Abstract
In human peripheral nerves, physiological evidence has been presented for a number of biophysical differences between cutaneous afferents and α motor axons. The differences in strength-duration properties for cutaneous afferents and motor axons in the median nerve have been attributed to greater expression of a persistent Na+ conductance (INa,P) on cutaneous afferents. However, it is unclear whether the biophysical properties of human group Ia afferents differ from those of cutaneous afferents. The present studies were undertaken to determine whether the properties of human group Ia afferents can be studied indirectly using ‘threshold tracking’ to measure the excitability changes in the H reflex, and to determine whether the excitability of group Ia afferents differs from that of cutaneous afferents. The strength-duration properties of the soleus H reflex and soleus motor axons were measured at rest and during sustained voluntary contractions. Similar experiments were performed on the median nerve at the wrist to study the strength-duration properties of cutaneous afferents, α motor axons and H reflex of the thenar muscles. In addition, the technique of ‘latent addition’ was used to determine whether there was a difference in a low-threshold conductance on soleus Ia afferent and motor axons. The present findings indicate that the strength-duration time constant (τSD) for the H reflex is longer than that for α motor axons, but similar to that for cutaneous afferents. There were no differences in τSD for the soleus H reflex at rest and during contractions, suggesting that τSD for the H reflex is largely unaffected by changes in synaptic or motoneurone properties. Finally, the difference in latent addition suggests that the longer τSD of the soleus H reflex may indeed be due to greater activity of a persistent Na+ conductance on Ia afferents than on soleus α motor axons.
In human peripheral nerves, there are a number of biophysical differences between cutaneous afferents and α motor axons (Bostock et al. 1998). Cutaneous afferents probably have greater expression of the hyperpolarization-activated cation conductance (IH) than human motor axons (Bostock et al. 1994; Lin et al. 2002). They also undergo smaller threshold changes during the relatively refractory, supernormal and late subnormal periods following a conditioning discharge (Kiernan et al. 1996). They have a longer strength-duration time constant (Panizza et al. 1994; Mogyoros et al. 1996), a longer time constant in experiments using ‘latent addition’ (Panizza et al. 1994, 1998) and lower rheobase (Mogyoros et al. 1996), all probably due to greater expression of a very slowly inactivating (‘persistent’) Na+ conductance (INa,P) (Bostock & Rothwell, 1997).
It is not known whether the properties of human group Ia muscle afferents are similar to those of large cutaneous afferents, though there are some data to suggest that time constants estimated using latent addition are similar (Panizza et al. 1994). On the other hand, Honmou et al. (1994) presented evidence for a difference in a slow Na+ conductance in the rat, there being greater expression on cutaneous afferents (and their neurones in the dorsal root ganglion; DRG) than on either α motor axons or muscle afferents (and their DRG neurones). This raises the possibility of differences in the expression of Na+ channels on human group I and cutaneous afferents. If any such difference involved INa,P, there might be differences in strength-duration properties, with a lower threshold for group I afferents. In this respect, muscle afferent effects following stimulation of a mixed nerve can often be recorded with very weak stimuli, 0.6 times the threshold for the most excitable motor axons, without the radiating paraesthesiae that would be expected if the stimulus also activated cutaneous afferents (see e.g. Pierrot-Deseilligny, 1996). This supports the view that group I afferents are more excitable than large-diameter cutaneous afferents.
Most human nerves innervating muscle are mixed nerves that also innervate skin, and it is difficult to study the properties of a group Ia afferent population in isolation. However, the excitatory input responsible for the H reflex is largely, if not exclusively, due to the synaptic effects of group Ia afferents on the motoneurone pool, and this raises the possibility of using the motoneurone as a ‘filter’ to allow the properties of muscle afferents to be studied. Of course, motoneurone discharge depends on more than just the properties of the excitatory input, and it would be necessary to consider whether synaptic processes (particularly those capable of altering the Ia input, such as homosynaptic depression and presynaptic inhibition) or changes in motoneurone properties contributed to the findings.
The latter can be minimized by using the technique of ‘threshold tracking’ (see Bostock et al. 1998) to clamp the size of the reflex discharge so that it remains the same percentage of maximum under all experimental conditions. With threshold tracking the stimulus is adjusted by computer to keep the response constant and, in studies on peripheral nerve axons, the findings reflect axonal excitability at the site of stimulation. As noted above, the properties of the Ia-motoneurone synapse and of the motoneurone pool could represent confounding factors when the measured response is a reflex discharge. Nevertheless, it has recently been demonstrated that the recovery cycle of the H reflex after a single conditioning volley subliminal for the H reflex can be explained by the changes in excitability of the afferent input for the reflex (Chan et al. 2002). The present studies were undertaken to determine whether the properties of human group Ia afferents can be studied indirectly using threshold tracking to measure the changes in stimulus current necessary to negate changes in the H reflex. Given this, the studies then addressed whether the strength-duration properties were different to those of cutaneous afferents at the same site in the mixed nerve.
Methods
Subjects
Thirty-four experiments were performed on 11 normal subjects (aged 23-66 years, 7 male, 4 female), without clinical or neurophysiological evidence of peripheral nerve disorders. All subjects gave written informed consent to the experimental procedures, which had been approved by the Committee on Experimental Procedures Involving Human Subjects of the University of New South Wales, in accordance with the Declaration of Helsinki.
Stimulation and recordings
All experiments were performed using a computerized threshold-tracking program (QTRAC; Professor Hugh Bostock, Institute of Neurology, Queen Square, London, UK; see Bostock & Baker, 1988; Bostock et al. 1998). With threshold tracking, the current required to produce the target potential is referred to as the threshold. ‘Proportional tracking’ was used, such that the extent to which the stimulus current increased or decreased was proportional to the difference between the target and the measured response (Bostock et al. 1998).
Five sets of studies were performed as outlined below.
H reflex and M wave of soleus
To determine the strength-duration properties of the soleus H reflex and of soleus motor axons, the thresholds required to produce an H reflex and an M wave that were each 50 % of their maxima (Hmax and Mmax, respectively) were measured. The soleus H reflex was produced by stimulating the tibial nerve at the popliteal fossa using bipolar electrodes, and the compound muscle action potential (CMAP) of soleus was recorded using surface electrodes 4 cm apart, in the midline over the lower third of the soleus muscle (Hugon, 1973). Stimuli were delivered at 0.5 and 1 Hz for H reflex studies, cycling through a sequence of test stimuli. The duration of the test stimuli was increased from 0.2 to 1 ms in steps of 0.1 ms. The strength-duration curves were measured for the H reflex and M wave, as in the left panels of Fig. 1 (Weiss, 1901; Bostock, 1983; Mogyoros et al. 1996). The strength-duration time constant (τSD) was calculated using Weiss’ formula (Weiss, 1901), according to which there is a linear relationship between stimulus charge and stimulus duration (illustrated in the right panels of Fig. 1). The τSD is given by the negative intercept of the regression line on the duration axis, and the rheobase by the slope of the regression line.
Figure 1. Strength-duration data for soleus H reflex and M wave.

A and B: left, strength-duration curves for 8 subjects (means ± s.e.m.) for soleus H reflex (10 % of maximum CMAP) with stimulus intervals of 1 s (•) and 2 s (○) (A) and for motor potentials (50 % of maximum CMAP) (B). A and B: right, corresponding charge-duration plots. Threshold charge (= stimulus strength ×stimulus duration) is linearly related to stimulus duration. The time constant (τSD) is given by the (negative) intercept of the linear regression line on the duration axis. Rheobase is given by the slope of the regression line. In A and B, the regression lines are based on the equations given in the panels on the right.
Effects of voluntary contraction
In six subjects, the effects of a sustained voluntary contraction on the soleus H reflex were measured. The intensity of the contraction was controlled by auditory and visual feedback of the EMG of soleus, the latter heavily low-pass filtered and expressed as a percentage of the contraction level produced by maximal tonic plantar flexion for 10 s. The first set of measurements involved not changing the size of the target H reflex. In this paradigm, the stimulus necessary to evoke the target reflex response was reduced (= ‘contraction, weaker stimulus’ in Fig. 2). The second set of measurements involved determining the current required at rest for the 1 ms stimulus, maintaining this current level for the 1 ms stimulus during the contraction, re-setting the target window to the larger H reflex, and then resuming tracking using this larger window. In this paradigm, the reflex response was larger but the afferent volleys were, presumably, comparable to those at rest (= ‘contraction, comparable stimulus’ in Fig. 2). To determine the strength-duration properties, the current required to produce an H reflex of constant amplitude was measured with test stimuli of five different durations: 0.2, 0.3, 0.5, 0.7 and 1 ms. For comparison, the thresholds were normalized to the 1 ms data. Using Weiss’ formula, the τSD for the soleus H reflex was then calculated at rest and during contractions.
Figure 2. Effects of voluntary contraction on τSD.
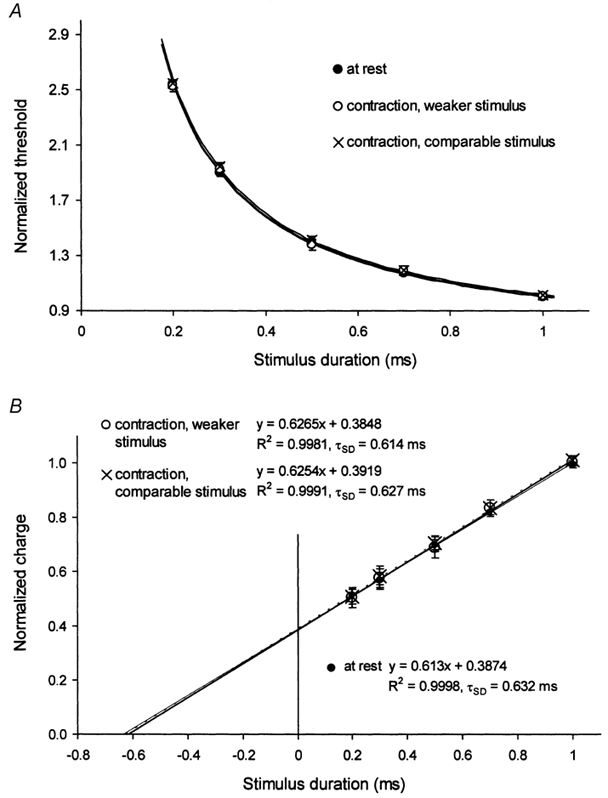
A, strength-duration data for the soleus H reflex for 6 subjects (means ± s.e.m.) normalized to the threshold for the 1 ms stimulus and plotted against stimulus duration, at rest (•) and during contractions using weaker stimuli (○) and comparable stimuli (×), as described in Methods. B, charge-duration plots for the data in A. The regression lines in A and B are derived from the equations in B.
Cutaneous afferent, H reflex and motor axon excitability in the median nerve
Experiments were performed on the median nerve at the wrist in six subjects to study the strength-duration properties of cutaneous afferents, α motor axons and H reflex of the thenar muscles evoked from the same site in the same experiment. Surface electrodes were used to stimulate the median nerve at the wrist. The antidromic compound sensory action potential (CSAP) was recorded from the index finger using ring electrodes 4 cm apart around the proximal phalanx and the CMAP was recorded using surface electrodes over the abductor pollicis brevis. The H reflex of the thenar muscle was measured during weak voluntary contractions producing an EMG level of 10-30 % of maximum. To determine the strength-duration properties of cutaneous and motor axons of the median nerve, the threshold currents required to produce a CSAP or CMAP of 50 % of maximum were measured with test stimuli of five different durations: 0.2, 0.3, 0.5, 0.7 and 1 ms. When studying the H reflex of the thenar muscles, the current required to produce a reflex response that was 5-10 % of maximal CMAP was tracked during a steady background voluntary contraction of the thenar muscles. The τSD values for cutaneous afferents, H reflex and motor axons were determined, based on Weiss’ formula (see Fig. 3B).
Figure 3. Comparison of the strength-duration properties of cutaneous afferents, H reflex and motor axons of the median nerve at the wrist.
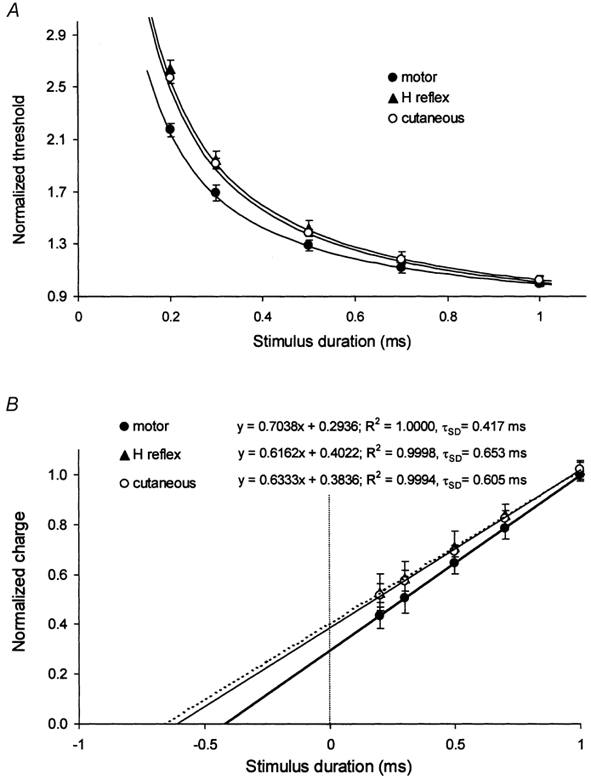
A, strength-duration curves for cutaneous afferents (○), H reflex (▴) and motor axons (•) of the median nerve at the wrist for 6 subjects (means ± s.e.m.). B, charge-duration plots for the data in A. In A and B, the regression lines are derived from the equations in B.
Latent addition
To determine whether the axons responsible for the H reflex and α motor axons express an appreciable low-threshold conductance that is active at rest, presumably mediated by ‘persistent’ Na+ channels, the technique of latent addition was used when recording the H reflex and M wave of the soleus muscle while stimulating the tibial nerve at the popliteal fossa. The threshold current required to produce the target response was determined using a test stimulus of 0.2 ms duration. A hyperpolarizing conditioning stimulus of 50 μs duration was delivered 0.2 ms before the test stimulus. Its intensity was derived from the linear regression equations obtained from the first two sets of experiments on soleus H reflex and M wave, and was fixed at ≈-50 % of threshold. The currents required to produce the target response at the 0.2 ms conditioning-test interval were measured separately for H reflex and α motor axons using a stimulus repetition rate of 2 Hz, and were compared with the unconditioned thresholds.
In all experiments, the temperature over the forearm or the calf was monitored continuously by skin sensors. It was kept above 32 °C using blankets and by applying radiant heat if necessary.
Results
Experiments were performed on the tibial nerve in the popliteal fossa, and this allowed comparison of the properties of the H reflex and the M wave responses to stimuli delivered at the same site in the same experiment. These experiments were performed at rest and during voluntary contractions of triceps surae. Attempts were made to record the antidromic CSAP from the sural nerve over the calf in response to stimuli in the popliteal fossa, so that the H reflex and motor axon data could be compared with those for cutaneous afferents at the same stimulus site. However, when the sural CSAP was sufficiently large to allow threshold tracking, the recordings were contaminated by low-threshold EMG potentials of the first recruited motor axons in the M wave. Accordingly, the properties of cutaneous afferents and α motor axons in the median nerve at the wrist were compared with those of the H reflex of the thenar muscles evoked from the same site in the same experiment but during a steady voluntary contraction (necessary to produce a usable H reflex in the thenar muscles). Finally, the technique of latent addition was used to determine whether the difference in strength-duration properties of the H reflex and of α motor axons could be attributed to a depolarizing process that activated rapidly but inactivated slowly (if at all), properties consistent with a persistent Na+ conductance (INa,P).
H reflex and M wave of soleus
In the same experiments, using the same stimulus site in the popliteal fossa, strength-duration curves were generated for the soleus H reflex and M wave by tracking the threshold for, respectively, an H reflex of 50 % of Hmax and an M wave of 50 % of Mmax. The left panels of Fig. 1 show the mean data (± s.e.m.) for eight subjects. The curves appear to be exponential but, as with single axons in the rat (Bostock et al. 1983) and sensory and motor axons in human subjects (Mogyoros et al. 1996), the data are hyperbolic and comply with Weiss’ Law (1901), with a linear relationship between stimulus charge and stimulus duration (Fig. 1, right panels). The τSD, or more correctly ‘chronaxie’, is given by the negative intercept of the regression line with the X-axis, and was 644 μs for the H reflex and 444 μs for the M wave. These values are remarkably similar to those reported by Mogyoros et al. (1996) for cutaneous afferents and α motor axons in the median nerve at the wrist (665 and 459 μs, respectively). Rheobase (the slope of the regression lines in the right panels of Fig. 1) was lower for the H reflex (5.249 mA) than for the M wave (7.834 mA).
The above measurements were made with successive stimuli delivered regularly at 1 s intervals. To assess whether presynaptic mechanisms, specifically the phenomenon of ‘homosynaptic depression’, affected the findings for the H reflex, the studies were repeated in the same experiment using an inter-stimulus interval of 2 s (Fig. 1A, open circles). At the lower stimulus repetition rate, the threshold for the 50 % H reflex was lower at each stimulus duration (Fig. 1A, left panel) and rheobase was lower (4.678 mA; Fig. 1A, right panel), but there was virtually no difference in τSD (634 μs).
Effects of voluntary contraction
In six subjects, the effects of a sustained voluntary contraction on τSD were documented. The intensity of the contraction was kept constant between 10 and 30 % of maximum, controlled by auditory and visual feedback of the EMG of soleus, the latter heavily low-pass filtered and expressed as a percentage of the contraction level produced by maximal plantar flexion. The contraction potentiated the H reflex, and two sets of measurements were made. The first set involved not changing the size of the target H reflex (open circles, ‘contraction, weaker stimulus’ in Fig. 2). These measurements would have involved the same percentage of the motoneurone pool as at rest, but the afferent volley necessary to produce the target reflex size would have been smaller. The second set of measurements involved determining the current required at rest for the 1 ms stimulus, maintaining this current level for the 1 ms stimulus during the contraction, re-setting the target window to the larger H reflex, and then resuming tracking using this larger window (× in Fig. 2, ‘contraction, comparable stimulus’). This procedure would keep the afferent volleys at rest and during contraction comparable, but the same afferent volley would activate a larger percentage of the motoneurone pool during the contraction.
To allow direct comparison, the data were normalized to the 1 ms threshold, of necessity eliminating differences in rheobase. Figure 2 shows that there were no differences in the estimates of τSD (632 μs at rest, 614 μs for the first set of measurements during contraction and 627 μs for the second). These findings suggest that τSD for the H reflex is largely independent of the properties of the motoneurone pool and of the processes that alter motoneurone excitability during a voluntary contraction.
Cutaneous afferent, H reflex and motor axon excitability
Experiments were performed on the median nerve at the wrist in six subjects to study the properties of cutaneous afferents, the H reflex and α motor axons in the same nerve at the same site in the same experiment. The properties of cutaneous and α motor axons were studied at rest in the same sequence, and the H reflex sequence was then recorded during a steady voluntary contraction (but this should not have introduced a confounding variable; see Fig. 2, above). The threshold data were normalized to the 1 ms threshold. As shown in Fig. 3, there was little difference in the curves for cutaneous afferents and for the H reflex (τSD 605 and 653 μs, respectively) but the τSD for α motor axons was shorter (417μs).
Latent addition
The technique of latent addition was used in experiments on the tibial nerve of six subjects to determine whether the difference in τSD for the H reflex and α motor axons was likely to reflect a difference in a slowly inactivating (persistent) Na+ conductance (INa,P). A difference in this conductance is probably responsible for the difference in strength-duration properties of cutaneous afferents and motor axons in the median nerve of human subjects (Bostock & Rothwell, 1997). In axons, the increase in threshold measured 0.2 ms after a hyperpolarizing pulse is largely, if not exclusively, due to a rapidly activating, slowly de-activating conductance that is depolarizing, properties expected of INa,P (Bostock & Rothwell, 1997; Bostock et al. 1998).
The histogram in Fig. 4A shows the increase in threshold measured 0.2 ms after a 50 % hyperpolarizing pulse for the soleus H reflex and M wave (each 50 % of their respective maxima). Figure 4B shows the τSD for the same subjects. There was a significant correlation between the physiological measure of Na+ channel activity in A and τSD in B (P + 0.003). Figure 4C shows the 12 data points (sensory and motor data for 6 subjects), with the two data points for the subject with high values for the H reflex indicated by arrows. When the two data points from this subject were eliminated, the relationship for the remaining five subjects was still significant (P + 0.046).
Figure 4. Relationship between τSD and threshold increase at the 0.2 ms interval.
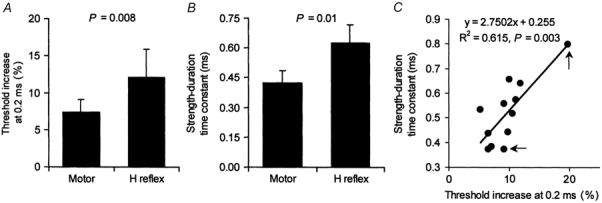
Histograms of threshold increase at the 0.2 ms conditioning-test interval in the latent addition paradigm (A) and τSD (B) for 6 subjects (means ± s.e.m.) for soleus H reflex and motor axons. In C, there is a significant relationship between τSD and the threshold increase at the 0.2 ms conditioning-test interval. The arrows indicate the data points for the subject with the most marked values (for the H reflex). The relationship was significant even when these two data points were eliminated (P + 0.046).
Discussion
The present findings demonstrate that τSD for the H reflex is longer than that for α motor axons in the same nerve, but is much the same as that for cutaneous afferents in the same nerve. The study also presents evidence that τSD for the H reflex is largely unaffected by changes in synaptic or motoneurone properties and, presumably, therefore reflects the properties of the afferent axons responsible for the reflex. Finally, evidence is presented that the longer τSD may indeed be due to greater activity of a persistent Na+ conductance (INa,P) on the afferents, much as is probably the case for the difference in strength-duration behaviour of cutaneous afferents and motor axons in the median nerve (Bostock & Rothwell, 1997).
Does τSD for the H reflex reflect afferent properties?
Group Ia afferents probably provide the major excitatory input for the H reflex, but other factors can modify reflex behaviour, and it cannot be automatically assumed that the properties of the reflex reflect the properties of the excitatory afferent input. First, the group Ia volley can be subjected to presynaptic modulation through two mechanisms, presynaptic inhibition with primary afferent depolarization and ‘homosynaptic’ or ‘post-activation’ depression, possibly due to transmitter depletion (see Hultborn et al. 1996; Wood et al. 1996). However, these factors are likely to have had a negligible influence on the measured parameter. The experimental paradigm was not one that would have altered presynaptic inhibition because presynaptic inhibition lasts < 300 ms and because there is no significant difference in presynaptic inhibition at rest and during tonic contractions (Meunier & Pierrot-Deseilligny, 1989; Nielsen & Kagamihara, 1993). In addition, there was no difference in τSD with different stimulus rates (once every second and once every 2 s), even though the reflex response was larger with the slower rate. Secondly, the H reflex is the net result of monosynaptic excitation and autogenetic inhibition through disynaptic non-reciprocal group I pathways (Burke et al. 1984; Marchand-Pauvert et al. 2002). It is also conceivable that recurrent inhibition due to the early recruited motoneurones can modify the reflex response of higher-threshold motoneurones (Burke et al. 1984; Marchand-Pauvert et al. 2002). However, post-synaptic influences on the motoneurone pool, whether excitatory or inhibitory, probably had little or no effect on the measured excitability parameter because τSD did not change when the motoneurone pool was activated during a voluntary contraction. Finally, the input-output relationship for the motoneurone pool can vary for motoneurones of different threshold (Crone et al. 1990) and can be altered by factors that trigger plateau potentials in motoneurones (Nielsen & Hultborn, 1993). However, factors affecting the motoneurone pool probably did not affect the measured parameter significantly because both non-reciprocal (Fournier et al. 1983) and recurrent (Hultborn & Pierrot-Deseilligny, 1979) inhibition are known to be altered during voluntary contractions, whereas τSD was similar with changes in stimulus rate and voluntary effort, whether H reflexes were large or small.
It is concluded that, even though many factors can shape the H reflex, τSD of the soleus H reflex probably reflects τSD of group Ia afferents in the tibial nerve, at least when this experimental paradigm is used.
Excitability of cutaneous and muscle afferents
As mentioned in the Introduction, there are biophysical differences between cutaneous afferents and motor axons, and these are presumably adaptive, possibly related to the different discharge patterns and discharge rates of the axons (Bostock et al. 1998). It might therefore be expected that the properties of motor axons of high and low-threshold motoneurones would differ. This question has not been addressed directly, but it is known that there is little change in properties such as refractoriness, supernormality and τSD as the size of the CMAP increases (Kiernan et al. 1996, 2000).
The present findings provide evidence that the strength- duration properties of group Ia afferents are similar to those of cutaneous afferents but differ from those of α motor axons. The differences in these properties for cutaneous afferents and motor axons in the median nerve have been attributed to greater expression of INa,P on cutaneous afferents (Bostock & Rothwell, 1997; Bostock et al. 1998). This raises the possibility that there may be a difference in INa,P on Ia afferents and α motor axons, a view supported by the difference in latent addition in Fig. 4A.
In the rat, there appear to be differences in non-classical Na+ conductances between cutaneous and muscle afferents, specifically involving a slowly activating Na+ conductance (Honmou et al. 1994). The present study sheds no light on this particular type of Na+ conductance. Baker & Bostock (1997) found evidence for a persistent Na+ conductance on some but not all large neurones in rat dorsal root ganglia; however, channels expressed on neurones are not necessarily expressed similarly on peripheral axons, and there are no data on this conductance for different populations of afferents.
It is still possible to draw some inferences from the present results for motor control studies, in which mixed nerve trunks are often stimulated. In human subjects the fastest cutaneous afferents have conduction velocities that are similar to or only slightly slower than those of group I muscle afferents (Burke et al. 1981; Macefield et al. 1989; Shefner & Logigian, 1994), and it is likely that they have similar axonal sizes. The present study demonstrates that rapidly conducting cutaneous and muscle afferents have similar strength-duration properties, and together these findings imply that even very weak stimulation of a mixed nerve is unlikely to activate group I muscle afferents selectively.
Implications
Differences in the strength-duration properties of human group Ia muscle afferents and α motor axons explain why the H reflex can usually be evoked by stimuli below threshold for the first activated motor axons (Paillard, 1955; Hugon, 1973; Veale et al. 1973; Panizza et al. 1992). Stimuli of relatively long duration (0.5-1.0 ms) are more effective for the H reflex, while stimuli of shorter duration (0.1-0.2 ms), producing a reflex discharge of the same size, will directly stimulate motor axons. This is largely because, as shown here, the rheobase of group Ia afferents is lower than that of α motor axons. With a 1.0 ms stimulus, threshold intensity approaches rheobase, so that a group Ia volley of sufficient size for a reflex response can be produced at stimulus intensities that activate few, if any, low-threshold α motor axons. That this is not possible with stimuli of short duration can be attributed to differences in the strength-duration time constants for afferent and efferent axons.
The lower rheobase implies that less current may be required to keep Ia afferents conducting, a property that could be important in disease states with a pathologically low safety margin for impulse conduction. Perception probably depends on the ability of axons to conduct impulses with, in general, little need for a highly synchronous afferent volley. On the other hand, the tendon jerk and H reflex are sensitive to pathology that could de-synchronize the afferent volley. A low rheobase for group Ia afferents would confer an ability to maintain conduction, albeit slowed. It is therefore not surprising that the perceptual consequences of muscle afferent inputs (i.e. kinaesthesia; see Goodwin et al. 1972; McCloskey, 1978) might not be impaired even when the dispersion of the afferent volley renders the H reflex or tendon jerk difficult to record. Finally, manoeuvres that alter the discharge rate of an axon and thereby its excitability (e.g. voluntary contraction, muscle stretch or vibration) may not affect muscle afferents and motor axons equally even when there are comparable changes in afferent and efferent discharge, or there is an identical M wave. In other words, controlling changes in the M wave does not guarantee that the Ia afferent volley is constant during an experimental manoeuvre.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia and Institut pour la Recherche sur la Moelle Épinière.
References
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. Journal of Neurophysiology. 1997;77:1503–1513. doi: 10.1152/jn.1997.77.3.1503. [DOI] [PubMed] [Google Scholar]
- Bostock H. The strength-duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. Journal of Physiology. 1983;341:59–74. doi: 10.1113/jphysiol.1983.sp014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Research. 1988;462:354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H, Burke D, Hales JP. Differences in behaviour of sensory and motor axons following release of ischaemia. Brain. 1994;117:225–234. doi: 10.1093/brain/117.2.225. [DOI] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle and Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. Journal of Physiology. 1997;498:277–294. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt RM. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. Journal of Physiology. 1983;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D, Skuse NF, Lethlean AK. Cutaneous and muscle afferent components of the cerebral potential evoked by electrical stimulation of human peripheral nerves. Electroencephalography and Clinical Neurophysiology. 1981;51:579–588. doi: 10.1016/0013-4694(81)90202-9. [DOI] [PubMed] [Google Scholar]
- Chan JHL, Lin C S-Y, Pierrot-Deseilligny E, Burke D. Excitability changes in human peripheral nerve axons in a paradigm mimicking paired-pulse transcranial magnetic stimulation. Journal of Physiology. 2002;542:951–961. doi: 10.1113/jphysiol.2002.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Experimental Brain Research. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Fournier E, Katz R, Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Research. 1983;288:375–377. doi: 10.1016/0006-8993(83)90122-1. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. Journal of Neurophysiology. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon M. Methodology of the Hoffmann reflex in man. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Vol. 3. Basel: S. Karger; 1973. pp. 277–293. [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Experimental Brain Research. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. Journal of Physiology. 1979;297:229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle and Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Burke D. Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain. 1996;119:1099–1105. doi: 10.1093/brain/119.4.1099. [DOI] [PubMed] [Google Scholar]
- Lin C S-Y, Grosskreutz J, Burke D. Sodium channel function and the excitability of human cutaneous afferents during ischaemia. Journal of Physiology. 2002;538:435–446. doi: 10.1113/jphysiol.2001.012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiological Reviews. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Conduction velocities of muscle and cutaneous afferents in the upper and lower limbs of human subjects. Brain. 1989;112:1519–1532. doi: 10.1093/brain/112.6.1519. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. Journal of Physiology. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. Journal of Physiology. 1989;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Hultborn H. Regulated properties of motoneurons and primary afferents: new aspects on possible spinal mechanisms underlying spasticity. In: Thilmann AF, Burke DJ, Rymer WZ, editors. Spasticity. Berlin: Springer-Verlag; 1993. pp. 177–192. [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. Journal of Physiology. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard J. Réflexes et Régulations d'Origine Proprioceptive chez l'Homme. Etude Neurophysiologique et Neuropsychologique. Paris: Arnette; 1955. [Google Scholar]
- Panizza M, Nilsson J, Roth BJ, Basser PJ, Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 1992;85:22–29. doi: 10.1016/0168-5597(92)90097-u. [DOI] [PubMed] [Google Scholar]
- Panizza M, Nilsson J, Roth BJ, Grill SE, Demirci M, Hallett M. Differences between the time constant of sensory and motor peripheral nerve fibers: further studies and considerations. Muscle and Nerve. 1998;21:48–54. doi: 10.1002/(sici)1097-4598(199801)21:1<48::aid-mus7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Panizza M, Nilsson J, Roth BJ, Rothwell J, Hallett M. The time constants of motor and sensory peripheral nerve fibers measured with the method of latent addition. Electroencephalography and Clinical Neurophysiology. 1994;93:147–154. doi: 10.1016/0168-5597(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Logigian EL. Conduction velocity in motor, cutaneous afferent, and muscle afferent fibers within the same mixed nerve. Muscle and Nerve. 1994;17:773–778. doi: 10.1002/mus.880170712. [DOI] [PubMed] [Google Scholar]
- Veale JL, Mark RF, Rees S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:75–86. doi: 10.1136/jnnp.36.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G. Sur la possibilité de render comparables entre eux les appareils servant a l'excitation électrique. Archives of Italian Biology. 1901;35:413–447. [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. Journal of Physiology. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]


