Abstract
The objective of this study was to describe the kinetics of voltage-dependent inactivation of native cardiac L-type Ca2+ currents. Whole-cell currents were recorded from guinea-pig isolated ventricular myocytes. Voltage-dependent inactivation was separated from Ca2+-dependent inactivation by replacing extracellular Ca2+ with Mg2+ and recording outward currents through Ca2+ channels. Voltage-dependent inactivation accelerated from slow monophasic decay at −30 mV to maximal rapid biphasic decay at +20 mV. Maximal voltage-dependent inactivation occurred with τf ≈30 ms and τs ≈300 ms, the fast component of decay accounted for 70 % of the current amplitude. In basal conditions Ca2+ current availability was sigmoid. Isoproterenol (isoprenaline) evoked a large increase in a time-independent component of the Ca2+ current which also increased with depolarisation. This was responsible for the apparent recovery of Ca2+ channel current availability at positive membrane potentials and thus a U-shaped availability-voltage (A-V) relationship. It is concluded that β-adrenergic stimulation altered the reaction of native cardiac L-type Ca2+ channels to membrane voltage. In basal conditions, voltage accelerated inactivation. In isoproterenol, voltage could also reduce inactivation.
A key to understanding the contribution of L-type Ca2+ currents (ICa,L) to the cardiac action potential and the process of excitation-contraction coupling is the mechanism of inactivation. Inactivation of L-type Ca2+ channels proceeds according to voltage- and Ca2+-dependent mechanisms (Pelzer et al. 1990). Recently a great deal of interest has centred upon the mechanism of Ca2+-induced inactivation with the implication of the association of the ubiquitous Ca2+-binding protein calmodulin with the intracellular C-terminus of the α1C subunit of the Cav1.2 channel (see Anderson, 2001 for recent review). The molecular basis of voltage-dependent inactivation of Ca2+ channels has also been extensively studied (reviewed in Stotz & Zamponi, 2001). In comparison with the dynamic regulation of inactivation induced by Ca2+ (Hamilton et al. 2000; Anderson, 2001; Bers, 2002), inactivation due to voltage has generally been considered to play a minor role in the decay of L-type Ca2+ channel currents in cardiac myocytes (Bechem & Pott, 1985; Imredy & Yue 1994; Linz & Meyer, 1998; Puglishi et al. 1999; Sun et al. 2000). Recent studies challenge this view (Mitarai et al. 2000; Findlay, 2002a,b).
Mitarai et al. (2000) provided the first clear evidence that β-adrenergic stimulation could profoundly affect the character of voltage-dependent inactivation of native cardiac L-type Ca2+ channels. They suggested that the apparent slowing of the voltage-dependent decay of Ca2+ channel currents induced by isoproterenol (isoprenaline) (Bean et al. 1984; Tsien et al. 1986; Tiaho et al. 1991; Findlay, 2002a) resulted from the conversion of channels from a rapidly inactivating to a slowly inactivating kinetic form. The consequence of this result is to suggest that models of cardiac muscle Ca2+ currents which have been developed to describe their behaviour under basal conditions, but which evaluated voltage-dependent decay following β-adrenergic stimulation, will have underestimated the contribution of voltage (Linz & Meyer, 1998; Puglisi et al. 1999; Sun et al. 2000). The general consensus of opinion which described the primary contribution of Ca2+-induced inactivation to the decay of Ca2+ currents will also have been overestimated since this also was measured under experimental conditions which were the equivalent of β-adrenergic stimulation (Bechem & Pott, 1985; Imredy & Yue, 1994). Thus a previous investigation from this laboratory found that whereas following β-adrenergic stimulation the kinetics of inactivation of the Ca2+ current were almost exclusively dependent upon Ca2+, under basal conditions a large part of inactivation was independent of Ca2+ (Findlay, 2002b). These results revealed the major contribution of voltage-dependent inactivation to the decay of L-type Ca2+ currents under basal conditions. That study examined voltage-dependent inactivation at one membrane voltage (+10 mV) in order to optimally separate the contributions of Ca2+ and voltage. The objective of the present study was to examine the process of voltage-dependent inactivation in native cardiac L-type Ca2+ channels over a more extensive range of membrane voltages. This information may then be used to develop models of the Ca2+ current and cardiac action potential which take full account of the contribution of voltage-dependent inactivation.
This investigation also addressed a second question. Previous studies have shown that under basal conditions the availability-voltage (A-V) relationship for ICa,L recorded in the absence of extracellular Ca2+ was sigmoid (Hadley & Hume, 1987; Findlay, 2002b). This suggested that under those conditions inactivation occurred via a voltage-dependent process with no interference from an ion-dependent inactivation mechanism (Ferreira et al. 1997; Findlay, 2002a). On the other hand, under the same experimental conditions following maximal β-adrenergic stimulation the A-V relationship was U-shaped with a clear ‘recovery’ of ICa,L following voltage-clamp steps to positive membrane potentials (Findlay, 2002b). Here it is shown that β-adrenergic stimulation resulted in a paradox where membrane voltage, rather than inducing inactivation, could induce a non-inactivating kinetic form of the channel. This mechanism could explain how a U-shaped relationship between inactivation and membrane voltage could arise in the absence of Ca2+-induced inactivation.
Methods
Cell preparation
All animal experiments were conducted according to the ethical standards of the Ministère Français de l'Agriculture (Licence number B37-261-4). Male guinea-pigs (250-400 g; purchased from Iffa-Credo Ltd, L'Abresle, France) were killed by cervical dislocation and the hearts were removed. Single ventricular myocytes were isolated using collagenase and protease digestion as described elsewhere (Le Guennec et al. 1993). Myocytes isolated from the left ventricle were aliquoted into 35 mm diameter plastic Petri dishes which served as experimental chambers. The storage solution consisted of the standard extracellular solution described below. Dishes which contained myocytes were kept on the laboratory bench and used within 6-8 h after isolation. Plastic Petri dishes which contained isolated myocytes were placed upon the stage of an Olympus CK2 inverted microscope. Isolated myocytes were superfused with experimental solutions via a parallel pipes system lowered into the vicinity of the cells. Fluid flow was maintained by gravity from syringe barrel reservoirs and the exchange of solutions was achieved by manual displacement of the pipes. Solution exchange around the myocyte was estimated to be complete in 5-6 s. All experiments were conducted at room temperature (≈23 °C) in a temperature-controlled laboratory.
Experimental procedures
Whole-cell current voltage-clamp experiments (Hamill et al. 1981) were conducted with an Axon Instruments 202A patch-clamp amplifier in resistive feedback mode (Axon Instruments, CA, USA). Pipettes were fabricated from thin walled borosilicate glass capillary tubes (Clark Electromedical Instruments, Pangbourne, UK) with a Narishige PB7 double-stage puller (Narishige Instruments, Tokyo, Japan). Pipettes were coated with Sylgard (Dow Corning, MI, USA) and then heat polished. Finished pipettes had a resistance of < 2 MΩ when filled with standard intracellular solution.
Experimental voltage-clamp protocols and data acquisition were controlled with Acquis1 software (Dipsi Industrie, Chatillon, France) installed upon a 386-20 PC computer. Data were filtered at either 1 or 2 kHz and acquired at 2 or 5 kHz, respectively. Cell capacitance and series resistance were compensated (≈80 %) with the Axon Instruments amplifier. Cell currents are expressed as cell current density in picoamps per picofarad (pA pF−1). Voltage-clamp protocols were delivered to the cells from the holding potential of −80 mV. Each voltage-clamp protocol was preceded by a voltage step to −50 mV for a period of 1000 ms. This prior voltage step served to inactivate any residual Na+ current remaining after the application of 10 μm TTX and to inactivate any T-type Ca2+ current in ventricular myocytes of the guinea-pig (Balke et al. 1992). This study utilised two voltage-clamp protocols. The first was a double-pulse protocol which consisted of pre-pulses to between −50 and +80 mV in 10 mV increments, a 10 ms interval at −50 mV and a test pulse voltage step to +80 mV. The duration of the pre-pulses was either 1000 or 5000 ms. Current-voltage (I-V) relationships and the kinetics of decay of ICa,L were evaluated from currents recorded during pre-pulse voltage steps. Availability-voltage (A-V) relationships were obtained by measuring the amplitude of the current evoked by the test pulse voltage step and normalising these data to that recorded following a pre-pulse voltage step to −50 mV. The second voltage-clamp protocol was an envelope-type of double-pulse protocol where the test pulse voltage step to +80 mV was preceded by voltage steps to a given voltage for durations which varied between 10 and 1000 ms (see Fig. 1). This protocol was used to follow the time course of the development of inactivation from the progressive loss of availability of ICa,L. Data analysis was performed with Acquis1 and Origin 4.1 (Microcal Software, MA, USA). The Boltzmann function (Origin 4.1) was fitted to values between the maximum and minimum of normalised data values for the A-V relationships. All results are presented as mean ± s.e.m. values of data obtained from n different myocytes.
Figure 1. The time course of the development of voltage-dependent inactivation.
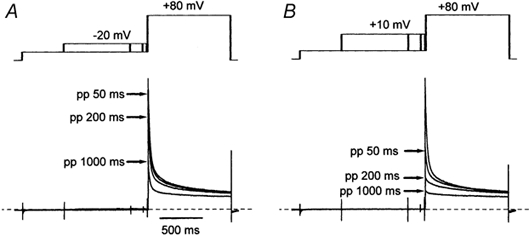
These normalised cell current traces were recorded from one representative isolated ventricular myocyte during pre-pulse voltage steps to −20 mV (A) and +10 mV (B) using the voltage-clamp protocol indicated schematically above the traces. For clarity only four traces representing currents recorded with no pre-pulse and pre-pulses of 50, 200 and 1000 ms duration (arrows) are shown here. The time scale is indicated by the horizontal bar in A. The dashed lines indicate the zero current level. Cell currents were recorded in the absence of extracellular Ca2+.
Experimental solutions
The standard extracellular solution which was used to fill the Petri dishes and store myocytes prior to experiments contained (mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 Hepes (pH 7.4 with NaOH). Tetrodotoxin (TTX citrate salt, 10 μm, from either Alomone Labs, Jerusalem, Israel or Latoxan, Valence, France) was added to this standard solution when it was used to superfuse cells during experiments. Calcium-free (zero calcium) extracellular solution contained 250 μm EGTA-NaOH, 3 mm MgCl2 and no added Ca2+. The standard intracellular solution which was used to fill the patch pipettes contained (mm): 140 KCl, 10 NaCl, 5 EGTA-KOH, 1.4 MgCl2, 0.1 CaCl2, 2 ATP-Mg2+, 10 glucose, 10 Hepes (pH 7.3 with KOH). The estimated free concentrations of Mg2+ and Ca2+ in this solution were 1 mm and 1 nm, respectively. In experiments where β-adrenergic stimulation was to be applied to the myocytes, the pipette solutions also contained 100 μm GTP. Isoproterenol was prepared daily as a 100 μm stock solution in distilled water and added to extracellular solutions to give a final concentration of 100 nm. Ryanodine was prepared as a 1 mm stock solution in distilled water and added to extracellular solutions to give a final concentration of 5 μm. Chemicals were purchsed from Sigma-Aldrich.
Voltage-gated currents through L-type Ca2+ channels were extracted from the ensemble whole-cell currents with 200 μm CdCl2. The limitations of this method are fully discussed by Linz & Meyer (1998). The cell current records obtained in the presence of Cd2+ were digitally subtracted from those recorded in the absence of Cd2+ with Acquis1.
Results
The characteristics of the L-type Ca2+ channel current which is recorded in isolated ventricular myocytes of the guinea-pig in the absence of extracellular Ca2+ have been described in Findlay (2002b). The results shown in this study were all obtained when myocytes were bathed in the zero calcium solution, which was described in Methods. It should be noted that the exposure of the myocytes to this solution was limited to the time necessary to achieve the voltage-clamp protocol; otherwise they were superfused with the normal extracellular solution which contained 2 mm Ca2+.
Voltage-dependent inactivation of L-type Ca2+ channels
This series of experiments sought to examine the time course of the development of voltage-dependent inactivation over a range of physiologically important membrane potentials. Since, between membrane potentials of −30 and +10 mV no current flowed through Ca2+ channels when Mg2+ had replaced Ca2+ in the extracellular medium (Fukushima & Hagiwara, 1985; Hess et al. 1986; Hadley & Hume, 1987; Findlay, 2002b), the inactivation of ICa,L was assessed indirectly by monitoring the availability of the outward Ca2+ channel current evoked by a voltage step to +80 mV (Hadley & Hume, 1987). Inactivation was induced by pre-pulse voltage steps of variable duration (Findlay, 2002b). Figure 1A illustrates the response of an isolated myocyte to pre-pulse voltage steps to −20 mV of 50, 200 and 1000 ms duration. Elongation of the pre-pulse, which evoked no current flow through Ca2+ channels, resulted in the progressive reduction of the amplitude of the outward Ca2+ channel current evoked by the test pulse to +80 mV. Pre-pulses to +10 mV provoked a more rapid and more extensive reduction of the test pulse current amplitude (Fig. 1B).
The effect of membrane potential upon the rate of inactivation of ICa,L recorded in the absence of Ca2+ is shown in Fig. 2A. The time course of the development of inactivation at each voltage could be fitted by exponential functions (lines in Fig. 2A) whose time constants are shown in Fig. 3A and B. The rate of inactivation accelerated with depolarisation of the membrane potential from −30 to ≈+20 mV. Voltage above ≈+20 mV had little or no further effect. The rate of inactivation recorded at −30 and −20 mV could be described by a single slow exponential function. The rate of inactivation recorded at −10 mV and more positive voltages required a double-exponential function. Maximal inactivation was biphasic with time constants of ≈30 and ≈300 ms. Figure 2B shows that a single and voltage-dependent process could account for these results, since, when the data from Fig. 2A were displayed upon a voltage rather than a time axis, a Boltzmann relation could be fitted to the data irrespective of the duration of the pre-pulse voltage step.
Figure 2. Exponential development of voltage-dependent inactivation.
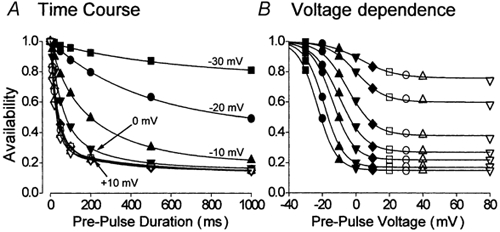
A, the time course of voltage-dependent inactivation of ICa,L at different membrane potentials. Lines represent the fitting of either a single or a double-exponential function to the data. B, the effect of pre-pulse duration upon the A-V relationship of the Ca2+ channel current. The data from A have been transposed onto a voltage scale. The lines represent fits of the Boltzmann function to these data which correspond to ICa,L availability recorded following pre-pulse steps of 10, 20, 50, 100, 200, 500 and 1000 ms duration reading from top to bottom. A and B, ▪, pre-pulse voltage (pp) −30 mV (n = 11). •, pp −20 mV (n = 19); ▴, pp −10 mV (n = 11); ▾, pp 0 mV (n = 11); ♦, pp +10 mV (n = 20); □, pp +20 mV (n = 12); ○, pp +30 mV (n = 11); ▵, pp +40 mV (n = 16); ▿, pp +80 mV (n = 13).
Figure 3. Voltage-dependent inactivation and membrane potential.
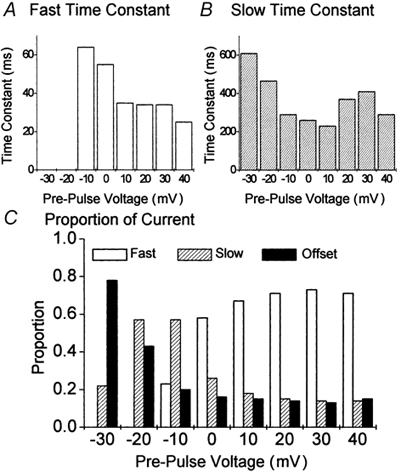
Analysis of the development of voltage-dependent inactivation was extracted from the details of the exponential functions fitted to the data in Fig. 2A. A, the fast time constant. B, the slow time constant. C, the initial amplitude of each component of either a single exponential function (slow decline (τ) and offset for data recorded following pre-pulse steps to −30 and −20 mV) or a double-exponential function (fast decline (τf), slow decline (τs) and offset for data recorded following pre-pulse steps to between −10 and +40 mV) was determined and expressed relative to their respective totals.
The inactivation of ICa,L in cardiac myocytes is generally considered to follow a biphasic time course because of the joint influence of voltage- and Ca2+-dependent inactivation (Pelzer et al. 1990). On the other hand, the results shown in Fig. 2 (and see also Mitarai et al. 2000; Findlay, 2002b) indicate that ICa,L could inactivate with a biphasic time course in the absence of Ca2+-dependent inactivation. This observation led Mitarai et al. (2000) to consider not only the time course of inactivation but also what proportions of the whole-cell current corresponded to such kinetically distinct components. The single exponential development of inactivation which occurred at −30 and −20 mV suggested that at these voltages whole-cell ICa,L would be composed of ion channels which either inactivated in a slow time-dependent manner or which showed no time-dependent behaviour on this time scale. The double-exponential development of inactivation which occurred at −10 mV suggested that some ion channels had now adopted a rapid form of time-dependent inactivation. These distinct kinetic forms of the L-type Ca2+ channel co-existed within the whole-cell population of channels and Fig. 3C shows that, from the starting point of −30 mV, depolarisation was associated with the progressive reduction of the time-independent component, at first the amplitude of the slow time-dependent component increased then declined, and the rapid time-dependent component first appeared at −10 mV and progressively increased. A steady-state was reached at +20 mV and further depolarisation had no further effect upon the distribution of ion channels between the different kinetic forms of behaviour within the whole-cell population.
The results shown in Figs 1–3 define the voltage-dependent behaviour of L-type Ca2+ channels over the physiologically important range of membrane potentials. Several conclusions could be drawn from this data. A voltage-dependent process was responsible for the graded time-dependent development of inactivation (Fig. 2B). The overall kinetic of inactivation was dependent upon the membrane potential (Fig. 1 and Fig. 3A and B). Individual L-type Ca2+ channels could be assigned to one of the three distinct kinetic groups (Fig. 3C). Membrane potential determined the distribution of ion channels between these kinetic groups (Fig. 3C). Maximal voltage-dependent inactivation was represented by ≈70 % of the channel population showing rapid inactivation with a time constant of ≈30 ms.
Figure 4 illustrates a similar analysis of the time course of the development of inactivation here applied to the total inactivation of ICa,L, which was recorded in the presence of extracellular Ca2+. The inactivation of ICa,L was recorded directly from the decay of inward Ca2+ currents. To allow a certain degree of comparison between these data and those of isolated voltage-dependent inactivation (Fig. 2 and Fig. 3), these experiments were conducted in the presence of 5 μm ryanodine in order to block Ca2+ release from the sarcoplasmic reticulum (Ca2+-induced Ca2+ release, CICR). The isolation of voltage-dependent inactivation by the exclusion of extracellular Ca2+ precluded a contribution of CICR to the time course of inactivation (Findlay, 2002b). In the context of this study it was therefore appropriate to also exclude the contribution of CICR to the total inactivation of ICa,L (Adachi-Akahane et al. 1996; Sham, 1997).
Figure 4. The time course of the development of total inactivation of ICa,L.
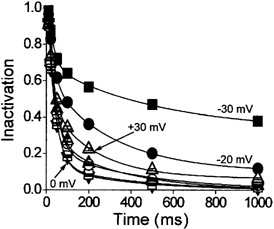
ICa,L was evoked by 1000 ms duration voltage-clamp steps to between −30 and +30 mV in myocytes which were bathed in extracellular solution which contained 2 mm Ca2+ and 5 μm ryanodine (n = 36). The inactivation of ICa,L was evaluated by measuring the amplitude of the inward Ca2+ current 10, 20, 50, 100, 200, 500 and 1000 ms following the onset of the voltage step. These values were then normalised to the amplitude of the peak inward current. ▪, voltage step to −30 mV; •, −20 mV; ▵, −10 mV; ▾, 0 mV; □, +10 mV; ○, +20 mV; ▿, +30 mV. The lines represent fits of double-exponential functions to the data.
By comparison with the data which described isolated voltage-dependent inactivation (Fig. 2A), two main points arose from the analysis of total inactivation (Fig. 4). First, the time course of decay of ICa,L recorded at −30 and −20 mV was described by double-exponential functions. Second, the rate of decay which increased with depolarisation to be maximal between 0 and +10 mV was then apparently slower at more positive voltages. Thus at negative membrane potentials a fast component of inactivation was visible in total inactivation (Fig. 5) which was absent from the isolated voltage-dependent inactivation (Fig. 3). Maximal inactivation at ≈+10 mV for voltage-dependent (Fig. 3) and total inactivation (Fig. 5) showed great similarity, which is in line with previous observations (Findlay, 2002a,b), though the amplitude of the component of fast inactivation was relatively greater in Ca2+ (Fig. 5C) than in its absence (Fig. 3C). At more positive membrane potentials certain differences were apparent. Thus total inactivation showed a reduction of the contribution of the component with rapid inactivation (Fig. 5C) and consequently an increase in the component with slow inactivation. The relationship between the kinetic components of isolated voltage-dependent inactivation were unchanged at these voltages (Fig. 3C).
Figure 5. Total inactivation of ICa,L and membrane potential.
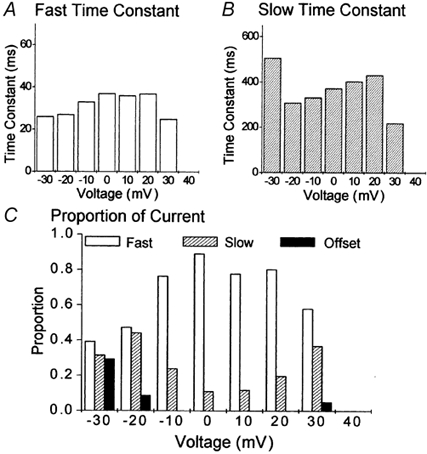
Analysis of the development of voltage-dependent inactivation was extracted from the details of the exponential functions fitted to the data in Fig. 4. A, the fast time constant of inactivation. B, the slow time constant of inactivation. C, the proportion of the total current occupied by fast inactivation, slow inactivation and an offset.
These results can be understood, and differences between results obtained for isolated voltage-dependent inactivation and total inactivation accounted for, now that it has been shown that biphasic decay of ICa,L results not only from the separate processes of voltage- and Ca2+-dependent inactivation (Pelzer et al. 1990), but that voltage-dependent decay also showed a biphasic time course (Mitarai et al. 2000; Findlay, 2002a,b). Thus total inactivation is the complex result of the superposition of the kinetics of Ca2+-induced inactivation, which was simplified in this study by the exclusion of the contribution of CICR, upon the biphasic time course of decay already imposed by the kinetic separation of the voltage-dependent behaviour of individual ion channels.
Voltage-dependent potentiation of ICa,L
The second question to be addressed in this study concerned the observation that, while the A-V relationship of ICa,L recorded under basal conditions in the absence of extracellular Ca2+ was sigmoid in form, following β-adrenergic stimulation it was U-shaped (Findlay, 2002b and see Fig. 6B). In the absence of extracellular Ca2+ and in the absence of cation influx under these experimental conditions there was no reason to consider that β-adrenergic stimulation had induced an ion-dependent inactivation mechanism. The cause of the recovery of availability of ICa,L following pre-pulse voltage steps to positive membrane potentials was investigated with a double-pulse voltage-clamp protocol with 5000 ms pre-pulse voltage steps. This protocol was considerably longer than those applied by either Mitarai et al. (2000) or Findlay (2002b) and it was hoped that this would approach as closely as possible the steady-state condition.
Figure 6. The effect of isoproterenol upon voltage-dependent inactivation of Ca2+ channel currents.
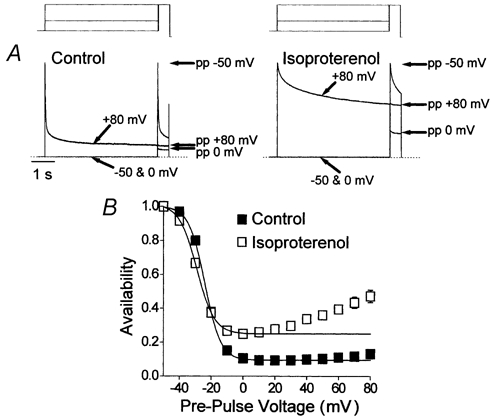
Ca2+ channel currents were recorded in the absence of extracellular Ca2+ with the double-pulse voltage-clamp protocol with 5000 ms duration pre-pulse voltage steps. Experiments were conducted first under basal conditions (lefthand traces in A and filled symbols in B) and then in the presence of 100 nm isoproterenol (righthand traces in A and open symbols in B). A, an illustration of voltage-dependent inactivation and recovery. These normalised records each represent three superimposed cell currents which had been evoked by the double-pulse voltage-clamp protocol with pre-pulse voltage steps to −50, 0 and +80 mV. The voltage-clamp protocol is shown schematically above the traces. The amplitude of the current evoked by the test pulse voltage step to +80 mV following each pre-pulse (pp) is indicated by the arrows to the right of the traces. The time scale on the left applies to both traces and the dotted lines indicate the zero current level. B, A-V relationships recorded following 5000 ms pre-pulse voltage steps (n = 10). The lines represent Boltzmann functions with the following parameters: for control data, maximum 1.00, minimum 0.09, V0.5 −24 mV, slope 4.9 mV; for data with isoproterenol, maximum 1.00, minimum 0.25, V0.5 −29 mV, slope 5.5 mV.
The cell current records shown in Fig. 6A illustrate the effects of β-adrenergic stimulation upon the Ca2+ channel currents recorded in the absence of extracellular Ca2+. The most obvious point is that isoproterenol provoked a drastic slowing of the decay of these currents (Mitarai et al. 2000; Findlay, 2002b) which was also reflected in the reduction of the minimum of the A-V relationship recorded in isoproterenol (Fig. 6B). In basal conditions the A-V relationship was essentially sigmoid (Fig. 6B, filled symbols), while following β-adrenergic stimulation the A-V relationship was U-shaped (Fig. 6B, open symbols), with a minimum at ≈0 mV and recovery with further depolarisation. The Boltzmann relation provided a close fit to the data recorded under control conditions, with a V0.5 of −24 mV; in isoproterenol the Boltzmann relation could only describe the descending limb of the relationship and evaluated a V0.5 of −29 mV. The minima of the A-V relationships were 0.09 in basal conditions and 0.25 in isoproterenol. It was therefore clear that a U-shaped A-V relationship did not result from the premature evaluation of availability. In fact, the comparison of Fig. 3D (50 ms pre-pulse duration) and 3B (1000 ms pre-pulse duration) in Findlay (2002b) with Fig. 6B shown here with a 5000 ms pre-pulse duration would actually suggest that the reverse was true; the extent of recovery of ICa,L during pre-pulse voltage steps to positive membrane potentials increased with time.
In order to resolve the question of the U-shaped A-V relationship which was recorded in the presence of isoproterenol (Fig. 6B) it was decided to analyse the decay of Ca2+ channel currents evoked by voltage steps to between +30 and +80 mV (Fig. 7), since it was following these that the major part of the ‘recovery’ of channel availability occurred (Fig. 6B). Within this range of voltages, under basal conditions the fast time constant of decay was essentially voltage independent, declining only slightly between +30 and +80 mV and isoproterenol slightly increased this at each voltage (Fig. 7A). The slow time constant of decay was clearly voltage dependent under basal conditions, accelerating with depolarisation (Fig. 7B). Isoproterenol accelerated the slow time constant at lower voltages while having no effect at higher voltages; thus in isoproterenol the slow time constant of decay was voltage independent (Fig. 7B). At each voltage the amplitude of the fast component of decay was reduced by β-adrenergic stimulation (Fig. 7C, squares) while the amplitudes of the slow (Fig. 7D) and time-independent (Fig. 7C, triangles) components of decay were increased. The initial amplitudes of the distinct components of decay were expressed relative to the amplitude of the total current. Under basal conditions, the composition of the current recorded between +30 and +80 mV was voltage independent and dominated (≈70 %) by fast decay (Fig. 7E). In the presence of isoproterenol, fast decay was reduced to ≈20 % of the total and the composition of the current was voltage dependent, with depolarisation increasing the contribution of the time-independent component (Fig. 7F). The data shown in Fig. 7C show that the amplitude of the fast decay (squares) and time-independent (triangles) components of ICa,L were interchanged between basal conditions and stimulation of the current by isoproterenol.
Figure 7. The effects of isoproterenol upon the voltage-dependent kinetic components of ICa,L.
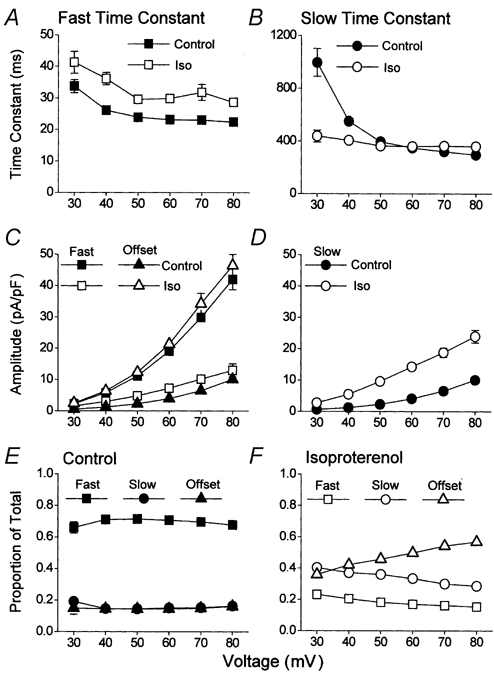
Cell currents had been evoked by 5000 ms duration voltage steps to between +30 and +80 mV (n = 10). The decay of each current was fitted with a double-exponential function. Filled symbols represent data obtained under basal conditions and open symbols represent data recorded following the application of 100 nm isoproterenol in the same cells. A, the fast time constant of inactivation. B, the slow time constant of inactivation. C and D, the initial amplitudes of the fast (C, squares), slow (D, circles) and offset (C, triangles) components of the Ca2+ channel currents. E and F, the initial amplitudes of the different components of decay were expressed relative to the total current recorded at each voltage under basal conditions (E) and in the presence of isoproterenol (F).
It was possible to draw some conclusions from the data shown in Fig. 6 and Fig. 7. Whilst under basal conditions the A-V relationship of the Ca2+ channel current could be adequately described by a single voltage-dependent process, while following β-adrenergic stimulation this was no longer possible (Fig. 6B). The rising phase of the β-adrenergic U-shaped A-V relationship was the reflection of the voltage-dependent increase in an inactivation resistant component of the Ca2+ channel current (Fig. 7F). Isoproterenol slowed the decay of Ca2+ channel currents, not by having a marked effect upon the time constants of decay (Fig. 7A and B), but by altering the composition of the whole-cell Ca2+ channel current (Fig. 7E and F, and see Mitarai et al. 2000; Findlay, 2002b). In absolute terms isoproterenol reduced the amount of current that decayed with a fast time course (Fig. 7C). This component which dominated the decay of Ca2+ channel currents under basal conditions (Fig. 7E) was replaced by Ca2+ channel currents which showed no inactivation upon this time scale (Fig. 7C).
Discussion
This study supports the suggestion that β-adrenergic stimulation profoundly influenced the manner in which the L-type Ca2+ channel reacted to membrane voltage (Mitarai et al. 2000; Findlay, 2002b). In basal conditions progressive depolarisation induced channels to adopt a rapid form of inactivation. β-Adrenergic stimulation was associated with the reduction of this form of inactivation and now progressive depolarisation induced channels to assume a form which did not show inactivation. The molecular basis of this switch in the response of the CaV1.2 channel to membrane voltage remains to be determined.
Visually, an unusual aspect of the results presented in this study concerns the representation of Ca2+ channel currents as the outward flow of intracellular monovalent cations which may be K+ or Cs+ (Findlay, 2002b; Macianskiene et al. 2002). One is more accustomed to observe Ca2+ current which flows from the extracellular environment into the cells. In physiological terms the L-type Ca2+ channel can be regarded as being inwardly rectified for the passage of Ca2+. There is a genuine reversal potential between Ca2+ influx and intracellular monovalent cation efflux (Tsien et al. 1987). That, when Mg2+ replaced extracellular Ca2+, outward currents through Ca2+ channels could be recorded from voltages as low as +20 mV resulted from the fact that, with a quasi-physiological cation gradient, monovalent cation permeation through Ca2+ channels had a reversal potential close to 0 mV (Tsien et al. 1987). The block of these channels by external Mg2+ is relieved by K+ or Cs+ efflux according to the voltage and the driving force (Fukushima & Hagiwara, 1985; Hess et al. 1986). This gave rise to outward currents through Ca2+ channels at voltages more positive than +10 mV, while no current flowed either inward or outward through the channels at voltages between −40 and +10 mV (Fig. 1).
The kinetics of voltage-dependent inactivation of ICa,L under basal conditions are complex. Notwithstanding that a single voltage-dependent process could describe the relationship between inactivation and membrane potential (Fig. 2B), the time course of inactivation was not monotonic and itself varied with the voltage (Fig. 2A). Analysis of the behaviour of single Ca2+ channel currents has shown that individual Ca2+ channels may adopt particular and distinct kinetic behaviours (Hess et al. 1984; Nowyck et al. 1985; Rose et al. 1992). Hess et al. (1984) described these as ‘modes’. The point of this concept is that, without changing conditions, for example when applying repetitively a given voltage step, a channel may switch apparently spontaneously from one to another particular pattern of opening and closing. The application of different stimuli such as more or less depolarised voltage steps or the application of an agonist may privilege the adoption of a particular kinetic pattern. But it should be emphasised that this would not be to the exclusion of other kinetic patterns or prevent an ion channel from switching between kinetic patterns, though the probability of such a switch might be reduced. The consequence of this behaviour is that the whole-cell current may show complex kinetics due to the fact that the behaviour of its component ion channels is not evenly distributed around one type of kinetic behaviour but divided between a number of different kinetic patterns. In this context therefore the effect of voltage upon the kinetics of inactivation of ICa,L may be seen to lie less in the time constant(s) of the process (Fig. 3A and B) and more in the re-distribution of ion channels between different kinetic behaviours or ‘modes’ (Fig. 3C). Models of the L-type Ca2+ channel current need to take account of this diversity of kinetic behaviour and its modulation by membrane potential.
Mitarai et al. (2000) suggested that the effect of β-adrenergic stimulation upon the voltage-dependent inactivation of ICa,L resulted from the shift of the majority of Ca2+ channels from a rapidly to a slowly inactivating form. This study suggests rather that the exchange might be between Ca2+ channels which inactivate rapidly and those which do not inactivate on this time scale (Fig. 7C). The absence of a time-independent component of ICa,L from the analysis of Mitarai et al. (2000) might have been the consequence of their applying 300 ms duration voltage steps. On this short time scale it would be difficult to illustrate a steady-state condition. Nevertheless this study did confirm the observation of Mitarai et al. (2000) that β-adrenergic stimulation provoked the loss of ion channels which showed rapid inactivation. Isoproterenol also resulted in the conversion of the sigmoid voltage-dependent A-V relationship, which was recorded under basal conditions, into a U-shaped relationship, even in the absence of Ca2+-dependent inactivation (Findlay, 2002b). It was shown that this resulted from the fact that, with β-adrenergic stimulation, membrane voltage, rather than inducing inactivation, actually promoted a non-inactivating kinetic form of the channel (Fig. 7F). Pietrobon & Hess (1990) and Hirano et al. (1999) both showed that strong depolarisation could evoke ‘mode 2′ type behaviour in single cardiac L-type Ca2+ channels and this form of behaviour has also been shown to be enhanced by β-adrenergic stimulation (Ochi & Kawashima, 1990; Yue et al. 1990; Hirano et al. 1999). It is therefore possible that voltage-dependent potentiation of L-type Ca2+ channel currents (see Kamp & Hell, 2000 for review) results from the loss of voltage-dependent inactivation.
Acknowledgments
I thank Alain Moreau and Helen Henri for isolating myocytes and Dr Claire Malecot for her constructive criticism of this text. This work was financed by grants from the Region Centre.
References
- Adachi-Akahane S, Leemann L, Morad M. Cross-signalling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. Journal of General Physiology. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Ca2+-dependent regulation of cardiac L-type Ca2+ channels: is a unifying mechanism at hand. Journal of Molecular and Cellular Cardiology. 2001;33:639–650. doi: 10.1006/jmcc.2000.1354. [DOI] [PubMed] [Google Scholar]
- Bean BP, Nowycky MC, Tsien RW. β-Adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bechem M, Pott L. Removal of Ca2+ current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflügers Archiv. 1985;404:10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Yi J, Rios E, Shirokov R. Ion-dependent inactivation of barium current through L-type calcium channels. Journal of General Physiology. 1997;109:449–461. doi: 10.1085/jgp.109.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Voltage- and cation-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. Journal of Physiology. 2002a;541:731–740. doi: 10.1113/jphysiol.2002.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. β-Adrenergic stimulation modulates Ca2+- and voltage-dependent inactivation of L-type Ca2+ channel currents in guinea-pig ventricular myocytes. Journal of Physiology. 2002b;541:741–751. doi: 10.1113/jphysiol.2002.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. Journal of Physiology. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RW, Hume JR. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. Journal of Physiology. 1987;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton SL, Serysheva I, Strasburg GM. Calmodulin and excitation-contraction coupling. News in Physiological Sciences. 2000;15:281–284. doi: 10.1152/physiologyonline.2000.15.6.281. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Journal of General Physiology. 1986;88:293–318. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Yoshinaga T, Murata M, Hiraoka M. Prepulse-induced mode 2 gating behaviour with and without β-Adrenergic stimulation in cardiac L-type Ca channels. American Journal of Physiology. 1999;276:C1338–1345. doi: 10.1152/ajpcell.1999.276.6.C1338. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circulation Research. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- Le Guennec J-Y, Peineau N, Esnard F, Lacampagne A, Gannier F, Argibay J, Gauthier F, Garnier D. A simple method for calibrating collagenase/pronase E ratio to optimise heart cell isolation. Biology of the Cell. 1993;79:161–165. doi: 10.1111/j.1768-322x.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Linz KW, Meyer R. Control of L-type calcium current during the action potential of guinea-pig ventricular myocytes. Journal of Physiology. 1998;513:425–442. doi: 10.1111/j.1469-7793.1998.425bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macianskiene R, Moccia F, Sipido KR, Flameng W, Mubagwa K. Channels involved in transient currents unmasked by removal of extracellular calcium in cardiac cells. American Journal of Physiology–Heart and Circulatory Physiology. 2002;282:H1879–1888. doi: 10.1152/ajpheart.00952.2001. [DOI] [PubMed] [Google Scholar]
- Mitarai S, Kaibara M, Yano K, Taniyama K. Two distinct inactivation processes related to phosphorylation in cardiac L-type Ca2+ channel currents. American Journal of Physiology–Cell Physiology. 2000;279:C603–610. doi: 10.1152/ajpcell.2000.279.3.C603. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist BAY K 8644. Proceedings of the National Academy of Sciences of the USA. 1985;82:2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R, Kawashima Y. Modulation of the slow gating process of calcium channels by isoprenaline in guinea-pig ventricular cells. Journal of Physiology. 1990;424:187–204. doi: 10.1113/jphysiol.1990.sp018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer D, Pelzer S, McDonald TF. Properties and regulation of calcium channels in muscle cells. Reviews of Physiology, Biochemistry and Pharmacology. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990;346:651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Puglisi JL, Yuan W, Bassani JWM, Bers DM. Ca2+ influx through Ca2+ channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circulation Research. 1999;85:1–10. doi: 10.1161/01.res.85.6.e7. [DOI] [PubMed] [Google Scholar]
- Rose WC, Balke CW, Wier WG, Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. Journal of Physiology. 1992;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JSK. Ca2+ release-induced inactivation of Ca2+ current in rat ventricular myocytes: evidence for local Ca2+ signalling. Journal of Physiology. 1997;500:285–295. doi: 10.1113/jphysiol.1997.sp022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz SC, Zamponi GW. Structural determinants of fast inactivation of high voltage-activated Ca2+ channels. Trends in Neurosciences. 2001;24:176–181. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- Sun L, Fan J-S, Clark JW, Palade PT. A model of the L-type Ca2+ channel in rat ventricular myocytes: ion selectivity and inactivation mechanisms. Journal of Physiology. 2000;529:139–158. doi: 10.1111/j.1469-7793.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaho F, Nargeot J, Richard S. Voltage-dependent regulation of L-type cardiac Ca channels by isoproterenol. Pflügers Archiv. 1991;419:596–602. doi: 10.1007/BF00370301. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydopyridine calcium agonists. Journal of Molecular and Cellular Cardiology. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation and block. Annual Review of Biophysics and Biophysical Chemistry. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Yue DT, Herzig S, Marban E. β-Adrenergic stimulation of calcium channels occurs by potentiation of high activity gating modes. Proceedings of the National Academy of Sciences of the USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]


