Abstract
This study examined the effect of caffeine on self-sustained firing (SSF) of human motor units. At physiological doses, caffeine acts as a competitive antagonist to the inhibitory effects of adenosine. This antagonism has many possible effects on the central nervous system. One of these effects is to increase the release of the excitatory neurotransmitters serotonin and noradrenaline. In addition, caffeine increases serotonin concentration in brainstem regions that have excitatory projections to spinal motor neurons. Since plateau potentials, which are responsible for SSF, are facilitated by these neurotransmitters, we hypothesized that caffeine would increase the frequency at which SSF occurs. A double-blind, repeated-measures design using either drug (6 mg kg−1 caffeine) or placebo (flour) was carried out on seven male subjects who reported ingesting less than 200 mg week−1 caffeine. We investigated the occurrence of SSF in tibialis anterior motor units (214 trials) and found a significant (P < 0.05) increase in the occurrence of SSF in the caffeine trial (87.0 ± 5.8 %) compared to the placebo (64.6 ± 9.7 %). These data further verify the presence of SSF in the tibialis anterior motor units of young men and provide indirect evidence of the facilitation of plateau potentials by monoamines in the human neuromuscular system.
Historically, α-motor neurons were thought to possess simple qualities, passively summing the synaptic input arriving at the dendrites and transferring the signal along the axon where it results in the activation of muscle. Recently, however, it has been shown in both invertebrates and vertebrates that α-motor neurons possess complex, specialized membrane properties capable of non-linear integration of synaptic inputs (Hounsgaard et al. 1984). Self-sustained firing (SSF) is an example of motoneuronal activity that arises as a result of these specialized membrane properties.
Self-sustained firing is the continued firing of a motor neuron following recruitment by a brief synaptic excitation during a constant, or even decreasing, level of synaptic drive. This excitation may be elicited in humans through the activation of Ia afferents by tendon vibration (Kiehn & Eken, 1997; Gorassini et al. 1998, 2002a) or stretch (Gorassini et al. 2002a). Other methods used in animal studies include electrical stimulation of the homonymous muscle (Eken & Kiehn, 1989) and intracellular current injection in reduced animal preparations (Hounsgaard et al. 1984, 1988; Conway et al. 1988). While a background level of excitability is not required to elicit SSF in animal studies (Hounsgaard et al. 1984, 1988; Conway et al. 1988; Crone et al. 1988) it is apparently required to elicit SSF in human studies (Kiehn & Eken, 1997; Gorassini et al. 1998). Thus, SSF may be more accurately described as self-facilitated firing. Nonetheless, SSF is the term adopted in the literature.
Self-sustained firing is dependent on plateau potentials which increase the excitability of the cell. Plateau potentials are triggered through the activation of voltage-sensitive, non-inactivating L-type Ca2+ channels that cause a persistent inward Ca2+ current in cat (Schwindt & Crill, 1980a,b; Hounsgaard et al. 1988) and turtle (Hounsgaard & Kiehn, 1989) preparations. SSF is observed when a plateau potential is activated at or below the recruitment threshold resulting in the sudden depolarization of the cell (Hounsgaard & Kiehn, 1989; Kiehn & Eken, 1997). Recent work on humans (Kiehn & Eken, 1997; Gorassini et al. 1998; Gorassini et al. 2002a,b) has revealed SSF characteristics, whereby motor units jump from rest to a stable firing frequency referred to as the ‘preferred firing range’ (Kiehn & Eken, 1997).
Plateau potentials are facilitated by the tonic activity of descending serotonergic and noradrenergic neurons (Crone et al. 1988). This relationship has been observed in reduced animal preparations (Crone et al. 1988; Hounsgaard et al. 1988), but has yet to be demonstrated in humans. One possible way to increase excitatory neurotransmitter release in humans is through the use of the ubiquitous plant alkaloid caffeine.
Caffeine, as an adenosine receptor antagonist, has diverse actions on the central nervous system (reviewed by Nehlig et al. 1992; Fredholm, 1995). At physiological dosages, caffeine increases neuronal activity by increasing excitatory neurotransmitter release and lowering the threshold for neuronal activation (Phillis et al. 1979) and causes an increase in spontaneous electrical activity in noradrenergic neurons (Grant & Redmond, 1982). In addition, caffeine increases serotonin concentration in the serotonergic neurons of the raphe nuclei (Berkowitz & Spector, 1971), which have excitatory projections to spinal motor neurons (Barasi & Roberts, 1974). Although caffeine has an effect on many neurotransmitters (reviewed by Nehlig et al. 1992), it is of particular interest that caffeine acts on serotonergic and noradrenergic neurons because these neurotransmitters have been shown to facilitate the activation of plateau potentials. The purpose of the present study was to attempt to alter the relative frequency of occurrence of SSF through the administration of caffeine. We hypothesized that caffeine would be associated with an increased occurrence of SSF in human motor units.
Methods
Subjects
Experiments were carried out on seven healthy males (26.5 ± 2.0 years, 178.9 ± 3.4 cm, 74.7 ± 4.3 kg, mean ± s.e.) who reported minimal caffeine consumption (< 200 mg week−1). Males were selected since the use of oral contraceptives increases the plasma half-life of caffeine by decreasing the activity of hepatic oxygenase, the enzyme which catalyses the breakdown of caffeine into paraxanthine (Abernethy & Todd, 1985). In addition the elimination of caffeine may be slowed in the luteal phase in the normal menstrual cycle (Lane et al. 1992). Subjects were non-smokers and of average body mass since both smoking and obesity increase the rate of caffeine degradation (Parsons & Neims, 1978; Kamimori et al. 1987). We selected only those subjects that reported using very little caffeine because habitual caffeine consumption results in the upregulation of adenosine receptors which diminishes the acute effects of caffeine (Bangsbo et al. 1992). In addition, subjects completed a questionnaire in which level of physical activity was assessed over the past year since intense physical activity increases the rate of clearance of caffeine (Graham et al. 1994). We only accepted subjects who participated in recreational activity no more than three times per week.
Applicants who met the above criteria attended an orientation session where all aspects of the protocol were explained and demonstrated. Each was asked to abstain from consuming caffeine-containing foods and beverages and medication of any sort for 1 week prior to and throughout the duration of the study, and to refrain from alcohol and physical activity the day before data collection. Experiments were conducted at the same time, both mornings, and subjects were asked to consume the same light breakfast and maintain the same sleep patterns before the experimental sessions. Each subject read and signed the informed consent document outlining the procedures of the experiment and possible side effects of the protocol, particularly those associated with acute caffeine consumption. Subjects were paid $10.00 per hour for their participation. This study was performed according to the Declaration of Helsinki and was approved by the York University Human Participants Review Committee guidelines.
Apparatus
Subjects were seated in a hydraulic chair, adjusted to position the hip angle at 90 deg. The ankle was strapped in a custom-made apparatus, which fixed the ankle joint at 5-10 deg plantarflexion. A padded clamp secured the knee at 90 deg and velcro straps secured the foot to a thin aluminium plate to which a strain gauge was attached.
Drug administration
Caffeine (U.S.P./F.C.C. grade caffeine, A&C American Chemicals Ltd, Montreal, Quebec) was administered in gelatin capsules (6 mg kg−1) immediately following the pre-test protocol. The average dose administered to subjects was 448 mg, comparable to four cups of strong coffee. Plasma caffeine was not measured directly in the present study, but was estimated based on previously published data, following the precedent of Jackman and colleagues (1996). Plasma caffeine concentrations are higher (30.4 μm) than those found in the cerebral spinal fluid (14.9 μm) following a 300 mg oral dose of caffeine (Soto et al. 1994). Placebo capsules were filled with all-purpose flour.
Protocol
A pre-test/post-test repeated-measures design was used for this study. Each subject came to the laboratory on two separate days which were at least 1 week apart. Each day began with a pre-test protocol consisting of three maximal isometric voluntary contractions (MVCs) followed by an investigation of SSF. Capsule administration (caffeine or placebo) took place immediately after the pre-test protocol. The post-test protocol followed 1 h after capsule ingestion to allow sufficient time to reach peak plasma caffeine concentrations (Bonati et al. 1982; Graham & Spriet, 1995). Since a previous study in our laboratory (Kalmar & Cafarelli, 1999) found no difference between placebo and control, a separate control trial was not used. A double-blind procedure was followed with a counterbalanced order of capsule administration.
Part A: maximal voluntary contractions and EMG
Three MVCs were performed in the ankle dorsiflexors, with 1 min rest intervals between each. Contractions were held until a plateau force was reached. Individual MVC values were averaged and used to provide an estimate of the degree of torque in the SSF trials.
Surface EMG was measured using bipolar silver-silver chloride electrodes (EQ Inc., Chalfont, PA, USA), with an inter-electrode distance of 2 cm, positioned in the direction of the muscle fibres over the belly of the tibialis anterior and its antagonist, the soleus. The skin underlying the electrodes was shaved, exfoliated and swabbed with a 70 % ethanol solution. The EMG signals were amplified at × 35 at the electrode and passed through a second-stage, variable-gain amplifier (Model D423A, York University Electronics Shop) before being stored on cassette tape.
Part B: self-sustained firing
We studied the occurrence of SSF in tibialis anterior muscle motor units. Although the soleus has been used previously for this purpose (Kiehn & Eken, 1997), that muscle has an average maximal firing frequency of 10.7 ± 2.9 Hz (mean ± s.d.) (Bellemare et al. 1983). In comparison, the mean firing frequency of the tibialis anterior is 28.2 ± 0.6 Hz (Macefield et al. 1993). Therefore, we chose to use the tibialis anterior since its larger range of firing frequencies would more readily reflect the changes in synaptic input necessary for the paired motor unit recording technique (see below), used to investigate SSF in human motor units.
Motor unit recordings were obtained using tungsten intramuscular electrodes (6-8 MΩ, FHC, Bowdoinham, ME, USA; Howarth Instruments, Cornwall, UK). Each electrode has approximately 10 μm of insulation removed from the tip, exposing the bare tungsten wire as a recording surface. Initially, a 25 gauge hypodermic needle was used to puncture the skin and fascia. The recording electrode was then inserted perpendicular to the muscle and was manually advanced after every trial. The reference, a second tungsten electrode, was inserted subcutaneously parallel to the long axis of the tibia over the patellar tendon. Both needle electrodes were removed prior to capsule ingestion, and reinserted prior to the post-test protocol. All microelectrodes were sterilized in an autoclave at 200 °C for 20 min before each experiment and were only used for a single subject.
Subjects were instructed to dorsiflex the ankle and maintain a low level isometric contraction (< 15 % MVC). This was necessary to avoid fatigue and to ensure clear recordings of individual motor units. Visual feedback of force output was provided on a computer monitor in front of the subject. Auditory feedback indicated to the experimenter when the control unit was firing steadily and hence, when the vibration could be applied.
To measure SSF, we used the paired-motor unit recording technique employed by both Kiehn & Eken (1997) and Gorassini and colleagues (1998, 2002a,b), whereby one low-threshold motor unit (the control unit) serves as a monitor of synaptic input into the motor neuron pool. Subjects were asked to dorsiflex the ankle until a single motor unit was recruited to serve as the control unit. No attempt was made to adjust the force of contraction to just below the recruitment threshold of the test unit. When the control unit was firing steadily, the vibrator (modified Dremel engraving tool, 175 Hz, 3 mm deflections) was placed on the distal tendon of the tibialis anterior for 1-3 s. Since the vibrator is only on for a short time, this method is preferred to strapping the vibrator on the subject, which can spread the vibration to other surrounding muscles (Bongiovanni & Hagbarth, 1990). An accelerometer was taped to the skin to record the time the vibrator was applied. Vibrating this tendon frequently recruited a second unit, the test unit. If the control unit exhibited a post-vibration firing frequency equal to or lower than its pre-vibration frequency, our interpretation was that synaptic input to the motor neuron pool had not increased. If, under these circumstances, the test unit fired for longer than 1 s after the vibrator was removed, the test unit was considered to exhibit SSF.
Since both force and EMG increase in response to the recruited SSF motor units (Kiehn & Eken, 1997; Gorassini et al. 1998), the firing rate of the control unit is the only reliable measure of descending drive. Before a trial was accepted as displaying SSF, the control unit had to demonstrate its sensitivity to changes in descending drive. To quantify this, an isometric ramp contraction was performed after every trial to ensure that the control unit was not firing at maximum frequency, and that the firing rate increased and decreased when force was varied above and below the level maintained in the SSF trial. Both conditions had to be satisfied before the trial was counted. Each isometric ramp contraction started from rest and gradually increased over 10 s to approximately 5 % MVC above the peak force from the previous run.
We calculated the relative frequency of occurrence of SSF as: (number of trials displaying SSF/total number of trials) × 100. A trial was counted each time a motor unit was recruited during vibration (test unit) and descending drive did not increase when vibration was removed. As many as four attempts to elicit SSF were performed on any single test unit, but each test unit was counted only once. For example, if the test unit displayed SSF in three of the four attempts, one would be added to the numerator, and one would be added to the denominator. After these four attempts, the recording microelectrode was advanced approximately 1-2 mm and the procedure repeated. No more than 12 motor units were recorded from an individual subject at one sitting. The relative occurrence of SSF was calculated and pre-test and post-test value for both caffeine and placebo conditions were compared.
Data processing
All signals were passed through preamplifiers and amplifiers and stored on videocassette (VCR model 500D, PCM model 4000A, Vetter, Rebersberg, PA, USA) for off-line analysis. Prior to analysis, signals were A/D converted and digitally filtered and analysed using Spike 2 for Windows (version 3.0, CED Ltd, Cambridge, UK).
Surface EMG
The surface EMG was digitized at a sampling rate of 1000 Hz, and high-pass filtered at 17 Hz. The root mean square amplitude of a 500 ms sample of maximal EMG and a 3 s sample during SSF was used to quantify the filtered surface signal.
Torque
Force records were digitized at a rate of 500 Hz and low-pass filtered at 50 Hz. Torque was expressed in newton metres (N m) or as a percentage of the maximum voluntary contraction (MVC).
Intramuscular EMG
The signal from the intramuscular electrode was amplified and bandpass filtered between 1.0 and 30 kHz (Neurolog, Digitimer, Medical Systems Corp, Greenvale, NY, USA) and then digitized at a sampling rate of 10 kHz. The action potential spikes were categorized first using the Spike 2 program for spike discrimination, then sorted manually based on differences in amplitude, shape and interspike interval. Once categorized, the mean firing frequency, mean interspike interval (ISI) and the ISI coefficient of variation were determined for both the control and test units.
The guidelines for analysing motor units were as follows. Only spike trains consisting of at least five spikes or more with an ISI coefficient of variation less than 20 % were used to calculate motor unit firing rates. In addition, average motor unit firing rates (1 s bins) of both control and test units were analysed during the same time frame, containing the greatest number of spikes and avoiding any gaps in the firing rates, and only included samples longer than 1 s in duration. Therefore, a test unit was only classified as SSF if the prolonged firing continued longer than 1 s after the vibration was removed. Recordings that recruited a test unit during vibration, but showed an increase in the firing frequency of the control unit after vibration, even by as little as 0.05 Hz, were discarded. These criteria ensured that only those recordings that displayed absolutely no increase in synaptic input were included in the analysis.
Statistical analysis
Statistics were performed using Statistica (release 5.1, Statsoft Inc., Tulsa, OK, USA). A 2-way (caffeine/placebo × pre-test/post-test) repeated-measures analysis of variance (ANOVA) was performed on the data for the relative occurrence of SSF and the Tukey post hoc test was used for individual comparisons when there were significant main effects. Data from SSF were then expressed as percentage change (pre- to post-test) for the placebo and caffeine conditions. The conditions were then compared using a dependent (within groups) paired t test, and P < 0.05 was deemed significant. Unless otherwise stated, data are presented as means ± s.e.m.
Results
The occurrence of SSF
We recorded 214 trials of which 139 displayed SSF according to our criteria. Sixteen of the 139 trials recruited two test units into SSF, but this was not condition specific. Therefore, we recorded from a total of 222 test units, and 214 control units. To compare our results with those in the literature, data from the pre-test trials of both treatments were combined. This gave a total of 103 trials, 57 of which showed evidence of SSF, with a relative frequency of occurrence of 55 %.
The mean firing frequencies of the control units were 8.72 ± 0.3 Hz (caffeine pre-test), 8.80 ± 0.16 Hz (caffeine post-test), 9.35 ± 0.17 Hz (placebo pre-test) and 9.08 ± 0.19 Hz (placebo post-test). There were no significant differences in control unit firing frequency across the four conditions. Averaged across all trials and subjects, the mean firing frequency of the control units was 9.0 ± 0.2 Hz, while the average of the test units was 7.9 ± 0.3 Hz. Figure 1 is an example of SSF from one of the trials with the corresponding isometric ramp contraction and mean firing frequency for the control unit shown in Fig. 2. All of the isometric ramp contractions performed at the end of each trial indicated that the control unit firing frequencies were sensitive to changes in descending drive. The average MVC pooled across all trials was 26.78 ± 1.49 N m and the relative torque maintained during the SSF trials had a range of 1-19 % MVC, but the average was 3.38 ± 0.23 % MVC.
Figure 1. Example of self-sustained firing.
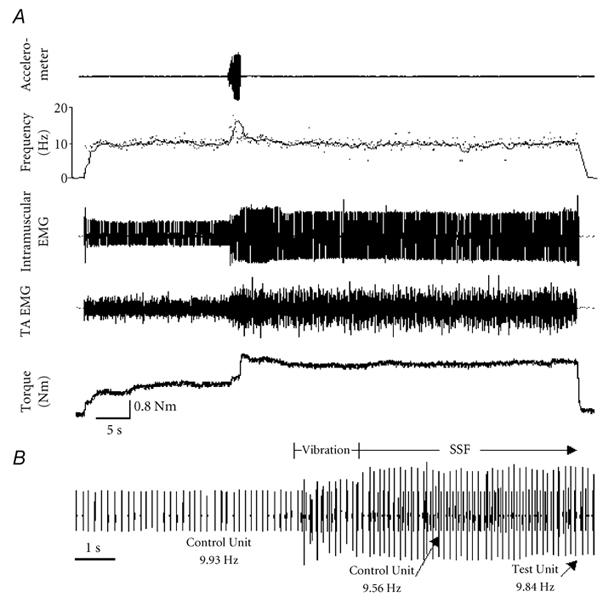
A, representative recording from one subject. The accelerometer channel indicates the application of the vibrator to the tendon. The frequency channel shows the instantaneous (dots) and average (line) firing rates of the control unit. The intramuscular channel reveals the recruitment of a motor unit (test unit) during vibration followed by prolonged firing. This recruitment coincides with an increase in the tibialis anterior EMG (TA EMG) and torque, since additional motor units are likely to be recruited elsewhere in the muscle. B, 13 s of recording on an expanded time base illustrating the period before and after vibration. The control unit fired steadily at 9.93 Hz before vibration and 9.56 Hz after vibration, indicating that descending drive to the motor neuron pool did not increase. The test unit (larger unit) recruited during vibration maintained a steady firing frequency of 9.84 Hz for 34 s after vibration was removed, and only terminated when the subject was instructed to relax. The amplitude of the test unit was attenuated during the vibration due to the depression of the skin for the 1.5 s the vibrator was applied (verified by a sham trial).
Figure 2. Isometric ramp contraction.
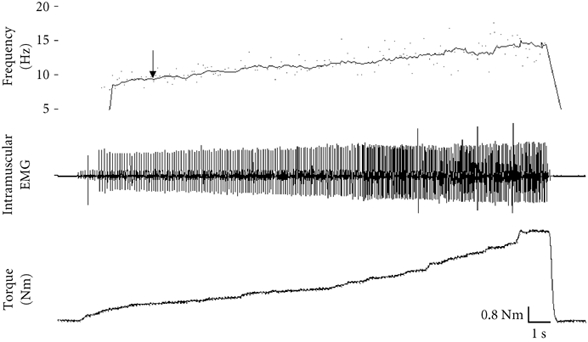
This was performed immediately after each SSF trial to test the sensitivity of the control unit. As torque increased, the firing frequency of the control unit also increased, indicating the sensitivity of the control unit to increased descending drive. The arrow denotes the firing frequency of the control unit (9.93 Hz) during the SSF trial. Since the control unit's firing frequency increases well beyond this point with further voluntary effort, we conclude that the control unit was not firing at a saturated frequency during the SSF trial.
Effect of caffeine on the occurrence of SSF
The results from the 2-way ANOVA (drug × pre-test/post-test) revealed that SSF occurred much more frequently (P < 0.05) in the caffeine post-test trials (87.0 ± 5.8 %), with 40 of the 46 trials displaying prolonged firing of the test unit after vibration (Fig. 3A). This was significantly higher than the other three trials (caffeine pre-test (20/43), 46.5 ± 9.8 %; placebo pre-test (37/60), 61.7 ± 10 %; placebo post-test (42/65), 64.6 ± 9.1 %). The caffeine pre-test value was lower than the placebo post-test value (P < 0.05), but there was no difference between the placebo and caffeine pre-test values.
Figure 3.
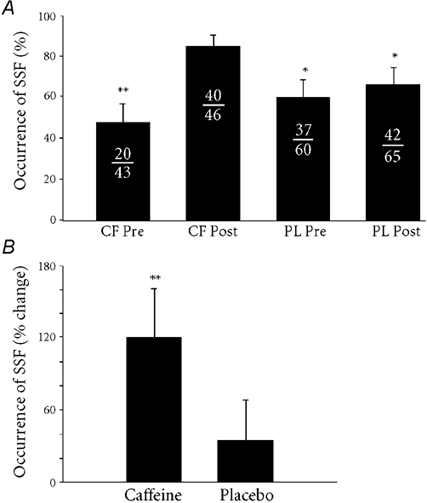
A, the occurrence of self-sustained firing (SSF) across all trials. The frequency of occurrence of SSF in the caffeine trial is significantly different from all other trials, but there were no differences between the two pre-test trials, or the placebo trials. Each fraction denotes the number of trials displaying SSF over the total number of trials recruited during vibration. Caffeine pre-test (CF Pre), 46.5 ± 9.8 %; caffeine post-test (CF Post), 87.0 ± 5.8 %; placebo pre-test (PL Pre), 61.7 ± 10.0 %; placebo post-test (PL Post), 64.6 ± 9.1 %. B, the effect of caffeine on the occurrence of self-sustained firing expressed as a percentage change from the pre-test for each condition. Caffeine caused a significant increase in the occurrence of SSF compared to placebo (*P < 0.05, **P < 0.01).
When expressed as a percentage change (pre-test to post-test) in each condition (Fig. 3B), caffeine increased the relative occurrence of SSF by 120 ± 41 %, which was significantly greater (P < 0.01) than that in the placebo condition (35 ± 33 %). From questionnaires that were filled out after each session, only two of the seven subjects correctly identified which capsule they ingested.
Discussion
The occurrence of SSF
The occurrence rates of SSF reported in previous investigations vary considerably from our own findings. Gorassini and colleagues (1998) observed SSF in 24 of 46 trials (52 %) and report that 13 of the 14 motor units (93 %) recorded in their study exhibited SSF. Kiehn & Eken (1997) observed SSF in 31 of 42 motor units (74 %). We observed SSF in 57 of 103 motor units (55 %) recorded in our pre-test (no treatment) trials.
There are a number of reasons why we observed a lower incidence of SSF than previously published values. The predominant explanation is that we did not select a background level of contraction that was just subthreshold to the test motor unit. Rather, subjects were asked to hold the contraction at a level that elicited a steady and consistent firing rate of the control unit. Setting the background synaptic input to just below that required for recruitment of the test unit increases the likelihood that a plateau potential will shift the test unit into its firing range and that SSF will be observed. Kiehn & Eken (1997) used this method of setting the background contraction level which may account for the higher incidence of SSF. In addition, Kiehn & Eken (1997) did not verify the sensitivity of the control unit. If the control unit was firing at a maximal frequency, it would not be a sensitive indicator of increased synaptic input. Thus some units may have been recruited by increased synaptic input rather than by a plateau potential, and SSF may have been overestimated.
Subjects in the study by Gorassini et al. (1998) increased the force of the contraction until a single motor unit was recruited and firing at a steady rate. The authors do not state that the contraction level is held just below the threshold of the test unit. Although they observed SSF in 13 of the 14 units tested, they observed SSF in only 52 % of their trials. This suggests that in that study (Gorassini et al. 1998), as well as our own, the test unit may have been too far below threshold for a plateau potential to elicit SSF in some cases.
Our data were obtained at very low force levels at which only a few motor units are recruited. In addition, motor units of a rate coding muscle such as the tibialis anterior (Clamann, 1993) are more sensitive to changes in descending drive. Plateau potentials are triggered primarily in low-threshold type I motor neurons with slow axonal conduction velocities (Lee & Heckman, 1998). According to the ‘Size Principle’ (Henneman, 1957), these units are recruited first, followed by the higher threshold more fatigable motor units. Plateau potentials triggered at these higher force levels would increase the gain of the response to excitatory input, thereby decreasing the drive necessary to maximally activate the motor units.
We cannot dismiss the possibility that a motor unit was counted more than once, but the following procedures minimized the likelihood of this occurring: (1) the needle was advanced slightly into the muscle after each trial; (2) spike shape and amplitude were compared for similarities between trials for each subject. Moreover, with 500 motor units in the tibialis anterior (Stein et al. 1992) and no more than 12 motor units recorded from an individual subject in each experiment, it is extremely unlikely the recordings were made from the same unit more than once.
Effect of caffeine on the occurrence of SSF
The occurrence of SSF was significantly greater in the caffeine trial compared to the other three trials (P < 0.05). While there was a significant difference between the caffeine pre-test and placebo post-test values, these data were collected on different days. More importantly, because the incidence of SSF was higher in the caffeine post-test condition than in any of the three ‘no caffeine’ conditions, and the percentage change in SSF was higher in the caffeine trial than in the placebo trial, we are confident that caffeine had an effect on the incidence of SSF.
Plateau potentials bring the membrane closer to threshold, increasing the likelihood of spike initiation and SSF. These plateau potentials are facilitated by serotonin and noradrenaline (Conway et al. 1988; Hounsgaard et al. 1988; Hounsgaard & Kiehn, 1989). This conclusion is based on the presence of bistable behaviour in the decerebrate cat (Hultborn et al. 1975) with the inability to evoke plateau potentials after an acute spinal transection and their reappearance after intravenous injection of the serotonin precursor, 5-HT or noradrenaline (Conway et al. 1988).
Caffeine has many effects on the central nervous system (see reviews Nehlig et al. 1992; Fredholm, 1995). Caffeine increases plasma noradrenaline (Vestal et al. 1983; Graham & Spriet, 1995), spontaneous electrical activity of noradrenergic neurons (Vestal et al. 1983), and serotonin concentration in the brainstem, the cerebral cortex and cerebellum (Berkowitz & Spector, 1971; Nehlig et al. 1992). Since the brainstem has dense serotonergic projections to the spinal motor neurons (Barasi & Roberts, 1974) we presume these levels are increased in the spinal column as well. Lee & Heckman (2000) established a dose-response relationship between monoaminergic input and persistent inward currents. Caffeine could therefore increase the occurrence of SSF by increasing the amplitude and frequency of the plateau potentials, or increasing excitatory synaptic input to the cell. In addition to neurotransmitter effects, caffeine stimulates Na+-K+ pump activity (Clausen, 1986; Lindinger et al. 1993). This causes an elevated intracellular [K+] similar to the blocking of K+ outward currents which are known to trigger plateau potentials (Lindinger et al. 1993). These issues are discussed further in recent reviews on motor neurons by Binder and colleagues (1996) and Rekling and colleagues (2000). To our knowledge, this is the first experiment that demonstrates the ability to manipulate the occurrence of SSF in human muscle. However, because we did not measure any of these parameters, it is not possible to isolate the mechanism of action of caffeine in this study.
Functional significance of self-sustained firing
Plateau potentials can raise the resting membrane potential by 5-15 mV, thereby functioning as a tool to modulate the excitability of the motor neuron (Conway et al. 1988; Eken et al. 1989). Thus, by amplifying the response to synaptic input, the motor neuron may reduce the need for constant synaptic drive for maintained muscle contractions, modifying the behaviour of the motor neuron, both in intensity and duration. The effects of central fatigue normally seen in low intensity, long duration activities (Davis & Bailey, 1997) could, in theory, be offset by SSF. The high serotonin concentrations that accompany central fatigue (Bailey et al. 1993) may trigger an increase in plateau potentials, and hence SSF, sparing excitatory drive to motor neuron pools and delaying the decline in neuromuscular drive. In addition, the extracellular accumulation of K+ that accompanies fatiguing contractions (Lindinger et al. 1993) would decrease the K+ gradient and limit the K+ outward current; this could also trigger plateau potentials. SSF could therefore participate in sustained low-level contractions that characterize endurance activities and assist in postural maintenance (Eken et al. 1989; Kiehn, 1991; Kiehn & Eken, 1997). The fact that motor nuclei innervating postural muscles with pronounced tonic activity have a very dense serotonergic innervation supports this hypothesis (Kojima & Sano, 1983). This was demonstrated in vivo by Kiehn and colleagues (1996) using neurotoxic destruction of monoaminergic input to rat spinal cords. Tonic electromyographic activity in motor behaviour was eliminated, forcing the muscle to rely on phasic bursts of activity. Self-sustained firing could help maintain tonic activity in a muscle with phasic synaptic excitation to the motor neuron pool (Conway et al. 1988; Eken et al. 1989; Kiehn, 1991; Kiehn & Eken, 1997). Since caffeine is associated with an increased occurrence of SSF, caffeine may act to increase the sensitivity of this system.
Involuntary repetitive contractions of single motor units known as fasciculations, which often accompany muscle cramps, have been compared to SSF (Baldissera et al. 1994). Both display the sudden onset triggered by a brief motor excitation through the Ia afferent fibres and are terminated by stretch, massage or electrical stimulation of the skin (Baldissera et al. 1991, 1994). It has been suggested that plateau potentials, activated in part by blocking K+ outward currents, alter K+ kinetics by increasing the extracellular [K+] around neighbouring motor neurons. This decreases the concentration gradient, slowing the transport of K+ out of the cell, thereby increasing intracellular [K+], which would ultimately result in further activation of plateau potentials (Baldissera et al. 1994). The result is the slow increase in motor unit depolarization known as the ‘staircase’ phenomenon (Crone et al. 1988), which initiates a positive feedback cycle leading to a stronger and stronger contraction (Baldissera et al. 1994). By increasing the occurrence of SSF, caffeine might contribute to cramping in individuals who suffer from myokymia. However, we did not see any evidence of cramping in the present experiment.
In summary, we were able to isolate SSF from the human tibialis anterior muscle of all seven male subjects. Caffeine ingestion was associated with a significant increase in the relative frequency of occurrence of SSF, possibly due to the alkaloid's effect on the serotonergic and noradrenergic systems. This study is the first to demonstrate the ability to manipulate the occurrence of SSF in humans. With caution, these data may be interpreted as indirect evidence of the facilitation of plateau potentials by neurotransmitters such as serotonin and noradrenaline previously observed using pharmacological manipulation in animal studies.
References
- Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose estrogen-containing oral contraceptives. European Journal of Clinical Pharmacology. 1985;28:425–428. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN. Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. Journal of Applied Physiology. 1993;74:3006–3012. doi: 10.1152/jappl.1993.74.6.3006. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Dworzak F. Cramps: a sign of motoneurone ‘bistability’ in a human patient. Neuroscience Letters. 1991;133:303–306. doi: 10.1016/0304-3940(91)90594-j. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Dworzak F. Motor neuron ‘bistability’. Brain. 1994;117:929–939. doi: 10.1093/brain/117.5.929. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Jacobsen K, Nordberg N, Christensen NJ, Graham T. Acute and habitual caffeine ingestion and metabolic responses to steady-state exercise. Journal of Applied Physiology. 1992;72:1297–1303. doi: 10.1152/jappl.1992.72.4.1297. [DOI] [PubMed] [Google Scholar]
- Barasi S, Roberts M. The modification of lumbar motoneurone excitability by stimulation of a putative 5-hydroxytryptamine pathway. British Journal of Pharmacology. 1974;52:339–348. doi: 10.1111/j.1476-5381.1974.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor unit discharge rates in maximal voluntary contractions of three human muscles. Journal of Neurophysiology. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Spector S. The effect of caffeine and theophylline on the disposition of brain serotonin in the rat. European Journal of Pharmacology. 1971;16:322–325. doi: 10.1016/0014-2999(71)90034-3. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda: American Physiological Society; 1996. pp. 3–53. chap. 2. [Google Scholar]
- Bonati M, Latini R, Galletti F, Young JF, Tognoni G, Garattini S. Caffeine disposition after oral doses. Clinical Pharmacology and Therapeutics. 1982;32:98–106. doi: 10.1038/clpt.1982.132. [DOI] [PubMed] [Google Scholar]
- Bongiovanni LG, Hagbarth KE. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. Journal of Physiology. 1990;423:1–14. doi: 10.1113/jphysiol.1990.sp018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamann HP. Motor unit recruitment and the gradation of muscle force. Physical Therapy. 1993;73:830–842. doi: 10.1093/ptj/73.12.830. [DOI] [PubMed] [Google Scholar]
- Clausen T. Regulation of active Na+/K+ transport in skeletal muscle. Physiological Reviews. 1986;66:542–580. doi: 10.1152/physrev.1986.66.3.542. [DOI] [PubMed] [Google Scholar]
- Conway B, Hultborn H, Kiehn O, Mintz I. Plateau potentials in α-motoneurones induced by intravenous injection of l-DOPA and clonidine in the spinal cat. Journal of Physiology. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigström H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. Journal of Physiology. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Medicine and Science in Sport and Exercise. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in α-motoneurones. Brain Research. 1989;80:257–266. doi: 10.1016/s0079-6123(08)62219-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, adenosine receptors and the actions of caffeine. Pharmacology and Toxicology. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett D, Yang J. Self-sustained firing of human motor units. Neuroscience Letters. 1998;247:13–16. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: Possible contribution to motor unit excitation. Journal of Neurophysiology. 2002a;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. Journal of Neurophysiology. 2002b;87:1859–1866. doi: 10.1152/jn.00025.2001. [DOI] [PubMed] [Google Scholar]
- Graham TE, Rush JWE, VanSoeren MH. Caffeine and exercise: Metabolism and performance. Canadian Journal of Applied Physiology. 1994;2:111–138. doi: 10.1139/h94-010. [DOI] [PubMed] [Google Scholar]
- Graham TE, Spriet LL. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. Journal of Applied Physiology. 1995;79:867–874. doi: 10.1152/jappl.1995.78.3.867. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Redmond DE. Methylxanthine activation of noradrenergic unit activity and reversal by clonidine. European Journal of Pharmacology. 1982;85:105–109. doi: 10.1016/0014-2999(82)90430-7. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neuron and their susceptibility to discharge. Science. 1957;126:1345–1346. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of α-motoneurones. Experimental Brain Research. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. Journal of Physiology. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. Journal of Physiology. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Wigström H, Wängberg B. Prolonged activation of soleus motoneurones following a conditioning train in soleus Ia afferents - a case for a reverberating loop. Neuroscience Letters. 1975;1:147–152. doi: 10.1016/0304-3940(75)90030-0. [DOI] [PubMed] [Google Scholar]
- Kalmar JM, Cafarelli E. Effects of caffeine on neuromuscular function. Journal of Applied Physiology. 1999;87:801–808. doi: 10.1152/jappl.1999.87.2.801. [DOI] [PubMed] [Google Scholar]
- Kamimori GH, Somani SM, Knowlton RG, Perkins RM. The effects of obesity and exercise on the pharmacokinetics of caffeine in lean and obese volunteers. European Journal of Clinical Pharmacology. 1987;31:595–600. doi: 10.1007/BF00606637. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Plateau potentials and active integration in the ‘final common pathway’ for motor behaviour. Trends in Neurosiences. 1991;14:68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons. Journal of Neurophysiology. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kojima M, Sano Y. The organization of serotonin fibers in the anterior column of the mammalian spinal cord. Anatomy and Embryology. 1983;167:1–11. doi: 10.1007/BF00304597. [DOI] [PubMed] [Google Scholar]
- Lane JD, Steege JF, Rupp SL, Kuhn CM. Menstrual cycle effects on caffeine elimination in the human female. European Journal of Clinical Pharmacology. 1992;43:543–546. doi: 10.1007/BF02285099. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: Systematic variations in persistent inward currents. Journal of Neurophysiology. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. Journal of Neuroscience. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger MI, Graham TE, Spriet LL. Caffeine attenuates the exercise-induced increase in plasma [K+] in humans. Journal of Applied Physiology. 1993;74:1149–1155. doi: 10.1152/jappl.1993.74.3.1149. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. Journal of Physiology. 1993;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Daval J, Debry G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Parsons WD, Neims AH. Effect of smoking on caffeine clearance. Clinical Pharmacology and Therapeutics. 1978;24:40–45. doi: 10.1002/cpt197824140. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Edstrom JP, Kostopoulos GK, Kirkpatrick JR. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Canadian Journal of Physiology and Pharmacology. 1979;57:1289–1312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiological Reviews. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. Journal of Neurophysiology. 1980a;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. Journal of Neurophysiology. 1980b;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Soto J, Sacristan JA, Alsar MJ. Cerebrospinal fluid concentrations of caffeine following oral drug administration: correlation with salivary and plasma concentrations. Therapeutic Drug Monitoring. 1994;16:108–110. doi: 10.1097/00007691-199402000-00017. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yong JF, Totosy de Zepetnek J, Belanger M. Optimal stimulation of paralyzed muscle after human spinal cord injury. Journal of Applied Physiology. 1992;72:1393–1400. doi: 10.1152/jappl.1992.72.4.1393. [DOI] [PubMed] [Google Scholar]
- Vestal RE, Eiriksson CE, Musser B, Ozaki LK, Halter JB. Effect of intravenous aminophylline plasma levels of catecholamines and related cardiovascular metabolic responses in man. Circulation. 1983;67:162–171. doi: 10.1161/01.cir.67.1.162. [DOI] [PubMed] [Google Scholar]


