Abstract
The responses of single-channel currents to capsaicin were recorded using the giga-seal patch-clamp technique in cell-attached and excised (inside-out/outside-out) patches from embryonic rat dorsal root ganglion (DRG) neurones in culture and in Xenopus oocytes heterologously expressing the rat vanilloid receptor (rVR1). Native and cloned vanilloid receptor (VR)-mediated currents exhibited outward rectification. In both the DRG neurones and oocytes expressing VR1, the chord conductances at −60 and +60 mV were ≈50 and ≈100 pS, respectively. At positive potentials, the channel exhibited a single conductance state. In contrast, at negative potentials, brief sojourns to subconductance states were apparent. The probability of the channel being open (Po) was dependent on the transmembrane voltage and the patch configuration (i.e. cell-attached vs. excised). In both DRG neurones and oocytes, the Po was greater at positive (+60 mV) than at negative (-60 mV) potentials. In cell-attached patches, the Po was approximately twofold higher, regardless of the applied potential. Most likely, the outward rectification observed in whole-cell currents is due to the voltage dependence of single-channel conductance and Po. The open-time distributions of single-channel currents recorded from native and cloned VRs in the presence of low agonist concentrations (0.01-0.03 μm) were best fitted with three exponential components. The closed-time distributions were best fitted by five exponential components. At higher concentrations (0.5-1 μm), an additional component was required to fit the open-time distribution, and the number of exponential components needed to fit the closed-time distributions was reduced to two. The overall mean open time at +60 mV was ≈4 ms, compared to ≈1.2 ms at −60 mV. However, the overall mean closed time was not voltage dependent. There were no significant differences between the native and cloned receptors. A comparison of single-channel properties of native and heterologously expressed VR channels indicates that expression of the rVR1 subunit alone can account for the single-channel behaviour of the majority of the native VRs. These results suggest that either native VRs are made up of VR1 subunits, or the incorporation of subunits other than VR1 does not influence the functional properties.
Capsaicin, the active ingredient of hot chilli peppers, induces a burning pain by activating certain receptors located at the sensory nerve endings (Wood et al. 1988; Oh et al. 1996; Szallasi & Blumberg, 1999). The capsaicin molecule has a vanilloid moiety; therefore, these receptors are interchangeably referred to as capsaicin or vanilloid receptors (VRs). VRs play an important role in transducing thermal and inflammatory pain. The VR is a non-selective cation channel that has a high permeability to Ca2+ and exhibits macroscopic outward rectification (Caterina et al. 1997). The activation of VRs depolarises the sensory nerve endings and evokes a train of action potentials that propagates to the spinal cord and brain. A number of VRs with structural and sequence homology have been cloned: TRPV1 (VR1; Caterina et al. 1997), TRPV2 (VRL1; Caterina et al. 1999), TRPV3 (VRL3; Peier et al. 2002; Smith et al. 2002; Xu et al. 2002) and TRPV4 (OTRPC4 or VR-OAC or VRL2; Strotmann et al. 2000). Stretch-inactivated channel (SIC), epithelial Ca2+ channels (ECaC1 and ECaC2) and a splice variant of VR1 (VR.5′ sv; Schumacher et al. 2000) are other members of this family (for a review see Gunthorpe et al. 2002). Generally, VRs are thought to be distributed only in peripheral sensory nerve endings, where they are involved in the perception of noxious stimuli. However, recent studies have shown the expression of VRs in regions other than sensory systems, which increases the scope and functional diversity of these receptors. The presence of VR1 has been confirmed in many parts of the brain (Mezey et al. 2000), blood vessels and colonic and urinary bladder nerve endings (Szallasi & Blumberg, 1999). VRs have been shown to be overexpressed in colonic nerve endings in inflammatory bowel disease (Yiangou et al. 2001). In the vasculature, VRs mediate the release of calcitonin gene-related peptide, (CGRP) which is a potent vasodilator (Zygmunt et al. 1999). Mice lacking the VR1 gene have deficits in thermal- or inflammation-induced hyperalgesia, but retain sensitivity to noxious heat. This confirms clearly the role of VR1 in certain modalities of nociception (Caterina et al. 2000; Davis et al. 2000).
Structurally, the VR is related to the voltage-gated K+ channels, cyclic-nucleotide-gated and transient receptor potential (TRP) family of ion channels, in that all have a topology of six transmembrane segments and a loop between the fifth and sixth segments (Caterina et al. 1997). Given this structural similarity, a tetrameric stoichiometry has been proposed (Kuzhikandathil et al. 2001), even though higher molecular-weight forms have been detected in immunoprecipitation studies (Kedei et al. 2001). However, in the native receptor, other TRPV subunits could co-assemble to form homo- or heteromultimeric complexes with functional specificity.
Given the possibility of a heteromultimeric stoichiometry and the multiple integrating modulatory processes, it is necessary to study the properties of the native and cloned VRs. In this study we have analysed the single-channel properties of native VRs in rat embryonic dorsal root ganglion (DRG) neurones grown in culture and the cloned VR1 heterologously expressed in Xenopus laevis oocytes.
Methods
Electrophysiology
Single-channel currents were recorded from rat DRG neurones in culture and from Xenopus laevis oocytes injected with VR1 cRNA. Animals were cared for according to the standards of the National Institutes of Health. All animal use protocols were approved by the Southern Illinois University School of Medicine Animal Care Committee. Oocytes were obtained by an abdominal incision after anaesthetising the animal by immersion in a 0.05 % solution of 3-aminobenzoic acid ethyl ester (MS222). Animals were killed by subcutaneous injection of a 2 % solution of MS222. After separating the oocytes from the follicular layer, 20–70 nl of VR1 cRNA (0.5-1 mg ml−1) was injected using a Drummond Nanoject (Drummond Scientific, Broomall, PA, USA). Oocytes were used for recording starting 3 days after injection. More than 90 % of the patches had single or multiple channels.
Primary DRG neuronal cultures were prepared from rat embryonic day 18 embryos. Adult pregnant rats were anaesthetised by exposing them to anhydrous ethyl ether for 4 min. Then the animals were killed by decapitation and embryos were removed. The embryos were killed by decapitation, DRGs were dissected and the cells were dissociated by triturating with a fire-polished glass pipette. Cells were cultured in Neurobasal medium (Life Technologies, Buffalo, NY, USA) supplemented with fetal bovine serum (FBS; 10 %), and grown on poly-d-lysine-coated glass coverslips. Cells were used from 3 to 15 days after plating. Small diameter (< 30 μm) rounded neurones were selected for patch-clamp experiments. Around 15 % of the patches exhibited single-channel current activity in response to capsaicin.
The giga-seal patch-clamp technique (Hamill et al. 1981) was used to record single-channel currents. For single-channel recording from DRG neurones, the bath solution contained (mm): potassium gluconate 140, KCl 2.5, MgCl2, 1; Hepes 5, EGTA 1.5, pH adjusted with NaOH to 7.3. For single-channel recordings from oocytes, the bath solution contained the same solution except that the concentration of monovalent cation was 100 mm. The patch pipettes were made from glass capillaries (Drummond, Microcaps), coated with Sylgard (Dow Corning, Midland, MI, USA). For cell-attached and inside-out patches, the patch pipettes were filled with a solution that contained (mm): sodium gluconate 140 (DRG neurones)/90 (oocytes), NaCl 10, MgCl2 1, Hepes 5, EGTA 1.5, pH adjusted with NaOH to 7.3. For outside-out patches, the pipette solution contained: (mm) sodium gluconate 140 (DRG neurones)/90 (oocytes), NaCl 10, BAPTA 10, Hepes 10, K2ATP 2, GTP 0.25, pH adjusted to 7.3 with NaOH. While recording from cell-attached patches, in order to avoid differences in the driving force because of the differences in the membrane potential, the external NaCl was replaced with an equal amount of KCl in order to nullify the membrane potential. All the experiments were performed at room temperature (21-23 °C). Agar-bridge electrodes were used to avoid changes in junction potential. The currents were recorded using a PC505A (Warner Instruments, Hamden, CT, USA) or Axopatch 2B (Axon Instruments, Foster City, CA, USA) patch-clamp amplifier. Data were collected with an open filter (PC505A) or with the filter set at 10 kHz (Axopatch 2B), digitized at 94 kHz (VR-10B, Instrutech, Great Neck, NY, USA), and stored on videotapes. For the analysis of amplitudes and open probabilities (Po), the data were filtered at 2.5 kHz (-3 db frequency with an eight-pole, low-pass Bessel filter, LP10, Warner Instruments) and digitized at 5 kHz. For dwell-time analysis, the data were filtered at 10 kHz and digitized at 50 kHz.
Single-channel analysis
Single-channel current amplitude and Po were estimated from all-point current-amplitude histograms (Channel 2 software kindly provided by Michael Smith, Australian National University, Canberra, Australia) and fitted to Gaussian densities (Microcal Origin, Northampton, MA, USA). For current-voltage (I-V) relationships, 10–50 single-channel openings were grouped and the amplitude was determined by fitting a Gaussian curve to an all-point histogram. Po was determined using unedited segments of data, which were typically 1–5 min long. For multiple-channel patches, mean Po was measured as nPo divided by n (where n is number of channels in the patch). Chord conductance was measured at +60 or −60 mV. Slope conductance was determined by linear fits to current versus voltage data between +20 and +60 mV or between −20 and −60 mV.
Patches that apparently had a single VR channel (assessed by the lack of overlapping events at +60 mV, when the Po was > 0.8) were used for dwell-time analysis. However, this criterion was not valid when lower concentrations of agonists were used. Single-channel currents were idealised (10 kHz band width) using a modified Viterbi algorithm (QUB software, www.qub.buffalo.edu). Dwell-time distributions were fitted with mixtures of exponential densities using a method of maximum likelihood. Additional exponential components were incorporated only if the maximum log likelihood increased by more than 2 log likelihood units (Chung et al. 1990; Qin et al. 1996; Premkumar et al. 1997). A dead time (τd) of 50 μs was imposed retrospectively, in that events shorter than 50 μs were ignored.
All the chemicals used in this study were obtained from Sigma (St Louis, MO, USA). The working concentration of capsaicin was prepared freshly before the experiments from a stock of 100 mm in ethanol. The final solution contained < 0.001 % ethanol. Data are given as means ± s.d., and statistical significance was evaluated using Student's t test.
Results
Single-channel conductance
Single-channel currents were recorded in the cell-attached, inside-out and outside-out patch configurations. Calcium-free (1.5 mm EGTA, 1 mm Mg2+) extracellular solutions were used in order to avoid calcium-induced desensitisation and tachyphylaxis (Docherty et al. 1996; Caterina et al. 1997; Koplas et al. 1997). Single-channel currents recorded at positive and negative potentials demonstrated that capsaicin (0.01-1 μm) activated currents in a reversible manner. Figure 1 shows single-channel currents recorded at −80 and +80 mV from an outside-out patch obtained from an oocyte heterologously expressing VR1. At positive potentials, one predominant conductance state was observed, whereas at negative potentials, fluctuations between conductance levels were apparent (seen as a broad peak in the all-point histogram). Well-defined single-channel bursts of lower amplitude were grouped, and from an all-point histogram it is clear that at least two additional conductance states could be resolved (Fig. 2). Possible factors that may give rise to current fluctuations, such as divalent cations, and capsaicin itself blocking the pore, were investigated. Removal of divalent cations by the addition of 1.5-10 mm EGTA in the extracellular solution or 5–10 mm BAPTA in the intracellular solutions did not affect the conductance levels, suggesting that residual divalent cations were not responsible for this effect (in the absence of added chelators, calcium- and magnesium-free solutions contained ≈3 μm Ca2+). Moreover, characteristics of single-channel currents did not change during washout of capsaicin or activation by anandamide (Premkumar & Ahern, 2000). In similar experimental conditions, the majority of patches obtained from DRG neurones exhibited single-channel currents that were similar to those recorded from the oocytes heterologously expressing VR1. In both DRG neurones and oocytes (Fig. 3), the slope conductances were ≈50 pS at −60 mV and ≈ 100 pS at +60 mV. The results of the analysis in different patch configurations and concentrations of agonists are shown in Table 1.
Figure 1. Single-channel currents activated by capsaicin.
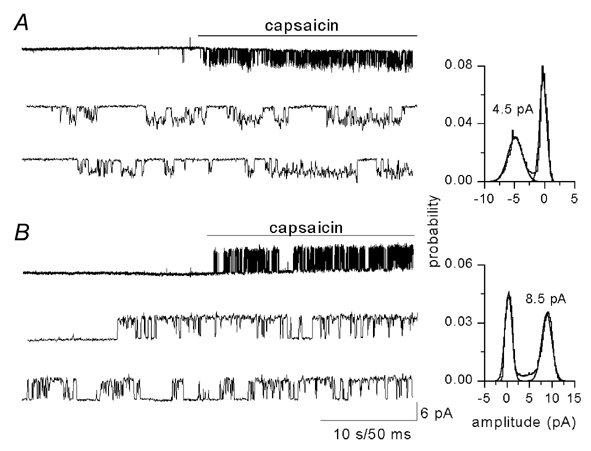
Application of capsaicin (1 μm) to an outside-out patch obtained from a Xenopus oocyte injected with vanilloid receptor (VR)1 cRNA activated single-channel currents. A, at −80 mV the current amplitude is 4.5 pA, corresponding to a conductance of 86 pS. B, at +80 mV the current amplitude was 8.5 mV, corresponding to single-channel conductance of 106 pS.
Figure 2. Subconductance levels of VR1 channel.
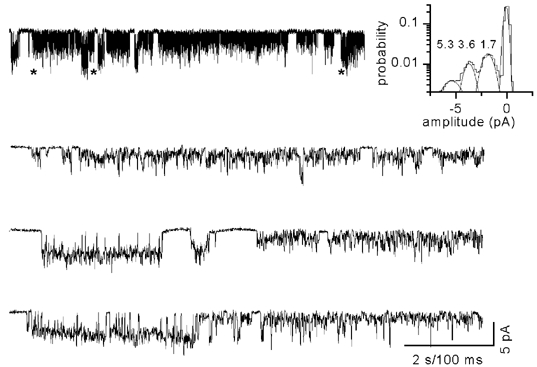
Single-channel currents recorded at −80 mV show sojourns to multiple conductance levels. Selected openings that show clear sublevels are grouped and the amplitude histogram (inset) shows two additional conductance levels (5.3, 3.6 and 1.7 pA corresponding to 66, 45 and 21 pS, respectively). * Regions that have been shown below in an expanded time scale.
Figure 3. The single-channel probability of opening (Po) is dependent on the patch configuration and transmembrane voltage.
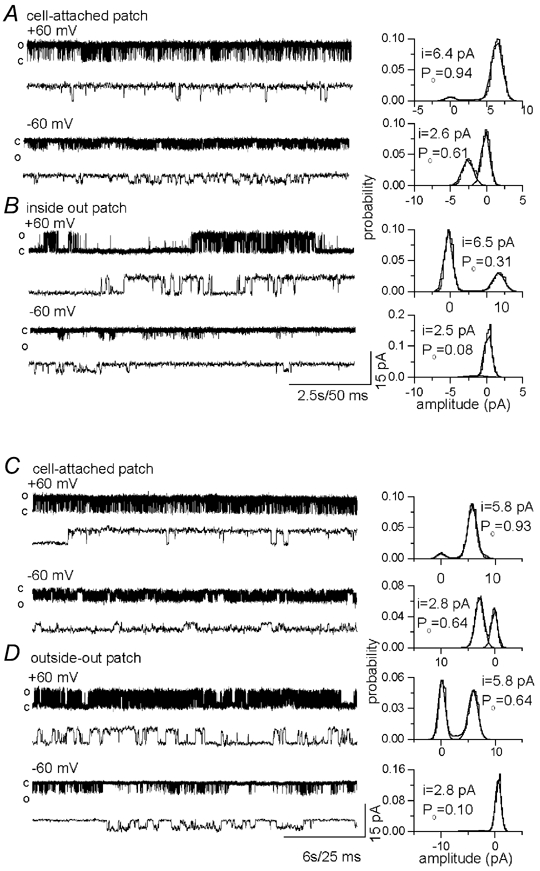
A, single-channel current recordings at +60 and −60 mV from dorsal root ganglion (DRG) neurones with the pipette containing 500 nm capsaicin. B, current recordings after forming an inside-out patch. C, in oocytes heterologously expressing VR1, single-channel currents activated by 500 nm capsaicin at +60 and −60 mV. D, current recordings after forming an inside-out patch. In cell-attached patches the Po was higher than in excised patches, and at positive potentials the Po was higher than at negative potentials.
Table 1.
Single-channel conductance and probability of opening (Po) in pttches obtained from native vanilloid receptors (VRs) from dorsal root ganglion (DRG) neurons in culture and from Xenopus oocytes heterologously expressing VR1
| Patch | Membrane potential (mV) | n | Concentration(μm) | Amplitude(pA) | Po | Slope conductance(pS) |
|---|---|---|---|---|---|---|
| DRG | +60 | 6 | 0.5–1 | 6.3 ± 0.58 | 0.80 ± 0.11 | 105 ± 10 |
| Cell-attached | −60 | 4 | 0.5–1 | 2.80. ± 0.37 | 0.40 ± 0.16 | 46 ± 6 |
| +60 | 4 | 0.01 | 605 ± 0.64 | 0.46 ± 0.09 | 101 ± 11 | |
| −60 | 4 | 0.01 | 3.1 ± 0.5 | 0.06 ± 0.06 | 51 ± 8 | |
| Excised | +60 | 6 | 0.5–1 | 5.9 ± 0.52 | 0.33 ± 0.15 | 98 ± 8 |
| −60 | 3 | 0.5–1 | 2.6 ± 0.21 | 0.12 ± 0.1 | 43 ± 4 | |
| Oocyte | +60 | 5 | 0.5–1 | 5.8 ± 0.44 | 0.70 ± 0.17 | 97 ± 7 |
| cell-attached | −60 | 6 | 0.5–1 | 2.6 ± 0.19 | 0.51 ± 0.22 | 43 ± 3 |
| Excised | +60 | 5 | 0.5–1 | 6.3 ± 0.27 | 0.36 ± 0.16 | 105 ± 5 |
| −60 | 4 | 0.5–1 | 2.8 ± 0.25 | 0.13 ± 0.1 | 46 ± 4 |
Values are presented as the mean ± s.d.
We explored the possibility that the reduced conductance at negative potentials could be due to loss of some second-messenger molecules or the phosphorylation state of the channel. Therefore, single-channel currents were recorded in cell-attached and excised patches. Although the Po was greater in cell-attached patches, the single-channel conductance showed no significant differences (Fig. 3 and Table 1).
Single-channel Po
Single-channel Po was dependent on the concentration of the ligand, the nature of the patch configuration (i.e. cell-attached vs. excised (inside/outside)) and the transmembrane voltage. As shown in other studies (Jung et al. 1999; Hwang et al. 2000), when the concentration of capsaicin was increased, the Po increased, attaining a maximum at 1–2 μm. The maximum Po in excised patches in response to application of capsaicin (0.5-1 μm) at +60 mV was 0.33 ± 0.15 in DRG neurones and 0.36 ± 0.16 in oocytes. In cell-attached patches held at +60 mV, capsaicin (0.5-1 μm) induced a maximum Po of 0.8 ± 0.11 in DRG neurones and 0.7 ± 0.17 in oocytes (Fig. 3 and Table 1). When an excised patch was formed after recording in the cell-attached mode, the Po showed a propensity to decrease over time, suggesting that factors such as phosphorylation and intracellular ATP play a role in inducing a higher Po. Figure 4 shows the change in the Po over time in the cell-attached configuration and after patch excision. Another observation was that the Po was voltage dependent. In DRG neurones, the Po was 0.8 ± 0.11 at +60 mV compared with 0.4 ± 0.16 at −60 mV. In oocytes, the Po was 0.7 ± 0.17 at +60 mV compared with 0.51 ± 0.22 at −60 mV. This was true for both cell-attached and excised patches, despite the Po being higher in cell-attached patches (Table 1).
Figure 4. Single-channel Po in cell-attached patches and after patch excision.
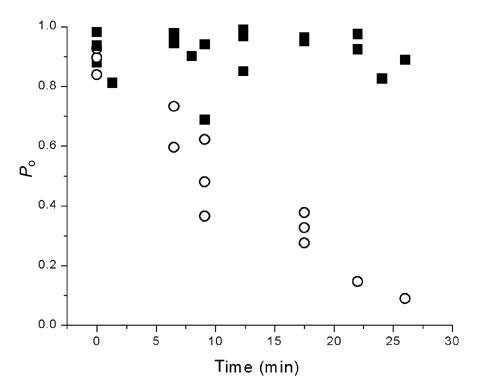
In the presence of 500 nm capsaicin, the Po was higher in cell-attached patches (> 0.7) and remained higher (▪), but upon patch excision, the Po decreased over time (○). Time zero represents the beginning time for both cell-attached as well as excised patches.
I-V relationship and rectification
Several studies have shown that whole-cell I-V relationships exhibit a profound outward rectification (Caterina et al. 1997; Tominaga et al. 1998; Piper et al. 1999). We have recorded single-channel currents at different membrane potentials to determine the factors that may be responsible for this effect. The outward limb of the single-channel I-V relationship has a slope conductance of ≈100 pS (between +20 and +60 mV) and the inward current limb has a slope conductance of ≈50 pS (between −20 and −60 mV; Table 1). Figure 5 shows the voltage dependency of single-channel current amplitude and Po in DRG neurones and oocytes. At potentials more negative than −60 mV, even though single-channel current amplitude increased in an ohmic fashion, the whole-cell current exhibited profound outward rectification. From this analysis it is clear that the single-channel conductance and Po are responsible for the outward rectification. At potentials more negative than −100 mV, a negative slope conductance was observed, which is possibly due to a voltage-dependent decrease in the Po. The combined effects of Po and single-channel conductance contribute to the rectification seen in the whole-cell experiments.
Figure 5. Outward rectification of the VR in DRG neurones and in oocytes heterologously expressing VR1.
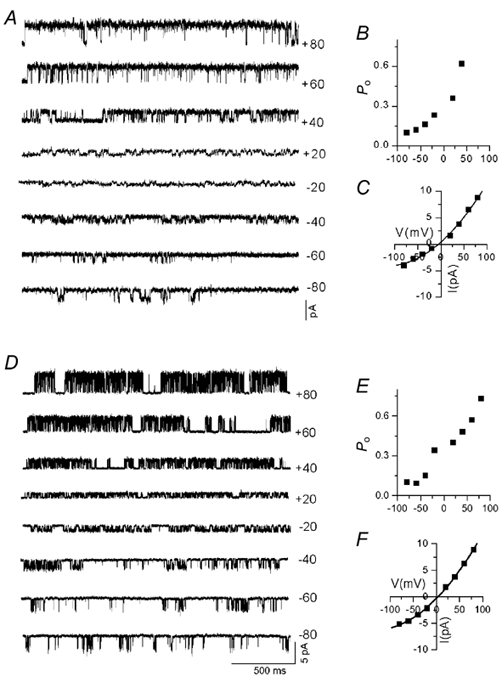
A, single-channel current recorded at different membrane potentials from DRG neurones. Both single-channel Po (B) and conductance (C) were reduced at hyperpolarised potentials. D, single-channel current recorded at different membrane potentials from an oocyte heterologously expressing VR1. E, progressive reduction in Po with hyperpolarisation. F, current-voltage relationship showing that the single-channel conductance was smaller at negative potentials. It is clear that both single-channel conductance and Po contributed to the macroscopic current rectification.
Kinetic properties
Patches that apparently had a single VR channel were used for kinetic analysis (see Fig. 3). In order to avoid calcium-dependent desensitisation and tachyphylaxis, single-channel currents were recorded in the absence of extracellular Ca2+ (1.5 mm EGTA). Single-channel currents were first idealised with two states, open and closed. Additional open or closed states were incorporated until the dwell-time histograms were well fitted with mixtures of exponential densities. A method of maximum log likelihood was used to determine the best fit to the data. Extensive analysis carried out by this method showed consistency in dwell-time distributions in segments of data within a patch as well as between patches (Tables 2 and 3). Since the single-channel conductance and Po were voltage dependent, the dwell-time distributions and the number of exponential components required to fit the data were also found to be voltage dependent. Although at both +60 and −60 mV the best fit of the open-time distribution required at least three open and four closed states, the time constants and the areas of relative distribution were statistically different (Tables 2 and 3, Fig. 6). Both native and cloned receptors exhibited similarities in the dwell-time distributions.
Table 2.
open-time distributions of native and cloned VRs
| Open time(ms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patch | Membrane Potential(mV) | Concentration of agonist(μm) | n | τ1 | τ2 | τ3 | τ4 | Mean open time (ms) |
| DRG | +60 | 0.01 | 3 | 0.05 ± 0.01 | 0.63 ± 0.4 | 7.76 ± 0.86 | 13.24 ± 0.68 | 3.23 |
| cell-attached | (0.67 ± 0.22) | 0.03 ± 0.02 | 0.12 ± 0.12 | 0.17 ± 0.17 | ||||
| +60 | 0.5–1 | 4 | 0.09 ± 0.007 | 1.69 ± 0.99 | 6.1 ± 2.9 | 18 ± 2.54 | 4.91 | |
| (0.07 ± 0.007) | (0.27 ± 0.17) | (0.64 ± 0.12) | (0.03 ± 0.02) | |||||
| −60 | 0.5–1 | 4 | 0.15 ± 0.1 | 0.83 ± 0.07 | 3.01 ± 0.72 | — | 1.36 | |
| (0.32 ± 0.20) | (0.31 ± 0.12) | (0.35 ± 0.1) | — | |||||
| Excised | +60 | 0.5–1 | 3 | 0.10 ± 0.06 | 1.26 ± 0.11 | 6.27 ± 0.69 | 16.2* | 5.30 |
| (0.1 ± 0.07) | (0.22 ± 0.19) | (0.67 ± 0.28) | (0.05) | |||||
| −60 | 0.5–1 | 3 | 0.14 ± 0.09 | 0.72 ± 0.05 | 2.30 ± 0.93 | — | 1.02 | |
| (0.36 ± 0.24) | (0.29 ± 012) | (0.33 ± 0.14) | — | |||||
| Oocyte | +60 | 0.03 | 3 | 0.05 ± 0.01 | 1.19 ± 0.7 | 4.85 ± 0.6 | — | 3.91 |
| Cell-attached | 0.5–1 | (0.11 ± 0.07) | (0.10 ± 0.01) | (0.78 ± 0.12) | — | |||
| +60 | 3 | 0.19 ± 0.16 | 1.21 ± 0.58 | 5.3 ± 0.48 | 10.68 ± 1.51 | 4.71 | ||
| (0.08 ± 0.01) | (0.22 ± 0.15) | (0.56 ± 0.15) | (0.14 ± 0.02) | |||||
| −60 | 4 | 0.08 ± 0.03 | 0.99 ± 0.10 | 2.8 ± 0.27 | — | 1.44 | ||
| (0.18 ± 0.16) | (0.48 ± 0.13) | (0.34 ± 0.19) | — | |||||
| Excised | +60 | 0.5–1 | 4 | 0.09 ± 0.04 | 0.44 ± 0.19 | 2.32 ± 1.15 | 9.12 ± 2.04 | 2.18 |
| (0.28 ± 0.08) | (0.22 ± 0.05) | (0.18 ± 0.01) | (0.18 ± 0.05) | |||||
| −60 | 0.5–1 | 3 | 0.08 ± 0.02 | 0.55 ± 0.11 | 2.63 ± 0.06 | — | 0.98 | |
| (0.33 ± 0.01) | (0.35 ± 0.01) | (0.29 ± 0.01) | — | |||||
Value are presented as the mena ± S.D τ1–τ4 are time constants.
Values from one experiment.
Table 3.
Closed-time distribution of native and cloned VRs
| Close time(ms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patch | Membrane Potential(mV) | Concentration of agonist(μm) | n | τ1 | τ2 | τ3 | τ4 | τ5 |
| DRG | +60 | 0.01 | 3 | 0.12 ± 0.08 | 0.64 ± 0.25 | 36.5 ± 0.33 | 98.1 ± 11.2 | — |
| cell-attached | (0.1 ± 0.1) | (0.1 ± 0.04) | (0.59 ± 0.40) | (0.36 ± 0.28) | — | |||
| +60 | 0.5–1 | 4 | 0.05 ± 0.01 | 0.22 ± 0.03 | 1.56 ± 0.44 | 7.06 ± 1.13 | 105 ± 105 | |
| (0.57 ± 0.22) | (0.22 ± 0.07) | (0.15 ± 0.13) | (0.05 ± 0.03) | (0.01 ± 0.004) | ||||
| −60 | 0.5–1 | 4 | 0.11 ± 0.02 | 0.60 ± 0.20 | 3.21 ± 2.97 | 21 ± 24.6 | 29.8* | |
| (0.58 ± 0.14) | (0.28 ± 0.11) | (0.03 ± 0.02) | (0.05 ± 0.07) | (0.001) | ||||
| Excised | +60 | 0.5–1 | 4 | 0.11 ± 0.03 | 1.30 ± 0.38 | 4.19 ± 1.74 | 22.8 ± 10.9 | 91.6 ± 89.6 |
| (0.37 ± 0.22) | (0.18 ± 0.11) | (0.21 ± 0.25) | (0.06 ± 0.07) | (0.01 ± 0.01) | ||||
| −60 | 0.5–1 | 3 | 0.05 ± 0.02 | 0.25 ± 0.07 | 1.95 ± 0.86 | 10.9 ± 9.5 | 97.7 ± 110.6 | |
| (0.53 ± 0.28) | (0.20 ± 0.07) | (0.2 ± 0.17) | (0.04 ± 0.04) | (0.01 ± 0.01) | ||||
| Oocyte | +60 | 0.03 | 3 | 0.06 ± 0.02 | 0.6 ± 0.28 | 20.7 ± 23.1 | 62.3 ± 42.3 | 294* |
| Cell-attached | (0.35 ± 0.02) | (0.39 ± 0.15) | (0.19 ± 0.14) | (0.04 ± 0.03) | (0.2) | |||
| +60 | 0.5–1 | 3 | 0.05 ± 0.02 | 0.23 ± 0.10 | 1.08 ± 0.15 | 10.0 ± 1.83 | 64.0 ± 10.2 | |
| (0.49 ± 0.23) | (0.26 ± 0.11) | (0.19 ± 0.11) | (0.02 ± 0.01) | (0.0008 ± 0.01) | ||||
| −60 | 0.5–1 | 4 | 0.008 ± 0.02 | 0.37 ± 0.13 | 1.1 ± 0.4 | 5.85 ± 2.7 | 36.7 ± 7.48 | |
| (0.76 ± 0.13) | (0.17 ± 0.17) | (0.14 ± 0.15) | (0.21 ± 0.23) | (0.02 ± 0.03) | ||||
| Excised | +60 | 0.5–1 | 4 | 0.11 ± 0.06 | 0.56 ± 0.17 | 6.5 ± 6.24 | 31.8 ± 18.6 | 54.12* |
| (0.33 ± 0.09) | (0.3 ± 0.06) | (0.24 ± 0.12) | (0.05 ± 0.05) | (0.2) | ||||
| −60 | 0.5–1 | 3 | 0.13 ± 0.01 | 0.78 ± 0.03 | 4.60 ± 0.66 | 24.2 ± 5.2 | 120.5 ± 12.02 | |
| (0.31 ± 0.01) | (0.26 ± 0.04) | (0.27 ± 0.01) | (0.11 ± 0.04) | (0.02 ± 0.01) | ||||
Values are presented sa the mean ± S.D.
Values from one or two patches.
Figure 6. Kinetic analysis of single-channel currents activated by capsaicin from native VRs in DRG neurones and cloned VR1 heterologously expressed in oocytes.
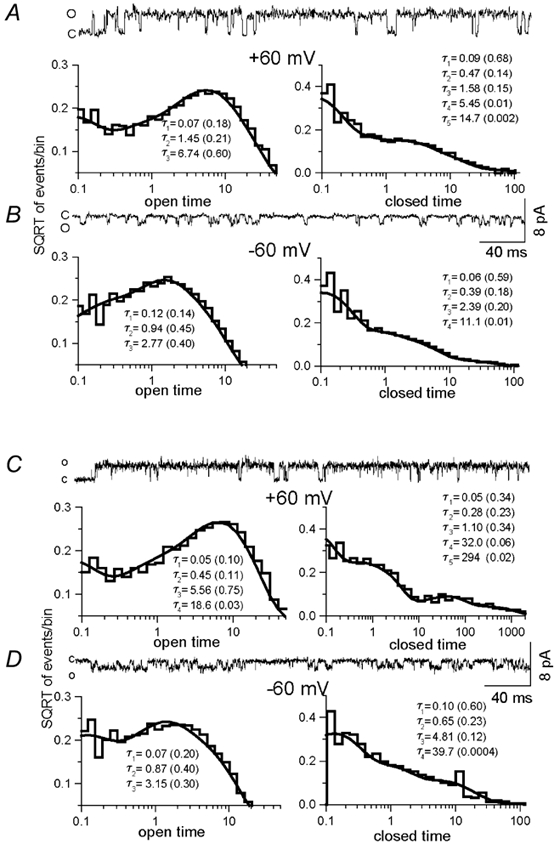
A, in DRG neurones, at positive potentials (+60 mV) the open- and closed-time distributions are well fitted with three and five exponential components, respectively. B, at negative potentials (-60 mV) the open- and closed-time distributions are well fitted with three and four exponential components, respectively. C, in oocytes expressing VR1, at positive potentials (+60 mV) open- and closed-time distributions are well fitted with four and five exponential components, respectively. D, at negative potentials (-60 mV), the open- and closed-time distributions are well fitted with three and four exponential components, respectively. The time constants of the exponential components and the relative fraction (in parenthesis) are shown.
At positive potentials (+60 mV), the number of exponential components required to fit the open-time distribution was dependent upon the concentration of the agonist. At lower concentrations (0.01-0.03 μm), the open-time distribution required three exponential components in both native and cloned VRs (Table 2). In DRG neurones, the time constants were 0.05, 0.63 and 7.76 ms, and in oocytes expressing VR1 the time constants were 0.05, 1.19 and 4.85 ms. However, when higher concentrations of capsaicin were used (0.5-1 μm), a fourth exponential component was needed to fit the data, which had a time constant of 13.2 and 18 ms in DRG neurones and 10.7 ms in oocytes. In DRG neurones, the fourth component was also seen at lower concentrations of capsaicin, possibly because of the phosphorylated state of the VR when the extracellular medium contained no Ca2+ (Vellani et al. 2001). Furthermore, at negative potentials (-60 mV), three exponential components were sufficient to fit the data both in DRG neurones and oocytes. The time constants were 0.15, 0.83 and 3.0 in DRG neurones and 0.08, 0.99 and 2.8 ms in oocytes. Although the Po was higher in cell-attached patches, the time constant of each component did not vary significantly, but the relative areas associated with each component did. The overall mean open times (0.5-1 μm capsaicin) were ≈4.5 ms in DRG neurones and ≈3.6 ms in oocytes at positive potentials compared to ≈1.2 ms in DRG neurones and ≈1.2 ms in oocytes at negative potentials (Table 2).
The closed-time distribution was well fitted with four or five exponential components (Fig. 6). The time constants of the two shortest exponential components changed little, if anything with the agonist concentration, suggesting that these components reflect fully liganded shut states (Table 3). At positive potentials, the time constants were 0.09 and 0.72 ms (mean of all experiments) in DRG neurones and 0.07 and 0.46 ms in oocytes. At negative potentials, the time constants were 0.08 and 0.43 ms in DRG neurones and 0.11 and 0.58 ms in oocytes (Table 3). This was seen clearly in patches in which the Po was higher than 0.9. The majority of the shut intervals was distributed within the two shortest components and the distributions at larger time constants were negligible (Table 3). Although the Po was higher, and the open-time distributions were significantly different at positive potentials compared to negative potentials, no detectable change was observed with respect to the first two components of the closed-time distributions. These results suggest that at positive potentials the channel tends to be more stable in the open state, compared to it being more stable in the closed state at negative potentials.
Discussion
The objective of this study was to characterise single-channel currents recorded from native (DRG neurones) and cloned (VR1 heterologously expressed in oocytes) VRs with respect to their conductance and kinetic properties. Several subunits with sequence homology to VR1 have been cloned, which raises the possibility that the native VRs could be either homo- or heteromultimers. The possible subunits that may contribute for the heteromultimeric stoichiometry include four members of the TRPV family. Furthermore, VRs are also known to be in direct association with receptor tyrosine kinase (TrkA) and phospholipase C (PLC; Chuang et al. 2001). It is probable that specific subunit combinations and associations with other proteins may affect the functional properties of the receptor.
Both native and cloned receptors exhibit macroscopic outward rectification (Caterina et al. 1997; Tominaga et al. 1998; Piper et al. 1999). From the results of single-channel analyses, it is clear that the rectification is due to the voltage dependence of both single-channel conductance and Po. The I-V curves from both native and cloned VRs have slope conductances of ≈100 pS (+20 to +60 mV) and ≈50 pS (-20 to −60 mV), respectively. The Po increased approximately two- to threefold from −60 to +60 mV, irrespective of the patch configuration. In cell-attached patches, the Po increased from 0.4 to 0.8 in DRG neurones and from 0.51 to 0.7 in oocytes. In excised patches, the Po increased from 0.12 to 0.33 in DRG neurones and from 0.13 to 0.36 in oocytes. The range of Po values acquired for native and cloned VRs is not statistically different (Table 1).
At negative potentials, single-channel currents exhibit more open-channel noise and brief sojourns to what appears to be subconductance states. This behaviour seems to be an inherent property rather than a result of divalent cations or capsaicin itself interfering with ion flow, because they also occur upon removal of the divalents or channels activated by anandamide. In a recent study (Chuang et al. 2001), Julius and coworkers have suggested that in the resting state phosphatidyl inositol 4,5, bisphosphate (PIP2) exerts a suppressive action on the channel. It is possible that the interaction of PIP2 may somehow contribute to the subconductance states. It is not clear whether capsaicin-induced conformational change of the VR relieves the inhibitory action of PIP2 or if it is independent of this action. In future experiments, it is necessary to investigate the role of PIP2 by recording currents in conditions where PIP2 no longer has an influence, either by removing it with an antibody or by stimulating the PLC pathway, which hydrolyses PIP2 to inositol-1,4,5-trisphosphate. The physiological relevance of rectification is not clear, but it may influence the rate of depolarisation that brings the membrane potential to threshold to initiate an action potential, which might be optimal for perceiving certain types of pain modalities. Once initiated, voltage- and time-dependent properties may come into play to regulate the firing pattern (Piper et al. 1999; Gunthrope et al. 2000; Ahern & Premkumar, 2002). For instance, if VR-mediated current were to exhibit a lesser degree of rectification in the presence of proinflammatory agents, it would be a plausible mechanism to explain hyperalgesia because the non-rectifying current may be able to depolarise the membrane readily to fire an action potential. It is worth noting that whole-cell ramp currents recorded in response to capsaicin from DRG neurones show a less pronounced outward rectification compared to cells heterologously expressing VR1 (Caterina et al. 1997; Reichling & Levine, 1997; Nagy & Rang, 1999). The discrepancy may be attributed to channels formed by subunits other than VR1 that exhibit less pronounced outward rectification.
Analyses of open- and closed-time distributions indicate that three to four exponential components for the open times and five exponential components for the closed times are required to fit the data. The presence of three to four open states suggests that the channel has a complex kinetic behaviour that may be related to the existence of multiple agonist binding-gating steps and modulation by second messengers. Indeed, the channel is probably a tetramer (Kuzhikandathil et al. 2001), and the different open components could correspond to the opening of the channel with various degrees of ligation. At negative potentials, the largest of the three open-time constants is ≈3 ms compared to ≈10 ms at the positive potentials. The overall mean open time at positive and negative potentials is ≈4 ms and ≈1.5 ms, respectively (see Nagy & Rang, 1999). Out of five closed-time constants, three get shorter as the concentration of agonist increases, suggesting that these reflect sojourns in closed states that are either unliganded or partially liganded.
The observation that the Po is higher in cell-attached patches compared to excised patches raises the possibility of modulation by second messengers (Premkumar, 2001). Phosphorylation by protein kinase A (PKA) or protein kinase C (PKC) and enhanced hydrolysis of PIP2 by PLC, which removes the suppressive action of PIP2 on the channels, have been shown to either directly activate or sensitise the VRs. The potentiation of VR responses by prostaglandins is mediated via PKA (Lopshire & Nicol 1997, 1998). Bradykinin (BK)-induced and extracellular-ATP-induced sensitisation of VR is mediated by PKC (Cesare et al. 1996; Premkumar & Ahern, 2000; Tominaga et al. 2001; Vellani et al. 2001). PKC also sensitises heat responses such that the threshold for VR activation is reduced from 42 to 35 °C, which means that VRs could be active at normal body temperature in certain conditions (Tominaga et al. 2001). Relieving the suppressive action of PIP2 on the channel function has been proposed to mediate the actions of nerve growth factor and BK, which renders the channel active even at 25 °C (Chuang et al. 2001). Elevation of intra-cellular Ca2+, extracellular ATP and metabolites of arachidonic acid have also been shown to potentiate the VR-mediated responses (Kress & Guenther 1999; Hwang et al. 2000; Tominaga et al. 2001). Finally, ATP binding to the Walker-domain region of the VR exerts a positive influence on the channel function (Kwak et al. 2000).
In the presence of extracellular Ca2+, VRs undergo desensitisation and tachyphylaxis, which has been shown to be due to the activation of calcium-dependent phosphatase, calcineurin (PP2B; Docherty et al. 1996). In a recent study, it was found that the VRs were in the phosphorylated state in the absence of extracellular Ca2+ because phorbol esters did not enhance whole-cell currents (Vellani et al. 2001). In this study, this is probably reflected in the cell-attached patches that exhibit a higher Po (≈0.8) compared to excised patches (≈0.3). Recent biochemical experiments have confirmed that there is indeed a substantial basal phosphorylation of VRs (Numazaki et al. 2002). Capsaicin has been shown to increase cAMP levels in DRG neurones (Liu et al. 2001), which could phosphorylate the VR and modify its functions. In order to study the basal activity without the influence of second messengers, it is necessary to study the properties of VRs when they are in a totally dephosphorylated state. Identification of the key residues (S502 and S800) by Numazaki et al. (2002) has made it possible to study the role of phosphorylation in detail. A variety of integrating stimuli activates or sensitises the VRs (Tominaga et al. 1998), and it is essential to know whether each of these stimuli activates the channel by binding to a specific site or simply by altering agonist affinity. After all, the quest for identifying an endogenous ligand may no longer be a priority because the multiple integrating stimuli are capable of synergistically activating the receptor maximally.
Acknowledgments
We thank S. Bhat for DRG cultures and C. Grosman for advice and critical reading of the manuscript. The VR1 clone was kindly provided by D. Julius. This work was supported by a grant from National Institute of Neurological Disorders and Stroke (NINDS) to L.S.P.
References
- Ahern GP, Premkumar LS. Voltage-dependent priming of vanilloid receptor: effects of agonists and phosphorylation state. Journal of Physiology. 2002 doi: 10.1113/jphysiol.2002.029561. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proceedings of the National Academy of the Sciences of the USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4, 5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chung SH, Moore JB, Xia LG, Premkumar LS, Gage PW. Characterization of single channel currents using digital signal processing techniques based on Hidden Markov Models. Philosophical Transaction of Royal Society of London B Sciences. 1990;329:265–285. doi: 10.1098/rstb.1990.0170. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) Journal of Physiology. 2000;525:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of the Sciences of the USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. Journal of Neuroscience. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. Journal of Biological Chemistry. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M, Guenther S. Role of [Ca2+]i in the ATP-induced heat sensitization process of rat nociceptive neurons. Journal of Neurophysiology. 1999;81:2612–2619. doi: 10.1152/jn.1999.81.6.2612. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. Journal of Neuroscience. 2001;21:8697–8706. doi: 10.1523/JNEUROSCI.21-22-08697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J, Wang MH, Hwang SW, Kim TY, Lee SY, Oh U. Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. Journal of Neuroscience. 2000;20:8298–8304. doi: 10.1523/JNEUROSCI.20-22-08298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. Journal of Neurophysiology. 2001;85:745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. Journal of Neurophysiology. 1997;78:3154–3164. doi: 10.1152/jn.1997.78.6.3154. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons, whole-cell and single-channel studies. Journal of Neuroscience. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proceedings of the National Academy of the Sciences of the USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. Journal of Neuroscience. 1999;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by PKCe and identification of two target serine residues. Journal of Biological Chemistry. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. Journal of Neuroscience. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Piper AS, Yeats JC, Bevan S, Docherty RJ. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. Journal of Physiology. 1999;518:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS. Interaction between vanilloid receptors and purinergic metabotropic receptors: pain perception and beyond. Proceedings of the National Academy of Sciences of the USA. 2001;98:6537–6539. doi: 10.1073/pnas.121190798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by PKC. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Qin F, Auerbach A. Subconductance states of a mutant NMDA receptor channel kinetics, calcium, and voltage dependence. Journal of General Physiology. 1997;109:181–189. doi: 10.1085/jgp.109.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophysical Journal. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proceedings of the National Academy of the Sciences of the USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Moff I, Sudanagunta SP, Levine JD. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. Journal of Biological Chemistry. 2000;275:2756–2762. doi: 10.1074/jbc.275.4.2756. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biology. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin). receptors and mechanisms. Pharmacological Reviews. 1999;51:159–212. [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proceedings of the National Academy of the Sciences of the USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. Journal of Physiology. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. Journal of Neuroscience. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, Distefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, DiMarzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


