Abstract
Depolarizing afterpotentials (DAPs) that follow action potentials in magnocellular neurosecretory cells (MNCs) are thought to underlie the generation of phasic firing, a pattern that optimizes vasopressin release from the neurohypophysis. Previous work has suggested that the DAP may result from the Ca2+-dependent reduction of a resting K+ conductance. Here we examined the effects of flufenamic acid (FFA), a blocker of Ca2+-dependent non-selective cation (CAN) channels, on DAPs and phasic firing using intracellular recordings from supraoptic MNCs in superfused explants of rat hypothalamus. Application of FFA, but not solvent (0.1 % DMSO), reversibly inhibited (IC50 + 13.8 μm; R + 0.97) DAPs and phasic firing with a similar time course, but had no significant effects (P > 0.05) on membrane potential, spike threshold and input resistance, nor on the frequency and amplitude of spontaneous synaptic potentials. Moreover, FFA did not affect (P > 0.05) the amplitude, duration, undershoot, or frequency-dependent broadening of action potentials elicited during the spike trains used to evoke DAPs. These findings suggest that FFA inhibits the DAP by directly blocking the channels responsible for its production, rather than by interfering with Ca2+ influx. They also support a role for DAPs in the generation of phasic firing in MNCs. Finally, the absence of a depolarization and increased membrane resistance upon application of FFA suggests that the DAP in MNCs may not be due to the inhibition of resting K+ current, but to the activation of CAN channels.
Hypothalamic magnocellular neurosecretory cells (MNCs) are responsible for the release of vasopressin (VP; the antidiuretic hormone) and oxytocin (OT) into the blood (Renaud & Bourque, 1991). In rats, VP-releasing MNCs respond to hyperosmolality (Brimble & Dyball, 1977) and hypovolaemia (Harris et al. 1975) by increasing their firing rate and adopting a phasic firing pattern comprising alternating periods of activity (7-15 Hz) and silence lasting tens of seconds each. Previous studies have shown that VP release is maximized by stimulation patterns mimicking phasic firing (Dutton & Dyball, 1979). The emergence of this pattern is therefore an important part of the response of MNCs during hyperosmolality and hypovolaemia.
Action potentials in MNCs are followed by a slow (2-3 s) depolarizing afterpotential (DAP; Andrew & Dudek, 1984; Armstrong et al. 1994). In phasically active cells, DAPs following consecutive action potentials summate temporally into a depolarizing plateau that sustains firing throughout the active period (Andrew & Dudek, 1984; Ghamari-Langroudi & Bourque, 1998). Conversely, the termination of each burst is associated with a repolarization of the plateau (Andrew & Dudek, 1984), a phenomenon which may result from activity-dependent inactivation of the conductance underlying the DAP (GDAP; Andrew & Dudek, 1984). Therefore DAPs appear to play a critical role in the generation of phasic firing. Interestingly, recent studies have suggested that modulation of GDAP by neurotransmitters may effectively regulate the expression of phasic firing in situ (e.g. Brown et al. 1999).
Studies in other cell types have shown that DAPs and plateau potentials can result from the activation of non-inactivating voltage-gated Na+ channels (Washburn, Anderson & Ferguson, 2000) or Ca2+ channels (Jung et al. 2001), or from Ca2+-activated non-selective cation (CAN; Partridge & Swandulla, 1988) or chloride channels (Martinez-Pinna et al. 2000). Interestingly, recordings made from MNCs in vitro under voltage clamp (Li & Hatton, 1997b) have suggested that the DAP in these cells may be due to a Ca2+-dependent decrease in resting K+ current. However, biophysical analysis of the DAP in MNCs is complicated by a variety of factors. For example, evaluation of the changes in membrane resistance accompanying the DAP may be misleading due to the steep voltage sensitivity of GDAP (Bourque, 1986). Moreover, the presence of multiple overlapping voltage- and calcium-dependent currents (see Hatton & Li, 1998; Ghamari-Langroudi & Bourque, 2001) can complicate the interpretation of membrane current relaxations observed under voltage clamp. Finally, the above factors and the dependence of the DAP on Ca2+ influx for activation (Andrew, 1987; Bourque, 1986; Li et al. 1995; Li & Hatton, 1997a) make it potentially difficult to compare DAPs evoked by trains of action potentials (e.g. Ghamari-Langroudi & Bourque, 1998, 2001) and those obtained by rectangular voltage commands under whole cell voltage clamp (e.g. Li & Hatton, 1997a,b). Indeed, DAPs evoked by action potentials during sharp microelectrode recordings are not blocked by tetrodotoxin (TTX; Andrew, 1987) or tetraethyl ammonium (TEA; Greffrath et al. 1998), but they are blocked by external Cs+ (Ghamari-Langroudi & Bourque, 1998). In contrast, DAPs evoked by depolarizing steps in whole cell voltage clamp are blocked by TTX or TEA, and appear to be insensitive to external Cs+ (Li & Hatton, 1997b). The ionic basis of the action potential-evoked DAP thus remains uncertain.
Recent studies in other cell types have indicated that CAN channels can be blocked by flufenamic acid (FFA; Partridge & Valenzuela, 2000). This compound has also been reported to effectively block DAPs and burst firing in cells in which CAN currents contribute to plateau potentials and bursting behaviour (e.g. Morisset & Nagy, 1999). In this study we reveal that action potential-evoked DAPs and spontaneous phasic firing are concertedly inhibited by FFA, suggesting the possible involvement of CAN channels in controlling the excitability of MNCs and hormone secretion from the neurohypophysis.
Methods
Preparation of superfused explants
Hypothalamic explants were prepared as described previously (Ghamari-Langroudi & Bourque, 1998). Male Long-Evans rats (150-300 g) were killed by decapitation according to a protocol approved by the McGill University Animal Care Committee. The brain was rapidly removed and a block of tissue (≈ 8 × 8 × 2 mm) comprising the basal hypothalamus was excised using razor blades and transferred to a temperature-controlled (31-33 °C) superfusion chamber. Explants were superfused (0.5-1 ml min−1) with an oxygenated (95 % O2-5 % CO2) artificial cerebrospinal fluid (ACSF, pH 7.4; 295 ± 1 mosmol kg−1) comprising (mm): NaCl, 121; MgCl2, 1.3; KCl, 3; NaHCO3, 26; glucose, 10; CaCl2, 2.5 (Fisher Scientific Company; Pittsburgh, PA, USA). Stock solutions of flufenamic acid (FFA) and meclofenamic acid (MFA; Sigma Chemical Co., Saint Louis MO, USA) were prepared by dissolving the drugs in dimethyl sulfoxide (DMSO; Sigma). All drugs were applied as dilutions of the stock in ACSF and the final concentration of DMSO did not exceed 0.2 %.
Electrophysiology
Intracellular recordings were obtained using sharp microelectrodes prepared from 1.2 mm o.d. glass capillary tubes (A.M. Systems Inc., Everett, WA, USA) pulled on a P87 Flaming-Brown pipette puller (Sutter Instruments Co., Novato, CA, USA) and filled with 2 m potassium acetate (yielding resistances of 70-150 MΩ). Recordings of membrane voltage and pipette current were obtained using an Axoclamp 2A amplifier (Axon Instruments Inc., Union City, CA, USA). Signals were displayed on a chart recorder and digitized (10 kHz) via a Digidata 1200B interface driven by Clampex 8.0 software (Axon Instruments Inc.).
Protocol used to elicit DAPs
The amplitude of DAPs following trains of action potentials is strongly affected by the number of spikes in the train (Andrew & Dudek, 1984) and the membrane potential from which it is evoked (VINITIAL; Bourque, 1986; Li & Hatton, 1997b). DAPs were therefore evoked using depolarizing current pulses (50-200 pA; 80 ms) eliciting constant numbers of action potentials (3-6, depending on the cell), from a constant VINITIAL (range for the group: −66 to −58 mV) which was maintained by DC current injection (0-100 pA), if necessary. Moreover, since DAP amplitude is attenuated by preceding action potential activity (Andrew & Dudek, 1984), successive trials were evoked at a low frequency (< 0.05 Hz) and spontaneous firing, when present, was prevented by holding VINITIAL below spike threshold.
Analysis of synaptic potentials
Continuous voltage segments were imported into Axograph 4.5 (Axon Instruments Inc.) running on a Macintosh computer. Spontaneous excitatory postsynaptic potentials (sEPSPs) and inhibitory postsynaptic potentials (sIPSPs) were detected using a cell-specific event template and a variable signal-to-noise threshold criterion. The amplitude of all events (relative to baseline) was measured automatically. Cumulative probability distributions of synaptic potential amplitude were constructed using Kaleidagraph 3.08 (Synergy Software, Reading, PA, USA). Differences between amplitude distributions were sought by comparing absolute amplitudes at 50 % probability (i.e. the median amplitude). Recording segments analysed under different conditions comprised between 176 and 608 events.
Statistics
Throughout the paper, averaged data are expressed as means ± s.e.m. Where indicated, differences between mean values recorded under control and test conditions were evaluated using a paired t test and were considered significant when P < 0.05. Possible correlations between changes in various spike parameters and DAP amplitude during FFA application were sought using the Pearson product moment method (SigmaStat 2.03, SPSS Science, Chicago, IL, USA) and significant correlations were deemed absent when P > 0.05.
Results
Intracellular recordings were made from supraoptic nucleus MNCs impaled with sharp microelectrodes in superfused explants of rat hypothalamus. All of these cells displayed characteristics that are specific to MNCs, but not to neighbouring non-neuroendocrine cells (Renaud & Bourque, 1991).
Effects of FFA on DAPs
Previous studies have shown that apamin, a selective blocker of the Ca2+-dependent K+ current underlying the post-train afterhyperpolarization (AHP) in MNCs, increases the amplitude of DAPs (Bourque & Brown, 1987; Armstrong et al. 1994; Kirkpatrick & Bourque, 1996). Conversely, inhibition of the current underlying the DAP by external Cs+ has been shown to increase the magnitude of the AHP (Ghamari-Langroudi & Bourque, 1998). Given this temporal overlap between the currents underlying the AHP and the DAP, we examined the effects of FFA on DAPs isolated by bath application of 100 nm apamin. Using the protocol described in the methods, post-train DAPs of consistent amplitude and time course could be recorded for up to 2 h. In each of 12 cells tested, bath application of FFA (0.3 μm-5 mm) reduced the amplitude of the post-train DAP (e.g. Fig. 1A and B). As shown in Fig. 1C, the effects of FFA were dose dependent, with an IC50 of 13.8 μm (R + 0.97). The onset of inhibition was slow, requiring 5-10 min to reach steady state. Recovery following washout occurred progressively and was completely achieved only in four cells, where the mean time required was 25.5 ± 2.7 min. Similar results were observed with the related compound meclofenamic acid (MFA; 100 μm), which inhibited the DAP by 87.5 % (2.4 ± 0.4 mV in control vs. 0.3 ± 0.3 mV with MFA; P + 0.017; n = 4). In contrast, bath application of vehicle alone (0.1-0.2 % DMSO) did not affect DAP amplitude (4.1 ± 0.4 mV in control vs. 5.1 ± 0.6 mV in DMSO; P + 0.74), membrane potential (-62.5 ± 1.2 mV in control vs. −64.1 ± 0.9 mV in FFA; P + 0.07; n = 8), input resistance (217 ± 10 MΩ in control vs. 201 ± 11 M Ω in FFA; P + 0.45; n = 4), or spike threshold (-46.3 ± 3.2 mV in control vs. −45.3 ± 2.1 mV in FFA; P + 0.52; n = 7).
Figure 1. Effects of FFA on post-train DAPs.
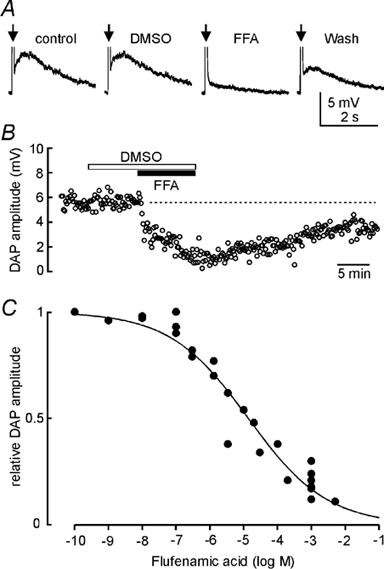
A, voltage recordings from an MNC. Individual traces show average (6 trials each) DAPs evoked by spike trains comprising 5 action potentials each (arrows; spikes not shown), evoked by a depolarizing current pulse. Vehicle (0.1 % DMSO) alone had no significant effect but application of 100 μm flufenamic acid (FFA) potently inhibited DAP amplitude. Note that 100 nm apamin was present throughout to inhibit the AHP. B, time course of the effects of 0.1 % DMSO and 200 μm FFA on another cell. Each point represents the amplitude of the DAP measured in an individual trial. Note the slow recovery of DAP amplitude following washout. C, effects of different concentrations of FFA on DAP amplitude. DAP amplitude was measured every 30-120 s from the average of 2-4 consecutive trials. Each point on the graph shows the maximal reduction of DAP amplitude observed in one cell at the corresponding FFA concentration. The continuous line is the best logistic fit through the data points computed using Sigmaplot 5.0 software (SPSS Science).
Effects of FFA on action potentials
The DAP has been shown to be dependent on action potential-mediated Ca2+ influx for its activation (Andrew, 1987; Li et al. 1995; Li & Hatton, 1997a). The inhibitory effect of FFA on DAPs, therefore, might be due to an effect on action potentials. As illustrated in Figure 2A and B, bath application of FFA (0.03-1 mm; n = 7) had no significant effect on the amplitude (83.1 ± 3.6 mV in control vs. 76.3 ± 4.9 mV in FFA; P + 0.16) or duration (1.1 ± 0.1 ms in control vs. 1.2 ± 0.2 ms in FFA; P + 0.21; n = 7) of the first action potential evoked in each spike train. Similarly, spike broadening during trains evoked in the presence of FFA (154 ± 0.4 %) was not different from the extent of broadening observed in control (156 ± 0.5 %; P + 0.78). When measured relative to baseline, the amplitude of the post-spike hyperpolarizing afterpotential was also insensitive to FFA (9.6 ± 0.8 mV in control vs. 8.5 ± 0.8 mV in FFA; P + 0.11; n = 7). In the same group of cells, however, the DAP was inhibited by more than 75 % (Fig. 2B). Finally, Pearson product moment analysis confirmed that the fractional decrease in DAP amplitude was not correlated with changes in any of the parameters examined (P > 0.1).
Figure 2. FFA does not affect action potentials that evoke DAPs.
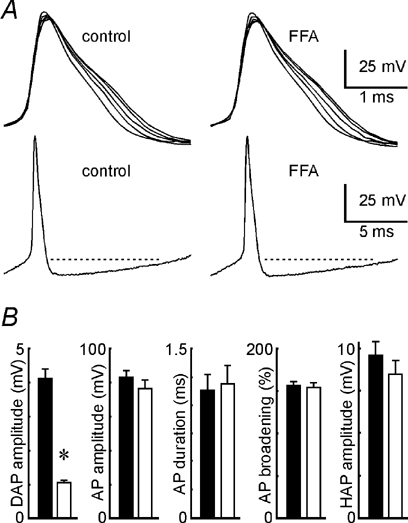
A, examples of action potentials elicited in spike trains used to evoke DAPs in one MNC under control conditions (left panels) and in the presence of 100 μm FFA (right panels). Top panels show all 5 action potentials evoked by a single train in each condition. Spikes are superimposed to show the progressive broadening of action potentials as the train progresses. Lower traces show the full extent of the first impulse to highlight the lack of differences in the post-spike hyperpolarizing afterpotential (HAP). B, bars represent mean values (± s.e.m.; n = 7) of DAP amplitude, first action potential (AP) amplitude, first AP duration, AP broadening and HAP amplitude recorded in the absence (filled bars) and presence (open bars) of FFA (30 μm-1 mm).
Effects of FFA on phasic firing
The effects of FFA and MFA were examined on five MNCs displaying spontaneous phasic firing. In one cell, bath application of 2 μm FFA, a concentration which reduces DAPs by ≈30 %, caused a 41 % decrease in burst length. In four other cells, application of 10-100 μm FFA (concentrations which block DAPs by ≈50 % or more) reversibly abolished the phasic pattern and provoked slow irregular firing (Fig. 3). On average, a period of 3.5 ± 1.2 min elapsed before the last spontaneous burst was recorded following the onset of drug application and 21.5 ± 2.1 min elapsed before a first clear burst (i.e. partial recovery) could be observed upon washout. A longer period of wash (> 30 min) was typically required for complete restoration of the original firing pattern. Inhibition of phasic firing was also observed in one cell exposed to 100 μm MFA, a concentration which inhibited the DAP by 87.5 %. Finally, we analysed the frequency and amplitude of spontaneous excitatory postsynaptic potentials (sEPSP) and spontaneous inhibitory postsynaptic potentials (sIPSP) recorded during 4.4-11 min segments taken under control conditions and in the presence of FFA. Neither the mean frequency (0.58 ± 0.07 Hz in control vs. 0.69 ± 0.14 Hz in FFA; P + 0.43) nor the mean median amplitude of sEPSPs (2.56 ± 0.42 mV in control vs. 2.38 ± 0.31 mV in FFA; P + 0.24) was affected by application of FFA (n = 4). Similarly, the frequency (0.81 ± 0.20 in control vs. 1.11 ± 0.58 Hz in FFA; P + 0.63) and median amplitude (-2.88 ± 0.45 in control vs. −3.12 ± 0.68 mV in FFA; P + 0.43) of sIPSPs was unaffected by the addition of FFA (n = 4).
Figure 3. FFA inhibits phasic firing in MNCs.
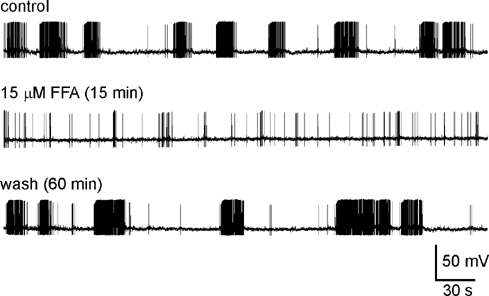
Excerpts of an intracellular recording obtained from an MNC showing spontaneous phasic firing. The traces shown were obtained before (control) and during application of 15 μm FFA, as well as following washout (wash). The time shown in parentheses corresponds to the time elapsed in each condition at the beginning of the corresponding trace. Note that FFA prevented the grouping of action potentials into phasic bursts, resulting in a slow irregular pattern of firing.
Discussion
Our study identifies FFA and MFA as blockers of the DAP that follows action potentials in rat MNCs. Blockade of the DAP by FFA had a slow onset, was dose-dependent (IC50 ≈14 μm), and reversed only slowly (> 25 min) upon washout. The slow time course of the effect of FFA on the DAP is similar to findings in hippocampal neurones (Partridge & Valenzuela, 2000), but is in stark contrast with the time required for its full blockade upon exposure to external Cs+ in the same preparation (≈2-3 min; Ghamari-Langroudi & Bourque, 1998). Recent experiments have shown that single CAN channels are blocked rapidly (< 20 s) when the drug is delivered directly to the cytoplasmic face of the channel (Guinamard et al. 2002). Thus, whereas Cs+ may block the channels underlying the DAP through a rapid action at an extracellular site (Ghamari-Langroudi & Bourque, 1998), the effects of FFA on the DAP may be slowed because of a requirement for transmembrane diffusion and access to an intracellular site.
Blockade of CAN channels by FFA is often reported to be preceded by a transient increase in current amplitude (e.g. Partridge & Valenzuela, 2000). This effect appears to be due to a potentiating effect of FFA-induced Ca2+ release from intracellular stores (Partridge & Valenzuela, 1999). However, in our experiments we did not observe an enhancement of the DAP upon applying FFA. One possibility is that channel potentiation following the onset of FFA application is short lived relative to the rate of onset of the blocking effect and that individual DAP measurement trials never coincided with a momentary enhancement. Alternately, channels underlying the DAP in MNCs may be already maximally potentiated under control conditions. Indeed, potentiation of CAN channels persists for several tens of seconds following an increase in internal [Ca2+] (Partridge & Valenzuela, 1999), a duration comparable to the inter-trial intervals used in our experiments. Finally, since not all cells show an increase in internal [Ca2+] in the presence of FFA (Harks et al. 2001), the absence of an enhancement of the DAP by FFA could have been due to the lack, or insufficient amplitude, of the FFA-induced increase in intracellular [Ca2+] in our preparation.
Fenamates are also inhibitors of the enzyme cyclooxygenase (COX; Ouellet & Percival, 1995). However, bath application of 100 μm indomethacin, another potent inhibitor of COX, did not affect DAPs (n = 2; data not shown). Therefore the effects of FFA and MFA on the DAP were not due to a modulatory effect of COX inhibition. Additionally, the effects of FFA were not accompanied by changes in membrane resistance, and thus inhibition of DAPs was not due to a shunting effect. Finally, FFA did not affect the post-spike hyperpolarizing afterpotential, spike amplitude, spike duration or frequency-dependent action potential broadening at concentrations which effectively inhibited the DAP (Fig. 2). It is therefore unlikely that the effects of FFA on DAPs were mediated by a decrease in action potential-dependent Ca2+ influx. These findings suggest that, like Cs+ (Ghamari-Langroudi & Bourque, 1998), FFA most likely inhibits DAPs through a direct blockade of the channels underlying GDAP.
Bath application of FFA reversibly blocked spontaneous phasic firing in MNCs. This effect occurred with a time course similar to its effect on the DAP. However, recent studies have shown that fenamates can inhibit gap junctions formed by connexin-43 (Harks et al. 2001), and that increased gap junctional coupling may facilitate phasic firing in MNCs (Yang & Hatton, 1999). It is therefore conceivable that the block of phasic firing by FFA might be due to a decrease in coupling. Increases in burst duration accompanying the enhancement of GAP junction coupling, however, were associated with membrane depolarization and decreased input resistance (Yang & Hatton, 1999). As pointed out above, loss of phasic firing in FFA was not associated with significant effects on membrane potential or resistance, suggesting that it did not result from a decrease in junctional coupling. Since FFA was without effect on spike threshold and on the frequency or amplitude of spontaneous postsynaptic potentials, we conclude that the loss of phasic firing was due to inhibition of the DAP. The inhibition of phasic firing by FFA thus provides further support for the involvement of DAPs in regulating the expression of phasic firing in MNCs (Ghamari-Langroudi & Bourque, 1998).
In addition to blocking CAN channels (e.g. Partridge & Valenzuela, 2000), fenamates have been reported to block a variety of Cl− channels (e.g. Eder et al. 1998). However, previous studies have shown that DAPs in MNCs are not due to a Cl− conductance (Andrew, 1987; Li & Hatton, 1997b). Moreover, we could find no report indicating that FFA could block resting K+ channels. If DAPs were due mainly to a decrease in resting K+ current, as proposed by Li & Hatton (1997b), one would expect that the blockade of GDAP would be associated with a depolarization of the membrane and with an increase in resistance. The inhibition of DAPs by FFA, however, was not associated with such changes, suggesting that DAPs may not be due to a decrease in resting K+ current, but to the activation of CAN channels.
The apparent discrepancy between the present results and those of Li & Hatton (1997b) may be due to the fact that DAPs examined here were evoked by trains of action potentials and were monitored via intracellular recording with sharp microelectrodes, whereas those studied by Li & Hatton were recorded by whole cell patch clamp and were evoked by rectangular voltage steps. As explained earlier, important pharmacological differences appear to distinguish DAPs evoked by these protocols. Different recording conditions might bias the relative contributions of different mechanisms to a similar phenomenon. Additional work will be required to resolve this issue.
Acknowledgments
This work was supported by CIHR Operating and Senior Scientist awards to C.W.B. We thank Drs Colin H. Brown and Robert Buss for helpful comments during the preparation of this manuscript.
References
- Andrew RD. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. Journal of Physiology. 1987;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. Journal of Neurophysiology. 1984;51:552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. Journal of Physiology. 1994;475:115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neuroscience Letters. 1986;70:204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Brown DA. Apamin and d-tubocurarine block the after-hyperpolarization of rat supraopic neurosecretory neurons. Neuroscience Letters. 1987;82:185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Brimble MJ, Dyball REJ. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. Journal of Physiology. 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Ghamari-Langroudi M, Leng M, Bourque CW. κ-Opioid receptor activation inhibits post-spike depolarizing after-potentials in rat supraoptic nucleus neurones in vitro. Journal of Neuroendocrinology. 1999;11:825–828. doi: 10.1046/j.1365-2826.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- Dutton DA, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. Journal of Physiology. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C, Klee R, Heinemann U. Involvement of stretch-activated Cl− channels in ramification of murine microglia. Journal of Neuroscience. 1998;18:7127–7137. doi: 10.1523/JNEUROSCI.18-18-07127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. Journal of Physiology. 1998;510:165–175. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Ionic basis of the caesium-induced depolarisation in rat supraoptic nucleus neurones. Journal of Physiology. 2001;536:797–808. doi: 10.1111/j.1469-7793.2001.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. Journal of Physiology. 1998;513:493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Rahmati M, Lenfant J, Bois P. Characterization of a Ca2+-activated nonselective cation channel during dedifferentiation of cultured rat ventricular cardiomyocytes. Journal of Membrane Biology. 2002;188:127–135. doi: 10.1007/s00232-001-0180-4. [DOI] [PubMed] [Google Scholar]
- Harks EGA, De Roos ADG, Peters PHJ, De Haan LH, Brouwer A, Ypey DL, Van Zoelen EJJ, Theuvenet APR. Fenamates: A novel class of reversible gap junction blockers. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1033–1041. [PubMed] [Google Scholar]
- Harris MC, Dreifuss JJ, Legros JJ. Excitation of phasically-firing supraoptic neurones during vasopressin release. Nature. 1975;258:80–82. doi: 10.1038/258080b0. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Li Z. Mechanisms of neuroendocrine cell excitability. Advances in Experimental Medicine and Biology. 1998;449:79–95. doi: 10.1007/978-1-4615-4871-3_8. [DOI] [PubMed] [Google Scholar]
- Jung H-Y, Staff NP, Spruston N. Action potential bursting in subicular pyramidal neurons is driven by a calcium tail current. Journal of Neuroscience. 2001;21:3312–3321. doi: 10.1523/JNEUROSCI.21-10-03312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. Journal of Physiology. 1996;494:389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Decavel C, Hatton GI. Calbindin-D28k : role in determining intrinsically generated firing patterns in rat supraoptic neurones. Journal of Physiology. 1995;488:601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Ca2+ release from internal stores: role in generating depolarizing after-potentials in rat supraoptic neurones. Journal of Physiology. 1997a;498:239–250. doi: 10.1113/jphysiol.1997.sp021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. Journal of Physiology. 1997b;505:95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinna J, McLachlan EM, Gallego R. Distinct mechanisms for activation of Cl− and K+ currents by Ca2+ from different sources in mouse sympathetic neurones. Journal of Physiology. 2000;527:249–264. doi: 10.1111/j.1469-7793.2000.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. Journal of Neuroscience. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet M, Percival MD. Effect of inhibitor time-dependency on selectivity towards cyclooxygenase isoforms. Biochemical Journal. 1995;306:247–251. doi: 10.1042/bj3060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Swandulla D. Calcium-activated non-specific cation channels. Trends in Neurosciences. 1988;11:69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Block of hippocampal CAN channels by flufenamate. Brain Research. 2000;867:143–148. doi: 10.1016/s0006-8993(00)02275-7. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Ca2+ stores dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. Journal of Physiology. 1999;521:617–627. doi: 10.1111/j.1469-7793.1999.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Progress in Neurobiology. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Washburn DL, Anderson JW, Ferguson AV. A subthreshold persistent sodium current mediates bursting in rat subfornical organ neurones. Journal of Physiology. 2000;529:359–371. doi: 10.1111/j.1469-7793.2000.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QZ, Hatton GI. Nitric oxide via cGMP-dependent mechanism increases dye coupling and excitability of rat supraoptic nucleus neurons. Journal of Neuroscience. 1999;19:4270–4279. doi: 10.1523/JNEUROSCI.19-11-04270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


