Abstract
For electrically stimulated muscles, it has been observed that maximal muscle force during and after stretch is substantially greater than the corresponding isometric force. However, this observation has not been made for human voluntary contractions. We investigated the effects of active muscle stretch on muscle force production for in vivo human adductor pollicis (n = 12) during maximal voluntary contractions and electrically induced contractions. Peak forces during stretch, steady-state isometric forces following stretch, and passive forces following muscle deactivation were compared to the corresponding isometric forces obtained at optimal muscle length. Contractions with different stretch magnitudes (10, 20, and 30 deg at a constant speed of 10 deg s−1) and different speeds (10, 20, and 60 deg s−1 over a range of 30 deg) were performed in triplicate in a random order, balanced design. We found three novel results: (i) there was steady-state force enhancement following stretch in voluntarily contracted muscles; (ii) some force enhancement persisted following relaxation of the muscle and (iii) force enhancement, for some stretch conditions, exceeded the maximum isometric force at optimal muscle length. We conclude from these results that voluntary muscle contraction produces similar force enhancement to that observed in the past with electrically stimulated preparations. Therefore, steady-state force enhancement may play a role in everyday movements. Furthermore, these results suggest that non-uniformities in sarcomere length do not, at least not exclusively, account for the force enhancement following active muscle stretch, and that the stretch magnitude-dependent passive force enhancement observed here may be responsible for the enhancement of force above the isometric reference force at optimal muscle length.
When an electrically stimulated muscle is stretched, its force will increase in accordance with Hill's (1938) force- velocity relationship and Huxley's cross-bridge theory (Huxley, 1957; Huxley & Simmons, 1971). However, in contrast to the classical force-velocity relationship and cross-bridge equations, the force of an actively stretched muscle remains greater than the purely isometric force at the corresponding muscle/fibre length. This so-called steady-state force enhancement following muscle stretch has been observed in a variety of muscles (e.g. Abbott & Aubert, 1952; Herzog & Leonard, 1997; Morgan et al. 2000) and fibre preparations (e.g. Sugi, 1972; Edman et al. 1978, 1982; Sugi & Tsuchiya, 1988; Edman & Tsuchiya, 1996; Linari et al. 2000). Force enhancement following stretch persists for up to 30 s in amphibian and mammalian muscles (Abbott & Aubert, 1952; Herzog et al. 2001), is typically independent of the speed of stretching (Edman et al. 1978; Sugi & Tsuchiya, 1988), but is directly related to the amount of stretch (Abbott & Aubert, 1952; Sugi, 1972; Edman et al. 1982; Sugi & Tsuchiya, 1988). Force enhancement has been associated with no change (Sugi & Tsuchiya, 1988) or a small change in muscle/fibre stiffness (Herzog & Leonard, 2000; Linari et al. 2000).
Despite the wealth of information on steady-state force enhancement following active muscle stretching, the mechanisms responsible for force enhancement are not known. The primary explanation for force enhancement has been the development of sarcomere length non-uniformities on the descending limb of the force-length relationship (e.g. Morgan, 1990, 1994; Morgan et al. 2000). However, force enhancement has been observed on the ascending limb of the force-length relationship (Sugi, 1972; van Atteveldt & Crowe, 1980; Ettema et al. 1992; Cook & McDonagh, 1995; Herzog & Leonard, 1997; de Ruiter et al. 2000); the steady-state forces have been found to exceed the isometric forces at the length from which the stretch was initiated (on the descending limb of the force-length relationship), including forces at optimal muscle/fibre length (Edman et al. 1978), and force enhancement has been associated with increased muscle/fibre stiffness (Herzog & Leonard, 2000; Linari et al. 2000). These are observations that are hard to reconcile with the sarcomere length non-uniformity theory.
Recently, two studies were performed to investigate the relevance of force enhancement in human skeletal muscle. Cook & McDonagh (1995) found force enhancement following stretch of electrically stimulated human first dorsal interosseus muscle. In agreement with data from animal muscles, they found that force enhancement following stretch was independent of stretch speed, but increased with increasing amounts of stretch. De Ruiter et al. (2000) observed force enhancement following stretch of electrically stimulated human adductor pollicis. They concluded that force enhancement was probably not associated with the cross-bridge kinetics, but was possibly related to the recruitment of structural proteins. De Ruiter et al. (2000) proposed titin as a possible protein to play this role, a suggestion that had been made on previous occasions to explain force enhancement in animal muscles (e.g. Edman et al. 1982; Noble, 1992; Edman & Tsuchiya, 1996).
Like all previous studies, the human experiments by Cook & McDonagh (1995) and de Ruiter et al. (2000) were performed with electrical stimulation of the target muscle. In order for force enhancement to have significance in the mechanism and control of everyday movements, force enhancement needs to be present during voluntary contractions. However, when stretching muscles during maximal voluntary contractions, eccentric forces are typically found to be similar to the corresponding maximal isometric contractions (Thomson & Chapman, 1988; Westing et al. 1988, 1990, 1991; Webber & Kriellaars, 1997), and force enhancement has not been demonstrated in these situations. The lack of force increase during stretch of voluntarily contracted muscle is typically associated with an inhibition of the neural drive (Westing et al. 1990; Webber & Kriellaars, 1997). Therefore, it is quite possible that force enhancement following stretch does not exist in human muscles that are activated voluntarily.
In this study, we wanted to address the following unresolved questions: (i) does force enhancement exist in human muscles that are voluntarily contracted, and if so, how does this force enhancement relate to that observed during electrical stimulation; (ii) what happens to muscle activation during and following stretching of voluntarily activated muscle and (iii) can we directly identify force enhancement through a non-cross-bridge, passive component, as suggested in the literature?
Methods
Subjects
Subjects (n = 12; 22–45 years of age; 8 male and 4 female) with no history of neuromuscular disorder and injury gave free, written informed consent to participate in this study. All experimental procedures were approved by the Conjoint Ethics Committee of the University of Calgary.
Apparatus
A custom-designed muscle testing apparatus was built to measure thumb adduction force and thumb angle at the carpometacarpal joint and to control thumb movements (Fig. 1).
Figure 1. Testing apparatus and experimental set up.
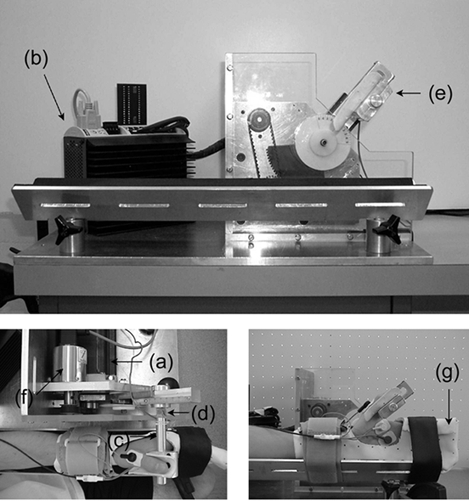
The testing apparatus used consists of a rotary stepper motor (a) with a digital stepper drive/controller (b), an aluminium rod (c) instrumented with two pairs of strain gauges (d) and an auxiliary piece for thumb placement (e), with an analog encoder (f). When the adductor pollicis contracts, the thumb presses against the rod and thumb adduction force is measured by the calibrated strain gauges. The forearm and the four digits of the left hand are restrained with a clinical cast (g) and secured on the apparatus using velcro straps. The thumb is placed and aligned on the rod as shown in the pictures. This experimental set up was designed to measure thumb adduction force during voluntary and electrically elicited, static and dynamic contractions.
A rotary stepper motor (Model TS42BP10, Parker Hannifin Corp., Cleveland, OH, USA) was connected to an aluminium rod (1.5 cm diameter and 15 cm long) via gears (1:4 gear ratio). The small gear was attached to a shaft of the motor that engaged the big gear. The big gear was cut to a quarter of a circle, so the amount of rotation was restricted to 90 deg. A rod was attached to the big gear perpendicular to the plane of the gear through a length-adjustable rail arm. One end of the aluminium rod was cut to a square cross section for mounting four strain gauges (Model no. CEA-06-125UN-350, Measurement Group, Inc. Raleigh, NC, USA). Two pairs of strain gauges were mounted on two opposing surfaces of the square-shaped end of the rod in a full wheatstone circuit. The surfaces on which the strain gauges were mounted were aligned so that the line of action of thumb adduction force was perpendicular to the surfaces. The other end was cut to form a groove for the attachment of an auxiliary piece for thumb placement and fixation. The auxiliary piece guided the thumb movement in the frontal plane. An analogue encoder (Series 03 rotary transducer, Hohner Corp., UK) was located beside the motor and was connected to the shaft of the motor through a conveyer belt for angle measurement. Thumb movements were controlled by moving the rod using user-defined programs through a digital controller (Model Gemini GT6-L8 Digital stepper driver/controller, Parker Hannifin Corp., Cleveland, OH, USA).
Experimental settings
The left hand was immobilized with a reusable clinical cast (Ezeform, Rehabilitation Division, Smith & Nephew Inc., Germantown, WI, USA) in a neutral position. Immobilization restricted movement of the wrist and fingers, except for the thumb. After immobilization, subjects sat on an adjustable chair with the forearm slightly abducted and the elbow flexed 90 deg. The forearm was placed in a V-shaped metal plate, and was secured with two velcro straps (Fig. 1). The centre of rotation of the motor was aligned with the carpometacarpal joint. The aluminium rod was placed horizontally between the thumb and the index fingers. By placing the distal end of the thumb on the auxiliary piece attached to the aluminium rod, thumb movement was restricted to the frontal plane (Fig. 1).
In order to maximize the range of motion, the fully abducted thumb angle was defined as 0 deg from which the adducted thumb angle was measured negative. This reference angle is on the plateau of the thumb adduction force-angle relationship (De Ruiter et al. 2000), that is, the active isometric force reaches a maximum at this angle. Subjects who could not move the thumb comfortably through the required range of motion (30 deg) in the testing apparatus were rejected from the study. Therefore, all participating subjects had a similar thumb adduction range of motion (approximately 38 deg), and the test range represented a relatively consistent percentage of the total range of motion for all subjects.
Experimental protocols
The basic idea of this study was to investigate the effects of muscle stretch conditions (i.e. the magnitude and speed of stretch) and muscle activation methods (i.e. maximal voluntary activation and electrical stimulation) on force enhancement following muscle stretch. We measured thumb adduction forces, joint angles and muscle activation for purely isometric contractions, and for isometric contractions following stretching of the thumb adductors.
Subjects attended three sessions on three different days, with at least one rest day between sessions. In the preparation session, subjects were familiarized with the testing procedures and the protocols involving electrical stimulation and maximal voluntary contraction. For the tests involving voluntary contractions, subjects were trained to perform consistent maximal voluntary contractions. Subjects were instructed to increase thumb adduction forces to the maximum level within 1–2 s, and then maintain the maximal force for the duration of the test. The start and finish of each contraction was clearly announced to each subject for every test contraction. Verbal encouragement was given during the maximal effort contractions. In the second and third test sessions, subjects performed the actual test protocols.
Subjects performed two different test protocols. Protocol 1 was aimed at investigating the effects of stretch amplitude and Protocol 2 was aimed at determining the effects of stretch speed on the forces during stretching and the isometric, steady-state forces following stretching. These two protocols were performed for electrically induced contractions (Fig. 2 and Fig. 4) and maximal voluntary contractions (Fig. 3 and Fig. 5).
Figure 2. Thumb adduction force and angle - time histories of test and reference contractions obtained for Protocol 1 during electrical stimulation.
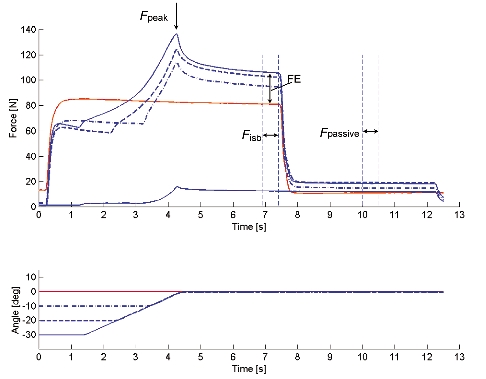
For the test contractions, peak force (Fpeak) was measured during the stretch phase. Isometric force (Fiso) was measured during the final, steady-state isometric phase for comparisons with the corresponding values obtained from the isometric reference contractions and used to assess force enhancement (FE). After deactivation of the muscle, passive force (Fpassive) was measured. Also, purely passive forces were measured for stretch contractions of the deactivated muscle (a stretch contraction over 30 deg stretch at 10 deg s−1 is presented).
Figure 4. Thumb adduction force and angle - time histories of test and reference contractions obtained for Protocol 2 during electrical stimulation.
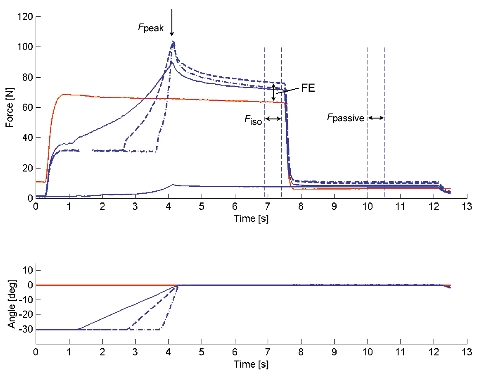
For the test contractions, peak force (Fpeak) was measured during the stretch phase. Isometric force (Fiso) was assessed during the final, steady-state isometric phase for comparisons with the corresponding values obtained from the isometric reference contractions and used to assess force enhancement (FE). After deactivation of the muscle, passive force (Fpassive) was measured. Also, purely passive forces were measured for stretch contractions of the deactivated muscle (a stretch contraction over 30 deg stretch at 10 deg s−1 is presented).
Figure 3. Thumb adduction force and angle - time histories and EMG signals of test and reference contractions obtained for Protocol 1 during voluntary contraction.
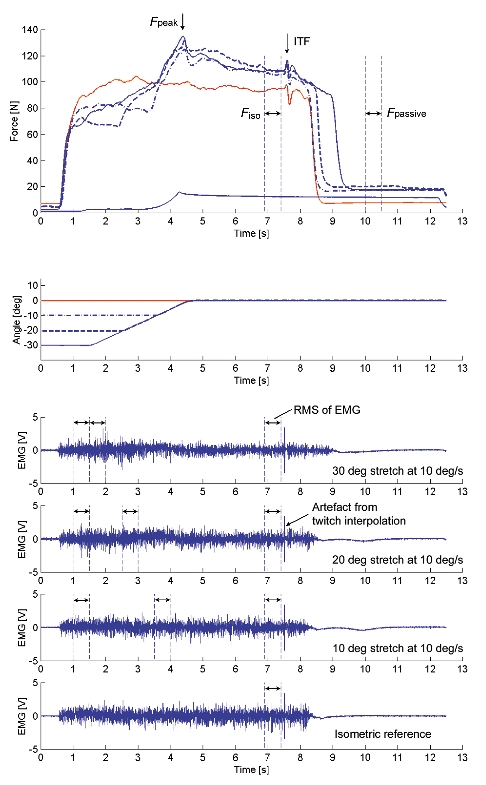
For the test contractions, peak force (Fpeak) was measured during the stretch phase, and isometric force (Fiso), EMG, and interpolated twitch force (ITF) were measured during the final isometric phase for comparisons with the corresponding values obtained from the isometric reference contractions. After deactivation of the muscle, passive force (Fpassive) was measured. Also, purely passive forces were measured for stretch contractions of the completely relaxed muscle (a stretch contraction over 30 deg stretch at 10 deg s−1 is presented).
Figure 5. Thumb adduction force and angle - time histories and EMG signals of test and reference contractions obtained for Protocol 2 during voluntary contraction.
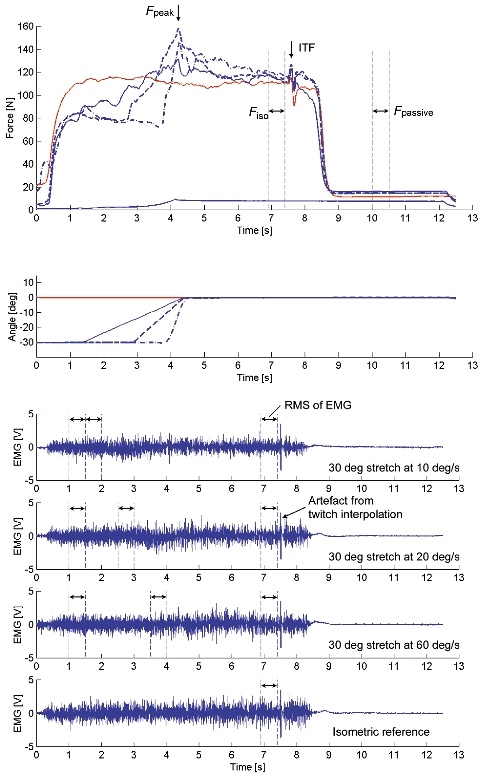
For the test contractions, peak force (Fpeak) was measured during the stretch phase, and isometric force (Fiso), EMG, and interpolated twitch force (ITF) were measured during the final isometric phase for the comparison with the corresponding values obtained from the isometric reference contractions. After deactivation of the muscle, passive force (Fpassive) was measured. Also, purely passive forces were measured for stretch contractions of the completely relaxed muscle (a stretch contraction over 30 deg stretch at 10 deg s−1 is presented).
In Protocol 1 (Fig. 2 and Fig. 3), subjects performed purely isometric contractions, and isometric contractions following muscle stretching (eccentric thumb adduction) of varying amplitudes. All purely isometric reference contractions were performed at a thumb angle of 0 deg. For the determination of possible force enhancement following stretch, subjects performed an isometric contraction at an initial thumb angle, followed by a stretch of the adductor pollicis, followed by a further isometric contraction at the final thumb angle. Stretching was always performed at an angular velocity of 10 deg s−1 over stretch amplitudes of 10, 20, and 30 deg to the final thumb angle of 0 deg (Fig. 2 and Fig. 3). Each stretch of a given amplitude was performed three times for a total of nine stretch experiments. Each set of three stretch tests, which consisted of three different stretch amplitudes, was preceded and followed by an isometric reference contraction at 0 deg. Following stretching, muscles were kept activated for a minimum of 3 s to ensure that a steady-state force was reached following the stretch-transient force response (Fig. 2 and Fig. 3). Similarly, following deactivation, the thumb was held passively for a minimum of 3 s at the final angle to determine possible history-dependent force effects of passive structures.
In Protocol 2 (Fig. 4 and Fig. 5), subjects performed purely isometric contractions, and isometric contractions following stretching (eccentric thumb adduction) at varying speeds. All purely isometric reference contractions were performed at a thumb angle of 0 deg. For the determination of possible force enhancement following stretch, subjects performed an isometric contraction at an initial thumb angle, followed by a stretch of the adductor pollicis, followed by a further isometric contraction at the final thumb angle. Stretches were always performed over an amplitude of 30 deg (from −30 deg to 0 deg) at stretch speeds of 10, 20, and 60 deg s−1 (Fig. 4 and Fig. 5). Each stretch at a given speed was performed three times for a total of nine stretch experiments. Each set of three stretch tests, which consisted of three different stretch speeds, was preceded and followed by an isometric reference contraction at 0 deg. Following stretching, muscles were kept activated for a minimum of 3 s to ensure that a steady-state force was reached following the stretch-transient force response (Figs 4 and 5). Similarly, following deactivation, the thumb was held passively for a minimum of 3 s at the final angle to determine possible history-dependent force effects of passive structures.
In order to minimize the possible effects of training or fatigue during the experimental protocol, all experimental conditions (type of activation, speed of muscle stretching, and magnitude of muscle stretching) were presented in a random but balanced design. Furthermore, each subject performed isometric reference contractions throughout the experimental protocol to monitor systematic changes in maximal voluntary force. Subjects were given as much rest as they required between contractions, however, a minimum rest of 3 min was enforced at all times.
Force and angle measurement
When the adductor pollics was activated, the thumb pressed against the aluminium rod, and thumb adduction force was measured using the calibrated strain gauges. Thumb angle was measured using an analog encoder and a goniometer.
Muscle activation measurements during voluntary contractions
During voluntary contractions, muscle activation was assessed using electromyography (EMG) for selected subjects (n = 4), and the interpolated twitch technique (Merton, 1954) for all subjects (n = 12).
Subjects thoroughly washed their left hand with warm water and cleaned the medial aspect of the wrist and the palmar side of the hand with alcohol. For EMG recordings, a pair of surface electrodes was placed over the palmar side of the hand on the adductor pollicis. One electrode was placed close to the base of the proximal phalanx, the other over the middle of the third metacarpal bone. The inter-electrode distance was about 2.5 cm. The reference electrode was placed on the posterior bony side of the thumb. EMG signals were amplified (×1000-5000) no further than 1 cm from the recording site, and were bandpass filtered (10 Hz and 1 kHz). EMG signals were collected at 2000 Hz.
For the twitch interpolation technique (Merton, 1954), a pair of carbon-impregnated rubber electrodes (4.5 × 4.5 cm), which were covered with a thin layer of conductive gel, were placed on the skin over the ulnar nerve. The stimulation electrodes were placed on the medial aspect of the wrist, approximately 2 and 7 cm proximal to the pisiform bone. A supramaximal doublet twitch (two square wave pulses of 0.8 ms duration and 8 ms inter-pulse interval) was delivered to the target muscle during the maximal voluntary contractions (Merton, 1954; Belanger & McComas, 1981). The interpolated twitch forces (ITF) were measured during the steady-state isometric phase of the isometric reference contractions and the isometric contractions following muscle stretching. Muscle inhibition was calculated by dividing the ITF values by the reference twitch force (RTF)(Belanger & McComas, 1981), which was measured in the relaxed muscle.
Tetanic contractions using electrical stimulation
In order to induce tetanic contractions, electrical stimulation was delivered to the adductor pollicis through the ulnar nerve using a constant voltage muscle stimulator with an isolation unit approved for human use (Grass S88, Quincy, MA, USA). A series of square wave pulses of 0.3-0.8 ms duration was delivered to the target muscle at a frequency ranging from 20–40 Hz. Tetanic electrical stimulation was systematically increased for each subject until a force was reached that was equal to the maximal voluntary force, or until subjects reached a level of stimulation that they could comfortably tolerate. During the tests with electrical stimulation, subjects were instructed to relax completely during and after the stimulation.
Data collection and analysis
During the experiments, thumb adduction forces, thumb angles and EMG signals were recorded simultaneously at a sampling frequency of 2000 Hz and stored on a microcomputer for off-line analysis.
Thumb adduction force was determined at three specific instances in time (Fig. 2): at the end of the stretch (FPeak), at 2.5-3 s (i.e. over a 500 ms interval) following the stretch (Fiso) to give the mean steady-state isometric force following stretching, and at 1.5-2 s (i.e. over a 500 ms interval) after the termination of muscle activation (Fpassive) to give the mean passive force following deactivation of the muscle. The Fpeak and Fiso values were normalized relative to the mean force of the isometric reference contractions that were performed immediately before and after each set of test contractions. Force enhancement following muscle stretch (FE) was determined by subtracting the mean steady-state forces of the isometric reference contractions from the corresponding force following active muscle stretching. The Fpassive values for each stretch condition were compared to the corresponding mean values of the isometric reference contractions.
For the voluntary contractions, the root mean square (RMS) values of the EMG signals (for a 500 ms period) were assessed just prior to the stretch, just after the stretch was started, and during the steady-state isometric phase following stretch (Fig. 3 and 5). The r.m.s values of the EMG signals from the stretch contractions were compared to the corresponding mean values of the isometric reference contractions. The ITF/RTF ratios for the test contractions were compared with the corresponding values for the isometric reference contractions.
Mean values of the measured variables for each contraction condition across all subjects were used for statistical analysis (α = 0.05) using repeated measures ANOVA and Bonferroni post hoc comparisons. In the Figures, the pooled variables for each contraction condition across all subjects are presented as means ± 1 s.e.m.
Results
We found steady-state force enhancement following adductor pollicis stretching for voluntary and electrically stimulated contractions, and for all stretch conditions (Figs 2-5). Force enhancement was found to depend positively on the amount of stretch, was independent of the speed of stretch and was associated with a substantial passive force component that could be quantified following deactivation of the muscle.
Peak force during stretch
The peak forces during stretching were significantly greater than the corresponding isometric reference forces (Figs 2-6). The mean peak force values reached during stretch were 138 ± 4 and 141 ± 2 % in Protocol 1 for voluntary and electrically induced contractions, respectively, and were 143 ± 5 and 149 ± 4 % in Protocol 2 for voluntary and electrically induced contractions, respectively.
Figure 6. Peak force reached during muscle stretch (Fpeak) for different conditions.
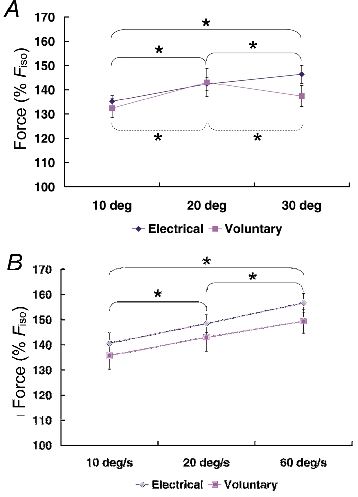
All peak forces were normalized relative to the corresponding isometric reference forces and pooled for each stretch condition and across all subjects. A, Protocol 1: the peak forces during stretch were greater for stretch amplitudes of 20 and 30 deg than those for 10 deg during electrical stimulation. Fpeak was found to be greatest for stretch amplitudes of 20 deg for the voluntary contractions. Significance (* P < 0.05) is presented separately for electrically induced (continuous line) and for voluntary (dashed line) contractions because a significant interaction (stimulation method × stretch condition) was found. B, Protocol 2: Fpeak increased with increasing speeds of stretch for the electrical and voluntary contractions. Means were collapsed across the stimulation methods and significance (* P < 0.05) is presented only for different stretch conditions, as no interaction (stimulation method × stretch condition) was found. Fpeak, peak force produced during active stretching.
The effect of stretch magnitude on peak force differed between voluntary and electrically induced contractions (Fig. 6A). For voluntary contractions, peak force was greatest for 20 deg of stretch. For electrically induced contractions, peak forces increased with increasing stretch magnitudes and were greatest for 30 deg of stretch.
Peak forces increased with increasing speeds of stretching for the voluntary and the electrically induced contractions (Fig. 6B).
Steady-state isometric force following stretch (Fiso)
The steady-state, isometric forces following stretching were significantly greater than the corresponding isometric reference forces for all stretch conditions in the voluntary and electrically stimulated muscles (Fig. 7). The mean force enhancement was 16 ± 3 and 17 ± 2 % for voluntary and electrically induced contractions in Protocol 1, respectively, and 12 ± 3 and 17 ± 2 % for voluntary and electrically induced contractions in Protocol 2, respectively.
Figure 7. Steady-state force enhancement (FE) following muscle stretch.
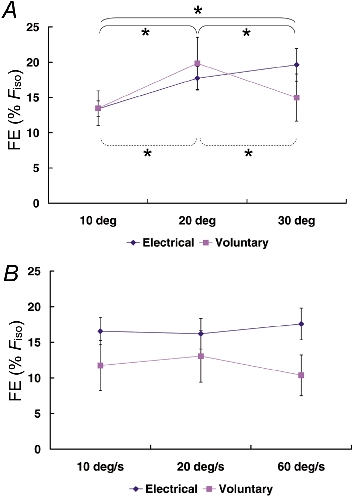
Steady-state force enhancement values were pooled for each stretch condition and across all subjects. A, Protocol 1: the steady-state force enhancement following 20 and 30 deg stretches was significantly greater than that following 10 deg stretches for the tests using electrical stimulation. Force enhancement was greatest following 20 deg of stretch for the voluntary contractions. Significance (* P < 0.05) is presented separately for electrically induced (continuous line) and for voluntary (dashed line) contractions because a significant interaction (stimulation method × stretch condition) was found. B, Protocol 2: force enhancement was not influenced by the speed of stretch regardless of the method of stimulation. FE: force enhancement following muscle stretching.
For the electrically stimulated muscles, force enhancement increased with increasing stretch magnitudes. The values for the 20 and 30 deg stretches were statistically greater than those for the 10 deg stretch amplitude (Fig. 7A). For the voluntary contractions, force enhancement was greater for the 20 deg stretches than that for the 10 and 30 deg stretches (Fig. 7A). Force enhancement during the steady-state isometric phase following stretch was independent of the speed of stretch (Fig. 7B).
Passive force after the contraction (Fpassive)
The passive forces following active muscle stretching were significantly greater than the passive forces following the purely isometric reference contractions (Fig. 2-5 and Fig. 8). We called this increased passive force following active muscle stretching ‘passive force enhancement’. Passive force enhancement was observed consistently, regardless of the muscle activation method and the stretch conditions.
Figure 8. Passive force (Fpassive) assessed after the termination of stimulation.
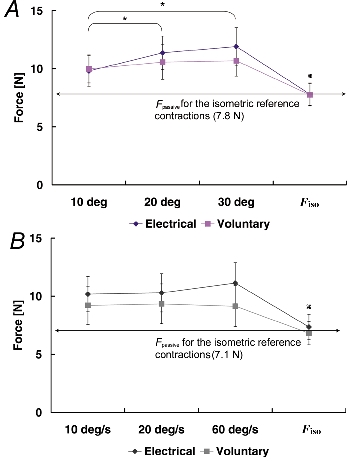
Passive force values were pooled for each stretch condition and across all subjects. A, Protocol 1: passive forces following the isometric reference contractions were significantly smaller than the passive forces following active muscle stretching († P < 0.05). There was no significant difference due to stimulation methods. Means were collapsed across the stimulation methods and significance (* P < 0.05) is presented only for different stretch conditions, as no interaction (stimulation method × stretch condition) was found. B, Protocol 2: passive forces following active muscle stretching were significantly greater than the passive forces following the isometric reference contractions († P < 0.05). There was no significant effect of the stimulation methods or the speed of stretching. Fpassive, passive force.
Passive force enhancement (FEpassive) was increased with increasing stretch amplitudes (Fig. 8A), but was found to be independent of the stretch speed (Fig. 8B).
Effect of stretch on muscle activation
Regardless of stretch condition, EMG signals before, during, and following active muscle stretching were smaller than those in the isometric reference contractions (Fig. 9). In accordance with this observation, the interpolated twitch forces following active muscle stretching were significantly greater than those obtained in the isometric reference contractions. There was no systematic change in muscle activation (EMG or twitch interpolation) as a function of stretch conditions.
Figure 9. Muscle activation determined with EMG and the interpolated twitch technique during voluntary contractions.
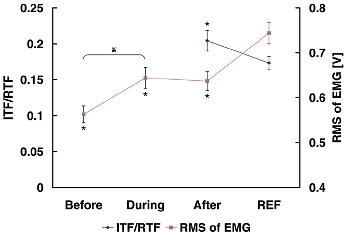
Muscle activation following active stretching was smaller than that during the isometric reference contractions as indicated by the smaller root mean square (RMS) values of the EMG and the greater interpolated twitch force (indicated with ITF/RTF) in the stretch tests compared to the reference contractions. Statistical significance (* P < 0.05) compared to the values of the isometric reference contractions. Muscle activation during active stretch was greater than just prior to stretch (†, P < 0.05). ITF, interpolated twitch force; RTF, resting twitch force; REF, isometric reference contraction, Before, RMS of EMG just before active stretch for 500 ms; During, RMS of EMG just during active stretch for 500 ms; After, RMS of EMG after active stretch for 500 ms during the steady-state isometric phase of the experimental stretch contraction.
Discussion
The primary purpose of this study was to investigate force enhancement following stretching of human adductor pollics during maximal voluntary contractions. New to the literature is the observation that steady-state force enhancement occurs during voluntary contractions. Force enhancement was independent of the stretch speed but was dependent on the stretch magnitude. Moreover, the eccentric force during stretch was substantially greater than the maximum isometric force during voluntary contractions, and force enhancement following stretching was associated with the contribution from a passive structure.
Peak force during stretch
It is well accepted that stretching of an electrically activated muscle produces force that is substantially greater than the corresponding (i.e. at the same length) isometric force. The peak eccentric force is known to depend on the magnitude and speed of the stretch (Katz, 1939; Edman et al. 1978, 1982; Cook & McDonagh, 1995; de Ruiter et al. 2000). Our results for the electrically stimulated adductor pollicis support the published results (Figs 2, 4 and 6A and B). Peak force during stretch for electrically induced contractions, averaged across all conditions, was 145 % of the corresponding isometric reference force. For trials with the greatest stretch amplitude (30 deg) and speed (60 deg s−1), peak force during stretch was, on average, 150 % of the corresponding isometric reference forces. These values agree well with those reported by de Ruiter et al. (2000, their Fig. 8B) for the same muscle, and also those found by Cook & McDonagh (1995) for the first dorsal interosseus muscle(their Fig. 5).
For voluntary contractions, it has been found that eccentric force is about the same as the corresponding isometric force (Thomson & Chapman, 1988; Westing et al. 1988, 1990, 1991; Webber & Kriellaars, 1997). These findings are in contrast to those when muscles are stimulated electrically. Although the reasons for this difference are not known, it has been speculated that it may be caused by neural inhibition during voluntary contractions (Dudley et al. 1990; Westing et al. 1990; Webber & Kriellaars, 1997). In contrast to results in the literature, we observed substantially greater peak forces during stretch than the corresponding isometric forces for all stretch amplitudes and speeds. On average, and across all stretch conditions, the peak voluntary forces during stretch exceeded the isometric forces by 41%. This result was observed despite a decrease in muscle activation (as assessed by EMG and twitch interpolation, Fig. 9) during stretch compared to the isometric reference contractions. Therefore, the peak forces during stretch are probably associated with the force- velocity properties of the adductor pollics, and not an increased activation caused by a stretch-activated reflex pathway, as sometimes observed for muscle stretch in submaximal contractions (Crago et al. 1976; Nakazawa et al. 2001).
Force enhancement following stretch
Although a steady-state force enhancement following stretching of an active muscle has been demonstrated many times for electrically stimulated preparations (Abbott & Aubert, 1952; Sugi, 1972; Edman et al. 1978, 1982; Sugi & Tsuchiya, 1988; Cook & McDonagh, 1995; Herzog & Leonard, 1997; de Ruiter et al. 2000; Linari et al. 2000; Morgan et al. 2000;), we found that such force enhancement can also occur in voluntarily contracting human skeletal muscle.
The mean steady-state force enhancement following active muscle stretching was 14 % for the voluntary contractions and 17 % for the electrically stimulated contractions. De Ruiter et al. (2000) found similar force enhancement values for the adductor pollicis following a small stretch (19 deg) of thumb adduction using electrical stimulation. One critical difference between these two studies is that the steady-state force enhancements were assessed 500 ms following the end of the stretch by de Ruiter et al. (2000), and 2.5-3 s following the end of the stretch in our study. Since it takes about 1.5-2 s to reach an approximate steady-state value following muscle stretching, it is likely that the force enhancement values given by de Ruiter et al. (2000) are slightly overestimated compared to the results reported here.
Force enhancement following stretch was independent of the speed of stretch for the electrically stimulated and the voluntarily activated contractions (Fig. 7). This result agrees with most of the findings published in the literature (Edman et al. 1978; Sugi & Tsuchiya, 1988). Similarly, force enhancement following stretch increased with increasing stretch amplitudes for the electrically stimulated tests, as has been described previously in the literature (Abbott & Aubert, 1952; Edman et al. 1982; Sugi & Tsuchiya, 1988). However, for the voluntary contractions, the steady-state force enhancement increased from the 10 deg to the 20 deg stretch amplitude, and then decreased from the 20 deg to the 30 deg stretch amplitude (Fig. 7A). To our knowledge, such a result has never been reported for electrically stimulated preparations, and future research is required to explain this observation.
Passive force enhancement
Edman et al. (1982) documented that the steady-state force enhancement following active muscle stretch was independent of the stretch speed but increased with increasing stretch amplitudes. They argued that force enhancement was not associated with a cross-bridge related mechanism, but proposed the existence of a parallel, passive elastic force that was engaged upon activation of the muscle. Herzog & Leonard (2000) and Lee et al. (2001) demonstrated that increasing amounts of shortening immediately preceding a stretch decreased the force enhancement in a dose-dependent manner, thereby supporting the argument put forward by Edman et al. (1982). Edman & Tsuchiya (1996) recorded the force transients in frog fibres that were shortened following isometric contractions and active stretches. They found that forces dropped more quickly in the initial transient phase after an active stretch compared to the corresponding isometric contractions. This result was explained by the recruitment of an elastic element during stretch. This elastic element was assumed to come into play because of non-uniformities in half-sarcomeres, rather than gross sarcomere non-uniformities, as Edman et al. (1982) had shown that force enhancement occurred to the same extent in ‘sarcomere clamped’ and ‘unclamped’ fibre preparations. Finally, de Ruiter et al. (2000) added to the indirect evidence for the existence of a parallel elastic element by demonstrating that force enhancement was not affected substantially by great variations in muscle force.
Accepting the above evidence for an elastic component associated with force enhancement and accepting that the elastic component is recruited because of gross non-uniformities in sarcomere or half-sarcomere lengths, the following two predictions can be made: (1) the steady-state force following active muscle stretch cannot exceed the isometric force at optimal muscle/fibre length and (2) once the muscle is deactivated and active force goes to zero (or to the normal parallel elastic force level at great muscle/fibre lengths), the corresponding passive force caused by the actively produced length non-uniformities must disappear.
Regarding the first prediction, we showed in single fibres of frog (n = 34) that the steady-state force following active stretch could exceed the isometric forces at optimal length in excess of 10 % (Rassier et al. 2001). Although not specifically tested, the shape of the force-angle relationship (de Ruiter et al. 2000), and the amount of force enhancement observed here (Fig. 7) suggest that the steady-state force enhancement was in excess of the peak isometric force. Therefore, gross sarcomere length non-uniformities, or non-uniformities in half-sarcomere length alone, may not fully explain these results.
Regarding the second prediction, we found that the passive forces (i.e. the force once the muscle was deactivated) following active muscle stretch were greater than the passive forces following an isometric reference contraction at the corresponding muscle length. This passive force enhancement was long lasting (Figs 2-5), was independent of the stretch speed (Fig. 8B), and was positively related to the stretch magnitude (Fig. 8A). The passive component also contributed significantly to the total force enhancement observed in these tests (Fig. 10). Since the passive force enhancement remains after deactivation of the muscle, it is unlikely that it is associated with sarcomere length non-uniformities or the development of non-uniformities in half-sarcomere lengths, as previously proposed (Morgan, 1990; Morgan, 1994; Edman & Tsuchiya, 1996). In both models, the sarcomere length non-uniformity, and the half-sarcomere non-uniformity model, passive force depends directly on the active force of the cross-bridges in series with the passive force (e.g. Edman & Tsuchiya, 1996; their Fig. 9). Once the muscle is deactivated, and active force goes to zero, the newly recruited passive force that contributes to the force enhancement in these models, has to go to zero as well.
Figure 10. The contributions of active and passive force enhancement to the total force enhancement (FE = FEactive + FEpassive).
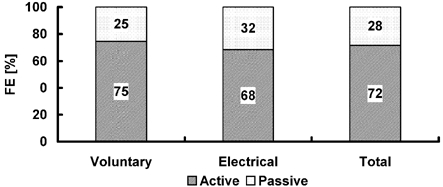
The contribution of force enhancement observed during contractions was estimated based on the assumption that the passive component of force enhancement, as determined here, subtracted from the total force enhancement would give the contribution of an ‘active’ component (i.e. a component that disappears following deactivation of the muscle). FE, force enhancement following muscle stretching; FEactive, force enhancement following muscle stretching due to active components; FEpassive, force enhancement following muscle stretching due to passive components.
One way to reconcile the current observation of a long lasting passive force enhancement that is not abolished with deactivation of the muscle is with a molecular spring of changing stiffness. Titin is such a spring in skeletal muscle and has been shown to critically influence passive force in muscle and fibre preparations (Horowits, 1992; Granzier & Wang, 1993; Granzier & Irving, 1995; Kellermayer et al. 1997). Therefore, titin could account for the observations made here.
Conclusions
We have demonstrated that steady-state force enhancement does not only exist in electrically stimulated muscle preparations, but also occurs in voluntary contractions. Peak forces during voluntary stretch were in excess of 40 % of the maximal isometric forces, indicating that muscle inhibition may play a smaller role in the small muscles of the hand compared to the large muscles of the limbs in which muscle stretch typically does not produce eccentric forces greater than the corresponding isometric forces. Finally, we have provided direct evidence for the existence of passive force enhancement following muscle stretch. We speculate that the passive force enhancement is associated with a molecular spring, such as titin, whose stiffness can be changed quickly and under physiological conditions.
Acknowledgments
This study was supported by the NSERC of Canada.
References
- Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. Journal of Physiology. 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. Journal of Applied Physiology. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Cook CS, McDonagh MJ. Force responses to controlled stretches of electrically stimulated human muscle-tendon complex. Experimental Physiology. 1995;80:477–490. doi: 10.1113/expphysiol.1995.sp003862. [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. Journal of Neurophysiology. 1976;39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- De Ruiter CJ, Didden WJM, Jones DA, De Haan A. The force-velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. Journal of Physiology. 2000;526:671–681. doi: 10.1111/j.1469-7793.2000.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Harris RT, Duvoisin MR, Hather BM, Buchanan P. Effect of voluntary vs. artificial activation on the relationship of muscle torque to speed. Journal of Applied Physiology. 1990;69:2215–2221. doi: 10.1152/jappl.1990.69.6.2215. [DOI] [PubMed] [Google Scholar]
- Edman KA, Elzinga G, Noble MI. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. Journal of Physiology. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KA, Elzinga G, Noble MI. Residual force enhancement after stretch of contracting frog single muscle fibers. Journal of General Physiology. 1982;80:769–784. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KA, Tsuchiya T. Strain of passive elements during force enhancement by stretch in frog muscle fibres. Journal of Physiology. 1996;490:191–205. doi: 10.1113/jphysiol.1996.sp021135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema GJ, Huijing PA, De Haan A. The potentiating effect of prestretch on the contractile performance of rat gastrocnemius medialis muscle during subsequent shortening and isometric contractions. Journal of Experimental Biology. 1992;165:121–136. doi: 10.1242/jeb.165.1.121. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophysical Journal. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier HL, Wang K. Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophysical Journal. 1993;65:2141–2159. doi: 10.1016/S0006-3495(93)81262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Lee HD, Wakeling J, Schachar R, Leonard TR. History dependent force properties of skeletal muscle: in vitro, in situ and in vivo considerations. Proceedings of the Congress of the 18th International Society of Biomechanics. 2001;211 [Google Scholar]
- Herzog W, Leonard TR. Depression of cat soleus-forces following isokinetic shortening. Journal of Biomechanics. 1997;30:865–872. doi: 10.1016/s0021-9290(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Herzog W, Leonard TR. The history dependence of force production in mammalian skeletal muscle following stretch-shortening and shortening-stretch cycles. Journal of Biomechanics. 2000;33:531–542. doi: 10.1016/s0021-9290(99)00221-3. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society B. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Horowits R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophysical Journal. 1992;61:392–398. doi: 10.1016/S0006-3495(92)81845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics and Biophysical Chemistry. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. Journal of Physiology. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer MS, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- Lee HD, Herzog W, Leonard TR. Effects of cyclic changes in muscle length on force production in in-situ cat soleus. Journal of Biomechanics. 2001;34:979–987. doi: 10.1016/s0021-9290(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Linari M, Lucii L, Reconditi M, Casoni ME, Amenitsch H, Bernstorff S, Piazzesi G, Lombardi V. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. Journal of Physiology. 2000;526:589–596. doi: 10.1111/j.1469-7793.2000.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. Journal of Physiology. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophysical Journal. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. An explanation for residual increased tension in striated muscle after stretch during contraction. Experimental Physiology. 1994;79:831–838. doi: 10.1113/expphysiol.1994.sp003811. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. Journal of Physiology. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Yamamoto SI, Ohtsuki T, Yano H, Fukunaga T. Neural control: novel evaluation of stretch reflex sensitivity. Acta Physiologica Scandinavica. 2001;172:257–268. doi: 10.1046/j.1365-201x.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- Noble MI. Enhancement of mechanical performance of striated muscle by stretch during contraction. Experimental Physiology. 1992;77:539–552. doi: 10.1113/expphysiol.1992.sp003618. [DOI] [PubMed] [Google Scholar]
- Rassier D, Herzog W, Wakeling J, Syme D. Stretch-induced, steady-state force enhancement in single skeletal muscle fibres exceeds the isometric force at the optimal fibre length. Journal of Biomechanics. 2001 doi: 10.1016/s0021-9290(03)00155-6. in the Press. [DOI] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. Journal of Physiology. 1972;225:237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. Journal of Physiology. 1988;407:215–229. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DB, Chapman AE. The mechanical response of active human muscle during and after stretch. European Journal of Applied Physiology. 1988;57:691–697. doi: 10.1007/BF01075990. [DOI] [PubMed] [Google Scholar]
- Van Atteveldt H, Crowe A. Active tension changes in frog skeletal muscle during and after mechanical extension. Journal of Biomechanics. 1980;13:323–331. doi: 10.1016/0021-9290(80)90011-1. [DOI] [PubMed] [Google Scholar]
- Webber S, Kriellaars D. Neuromuscular factors contributing to in vivo eccentric moment generation. Journal of Applied Physiology. 1997;83:40–45. doi: 10.1152/jappl.1997.83.1.40. [DOI] [PubMed] [Google Scholar]
- Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. European Journal of Applied Physiology. 1991;62:104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]
- Westing SH, Seger JY, Karlson E, Ekblom B. Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. European Journal of Applied Physiology. 1988;58:100–104. doi: 10.1007/BF00636611. [DOI] [PubMed] [Google Scholar]
- Westing SH, Seger JY, Thorstensson A. Effects of electrical stimulation on eccentric and concentric torque-velocity relationships during knee extension in man. Acta Physiologica Scandinavica. 1990;140:17–22. doi: 10.1111/j.1748-1716.1990.tb08971.x. [DOI] [PubMed] [Google Scholar]


