Abstract
Epithelial release of adenosine triphosphate (ATP), an important autocrine and paracrine signalling molecule, is acutely mechanosensitive and therefore difficult to study. We describe here a novel preparation that minimizes mechanical and metabolic perturbations, and use it to examine ATP secretion by epithelial cells. The Calu-3 cell line derived from human airway sub-mucosal glands was cultured in a hollow fibre bioreactor on porous capillaries that were perfused by a re-circulating medium pump. Cells became polarized and cultures were stable for > 5 months, as evidenced by microscopy and lactate production (≈250 μg (108 cells)−1 day−1). Elevating apical flow rate 5-fold increased ATP secretion from ≈200 to 6618 fmol min−1. Reducing apical osmolarity by 25–43 % also increased ATP secretion, which then declined spontaneously to a plateau rate that persisted as long as hypotonic perfusion was maintained. Release deactivated rapidly after shear and osmotic stresses were terminated, and was not associated with detectable cell lysis. Lowering apical [Ca2+] to increase connexin hemichannel permeability also stimulated ATP release and increased secretion during both hyposmotic and shear stress; however, the connexin 43 blocker flufenamic acid inhibited shear-induced ATP release only in low-Ca2+ solution, and therefore another secretory pathway may operate with physiological (i.e. mm) calcium. Regardless of the mechanism, the present results quantify ATP responses to mechanical and osmotic stimuli and demonstrate the usefulness of capillary cultures for studying epithelial secretion.
Extracellular nucleotides mediate signalling in a vast array of physiological processes that includes synaptic transmission (Holton & Holton, 1954), platelet aggregation (Detwiler & Feinman, 1973), regulation of blood flow (Milner et al. 1990), constriction of hepatic canaliculi (Kitamura et al. 1991), nociception (Burnstock, 1996), cell proliferation (Ishikawa et al. 1996), bone remodelling (Bowler et al. 2001), and host responses to infection (McNamara et al. 2001). In the airways, nucleotides contribute to mucociliary clearance by stimulating ion transport, ciliary beat frequency and mucus secretion (for review, see Fedan, 1997). ATP is released in vitro when epithelial cells are distended on a flexible substrate (Grygorczyk & Hanrahan, 1997) or exposed to hypotonic solutions (Taylor et al. 1998), and may function in cell volume regulation (Wang et al. 1996; Hazama et al. 2000) and sensing epithelial stretch (Ferguson et al. 1997; Knight et al. 2002). While intriguing, mechano-sensitivity also complicates in vitro studies since small perturbations induced by tilting cultures or collecting samples can produce large ATP effluxes that obscure responses to other stimuli (Grygorczyk & Hanrahan, 1997).
We describe here a novel approach for studying the release of epithelial factors under controlled conditions. Calu-3, a human airway sub-mucosal gland cell line, was cultured in a hollow fibre bioreactor that allows independent control over solutions bathing the apical and basolateral sides of the epithelium. Apical perfusate was collected under various conditions and assayed for [ATP] using the luciferin-luciferase reaction. ATP was released during increases in apical flow rate (i.e. shear stress) and exposure to hypotonic solution. Capillary cultures should be useful for studying the secretion of other epithelially derived substances, such as those involved in the innate immunity of mucosal surfaces. A preliminary report of these results has appeared (Guyot et al. 2000).
Methods
Cell culture
The human lung adenocarcinoma cell line Calu-3 was obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in minimal essential medium (MEM) supplemented with MEM non-essential amino acids (0.1 mm), sodium pyruvate (1 mm), 15 % fetal bovine serum, and penicillin-streptomycin (100 U ml−1). Medium and supplements were from Life Technologies (Burlington, ON, Canada). A hollow fibre bioreactor (CellMax, Spectrum Labs, Inc., Rancho Dominguez, CA, USA) was used to culture the cells, which were seeded on 50 polypropylene capillaries running axially through a sterile cartridge. Each porous capillary had an outside diameter of 630 μm and wall thickness of 150 μm. The extracapillary space (i.e. apical perfusate volume bathing the cells) was 1.4 ml (area available for cell attachment = 100 cm2, average depth = 100 μm; Fig. 1A). Cartridges were equilibrated with recirculating medium for 48 h before introducing ≈5 × 107 cells into the extracapillary space (ECS) under sterile conditions with gentle agitation and slow rotation of the cartridge to promote homogeneous distribution on the capillaries. Luminal perfusion of the capillaries with culture medium was restarted after allowing cells to attach for 1 h. Lactate production, which was assayed (Sigma) daily during the first week and at 2–5 day intervals thereafter, reached ≈250 μg day−1 within 2 days and remained constant until the experiment was terminated (typically > 3 months; Fig. 1B). Dye experiments indicated there was no mixing of culture medium with luminal perfusate once the culture was established. The medium was replaced when lactate concentration exceeded 1 mg ml−1 and serum was gradually reduced from 15 to 2 % during the first month to reduce cost and because endogenous growth factors made a high serum concentration unnecessary. Trypsinization of mature cultures yielded ≈108 cells.
Figure 1. Apparatus used for cell culture and ATP collection.
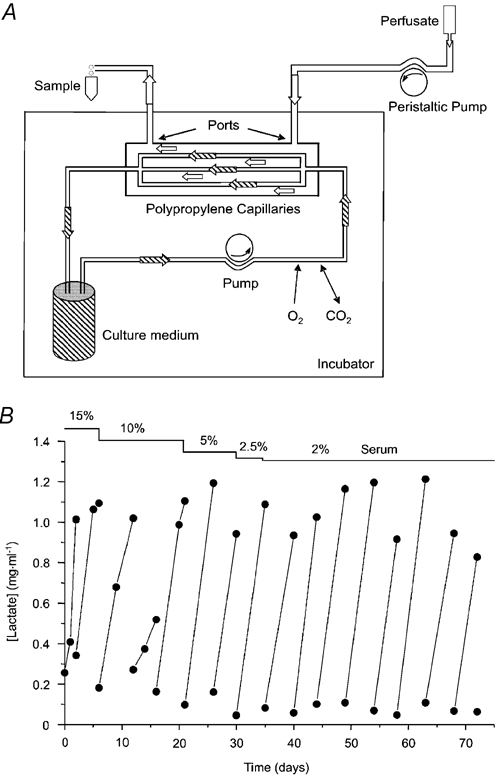
A, schematic drawing of the preparation showing cartridge with inlet and outlet ports, polypropylene capillaries, culture medium reservoir (500 ml), and recirculating pump connected by gas-permeable tubing and housed in a 5 % CO2 incubator at 37 °C. Perfusate is pumped by an independent eight channel peristaltic pump through the extracapillary space (over the apical surface of the cells), and collected in Eppendorf tubes at 4 °C. B, example of lactate production by a long-term capillary culture of Calu-3 cells.
Microscopy
Cells were fixed in situ by perfusing the extracapillary space (ECS) with 2.5 % glutaraldehyde solution buffered with 0.1 m sodium cacodylate (pH 7.4) for 1 h at 4 °C. This solution was replaced with 0.1 m sodium cacodylate buffer (pH 7.4) for 10 min and rinsed twice to wash out traces of glutaraldehyde. The bundle of hollow fibres was removed, cut transversely into short pieces, and post-fixed for 1 h at 4 °C in 1 % osmium tetroxide buffer containing 0.1 m sodium cacodylate. Pieces of the capillary bundle were dehydrated through a graded series of ethanol solutions and propylene oxide baths, and embedded in Epon at 60 °C for 48 h. Thick sections (0.5 μm) were cut, stained with 1 % toluidine blue for several seconds, and observed using a light microscope. Thin sections of selected regions were cut from each block using a diamond knife, placed on copper one-slot grids, stained with uranyl acetate and lead citrate, and examined using a Philips EM 410 electron microscope.
Solutions
The control solution used to perfuse the extra-capillary space (i.e. the apical aspect of the culture) contained (mm): 140 NaCl, 5 KCl, 1.2 MgCl2, 1.2 CaCl2, 10 glucose, and 10 N-tris(hydroxymethyl)-methyl-2-aminoethanesulfonic acid (TES), and was adjusted to pH 7.4 with NaOH. Hyposmotic solutions (in which osmolarity was reduced by 25, 38 or 43 %) were prepared by reducing [NaCl] to 100, 80 or 70 mm, respectively. Control and 70 mm NaCl solutions were made nominally Ca2+ free by replacing CaCl2 with 1 mm EGTA + 2.6 mm mannitol. A stock 50 mg ml−1 solution of flufenamic acid (Aldrich) was prepared in ethanol and diluted in perfusate (final concentration 100 μm). All solutions were sterilized by filtration (0.22 μm, Millipore Corp., Bedford, MA, USA). The ECS was perfused with sterile 140 mm NaCl saline at 1 ml min−1 for 1 h before each experiment. Samples (100 μl) from the outlet port were collected on ice at timed intervals (see Fig. 1A), spun briefly in a micro-centrifuge, and assayed for ATP concentration.
ATP assays
Samples were mixed in a cuvette with 100 μl of reaction mix (Sigma) to yield final luciferin and luciferase concentrations of 20 and 33 nm, respectively, and assayed using a TD 20/20 luminometer (Turner Designs, USA). The light signal (in relative light units; RLU) was integrated for 10 s after a delay of 1 s. ATP concentration was calculated from standard curves of bioluminescence vs. [ATP] in each perfusate used during experiments. ATP standards were prepared immediately before use by serial dilution of a 1 mm stock solution. Flufenamic acid (100 μm) had no effect on the standard curve which ranged from 10−6 to 10−12m ATP. The activity of lactate dehydrogenase (LDH), a cytoplasmic marker enzyme, was also assayed (Sigma) in samples of the perfusate but was below detectable levels (i.e. < 19.2 units), suggesting ATP release was not due to cell lysis.
Calculations and statistics
Shear stress in the extra-capillary space (ECS) was roughly estimated using the dimensions of the cartridge and hollow fibres. The total cross-sectional area of the extracapillary space (0.127 cm2) was calculated by summing the cross-sectional areas of the 50 capillaries (calculated from their outside diameters) and subtracting that value from the inside area of the cartridge, which was determined by measuring the internal diameter of the empty cartridge using calipers. This ECS was assumed to be distributed equally around the hollow fibres, forming hypothetical tubes of radius r = 2.84 × 10−2 cm. At an ECS perfusion rate of 1 ml min−1, the flow rate Q in each hypothetical tube was 3.4 × 10−4 ml s−1, the viscosity of the perfusate was 0.01 poise, and the mean shear stress (τs) estimated from Poiseuille’ s law was:
| (1) |
The fibres are distributed throughout the cartridge but are not identically spaced, and therefore this provides an estimate of the mean shear stress averaged over all the capillaries. Perfusate flow was slightly pulsatile due to the use of a peristaltic pump; however, the same oscillations were present during interventions and control periods, and therefore they probably contributed little to the large responses described here. Moreover, such oscillations caused little ATP release, as evidenced by the negligible basal release rates measured with the same pulsatile flow (see Fig. 3B below). The rates of ATP release (units: pmol min−1) are plotted as means ± standard errors of the mean (s.e.m.). When comparing responses to shear stress in the absence or presence of flufenamic acid, the total amount of ATP released during the test period was calculated by integrating under the curve. Differences were evaluated using Student's t test, with P < 0.05 considered significant.
Figure 3. Effect of flow rate on ATP release.
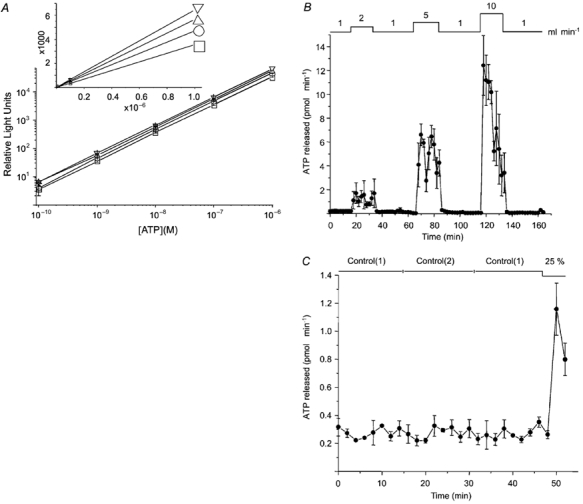
A, calibration curves for the luciferin-luciferase reaction in the same saline used to superfuse cells. □, control (140 mm NaCl); ○, 25 % hypotonic (100 mm NaCl); ▵, 38 % hypotonic (80 mm NaCl); ▿, 43 % hypotonic (70 mm NaCl). Note the downward shift as the NaCl concentration is increased. Values shown are the means ± s.e.m. of three experiments, each done in duplicate. B, ATP released during perfusion of the extra-capillary space at different flow rates (1-10 ml min−1; estimated shear stress 0.19-1.9 dyn cm−2). Cells were equilibrated by perfusing at 1 ml min−1 for 1 h before ‘Time 0′. Note that ATP release at 2 and 5 ml min−1 remained elevated until control flow rate was restored. Mean ± s.e.m. of 3 experiments. C, effect of switching perfusates on ATP release. Switching between identical solutions did not affect ATP secretion, although it was released during subsequent exposure to hypotonic solution. These cells had been cultured in bioreactors for 2.5 months prior to expermiments.
Results
Calu-3 cultures had a smooth texture without cell ‘nodules’ or necrotic regions. Low magnification revealed a continuous layer of cells (Fig. 2Ai), with multi-layered regions where adjacent capillaries were close enough to allow the cells to form ‘bridges’, as described previously for human choriocarcinoma cells (Knazek et al. 1972). Cells were cuboidal (Fig. 2Aii and B). Tight junctions (zo, zonula occludens), adherens junctions (za, zonula adherens) and spot desmosomes (mo, macula occludens) were observed at high magnification (Fig. 2C) as were apical microvilli (mv, microvillae) and occasional sub-apical vesicles (ccv, clathrin-coated vesicle; Fig. 2D). The micrographs and lactate assays suggest that Calu-3 cells polarize and form stable, long-term cultures on hollow fibres.
Figure 2. Polarization of Calu-3 cells cultured on hollow fibres.
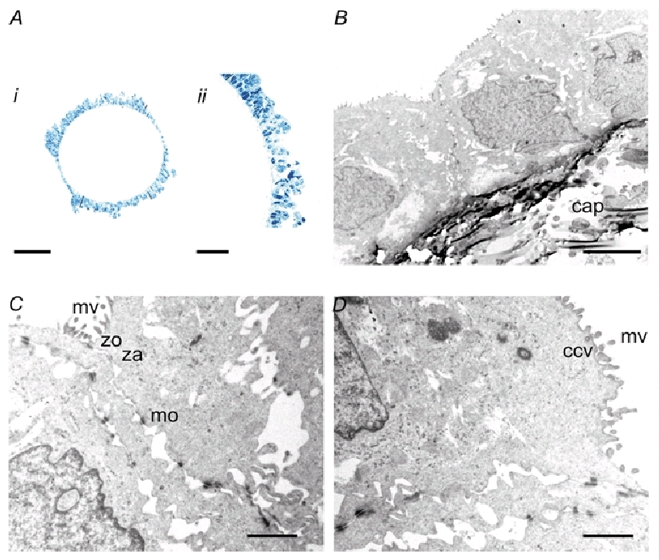
A, light micrographs taken at (i) ×25 and (ii) ×63 magnification showing mono- and multilayered cultures of Calu-3 cells in a long-term culture (1.5 months). The scale bars indicate 200 and 5 μm, respectively. No cell nodules or necrotic regions were observed. B, low-power electron micrograph showing cuboidal cells with apical microvillae growing on the external surface of a hollow fibre (‘cap’ indicates the polypropylene capillary wall). Scale bar = 5 μm. C and D, electron micrographs showing microvillae (mv), tight junctions (zo), adherens junctions (za), desmosomes (mo) and sub-apical vesicle (ccv). Scale bar = 1 μm.
In the assays used to generate standard curves, luminescence was proportional to [ATP] over a wide range as expected but was shifted upwards in hypotonic solutions (Fig. 3A). This was more obvious from the slopes of linear plots, which increased by 33, 60 and 81.5 % as [NaCl] was reduced from 140 to 100, 80 and 70 mm, respectively (Fig. 3A, inset). Consequently standard curves were calculated for each perfusate used during the experiment. Doubling the flow rate within the extracapillary space stimulated ATP release from 208 ± 5 to ≈1266 ± 120 fmol min−1 (Fig. 3B). Elevations to 5 and 10 ml min−1 increased peak secretion rate further to ≈6618 ± 900 and 12420 ± 2510 fmol min−1, respectively. Interestingly, ATP release was sustained during moderate shear stress but declined steadily at the highest flow rate examined (i.e. 1.89 dyn cm−2 shear stress at 10 ml min−1), suggesting depletion of the releasable ATP pool. Secretion returned to the baseline within 2 min after control perfusion rate was restored regardless of the shear stress applied. Since mechanical artefacts induced by switching solutions might account for the ATP release at low flow rates; i.e. when the pump rate was increased from 1 to 2 ml min−1, control experiments were performed by switching between two identical solutions while keeping the perfusion rate constant (Control(1) and (2); Fig. 3C). This did not affect release (227 ± 13 and 267 ± 15 fmol min−1 with Control(1) solution vs. 274 ± 15 fmol min−1 with Control(2) solution), although the cells were clearly capable of responding since reducing osmolality by 25 % caused a 5-fold stimulation (Fig. 3C). We conclude that the shear stress produced by flow rates of 5 ml min−1 causes sustained release of ATP at high rates.
The effect of hypotonicity, which should induce both cell swelling (a mechanical stimulus) and dilution of intracellular contents, was also examined. Reducing apical osmolarity by 25 % (100 mm NaCl) markedly stimulated ATP release (325 ± 14 fmol min−1 to 1248 ± 72 fmol min−1; n = 4; Fig. 4A). Further reductions of 38 % (80 mm NaCl) or 43 % (70 mm NaCl) elicited larger responses, although ATP efflux always reached a peak during the first 2 min and declined spontaneously to a plateau rate that was 30–50 % of the maximum. The plateau was maintained as long as cells were exposed to hypotonic solution (i.e. ≥ 30 min), and the rate of ATP release returned to the baseline level within 2 min when cells were superfused again.
Figure 4. Effect of hypotonic stress and modulators of connexin hemichannel permeability.
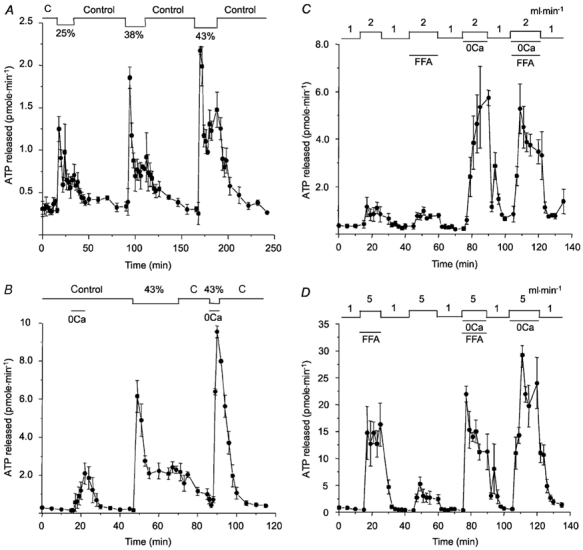
A, reducing osmolarity rapidly increased ATP release during the first 2 min, which then declined spontaneously to a plateau rate that was sustained for at least 30 min. ATP release returned to the basal rate when isosmotic control solution was restored. Percentage values indicate reductions in osmolality; ‘C’ indicates isosmotic control perfusate. B, low Ca2+ stimulates apical ATP release in isosmotic solution and potentiates the initial response to hypotonicity. C, effect of flufenamic acid (FFA; 100 μm) on ATP released in response to shear stress, and the combined action of shear stress and low Ca2+. Note that FFA had no effect on shear-induced release, but partially inhibited the release induced by shear stress + low Ca2+. D, same interventions as C but in reverse order. Note that a higher flow rate (5 ml min−1) was used to generate shear stress so that any inhibition by FFA would be detected more easily. At least two cartridges were used for each panel, which show the means ± s.e.m. of 3–4 experiments. The cells had been cultured in bioreactors for 1 month (Fig. 4A-C) or 2 months (Fig. 4D).
Recent evidence suggests that connexin hemichannels mediate ATP release from astrocytes (Stout et al. 2002). We investigated their role in airway epithelial cells by perfusing the extracapillary space with low-Ca2+ solution to increase connexin permeability, both in the absence and presence of hypotonic stress (Fig. 4B). Under isosmotic conditions, low Ca2+ increased the rate of ATP secretion by ≈10-fold (from 215 ± 28 to 2082 ± 55 fmol min−1; n = 4), and the maximum rate of secretion under these conditions would have been higher except that exposure to low Ca2+ was kept brief to avoid damaging the monolayer. Integrity of the cells after low Ca2+ was subsequently confirmed by their normal, biphasic ATP response to hypotonic challenge. Note that hypotonically induced release was greatly enhanced by low Ca2+, although the exposure to hypotonic low-Ca2+ solution was too brief to show the plateau phase (Fig. 4B). The next series of experiments tested the effect of the connexin 43 inhibitor flufenamic acid (100 μm) and low Ca2+ on ATP release induced by mild shear stress (i.e. by increasing flow rate to 2 or 5 ml min−1 in Fig. 4C or D, respectively). Total ATP released during 15 min exposure to mild shear stress was 11.2 ± 3.6 pmol in control medium, and this was not inhibited by 100 μm flufenamic acid (10.0 ± 0.43 pmol). Flufenamic acid also did not inhibit total ATP release (43.4 ± 2.1 vs. 46.1 ± 11.2 pmol) when integrated ATP responses were compared during simultaneous shear stress and 0 Ca2+, although there was a clear inhibitory trend during the response. Most striking was the large (> 4-fold) increase in shear-induced ATP release to 5740 ± 32 fmol min−1 in low-Ca2+ solution, a level that greatly exceeds the summed responses to flow (Fig. 4C) and low Ca2+ (Fig. 4B). Release rates were also obtained when the order of controls and FFA exposures were reversed (Fig. 4D). Again, FFA did not inhibit shear-induced ATP release. Indeed, the subsequent control response was actually reduced by 75 % compared to that seen with FFA present, presumably due to depletion of the releasable ATP pool. This provides further evidence against connexin-mediated release from the cytoplasm during apical shear stress, since the ATP released during the response (164 ± 18 pmol) should be less than 1 % of that present in the cytoplasm of 108 cells (≈20 000 pmol). Nevertheless, FFA did inhibit ATP release by 35 % in low Ca2+ medium, and the subsequent control response was actually larger than the initial response with 0 Ca2+, suggesting no depletion of the releasable pool under these conditions. FFA sensitivity suggests that connexin hemichannels contribute to ATP release when Ca2+ is low, and this may explain why there is no diminution of the second response to 0 Ca2+, since connexin-mediated ATP release would be derived from the large cytoplasmic pool. This contrasts with shear stress-induced ATP secretion in the presence of millimolar extracellular [Ca2+], which is insensitive to FFA, suggesting little involvement of connexin 43.
Discussion
This paper describes ATP release from human airway epithelial cells during shear and osmotic stress using a novel flow-through preparation. The responses have distinct time courses and are strongly enhanced by lowering extracellular calcium. Flufenamic acid, which inhibits ATP release through connexin hemichannels in astrocytes (Stout et al. 2002), was partially effective only under conditions of low extracellular calcium, suggesting other pathways, perhaps connexins that are resistant to this inhibitor, may mediate ATP secretion by Calu-3 cells exposed to shear stress in solutions that contain physiological [Ca2+].
Calu-3 cells released 2-3.6 fmol ATP min−1 (106 cells)−1 when the apical compartment of the hollow fibre bioreactor was superfused at a low rate. This is less than the constitutive release reported for static cultures of transformed bronchial epithelial cells (16HBE14o−; 20–200 fmol min−1 (106 cells)−1), which was estimated from steady-state [ATP] in the medium and the half-life of exogenously added [γ-32P]ATP (Lazarowski et al. 2000). The different basal release rates may reflect either the cell lines or methodologies used. Small mechanical perturbations when mixing [γ-32P]ATP or collecting samples might explain higher constitutive ATP release from 16HBE14o− cells, despite the small chronic shear stress on Calu-3 cells under control conditions induced by perfusion at 1 ml min−1. Alternatively, only apical ATP release was collected from polarized Calu-3 cells in the present study, whereas constitutive release from 16HBE14o− cells was studied using unpolarized cells cultured on plastic, which might release ATP via apical and basolateral mechanisms. Culture conditions that affect the expression of adhesion molecules, cytoskeletal elements, or connexins (Maroto & Hamill, 2001; Stout et al. 2002) could also contribute to the different rates of ATP release obtained.
The present results confirm and quantify the sensitivity of epithelial ATP release to mechanical stimuli reported previously (Grygorczyk & Hanrahan, 1997), although we can only speculate regarding its physiological role in vivo. Extracapillary shear stress applied during the present experiments was low (0.19-1.9 dyn cm−2) compared to those typically used to study endothelial ATP release (e.g. 25 dyn cm−2; (Yegutkin et al. 2000) or those found in vivo, for example, in veins and arteries (1-6 and 10–70 dyn cm−2, respectively). If ATP release is normally triggered when submucosal glands become distended with mucus, it may serve to stimulate local Cl− and fluid secretion and thus facilitate mucus delivery onto the airway surface. Hypotonically stimulated ATP release has been reported in many cell types including airway epithelia (Taylor et al. 1998), and has been implicated in cell volume regulation (Wang et al. 1996; Hazama et al. 2000). The biphasic response may reflect the time course of mechanical stimulation, since most cells have a regulatory volume decrease after hypotonic swelling. The same mechanical forces could potentially mediate both types of ATP release responses, and therefore further studies are needed to establish the mechanism of ATP release. The results in this paper are consistent with a role for connexin 43 hemichannels under some experimental conditions, since ATP release during shear stress was greatly enhanced in low-Ca2+ solution and was partially inhibited by flufenamic acid (FFA). However other routes for mechano-sensitive ATP efflux apparently exist since FFA did not affect the release induced by shear-stress in the presence of normal extracellular [Ca2+]. Future studies should investigate whether reversible opening of tight junctions (despite normal basolateral [Ca2+]), and consequent leakage of ATP from the lateral intercellular spaces, contributes to ATP secretion induced by low Ca2+.
Hypotonic solutions caused step-like increases in extracellular ATP concentration in previous studies (e.g. Taylor et al. 1998) rather than the biphasic response observed in the present work. This difference can be attributed to the methodologies used, since previous assays with luciferase reagents in the bath monitored extracellular ATP concentration continuously rather than ATP efflux and therefore reported the integrated response (i.e. ATP accumulation). The flow-through system used here measures ATP flux, and therefore it detects the biphasic time course of osmotically induced release. Measuring ATP ‘off-line’ under ideal conditions is also advantageous when experimental interventions, such as the use of hypotonic solutions, affect the luciferase reaction. The high cell density and surface area-to-volume ratio in this preparation simulate the environment in native tissue, and continuous luminal perfusion of the fibres (i.e. the basolateral side of the cells) with medium ensures that metabolism can be maintained during sustained ATP release. Capillary cultures have been used extensively in the immunology and biotechnology fields (e.g. Quarles et al. 1980; Hillman et al. 1994); however, to our knowledge they have not been used previously to study epithelial secretion. The present results demonstrate their utility for studies of ATP release, which is crucial to understanding regulation of transepithelial salt transport (Mason et al. 1991), mucus secretion (Kim & Lee, 1991), and ciliary beat frequency (Saano et al. 1992) in the airways. ATP also functions as a growth factor and modulator of signal transduction (Ostrom et al. 2000) and is metabolized to adenosine, a secretagogue (Lazarowski et al. 1992) that regulates cystic fibrosis transmembrane conductance regulator (CFTR) channels through local activation of A2B adenosine receptors (Huang et al. 2001). A more general application of capillary cultures would be in studies of epithelial-derived products such as those involved in host defence at mucosal surfaces (Hancock & Diamond, 2000). For example, comparing secretions produced by longterm capillary cultures of normal and cystic fibrosis cells under well-controlled conditions may reveal abnormalities which lead to the recurrent infections by specific pathogens that characterize cystic fibrosis.
Acknowledgments
We thank J. Ouellette (Pharmacology, McGill University) for microscopy, H. Goldsmith (McGill University), D. Laird (University of Western Ontario) and J. Cadwell (FiberCell Sytems, Inc.) for helpful discussions, and R. Grygorczyk (Université de Montréal) for comments on the manuscript. This work was supported by the Canadian Cystic Fibrosis Foundation and Canadian Institutes of Health Research (CIHR). A.G. was supported by postdoctoral fellowships from Vaincre la mucoviscidose (France) and the Canadian Lung Association/ CIHR. J.W.H. is a CIHR senior scientist.
References
- Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: A mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28:507–512. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Detwiler TC, Feinman RD. Kinetics of the thrombin-induced release of adenosine triphosphate by platelets. Comparison with release of calcium. Biochemistry. 1973;12:2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- Fedan JS. Nucleotides and nucleosides in pulmonary function. In: Jacobson KA, Jarvis MF, editors. Purinergic Approaches in Experimental Therapeutics. Wiley-Liss, Inc.; 1997. pp. 333–356. [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism. Journal of Physiology. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. American Journal of Physiology. 1997;272:C1058–1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Guyot A, Tessier M-C, Berthiaume Y, Grygorczyk R, Hanrahan JW. A novel system for studying ATP release by airway epithelial cells. Pediatric Pulmonology Supplement. 2000;20:119. [Google Scholar]
- Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends in Microbiology. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl− conductances in murine C127 cells. Journal of Physiology. 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman GG, Wolf ML, Montecillo E, Younes E, Ali E, Pontes JE, Haas GP. Expansion of activated lymphocytes obtained from renal cell carcinoma in an automated hollow fiber bioreactor. Cell Transplantation. 1994;3:263–271. doi: 10.1177/096368979400300402. [DOI] [PubMed] [Google Scholar]
- Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. Journal of Physiology. 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proceedings of the National Academy of Sciences of the USA. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Higashiyama M, Kusaka I, Saito T, Nagasaka S, Fukuda S. Extracellular ATP promotes cellular growth of renal inner medullary collecting duct cells mediated via P2U receptors. Nephron. 1996;76:208–214. doi: 10.1159/000190170. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. British Journal of Pharmacology. 1991;103:1053–1056. doi: 10.1111/j.1476-5381.1991.tb12299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Brauneis U, Gatmaitan Z, Arias IM. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991;14:640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- Knazek RA, Gullino PM, Kohler PO, Dedrick RL. Cell culture on artificial capillaries: an approach to tissue growth in vitro. Science. 1972;178:65–66. doi: 10.1126/science.178.4056.65. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. American Journal of Physiology - Renal, Fluid and Electrolyte Physiology. 2002;282:F281–88. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. Journal of Biological Chemistry. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. British Journal of Pharmacology. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. Journal of Biological Chemistry. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. British Journal of Pharmacology. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proceedings of the National Academy of Sciences of the USA. 2001;98:9086–9091. doi: 10.1073/pnas.161290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P, Bodin P, Loesch A, Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochemical and Biophysical Research Communications. 1990;170:649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. Journal of Biological Chemistry. 2000;275:11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- Quarles JM, Morris NG, Leibovitch A. Carcinoembryonic antigen production by human colorectal adenocarcinoma cells in matrix perfusion culture. In Vitro. 1980;16:113–118. doi: 10.1007/BF02831502. [DOI] [PubMed] [Google Scholar]
- Saano V, Virta P, Joki S, Nuutinen J, Karttunen P, Silvasti M. ATP induces respiratory ciliostimulation in rat and guinea pig in vitro and in vivo. Rhinology. 1992;30:33–40. [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. Journal of Biological Chemistry. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. American Journal of Physiology. 1998;275:C1391–1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proceedings of the National Academy of Sciences of the USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. British Journal of Pharmacology. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]


