchiÖTH HB. Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Research Bulletin. 2001a;54:521–525. doi: 10.1016/s0361-9230(01)00442-7. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Yoneda M, Kohsaka A, Kakizaki Y, Suda T, SchiÖTH HB. Normalization of circulating leptin levels by fasting improves the reproductive function in obese OLETF female rats. Neuropeptides. 2001b;35:45–49. doi: 10.1054/npep.2000.0842. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Morash B, Ur E. The brain is a source of leptin. Frontiers in Hormone Research. 2000;26:106–125. doi: 10.1159/000061018. [DOI] [PubMed] [Google Scholar]
- Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proceedings of the National Academy of Sciences of the USA. 1997a;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997b;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65:223–228. doi: 10.1159/000127276. [DOI] [PubMed] [Google Scholar]


