Abstract
Capsaicin-sensitive primary afferents (CSPA) are known to be involved in nociception and neurogenic inflammation. Extensive research has been devoted to the sensory role of these fibres but less attention has been paid to their local effector function. This study aimed at gaining more insight into the molecular me xchanisms underlying the neurogenic inflammation induced by this special group of afferent fibres. Different groups of rats (n = 5 in each group), either naive or subjected to selective ablation of their CSPA, received individual intraplantar injections of saline, capsaicin, its vehicle or capsaicin preceded by its antagonist, capsazepine. Acute tests for nociception were used to assess the variations of the nociceptive thresholds. Variations of the levels of proinflamamtory cytokines and nerve growth factor (NGF) were measured by enzyme-linked immunosorbant assay (ELISA). Intraplantar injection of capsaicin (10 μg in 50 μl) produced a sustained thermal and mechanical hyperalgesia that peaked at 3–6 h and disappeared 24 h following the injection. Similar capsaicin injection in further groups of rats produced an early upregulation of the proinflamamtory cytokines and NGF, which peaked at 30–60 min and returned to control levels within 2–5 h. Similar effects were observed following the application of either capsaicin or intense electrical stimulation on the cut end of the distal portion of the sciatic nerve. The effects of capsaicin were abolished in rats subjected to selective ablation of their CSPA. These results demonstrate that CSPA can simultaneously challenge the immune system through the release of proinflammatory mediators and the central nervous system through nociceptive signalling and can therefore serve as a common afferent pathway to both immune and nervous systems.
The existence of peripheral nerve terminals subserving a dual sensory-effector function was first demonstrated by Bayliss in 1901, who showed that antidromic excitation of sensory nerves produced local vasodilatation independent from the central nervous system. A nocifensive role, in addition to the afferent signalling of nociception, has been assigned by Lewis (1937) to the effector property of this special group of sensory fibres.
The demonstration by Jancso et al. (1967) that capsaicin can selectively activate a special group of sensory fibres that induce neurogenic inflammation provided a new important chemical tool for the exploration of the physiological role of these fibres, which are called capsaicin-sensitive primary afferents (CSPA).
During the past three decades, research efforts have been focused on the role of capsaicin in pain and inflammation. For example, extensive research has been devoted to the study of the sensory effects of capsaicin when applied on the skin (Carpenter & Lynn, 1981; Simone & Ochoa, 1991) or perineurally (Wall & Fitzgerald, 1981; Holzer, 1991) or when injected intradermally into human volunteers (Culp et al. 1989; Simone et al. 1989; LaMotte et al. 1992) or experimental animals (Sakurada et al. 1992; Gilchrist et al. 1996). In summary, these reports agreed that capsaicin could activate nociceptors and produce central and peripheral hyperalgesias, which were often followed by transient or long-lasting desensitization (as reviewed by Fitzgerald, 1983; Buck & Burks, 1986; Holzer, 1991; Szolcsanyi, 1993).
However, several studies have documented the contribution of CSPA to inflammatory reactions, using various methods to induce inflammation, such as adjuvant arthritis (Colpaert et al. 1983; Hara et al. 1984) or acute injection of histamine (Amann et al. 1995) or mustard oil (Inoue et al. 1997).
Most of the published studies, when addressing the irritation of peripheral nociceptors by capsaicin, aimed at understanding its impact on the function of the central nervous system. Similarly, most of the studies of the role of CSPA in inflammation have highlighted its contribution to nociception. Although the dual role of the CSPA fibres has been known for some time, the progress in our understanding of the local effector role of these fibres has lagged behind that of their afferent (or centripetal) function. Furthermore, despite major studies on the local effector functions of the CSPA (reviewed by Holzer, 1988; Maggi, 1993; Szolcsanyi, 1996), the concept of neurogenic inflammation and the attenuation of inflammatory hyperalgesia by selective ablation of the CSPA fibres has led to the erroneous impression that their role was to add pain to the injury and to increase the inflammation. This assumption could serve to explain the treatment of chronic inflammatory diseases by gold therapy in traditional medicine, since gold salts have neurotoxic effects on small unmyelinated afferent fibres that contain substance P (Basbaum & Levine, 1991).
The aim of this study was to gain further insight into the molecular mechanisms underlying neurogenic inflammation and to elaborate further on the physiological role of this phenomenon. For this purpose, we demonstrate that local activation of CSPA fibres upregulates the levels of proinflammatory mediators known to be released primarily by immune cells. We also describe the differential regulation of these mediators by capsaicin, following ablation of the CSPA. The results suggest a physiological role played by CSPA fibres, consisting of a simultaneous activation of the central nervous system through nociceptive signalling and of the immune system through the local release of proinflammatory mediators.
Methods
This study was performed on adult female rats (250-300 g) kept in groups of five in clear plastic cages with floors covered with sawdust. During the experimentation period, rats were maintained under standard colony conditions including 12 h-12 h light-dark cycle, 22 ± 2 °C temperature and free access to food and water. All experimental procedures were carried out with strict adherence to the ethical guidelines for the study of pain in conscious animals (Zimmermann, 1983) and were approved by the University Animal Care Committee. Surgical procedures for acute experimentation or for tissue biopsies were carried out under deep anaesthesia with pentobarbitone (35 mg kg−1, i.p.). Lethal injection of the same anaesthetic (100 mg kg−1) was given to each rat at the end of the experimentation period.
Procedures for activation or ablation of CSPA fibres
Intraplantar injections
Capsaicin (8-methyl-N-vanillyl-nonanamide; cat. no. M1022, Sigma, St Louis, MO, USA) was dissolved in a 10 % Tween and 90 % olive oil solution and injected at one of four concentrations (1, 10, 25 and 50 μg in 50 μl) into four groups each comprising five rats. Each rat was gently restrained in a plastic cone (Decapicone, Braintree Inc., Braintree, MA, USA) and a volume of 50 μl of a solution of capsaicin or its solvent was injected into the dorsal aspect of the left hind paw, using a sterile insulin syringe (U-40 micro five-IV, Becton Dickenson, Franklin Lakes, NJ, USA).
Perineural application
Based on previous observations in our laboratory (Nassar et al. 1995), capsaicin (10 mg in 1 ml) was applied perineurally in three groups each comprising five rats. Under deep anaesthesia, the sciatic nerve in the left hind leg was isolated proximal to its trifurcation, ligated and cut, and the distal portion of the nerve was covered by small pieces of cotton wool soaked with capsaicin for a period of 30 min. Each nerve received a volume of 0.05-0.1 ml of the solution. Similar procedures were performed on the sciatic nerve of the right hind leg but the cut end of the distal portion of the nerve was soaked with the solvent or paraffin oil. The treated nerves were carefully isolated from the neighbouring tissues by pieces of Parafilm. At the end of treatment, the pieces of cotton were removed and the nerves were thoroughly washed with saline. Skin tissues from both legs were isolated at 1, 2 and 3 h post treatment (as 1 group per time interval).
Electrical stimulation
This was performed on the sciatic nerves isolated as described for perineural capsaicin treatment. The cut end of the distal portion of the sciatic nerve of the left leg was mounted on bipolar platinum electrodes, connected through an isolation unit (CCU1, Grass) to a stimulator (58800, Grass). Constant current stimulation (5-10 mA, 0.5 ms, each pulse) was delivered at a rate of 0.25 Hz for a period of 30 min. These stimulation parameters have been shown to elicit afferent C fibre volleys and related reflexes (Fitzgerald, 1982; Saadé et al. 1999). Muscle twitches due to electrical stimulation were alleviated by local injection of 2–5 μg of gallamine (Flaxedil, Specia, Paris, France) diluted in 25 μl saline. The right leg was subjected to similar treatment for the same time duration but without receiving electrical stimulation.
Selective ablation of CSPA fibres
This was performed in newborn and adult rats. For neonatal treatment at day 2 after birth, each rat pup received an i.p. injection of capsaicin (50 mg kg−1) dissolved in a solution of 10 % Tween 80, 10 % alcohol and 80 % saline. This procedure is supposed to destroy all CSPA in adult animals (Nagy et al. 1983). The success of treatment was assessed by the eye-wiping test (Hammond & Ruda, 1991). Briefly, one small drop of 0.01 % capsaicin was instilled using a 27 gauge needle into an eye of each treated rat, and the wiping movements were monitored for 1–2 min. The few rats that showed moderate to frequent eye wiping, suggesting incomplete ablation of their CSPA, were immediately anaesthetized with ether and killed by cervical dislocation.
The protocol described by McCafferty et al. (1997) was followed for the ablation of CSPA fibres in adult rats. Under light ether anaesthesia, each rat received repetitive subcutaneous injections of capsaicin with the following schedule: 25 mg kg−1 as first injection, 50 mg kg−1 as second injection given after 8 h and 50 mg kg−1 as third and last injection given at 32 h after the first injection. Treated animals were left to recover for 2 weeks, after which they were subjected to the eye-wiping capsaicin test to assess the successful ablation of CSPA (Hammond & Ruda, 1991). Animals that showed moderate or frequent eye wiping were not included in this study because the CSPA ablation was considered incomplete and were killed by cervical dislocation under deep anaesthesia.
Pretreatment with capsazepine
Capsazepine (C-191, RBI), the selective antagonist of capsaicin (Bevan et al. 1992), was dissolved in a solution of 0.5 % methanol in phosphate-buffered saline and administered by intraplantar injection at the dosages of 0.05, 0.1 and 0.5 μg in 25 μl, 30 min before the injection of capsaicin (10 μg in 50 μl) in the same hind paw.
Experimental groups
Behavioural tests were performed on eleven groups (n = 5 rats per group) of rats distributed as follows. Five groups of normal rats received intraplantar injections of either capsaicin at different dosages (4 groups) or the capsaicin solvent (1 group). In another four groups, rats received either capsazepine only (0.5 μg in 25 μl, intraplantar) or capsaicin injection (10 μg in 50 μl, intraplantar) preceded by intraplantar injection of capsazepine at the dosage of 0.05, 0.1 and 0.5 μg, as one group per dose. The remaining two groups were subjected to selective ablation of CSPA fibres neonatally (1 group) or as adults (1 group).
Determination of the variation of the levels of cytokines in the skin of the hind paws was carried out on another nineteen groups (n = 5 rats per group) of rats based on the following distribution. Eight groups of normal rats received intradermal injection of saline (1 group), solvent (1 group) or capsaicin 10 μg (6 groups). Rats receiving the capsaicin treatment were killed at 30 min, 1, 2, 3, 4 and 6 h after the injection, as one group per time interval. Two groups subjected to CSPA ablation, either as adults (1 group) or as neonates (1 group), received intraplantar injection of capsaicin and were killed 1 h following the injection. Nine groups of rats were subjected to isolation of their sciatic nerves and to either perineural capsaicin (3 groups) or electrical stimulation of the cut end of the distal portion of the sciatic nerve (6 groups). Following the capsaicin application, rats were killed at 1, 2 and 3 h, as one group per treatment and per time interval. For electrical stimulation of the sciatic nerve, five groups of normal rats were killed at 1, 2, 3, 4 and 6 h after the end of stimulation. Another group of rats subjected to CSPA ablation as adults was also subjected to electrical stimulation and killed after 3 h.
Behavioural tests
Variations of withdrawal latencies, due to intraplantar injections of capsaicin, were assessed by using three tests for acute nociception: the paw pressure (PP), the paw immersion (PI) and the hot plate (HP) tests. These tests were performed according to the methods described by Kanaan et al. (1996).
The PP test was used for the assessment of the variations of mechanical nociception. Rats were gently handled with a restraining plastic cone (Decapicone) and a constant pressure (0.2 kg cm−2) was applied through a blunt probe (surface 0.1 cm2) on either the left or the right hind paw. The pressure was discontinued when a rat displayed a clear attempt to withdraw its leg, and the time lapse between the application of pressure and paw withdrawal was measured as the latency or threshold of the test. This test was performed alternately on both hind paws with a minimum time interval of 3 min between two consecutive tests on the same leg.
For the PI test, the hind paws of a gently restrained rat were dipped alternately in a beaker filled with distilled water heated at 48 °C. A minimum time interval of 3 min was observed between the two consecutive tests on the same paw. The latency of withdrawal of the leg from the hot water was measured and the threshold of the test was based on the average of three measurements on each leg.
For the HP test, each rat was placed on a heated metal plate at 53 ± 0.2 °C. The time for the first sign of paw licking or jumping was monitored and considered as the withdrawal latency of this test.
The standard protocol followed for the execution of behavioural tests was to bring the rats in their cages (1 group of 5 per cage) to the laboratory daily, starting 3 days before the injections were made. During this period of time, rats were familiarized with the environment and to frequent handling. The baseline latency for each nociceptive test was established by averaging all measurements made for each test during the days preceding the injection.
Intraplantar injections of capsaicin were performed in the morning of day 4 and individual reactions of each rat to each pain test were assessed at 3, 6, 9 and 24 h following the injection. Since intraplantar injection of capsaicin produced paw swelling, it was not possible to follow the double-blind method in the execution of behavioural tests. However, these tests were carried out by operators not informed of the type of injection or the aim of the experiments.
Determination of the levels of cytokines
The two-site ELISA technique was used for the determination of the concentration of the various cytokines and nerve growth factor (NGF) in the skin tissues of animals subjected to capsaicin injections and other treatments.
Under deep anaesthesia with pentobarbitone (50 mg kg−1), the entire hind paw skin was removed from the injected and the non-injected hind legs. At the end of this procedure, the rats received a supplementary dose of the anaesthetic and were killed by cervical dislocation. The tissue samples were weighed, snap frozen on dry ice and stored at −70 °C to be processed for the determination of the levels of interleukin (IL) 1β and IL-6, tumour necrosis factor-α (TNF-α) and NGF. Skin tissues were homogenized in phosphate-buffered saline (pH 7.4) containing 0.4 m NaCl, 0.05 % Tween 20, 0.5 % bovine serum albumin, 0.1 mm phenyl-methyl-sulphonyl-fluoride, 0.1 mm benzethonium chloride, 10 mm EDTA and 20 IU aprotinin. The homogenates were then centrifuged at 12000 g for 60 min at 4 °C. The levels of various cytokines and NGF in the supernatant of the homogenized tissues were measured by ELISA, as described in detail by Saadé et al. (2000b).
Briefly, the procedure used for each cytokine was performed as follows for IL-1β. Standards and samples (100 μl of each) were added to Nunc maxisorp immunoplates, in duplicate, coated with affinity-purified polyclonal sheep anti-rat IL-1β antibody. After overnight incubation (4 °C) and washing, biotinylated polyclonal sheep anti-rat IL-1β (100 μl) was added to all the wells and the plates incubated overnight (4 °C). Following washing of the plates, 100 μl of IgG-HRP conjugate was added to all the wells and the plates incubated for 2.5 h at room temperature. After washing, colour was developed using tetramethylbenzidine and optical density was measured at 450 nm.
Similar procedures were performed for IL-6 and TNF-α using their corresponding antibodies. Reagents for IL-1β, IL-6 and TNF-α were provided by Dr Stephen Poole (the National Institute for Biological Standards and Control (NIBSC), Potters Bar, Herts, UK).
NGF was measured using an immunoassay kit (Promega, Madison, WI, USA) as described by the manufacturer and following the above-mentioned procedures for cytokines.
Data analysis
The nociceptive threshold for each test was measured by averaging individual values in each group before any treatment. Variations of nociceptive thresholds following each treatment were compared to the baseline of each test using repetitive parametric measure ANOVA followed by Bonferroni post hoc test.
The variations in the levels of cytokines were assessed by averaging the values obtained from each animal group for each cytokine and at the indicated time intervals. The basal level of each cytokine was determined in animals injected with saline or solvent. The measured values in each of the injected and non-injected legs were averaged and compared to the level measured in the non-injected leg or in the saline-injected animals using ANOVA followed by Bonferroni post hoc test.
GraphPad Prism 3 and Instat 3 software were used for statistics and graphics (GraphPad Software Inc., San Diego, CA, USA).
Results
Nociceptive effects of intraplantar injections of capsaicin
Intraplantar injections of different doses of capsaicin, ranging from 1 to 50 μg in 50 μl of solution, produced evident redness and swelling of the injected leg, starting 30–60 min after the injection. The lowest dose (1 μg in 50 μl) did not produce any significant hyperalgesia and the 5 μg dose had only mild effects. The high dose of capsaicin (50 μg) produced a complex effect, including hyperalgesia as assessed by the paw pressure and paw immersion tests when performed on the contralateral leg, while the withdrawal latency of the injected leg was not different from that of the control group. The 25 μg dose elicited only mechanical hyperalgesia (paw pressure test) in the injected leg. However, the latency of the hot plate test was decreased with all the injected doses of capsaicin since this test involves the injected and non-injected legs (Fig. 1).
Figure 1. Dose-dependent effect of intraplantar injections of increasing doses of capsaicin on the various nociceptive tests.
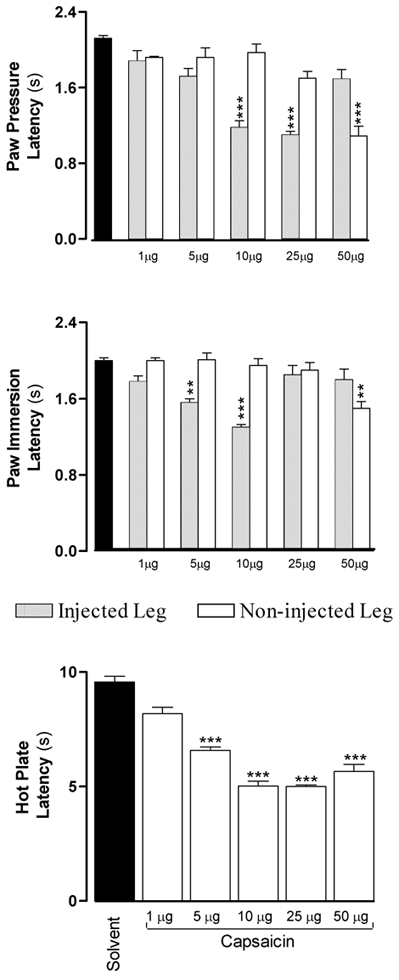
Withdrawal latencies were measured 6 h after the injection, which corresponds to the peak effects of the lowest dose of capsaicin. Note that with doses greater than 25 μg the injected legs became desensitized and the hyperalgesia became evident in the non-injected paw. The significance for each bar was calculated compared to solvent injections (▪). * P < 0.05, ** P < 0.01, *** P < 0.001.
The 10 μg dose of capsaicin produced significant hyperalgesia, redness and oedema in the injected leg without any effect on the non-injected leg. This dose produced mechanical and thermal hyperalgesia that peaked at 6 h post injection and declined progressively to reverse by 24 h (Fig. 2). At peak time, the PP latency decreased from 2.12 ± 0.03 to 1.18 ± 0.07 s (P < 0.001), the PI latency decreased from 2.00 ± 0.03 to 1.30 ± 0.03 s (P < 0.001) and the HP latency decreased from 9.56 ± 0.25 to 5.02 ± 0.22 s (P < 0.001). Accordingly, this dose was used for all subsequent experiments. A second injection of capsaicin (10 μg) on the same leg made after 1 or 2 days produced comparable effects to the first injection (data not shown).
Figure 2. Time courses of the effects of intraplantar injections of either capsaicin (10 μg in 50 μl) or its solvent (50 μl) on the latencies of nociceptive tests performed on injected and non-injected legs of two groups of rats (n = 5 each).
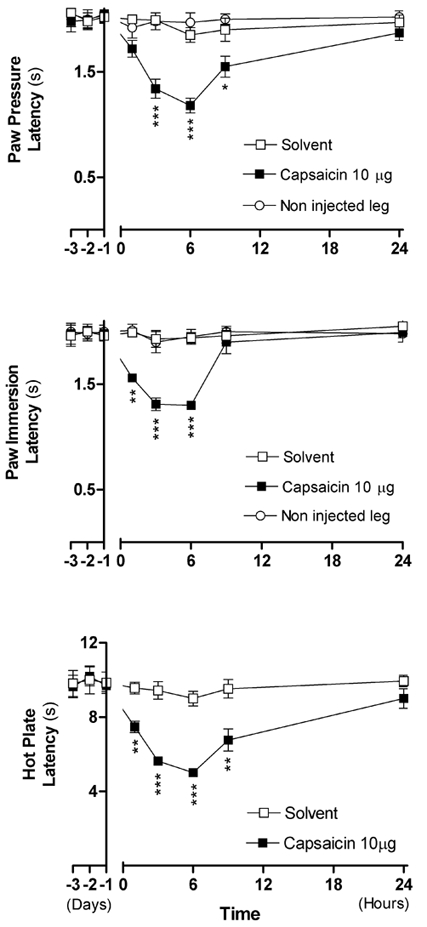
Each point in each curve corresponds to the mean ± s.e.m. of 5 measurements. Note that the hyperalgesia did not spread to the non-injected leg. The negative values on the X-axis represent the means of measurements made over 3 days before the injection. The P value for each point was calculated in comparison to the value obtained with solvent injection. * P < 0.05, ** P < 0.01, *** P < 0.001.
Effects of intraplantar injection of capsaicin on cytokine and NGF levels
Capsaicin (10 μg in 50 μl, intraplantar) was injected into different groups of rats that were killed after various time intervals, and cytokine levels were determined in the skin of the injected and non-injected paws.
As shown in Fig. 3, the levels of all the cytokines and NGF showed significant increases that peaked at 1 h and recovered to their basal levels at 2–3 h post injection, with the exception of IL-1β, which showed recovery after 6 h. Compared to their levels in naïve animals, the IL-1β level increased from 1278 ± 131 to 4012 ± 335 pg per hind paw (P < 0.001), the IL-6 level increased from 596 ± 82 to 1297 ± 127 pg per hind paw (P < 0.001), the TNF-α level increased from 284 ± 34 to 876 ± 25 pg per hind paw (P < 0.001) and the NGF level increased from 8.5 ± 0.5 to 19.5 ± 2 ng per hind paw (P < 0.01).
Figure 3. Time courses of the variations of the levels of proinflammatory cytokines and NGF induced by capsaicin compared to various treatments.
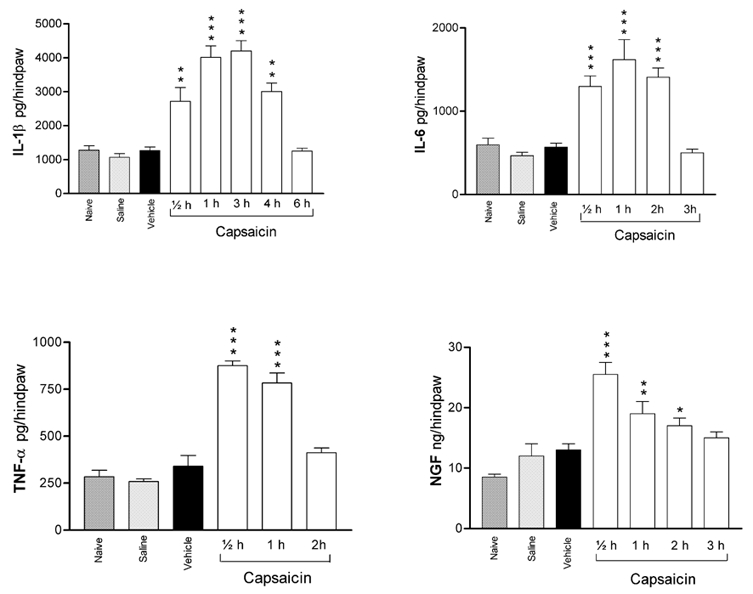
Each bar represents the mean ± s.e.m. of measurements made on a separate group of animals for each treatment or at each time interval following intraplantar injection of 10 μg of capsaicin. The P values were determined with reference to the mean of measurements made on naïve, saline- and vehicle solvent-injected rats. * P < 0.05, ** P < 0.01, *** P < 0.001.
All comparisons have been made with reference to values observed in naïve animals, which were not significantly different from those observed in animals injected with saline or solvent. Therefore, for the purpose of clarity in illustrations, results of solvent injections are only shown in Figs 1, 2 and 3. The levels of all the cytokines measured in the non-injected paws were not significantly different from those observed in naïve animals (data not shown).
Effects of intraplantar injections of capsaicin following ablation of CSPA or pretreatment with capsazepine
Both neonatal and adult animals treated with capsaicin to ablate the CSPA did not show any alteration of their nociceptive thresholds following intraplantar injections of capsaicin (10 μg in 50 μl), as shown by the PP, PI and HP tests (Fig. 4). It is worth noting that these animals did not exhibit evident redness or swelling, which are important features of capsaicin-induced neurogenic inflammation. Pretreatment with capsazepine produced a dose-dependent attenuation of the capsaicin-induced hyperalgesia (data not shown). At a dosage of 0.5 μg, capsazepine abolished the effects of capsaicin (Fig. 4).
Figure 4. Prevention of capsaicin (CAP)-induced hyperalgesia either by ablation of the CSPA fibres or by pretreatment with capsazepine (Capsaz).
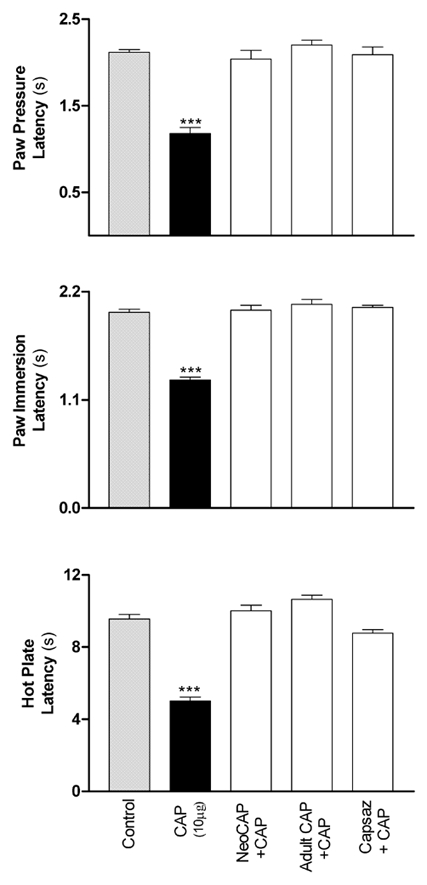
Each bar represents the mean measurements of test withdrawal latencies made on a separate group of rats (n = 5) which received one of the following treatments: saline injection (control), intraplantar injection of capsaicin (10 μg in 50 μl) into naïve rats or into rats subjected to CSPA ablation either neonatally (NeoCAP) or as adults (Adult CAP) or pretreatment with capsazepine (0.5 μg in 20 μl, intraplantar; Capsaz + CAP). The significance of differences was determined with reference to the control group. * P < 0.05, ** P < 0.01, *** P < 0.001.
In adult rats subjected to CSPA ablation, the levels of the cytokines, measured 2–3 weeks after treatment, were not significantly different from those observed in naïve rats. The level of NGF, however, was significantly increased following CSPA ablation (Fig. 5). Intraplantar injection of capsaicin (10 μg in 50 μl) did not produce significant alteration in the levels of any of the cytokines or NGF (Fig. 5).
Figure 5. Selective ablation of CSPA fibres in adult rats abolishes the effects of intraplantar injection of capsaicin on cytokines and NGF.
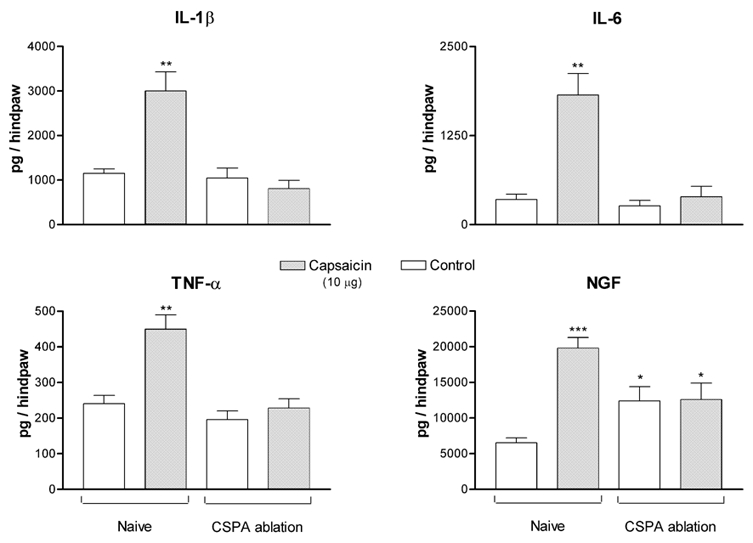
Each bar represents the mean measurement for each cytokine made on a separate group of rats. Control measurements were made on either naïve rats or on rats subjected to CSPA ablation, both injected with saline. Capsaicin measurements were made on similar groups of rats that were injected with capsaicin. The significance of differences was determined with reference to values observed in control naïve rats. * P < 0.05, ** P < 0.01, *** P < 0.001.
Effects of electrical stimulation of the sciatic nerve
Repetitive electrical stimulation of the cut end of the distal portion of the sciatic nerve for 30 min produced a significant elevation of cytokine levels in the skin of the stimulated leg when compared to their levels in the skin of the contralateral leg. This elevation was evident at 2 h, reached a peak at 3 h following the beginning of stimulation and disappeared after 6 h (Fig. 6). The concentrations of all the mediators in the contralateral leg showed a moderate increase with time that did not reach significant levels (Fig. 6). Electrical stimulation, with similar parameters, applied to the cut end of the distal portion of the sciatic nerves of a group of rats subjected to ablation of their CSPA as adults, did not elicit significant alteration in the levels of any of the mediators (Fig. 7).
Figure 6. Variation of the levels of cytokines and NGF in the leg skin tissues following electrical stimulation applied on the cut end of the distal portion of the sciatic nerve.
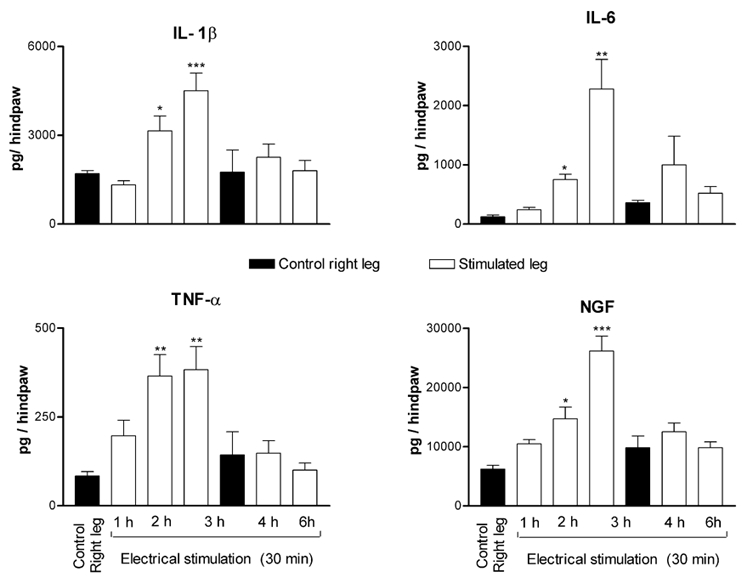
Each bar represents the mean ± s.e.m. of measurements made on separate groups of rats at the indicated time interval following the initiation of stimulation. The control right leg corresponds to the measurements made on skin tissues of the right legs of rats killed after 1 and 3 h. The significance of differences was determined with reference to the control group at 1 h. * P < 0.05, ** P < 0.01, *** P < 0.001.
Figure 7. Effects of electrical stimulation applied on the cut end of the distal portion of the sciatic nerve in control rats compared to rats subjected to CSPA ablation as adults.
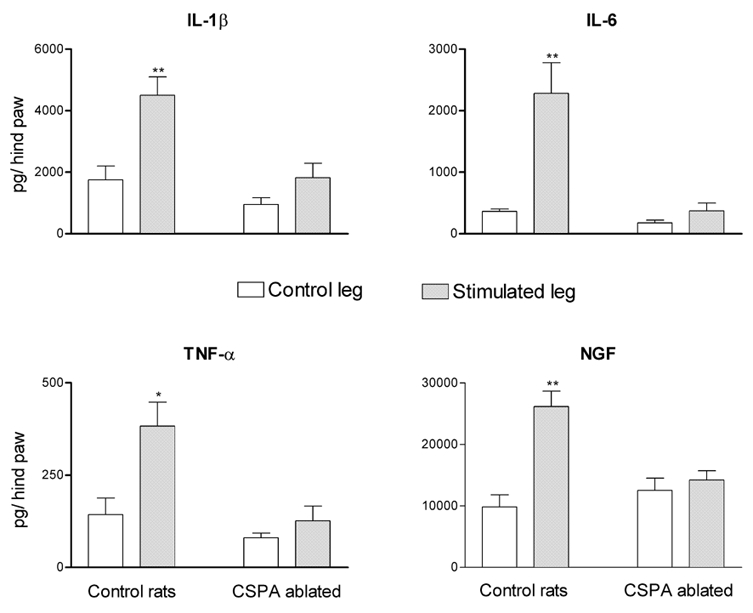
Electrical stimulation was applied on the sciatic nerve supplying the left leg. Each bar represents the mean ± s.e.m. of measurements made 3 h after the application of stimulation. This time corresponds to the peak effects of electrical stimulation on the levels of cytokines and NGF. The significance of differences was measured with reference to the control group. * P < 0.05, ** P < 0.01.
Effects of perineural application of capsaicin
Application of capsaicin (1 mg in 0.1 ml) onto the cut end of the distal portion of the sciatic nerve produced a 200–300 % increase in the levels of IL-1β, IL-6 and NGF in the skin of the leg supplied by this nerve. A less pronounced but significant increase of the level of TNF-α was also observed in the skin of the same leg when compared to levels measured in the skin of the contralateral leg supplied by the sciatic nerve soaked with solvent or with paraffin oil (Fig. 8). As shown in Fig. 8, this increase peaked within 30 min to 1 h and persisted over 1–2 h for TNF-α and for 2–3 h for IL-6, NGF and IL-1β, following the application of capsaicin.
Figure 8. Upregulation of cytokines and NGF levels by perineural application of capsaicin (1 mg in 0.1 ml) on the cut end of the distal portion of the sciatic nerve.
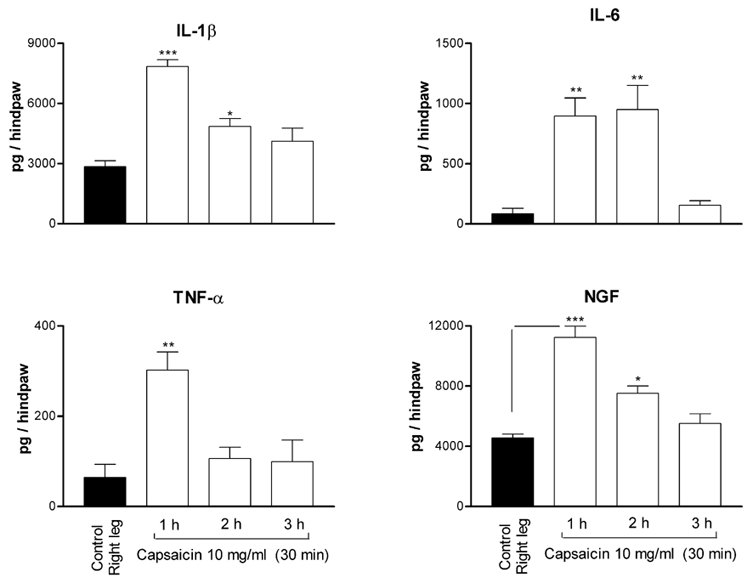
Each bar represents the mean ± s.e.m. of measurements made on separate groups of rats (n = 5) at the indicated time following application of capsaicin. The control corresponds to the measurements in the skin tissues of the opposite leg (right leg) at 1 h following application of paraffin oil onto the cut sciatic nerve supplying this leg. The significance of differences was determined with reference to the control (right leg). * P < 0.05, ** P < 0.01, *** P < 0.001.
Discussion
Intraplantar injections of various doses of capsaicin produced significant alterations in both thermal and mechanical nociceptive thresholds, as assessed by the variations in the latencies of the HP, PI and PP tests. At low doses, capsaicin effects were clearly dose dependent. The 5 and 10 μg injections produced reversible and significant hyperalgesia restricted to the injected leg, with no effects on the non-injected leg. The capsaicin dose-dependent effect was still evident up to the 25 μg dose on the PP test; however, this dose had no discriminative effect on the PI test (i.e. injected and non-injected legs gave normal results). At higher doses of capsaicin, the effect of the injection became more complex and spread to the non-injected leg. This observation is in line with the bilateral alterations of the behaviour of dorsal horn neurons, reported by Fitzgerald (1982), following application of a high dose of capsaicin onto one sciatic nerve. Further confirmation to this finding came from the work of Levine et al. (1985) describing contralateral reflex neurogenic inflammation due to the activation of capsaicin-sensitive afferents. These findings, which were attributed to a possible spinal and supraspinal cross-activation during excessive stimulation, can justify the use of smaller amounts of capsaicin to secure effects localized to the injected leg.
The 10 μg capsaicin injection produced significant and long lasting thermal and mechanical hyperalgesia that was paralleled by redness and oedema involving the whole leg. These effects were localized to the injected leg and disappeared within 24 h following the injection. The reversibility of the effects of intraplantar injection of 10 μg of capsaicin was ascertained by the ability of a second injection at the same dosage to produce effects comparable to those of the first injection.
The use of intraplantar injection of capsaicin to induce hyperalgesia in experimental animals has been described in several studies. The methods used for the assessment of hyperalgesia ranged from counting of the number of licks to the paw, as in the formalin test (Sakurada et al. 1992), to the application of Von Frey hairs (Kim et al. 2001) and radiant heat (Gilchrist, 1996). All cited works reported hyperalgesia with doses ranging from 10 to 40 μg of capsaicin and variable duration from a few minutes to a few hours. In the present study, capsaicin injection at the dose of 10 μg produced more pronounced and durable hyperalgesia. This difference can be attributed to the difference in the methods used for the assessment of the variations of nociceptive thresholds. The method we used, however, did not allow for a discrimination between primary and secondary hyperalgesia. This discrimination was not among the main purposes of this study, since our main aim was to investigate the local inflammatory effects produced by the activation of the CSPA fibres. The observed effects of intraplantar injection of capsaicin appear to be produced by the activation of these fibres, since these effects were abolished by either selective ablation of the CSPA fibres or pretreatment with the selective capsaicin antagonist, capsazepine.
The ability of the dose of 10 μg of capsaicin to produce hyperalgesia and oedema and the reversibility and reproducibility of its effects provided enough justification for the investigation of the effects of this dose of capsaicin on the levels of cytokines and NGF, which are known to be involved in the inflammatory cascade. As shown in the Results, intraplantar injection of 10 μg capsaicin produced significant upregulation of the levels of proinflammatory cytokines and NGF. This upregulation was restricted to the injected leg and was comparable to that observed with intraplantar injection of endotoxin (Safieh-Garabedian et al. 1997). It is, however, noteworthy that the upregulation of cytokines started earlier, i.e. at 30–60 min after capsaicin injection, while it was observed 3–4 h following intraplantar injection of endotoxin.
Furthermore, the hyperalgesia produced by intraplantar injection of either capsaicin or endotoxin showed a delayed peak (6-9 h) and recovery (24 h) when compared to those shown by the cytokine levels. As previously discussed (Safieh-Garabedian et al. 1997), the hyperalgesia can be considered as the end result of the inflammatory cascade triggered by the proinflammatory mediators and consequently, like other signs of inflammation (redness, oedema and fever), it takes more time to reach full development and recovery.
Although it was recently shown that capsaicin, under special in vitro conditions, can activate non-neuronal tissues (Biro et al. 1998), the observed upregulation of cytokines by capsaicin can be attributed to the activation of the CSPA fibres. This assumption is based on three experimental findings: first, intraplantar injection of capsaicin into adult rats subjected to CSPA ablation failed to produce hyperalgesia and upregulation of cytokines; second, perineural application of capsaicin on the distal end of the cut sciatic nerve produced an early and significant upregulation of cytokines in skin tissues of the leg supplied by the cut nerve; and third, electrical stimulation, at C fibre strength, of the distal part of the cut sciatic nerve, produced oedema and upregulation of cytokines in the skin tissues of the leg. It is worth noting here that, despite the fact that the sciatic nerve contains all groups of afferent and efferent nerve fibres, capsaicin application affects only the CSPA group (Cervero & McRitchie, 1982). Furthermore, similar results were obtained by electrical stimulation or capsaicin application to the distal end of the cut saphenous nerve, which is known to contain essentially cutaneous sensory afferent fibres (N. E. Saadé, C. A. Massaad, S. J. Jabbur, B. Safieh-Garabedian & S. F. Atweh, unpublished data). The observed moderate increase of cytokine levels in the contralateral leg could be due to the massive discharge produced by cutting its sciatic nerve; however, this did not reach the level of significance. Thus, the neurogenic inflammation, induced by either antidromic electrical stimulation of afferent fibres or by application of capsaicin, is characterized by the upregulation of proinflammatory cytokines traditionally observed in inflammatory conditions induced by bacterial toxins and other inflammatory agents (Dray & Bevan, 1993; Safieh-Garabedian et al. 1997; Poole et al. 2000).
It is important to note that perineural application of capsaicin, like intraplantar injection, resulted in an early peak of the cytokine upregulation, while the peak effect and recovery following electrical stimulation were delayed for 1–2 h. This temporal discrepancy can be attributed to one or both of the following. First, the high dosage of capsaicin (used in perineural application) can produce a stronger and faster recruitment of the CSPA fibres involved in neurogenic inflammation. Furthermore, the release of neuropeptides and consequently the inflammatory cascade can be triggered directly by capsaicin without necessarily involving action potential mechanisms in the peripheral terminals of afferent fibres. Second, electrical stimulation, in contrast, can recruit the entire spectrum of afferent and efferent fibres in the sciatic nerve. Some of these additionally recruited fibres can exert opposite effects to those induced by capsaicin, such as vasoconstriction (induced by sympathetic efferents) in contrast to vasodilatation and extravasation (induced by capsaicin).
As predicted by Sir Thomas Lewis in 1937 (Lembeck, 1983), the nocifensor afferent system is characterized by the local release of neuropeptides such as substance P, calcitonin gene-related peptide (CGRP) and vasoactive intestinal peptide (VIP) (Siney & Brain, 1996; for a review, see Holzer, 1988; Maggi, 1991). Each of these peptides is known to interfere with one or more of the cellular mechanisms involved in the inflammatory reaction. For instance, substance P can contribute to vasodilatation, to histamine discharge by mast cells (Weihe et al. 1991; Ansel et al. 1993) and to the modulation of the function of immune cells displaying substance P receptors on their cytoplasmic membranes (Payan et al. 1983; Helme et al. 1987; Morley et al. 1987; Peck, 1987; Marriott & Bost, 2001). The CGRP is well known for its vasoactive action (Escott & Brain, 1993; Kress et al. 1999) and for its effects on immune cells (Payan et al. 1987; Umeda, 1992; Wang et al. 1992; Hosoi et al. 1993). Several experimental observations also point out the immunoactive properties of VIP (reviewed by Bellinger et al. 1996). Furthermore, Kress et al. (1999) have shown that antidromic nerve stimulation may lead to the release of prostaglandin E2 (PGE2) from rat skin in vitro. This observation is in line with recent data from our group showing significant and sustained upregulation of PGE2 levels following intraplantar injection of capsaicin (Saadé et al. 2000a; N. E. Saadé, C. A. Massaad, S. J. Jabbur, B. Safieh-Garabedian & S. F. Atweh, unpublished data). PGE2, which can be produced by almost all types of cells, is a well-known key player in the inflammatory cascade and in the resulting inflammatory hyperalgesia (for review see Dray, 1994; Tilley et al. 2001). In line with these findings, preliminary results from our laboratory (Massaad et al. 2000; Saadé et al. 2000a) demonstrate that pretreatment with antagonists to neuropeptides (substance P and CGRP), histamine and adrenaline can alter the hyperalgesic effects of intraplantar injections of capsaicin and display differential effects on the upregulated cytokines. All the presented observations, taken together, allow the conclusion that CSPA fibres can induce the upregulation of proinflammatory cytokines and NGF through the release of neuropeptides by their peripheral terminals.
In conclusion, the results of the present study provide a possible answer to the question raised by Bayliss (1901) about the physiological significance of the sensory fibres with efferent function. It appears that the normal activation of these fibres involves two simultaneous mechanisms: a local peripheral release of neuropeptides in parallel with afferent nociceptive signalling. The function of the release of neuropeptides, a local mechanism, is to trigger an inflammatory cascade, as a first defence reaction, while the nociceptive signalling can lead ultimately to the activation of the hypothalamus-pituitary-adrenal axis (Green et al. 1995; Pan et al. 1997), which contributes further to the control of the inflammatory reaction. Thus, one can speculate that the CSPA fibre system can serve as a common afferent pathway to both immune and nervous systems.
Acknowledgments
The authors wish to thank Mrs Sawsan Sharrouf and Mr Riad Maalouf for their technical assistance. This project was supported by a grant from the Lebanese National Council for Scientific Research.
References
- Amann R, Schuligoi R, Lanz I, Donnerer J. Histamine-induced edema in the rat paw - Effect of capsaicin denervation and a CGRP receptor antagonist. European Journal of Pharmacology. 1995;279:227–231. doi: 10.1016/0014-2999(95)00169-l. [DOI] [PubMed] [Google Scholar]
- Ansel JC, Brown JR, Payan DG, Brown M. Substance P selectively activates TNF-α gene expression in murine mast cells. Journal of Immunology. 1993;150:4478–4485. [PubMed] [Google Scholar]
- Basbaum AI, Levine JD. The contribution of the nervous system to inflammation and inflammatory disease. Canadian Journal of Physiology and Pharmacology. 1991;69:647–651. doi: 10.1139/y91-096. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. Journal of Physiology. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Brouxhon S, Felten S, Felten DL. The significance of vasoactive intestinal polypeptide (VIP) in immunomodulation. Advances in Neuroimmunology. 1996;6:5–27. doi: 10.1016/s0960-5428(96)00008-3. [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CSJ, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. British Journal of Pharmacology. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro T, Maurer M, Modarres S, Lewin NE, Brodie C, Acs G, Acs P, Paus R, Blumberg PM. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- Buck SM, Burks TF. The neuropharmacology of capsaicin. Review of some recent observations. Pharmacological Reviews. 1986;38:179–226. [PubMed] [Google Scholar]
- Carpenter SE, Lynn B. Vascular and sensory responses of human skin to mild injury after topical treatment with capsaicin. British Journal of Pharmacology. 1981;73:755–758. doi: 10.1111/j.1476-5381.1981.tb16812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, McRitchie HA. Neonatal capsaicin does not affect unmyelinated efferent fibers of the autonomic nervous system: functional evidence. Brain Research. 1982;239:283–288. doi: 10.1016/0006-8993(82)90853-8. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Donnerer J, Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sciences. 1983;32:1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Culp WJ, Ochoa JL, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Brain. 1989;112:1317–1331. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- Dray A. Tasting the inflammatory soup: the role of peripheral neurones. Pain Reviews. 1994;1:153–171. [Google Scholar]
- Dray A, Bevan S. Inflammation and hyperalgesia: highlighting the team effort. Trends in Pharmacological Sciences. 1993;14:287–290. doi: 10.1016/0165-6147(93)90041-H. [DOI] [PubMed] [Google Scholar]
- Escott KJ, Brain SD. Effect of a calcitonin gene-related peptide antagonist (CGRP8-37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. British Journal of Pharmacology. 1993;110:772–776. doi: 10.1111/j.1476-5381.1993.tb13878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Alterations in the ipsi- and contralateral afferent inputs of dorsal horn cells produced by capsaicin treatment of one sciatic nerve in the rat. Brain Research. 1982;248:97–107. doi: 10.1016/0006-8993(82)91151-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Capsaicin and sensory neurons - a review. Pain. 1983;15:109–130. doi: 10.1016/0304-3959(83)90012-x. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Green PG, Miao FJP, Janig W, Levine JD. Negative feedback neuroendocrine control of the inflammatory response in rats. Journal of Neuroscience. 1995;15:4678–4686. doi: 10.1523/JNEUROSCI.15-06-04678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Ruda MA. Developmental alterations in nociceptive threshold, immunoreactive calcitonin gene-related peptide and substance-P, and fluoride-resistant acid phosphatase in neonatally capsaicin-treated rats. Journal of Comparative Neurology. 1991;312:436–450. doi: 10.1002/cne.903120310. [DOI] [PubMed] [Google Scholar]
- Hara A, Sakurada T, Sakurada S, Matsumura H, Kensuke K. Antinociceptive effects of neonatal capsaicin in rats with adjuvant arthritis. Archives of Pharmacology. 1984;326:248–253. doi: 10.1007/BF00505326. [DOI] [PubMed] [Google Scholar]
- Helme RD, Eglezos A, Dandie GW, Andrews PV, Boyd RL. The effect of substance P on the regional lymph node antibody response to antigenic stimulation in capsaicin-pretreated rats. Journal of Immunology. 1987;139:3470–3473. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tackykinins, calcitonin gene related peptide and other neuropeptides. Neuroscience. 1988;24:739–769. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin neurons. Pharmacological Reviews. 1991;43:143–201. [PubMed] [Google Scholar]
- Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Inoue H, Asaka T, Nagata N, Koshihara Y. Mechanism of mustard oil-induced skin inflammation in mice. European Journal of Pharmacology. 1997;333:231–240. doi: 10.1016/s0014-2999(97)01040-6. [DOI] [PubMed] [Google Scholar]
- Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. British Journal of Pharmacology. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan SA, Saadé NE, Haddad JJ, Abdelnoor A, Atweh SF, Jabbur SJ, Safieh-Garabedian B. Endotoxin induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996;66:373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- Kim HT, Park SK, Lee SE, Chung JM, Lee DH. Non-noxious A fiber afferent input enhances capsaicin-induced mechanical hyperalgesia in the rat. Pain. 2001;4:169–175. doi: 10.1016/S0304-3959(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Kress M, Gutiimann C, Averbeck B, Reeh PW. Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin in vitro. Neuroscience. 1999;89:303–310. doi: 10.1016/s0306-4522(98)00280-2. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LER, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. Journal of Physiology. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F. Sir Thomas Lewis' nocifensor system, histamine and substance-P-containing primary afferent nerves. Trends in Neurosciences. 1983;6:106–108. [Google Scholar]
- Levine JD, Dardick SJ, Basbaum AI, Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. Journal of Neuroscience. 1985;5:1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. The nocifensor system of nerves and its reactions. British Medical Journal. 1937;194:431–435. 491–494. doi: 10.1136/bmj.1.3973.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty D-M, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. American Journal of Physiology. 1997;272:G272–280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The pharmacology of the efferent function of sensory nerves. Journal of Autonomic Pharmacology. 1991;11:173–208. doi: 10.1111/j.1474-8673.1991.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. Journal of Neuroimmunology. 2001;114:131–141. doi: 10.1016/s0165-5728(00)00466-5. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Safieh-Garabedian B, Ochoa-Chaar CI, Jabbur SJ, Atweh SF, Saadé NE. Differential modulation of capsaicin (CP)-induced hyperalgesia and cytokine upregulation by sympathetic antagonists. Society of Neuroscience Abstracts. 2000;26:1695. [Google Scholar]
- Morley JE, Kay NE, Solomon GF, Plotnikoff NP. Neuropeptides: conductors of the immune orchestra. Life Sciences. 1987;41:527–545. doi: 10.1016/0024-3205(87)90405-x. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. Journal of Neuroscience. 1983;3:399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar CF, Barada KA, Abdallah LE, Hamdan WS, Taha AM, Atweh SF, Saadé NE. Involvement of capsaicin sensitive primary afferent fibers in the regulation of jejunal alanine absorption. American Journal of Physiology. 1995;268:G695–699. doi: 10.1152/ajpgi.1995.268.4.G695. [DOI] [PubMed] [Google Scholar]
- Pan B, Castrolopes JM, Coimbra A. Chemical sensory deafferentation abolishes hypothalamic pituitary activation induced by noxious stimulation or electroacupuncture but only decreases that caused by immobilization stress. A c-fos study. Neuroscience. 1997;78:1059–1068. doi: 10.1016/s0306-4522(96)00661-6. [DOI] [PubMed] [Google Scholar]
- Payan DG, Brewster DR, Goetzl EJ. Specific stimulation of human T lymphocytes by substance P. Journal of Immunology. 1983;131:1613–1615. [PubMed] [Google Scholar]
- Payan DG, McGillis JP, Renold FK, Mitsuhashi M, Goetzl EJ. Neuropeptide modulation of leukocyte function. Annals of the New York Academy of Sciences. 1987;496:182–191. doi: 10.1111/j.1749-6632.1987.tb35764.x. [DOI] [PubMed] [Google Scholar]
- Peck R. Neuropeptides modulating macrophage function. Annals of the New York Academy of Sciences. 1987;496:264–270. doi: 10.1111/j.1749-6632.1987.tb35774.x. [DOI] [PubMed] [Google Scholar]
- Poole S, Cunha FQ, Ferreira SH. Bradykinin, cytokines and inflamatory hyperalgesia. In: Saadé NE, Apkarian AV, Jabbur SJ, editors. Pain and Neuroimmune Interactions. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 31–54. [Google Scholar]
- Saadé NE, Atweh SF, Privat A, Jabbur SJ. Inhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexes. Brain Research. 1999;846:72–86. doi: 10.1016/s0006-8993(99)02003-x. [DOI] [PubMed] [Google Scholar]
- Saadé NE, Massaad CA, Ochoa-Chaar CI, Atweh SF, Safieh-Garabedian B, Jabbur SJ. Possible contribution of neuropeptides and histamine to the hyperalgesia induced by intraplantar injection of capsaicin. European Journal of Neuroscience. 2000a;12:123. [Google Scholar]
- Saadé NE, Nasr IW, Massaad CA, Safieh-Garabedian B, Jabbur SJ, Kanaan SA. Modulation of ultraviolet-induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. British Journal of Pharmacology. 2000b;131:1317–1324. doi: 10.1038/sj.bjp.0703699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Kanaan SA, Haddad JJ, Abou Jaoude P, Jabbur SJ, Saadé NE. Involvement of interleukin-1β, nerve growth factor and prostaglandin-E2 in endotoxin induced localized inflammatory hyperalgesia. British Journal of Pharmacology. 1997;121:1619–1626. doi: 10.1038/sj.bjp.0701313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31:1279–1285. doi: 10.1016/0028-3908(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–108. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Ochoa JL. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- Siney L, Brain SD. Involvement of sensory neuropeptides in the development of plasma extravasation in rat dorsal skin following thermal injury. British Journal of Pharmacology. 1996;117:1065–1070. doi: 10.1111/j.1476-5381.1996.tb16698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsanyi J. Actions of capsaicin on sensory receptors. In: Wood J, editor. Capsaicin in the Study of Pain. San Diego: Academic Press; 1993. pp. 1–26. [Google Scholar]
- Szolcsanyi J. Neurogenic inflammation: reevaluation of axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. London: CRC Press; 1996. pp. 33–42. [Google Scholar]
- Tilley SJ, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. Journal of Clinical Investigation. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda Y. Inhibition of immune responses by calcitonin gene-related peptide. Annals of the New York Academy of Sciences. 1992;657:552–554. doi: 10.1111/j.1749-6632.1992.tb22832.x. [DOI] [PubMed] [Google Scholar]
- Wall PD, Fitzgerald M. Effects of capsaicin applied locally to adult peripheral nerve. I. Physiology of peripheral nerve and spinal cord. Pain. 1981;11:363–377. doi: 10.1016/0304-3959(81)90636-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Millet I, Bottomly K, Vignery A. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. Journal of Biological Chemistry. 1992;267:21,052–21,057. [PubMed] [Google Scholar]
- Weihe E, Nohr D, Muller S, Buchler M, Friess H, Zentel HJ. The tachykinin neuroimmune connection in inflammatory pain. Annals of the New York Academy of Sciences. 1991;632:283–295. doi: 10.1111/j.1749-6632.1991.tb33116.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


