Abstract
This study examined the strength of motor unit synchronisation based on time- and frequency-domain measures during postural, shortening and lengthening contractions of a hand muscle in young adults. Single motor unit activity was recorded with intramuscular electrodes in the left first dorsal interosseus muscle as the subject held the index finger at a constant position while supporting a light load for 2-5 min. The subject then performed slow (1.7 deg s−1) shortening and lengthening contractions to lift and lower the load. The movement required subjects to perform 10-25 constant-velocity contractions with the index finger over a 10 deg range of motion by using 6 s shortening and lengthening contractions. Individual discharge times were obtained from 23 pairs of motor units in 14 subjects to assess the strength of motor unit synchronisation and coherence during the three tasks. The strength of motor unit synchronisation was approximately 50 % greater during the lengthening contractions compared with the postural and shortening contractions, and the width of the central synchronous peak in the cross-correlation histogram was ≈4 ms narrower during shortening contractions. These findings reveal that there is an increase in common input to motoneurones during lengthening contractions and a greater relative contribution of direct common inputs to motoneurones during shortening contractions compared with postural tasks. Furthermore, the amount of motor unit coherence in the low-frequency band (2-12 Hz) was reduced during shortening contractions compared with postural and lengthening contractions. These data indicate that the timing of inputs received by the motoneurones innervating the first dorsal interosseus of young adults differs during postural, shortening and lengthening contractions against a light load.
The correlated discharge of action potentials by motoneurones is caused by common synaptic input that is delivered either by branched presynaptic neurones or by rhythmic drive from supraspinal sources (Sears & Stagg, 1976; Kirkwood & Sears, 1978; Farmer et al. 1993a; Türker & Powers, 2002). The effect of the common input on motor unit activity is quantified as motor unit synchronisation. The two sources of common input can be distinguished by time- and frequency-domain analyses of the discharge times of pairs of motor units. Although technically challenging, the measurement of motor unit synchronisation provides novel information about connections in the human central nervous system (CNS) during voluntary muscle contractions.
Variation in the amount of synchronisation is often interpreted as indicating changes in the CNS strategy used to perform a task, as described in discussions on the adaptability of the neuromuscular system (Semmler & Enoka, 2000; Semmler, 2002). For example, motor unit discharge is more synchronised in the hand muscles of weight-lifters compared with control subjects (Milner-Brown et al. 1975; Semmler & Nordstrom, 1998), and is less synchronised in the hand muscles of musicians (Semmler & Nordstrom, 1998). Such findings are assumed to reflect changes in the corticospinal expression of muscle strength (Milner-Brown et al. 1975; Semmler & Enoka, 2000). Similarly, motor unit synchronisation increases during the performance of attention-demanding tasks (Schmied et al. 2000), as does the level of synchronous activity in the motor cortex (Murthy & Fetz, 1996a,b).
Due to technical limitations, most assessments of motor unit synchronisation have been performed during low-force isometric contractions. Some investigators, however, have been able to measure motor unit synchronisation during movement. For example, Hansen et al. (2001) used a cross-correlation technique based on multiunit EMG recordings to show that motor unit synchronisation is present in the leg muscles of humans during locomotion. Furthermore, Kakuda et al. (1999) found common modulation at 6-12 Hz in the discharge of pairs of motor units in extensor carpi radialis during slow shortening contractions, but not during the maintenance of position. The purpose of our study was to quantify the strength and coherence of motor unit synchronisation during postural, shortening and lengthening contractions of a hand muscle. Some of these results have been published previously in abstract form (Semmler et al. 2000, 2002).
Methods
Seventeen healthy subjects (12 men and five women, aged 22-45 years) volunteered to participate in the study. All subjects were moderately active and 16/17 were right-handed, as identified by the Edinburgh Handedness Inventory (Oldfield, 1971). The Human Research Committee at the University of Colorado approved the experimental procedures and the experiments were performed in accordance with the Declaration of Helsinki. Written consent was obtained from each subject prior to beginning the experiment.
Each subject participated in one to three experimental sessions that were performed on separate days. Each experiment involved recording the discharge of one to four pairs of single motor units from the first dorsal interosseus muscle of the left hand. Single motor unit recordings were obtained from the same pairs of motor units as the subject performed a series of isometric (restrained and sustained posture) and anisometric (shortening and lengthening) contractions with the first dorsal interosseus muscle. The experiment was terminated if one of the motor units was lost during a trial. This occurred most often during the anisometric contractions, presumably because the continual shortening and lengthening of the first dorsal interosseus muscle changed the geometry between the muscle fibre and the recording electrode and, hence, altered the shape of the motor unit waveform. For this reason, approximately 60 experiments were performed to obtain motor unit discharge characteristics from 23 pairs of motor units during the anisometric contractions. This low yield underscores the difficulty in recording the discharge characteristics from pairs of motor units during contractions that involved movement of the index finger.
Experimental arrangement
The experiments were conducted with the subject seated in an adjustable chair facing a 14 inch computer monitor, which was positioned 1.5 m away, at the level of the subject's eyes. The monitor displayed either index finger force or position. The left arm was placed prone on a manipulandum and the elbow joint was flexed to ≈90 deg. The index finger was placed in a semi-circular polyethelene splint that was positioned on the lateral surface of the index finger to keep the interphalangeal joint extended. The third to fifth digits were flexed around a handle located on the manipulandum and the thumb was kept extended by a support. With this arrangement, abduction of the index finger at the metacarpophalangeal joint in the horizontal plane was produced almost exclusively by contraction of the first dorsal interosseus muscle.
The index finger was abducted 5 deg from the neutral position for the restrained-isometric contractions and a force transducer (Sensotec model 13, Columbus, OH, USA) was attached to the splint to measure force at the proximal interphalangeal joint. The force exerted during maximum voluntary contractions (MVCs) was measured with a low-sensitivity force transducer (0-220 N), whereas a more sensitive transducer (0-10 N) was used during the motor unit trials. A low-friction, linear variable displacement transducer (LVDT; Novotechnik, Stuttgart, Germany) was used to detect the adduction-abduction displacement of the index finger about the metacarpophalangeal joint during the anisometric contractions. One end of the LVDT was attached to the finger splint by low-compliance line that ran over a pulley. The load lifted by the subject was attached to the other end of the LVDT. This set-up allowed the index finger to move freely throughout its range of motion. The LVDT was calibrated over a 10 deg range of motion for each subject and each recording session.
The electromyogram (EMG) of the first dorsal interosseus muscle was recorded with bipolar surface electrodes (4 mm diameter; silver-silver chloride) that were placed 1-2 cm apart (centre-to-centre) on the skin overlying the muscle. A reference electrode was placed over a bony prominence on the dorsal aspect of the hand. The surface EMG signals were amplified (1000-2000 ×), bandpass filtered (20-800 Hz), displayed on an oscilloscope and stored on tape.
Two to three fine-wire electrodes were inserted percutaneously into the first dorsal interosseus muscle to obtain bipolar recordings of the discharge of single motor units. Each electrode consisted of three Formvar-insulated, stainless-steel wires (two 50 μm and one 25 μm diameter, or three 50 μm diameter; California Fine Wire, Grover Beach, CA, USA). Fabrication of the electrodes consisted of fixing the three wires together at the recording tip with medical-grade cyanoacrylate glue and tightly coiling this tip around a 0.13 mm diameter wire for ≈3 mm with a custom coiling apparatus (Laidlaw et al. 2000). The three wires were threaded through a disposable 27 gauge hypodermic needle and a hook of ≈2 mm in length was created at the tip of the recording end of the electrode. The electrode was impaled into the muscle to a depth of 1-2 cm and the needle withdrawn, leaving the wires within the belly of the muscle. Recordings were obtained from two wires within each electrode; the third wire was used as an alternative bipolar configuration to sample from other motor units within the same muscle. The location of the electrode could be altered by fine manual adjustments of the wires to optimize the detection of action potentials from a single motor unit. The distance between the two intramuscular electrodes was usually 1-2 cm. Motor units were detected on-line using an amplitude window discriminator (DIS-1; BAK Electronics, Inc., Rockville, MD, USA) to provide audio-feedback of motor unit discharge during the contractions. Single motor unit recordings were amplified (1000-2000 ×), bandpass filtered (20 Hz-8 kHz), displayed on an oscilloscope and stored on tape.
Experimental procedures
Each subject was asked to perform five tasks: (1) a contraction of the first dorsal interosseus muscle to determine the maximum load that could be lifted with the index finger once (1-RM load); (2) an isometric MVC to determine the strength of the first dorsal interosseus muscle; (3) a low-force isometric contraction of the first dorsal interosseus muscle against a force transducer (restrained condition); (4) a low-force isometric contraction of the first dorsal interosseous muscle while supporting a light inertial load and holding the index finger in a constant position (postural condition); and (5) anisometric (shortening and lengthening) contractions of the first dorsal interosseus muscle by lifting and lowering a light inertial load. The discharge of single motor units was recorded during tasks 3-5. The tasks were performed in a pseudo-random order. The number of tasks completed by each subject depended on the duration of the experiment, the order in which the tasks were performed and whether the same motor units could be detected in subsequent contractions.
1-RM load
Subjects were instructed to lift and lower an inertial load slowly with the index finger throughout a range of motion of ≈20 deg in a horizontal abduction-adduction plane. The load was increased after each repetition until the subject could no longer complete the task. The maximal load that could be lifted over the 20 deg range of motion was identified as the 1-RM load. Subjects were given a 60 s rest between each attempt. On average, four trials were required to determine the 1-RM load for each subject.
MVC force
The force exerted by the index finger during an MVC was measured in subjects who performed the restrained-isometric contractions. The task involved a gradual increase in the abduction force to its maximum value over 2-3 s, after which the maximal force was maintained for another 2-3 s. Subjects were aided in this task by visual display of the index finger force on the monitor and by a verbal count given at 1 s intervals throughout the contraction. Strong verbal encouragement was provided once a plateau in the force trajectory was achieved. After several practice trials, two to four MVC trials were recorded. The trial with the greatest MVC force was selected for analysis. Rest periods of ≈60 s were given between each MVC trial.
Restrained-isometric contractions
The discharge of pairs of single motor units was recorded while subjects exerted a steady abduction force with the index finger. Subjects were asked to slowly increase the index finger force until each electrode detected at least one motor unit that discharged action potentials repetitively. The force required to sustain the discharge was maintained for 2-5 min with the aid of a target line that was displayed on the computer monitor. The target force was occasionally adjusted during these trials so that at least one motor unit could be readily identified with each electrode. The subject was provided with audio feedback of the discharge from one motor unit. The mean force used to activate the motor units throughout the trial was noted and the strength of the contraction relative to maximum force (%MVC) was determined.
Postural contractions
A weight that matched the target force for the isometric task was attached to one end of the LVDT. The weight provided a load in the adduction direction that was opposed by abduction of the index finger with the first dorsal interosseus muscle. The angular position of the index finger was displayed on the monitor along with a target line corresponding to 5 deg of abduction from the neutral position. The task was to hold the index finger at 5 deg while supporting a load that required activation of the same motor units recorded in the restrained-isometric task. The contraction was sustained for 2-5 min.
Anisometric contractions
The same load used for the postural contraction was raised and lowered with anisometric contractions of the first dorsal interosseus muscle. The angular position of the index finger was displayed on the feedback monitor along with a triangular template representing the index finger displacement to be performed. The template represented a constant-velocity contraction of 1.7 deg s−1 in both the abduction and adduction directions. Each subject was instructed to match the template by moving the index finger through a 10 deg range of motion from the starting position (5 deg abduction). The subjects raised the load during 6 s of abduction (shortening contraction) and lowered the load during 6 s of adduction (lengthening contraction). Each subject performed 10-25 contractions, with a brief rest after 3-4 contractions.
Data analysis
All data collected during the experiments were stored on tape (Sony PC 116 DAT recorder; Sony Magnescale Inc., Montvale, NJ) and subsequently digitised (CED 1401, Cambridge Electronic Design, Cambridge, UK) to a computer and analysed off-line. The sampling rate was 200 Hz for force and position, 2 kHz for the surface EMG and 20 kHz for the single motor unit recordings.
Single motor unit discharges were discriminated using a computerized, spike-sorting algorithm (Spike2, version 3; Cambridge Electronic Design), which identified the action potentials belonging to a particular motor unit based on waveform shape. To ensure discrimination accuracy, the interspike intervals of identified motor units were examined for every trial. Trials that contained abnormally short and long interspike intervals due to discrimination error were re-analysed on a spike-by-spike basis. The mean, standard deviation and coefficient of variation of the discharge times were determined using custom-designed software written in Matlab (Mathworks Inc., Natick, MA, USA). For the anisometric contractions, the slope of a linear regression line was subtracted from the data and the standard deviation and coefficient of variation were calculated from the de-trended data.
Motor units detected with separate electrodes during the same trial were paired for cross-correlation analysis to assess the amount of motor unit synchronisation. The 201 bins in each cross-correlation histogram characterized the associated activity between the two motor units for 100 ms before and 100 ms after the discharge of the reference motor unit. The cumulative sum (CUSUM; Ellaway, 1978) technique was used to estimate the location and width of the central synchronous peak. Cross-correlation histograms with a mean bin count < 4 were not analysed. The magnitude of the central synchronous peak in the cross-correlogram was quantified using two commonly used indices: (1) the index CIS (Common Input Strength), which indicates the number of synchronous discharges in excess of chance divided by the duration of the trial when both motor units were tonically active. This index represents the frequency of extra synchronous discharges, and has been shown to be mathematically independent of discharge rate (Nordstrom et al. 1992); (2) the index E, which corresponds to the total number of extra counts within the peak relative to the number of discharges by the motor unit with the lowest mean discharge rate (Datta & Stephens, 1990). This index represents the probability of extra synchronous discharges for every discharge by the reference motor unit. The index E is independent of discharge rate in rat hypoglossal motoneurones when they are induced to discharge repetitively due to common input of 1-2 mV excitatory postsynaptic potentials (Türker & Powers, 2002).
The frequency-domain characteristics of common inputs to motoneurones were estimated from the coherence spectrum between the discharge times of pairs of motor units. The method used was similar to that developed by Rosenberg et al. (1989) and was implemented using Matlab. The discriminated motor unit data were divided into contiguous, non-overlapping epochs of 1.28 s that comprised 256 bins. Each 5 ms bin was assigned a value of 1 when it contained a discharge and a value of 0 when it did not. The time-series data from each disjoint section were transformed to the frequency domain, resulting in a frequency resolution of 0.78 Hz. Auto- and cross-spectra were estimated by averaging over the disjoint sections, and coherence estimates for two concurrently recorded motor unit signals were computed. The sample coherence indicates the degree of linear correlation in the frequency domain between two signals on a scale from zero to one. Values of coherence exceeding the 95 % confidence level (Rosenberg et al. 1989) for the frequencies of interest (0-100 Hz) were regarded as significant. To calculate the incidence of motor unit coherence at each frequency, a value of 1 was placed in the frequency bin if it exceeded the 95 % confidence interval, or zero if it did not. When comparing the strength of coherence between two motor units across tasks, the data were transformed into a standard distribution using z-scores.
Statistical analysis
The dependent variables for the strength tasks were the 1-RM load and the peak force during the MVC. A one-factor, repeated-measures ANOVA was used to compare dependent variables for each type of contraction. For the single motor unit experiments, the dependent variables were: (1) mean discharge rate; (2) geometric mean discharge rate; (3) mean coefficient of variation for discharge rate; (4) geometric mean coefficient of variation for discharge rate; (5) synchronisation strength (CIS and E); (6) synchronisation peak width; and (7) strength of coherence at a given frequency. Post hoc analyses (Scheffé‘s F test) were performed as necessary. The data are reported as means ± s.d. in the text and means ± s.e.m. in the figures.
Results
Motor unit discharge properties
Discharge properties of individual motor units during all four tasks are shown in Table 1. From a total of 170 motor units, the discharge characteristics were similar across tasks except that the mean discharge rate was greater during shortening contractions. Furthermore, there was a positive trend in the mean discharge rate during the shortening contractions that was significantly different to the negative trend observed during lengthening contractions. No differences were observed in the standard deviation and coefficient of variation for discharge rate between the different types of contractions.
Table 1.
Motor unit discharge properties during different contractions
| Restrained isometric | Postural | Shortening | Lengthening | |
|---|---|---|---|---|
| Number of motor units | 36 | 56 | 40 | 38 |
| Number of discharges | 1796 ± 594*† | 1390 ± 630† | 836 ± 234 | 702 ± 236 |
| Mean frequency (Hz) | 10.4 ± 1.5 | 10.3 ± 1.4 | 12.3 ± 1.8‡ | 10.3 ± 1.7 |
| s.d. (Hz) | 2.1 ± 0.6 | 2.1 ± 0.6 | 2.3 ± 0.7 | 2.2 ± 0.7 |
| CV(%) | 19.7 ± 4.8 | 20.5 ± 5.4 | 19.1 ± 5.3 | 21.2 ± 4.5 |
| Trend (Hzs−1) | — | — | 0.49 ± 0.31 | −0.40 ± 0.24§ |
Data represent means ± s.d. for the total duration of the restrained-isometric and postural tasks, and averages for each 6 s phase for the shortening and lengthening contractions.
P < 0.01 compared with postural (Scheffé's F test).
P < 0.001 compared with shortening and lengthening contractions.
P<0.0001 compared with all other tasks.
P < 0.0001 compared with shortening contractions.
Restrained-isometric and postural tasks
Motor unit discharge characteristics were measured from 18 motor unit pairs in 10 subjects during restrained-isometric and maintained postural contractions. The mean force during the restrained task was 4.4 % MVC (1.7 N) and the mean load for the postural task was 3.8 % of 1-RM (0.09 kg; paired t test, P > 0.05). The motor unit discharge characteristics and the extent of motor unit synchronisation were similar for the two contractions. The strength of motor unit synchronisation measured from the cross-correlogram was similar for the restrained (index CIS, 0.96 ± 0.63 impulses s−1; index E, 0.10 ± 0.06 impulses trigger−1) and postural (index CIS, 1.21 ± 0.92 impulses s−1; index E, 0.14 ± 0.10 impulses trigger−1) isometric contractions (paired t tests, P > 0.05). The width of the central synchronous peak in the cross-correlogram was 19.9 ± 5.0 ms for the restrained task and 18.0 ± 4.7 ms for the postural task (paired t test, P > 0.05). Furthermore, the geometric mean discharge rate (restrained, 10.2 ± 0.4 Hz; postural, 10.1 ± 0.3 Hz) and coefficient of variation for discharge rate (restrained, 19.6 ± 1.1 %; postural, 20.8 ± 1.2 %) for the two motor units contributing to each cross-correlogram were similar for the two tasks (paired t tests, P > 0.05). Because there was no statistical difference in motor unit discharge characteristics between the restrained and postural isometric contractions, all subsequent comparisons of isometric and anisometric contractions have used the data from the postural isometric task as representative of an isometric contraction.
Postural and anisometric contractions
Figure 1 shows motor unit recordings during the performance of postural and anisometric (shortening and lengthening) contractions in one subject. The load during the postural and anisometric contractions was 0.03 kg (1 % 1-RM load). The same motor units were examined in each contraction, as shown by the similar motor unit waveforms in the two tasks. The geometric mean discharge rate for the two discriminated motor units was 12.1 Hz for the postural contraction, 10.8 Hz for the shortening contractions and 9.0 Hz for the lengthening contractions. The geometric mean coefficient of variation was 21.6 % for the postural contraction, 22.5 % for the shortening contractions and 22.6 % for the lengthening contractions.
Figure 1. Single motor unit and performance data during postural and anisometric contractions in one subject.
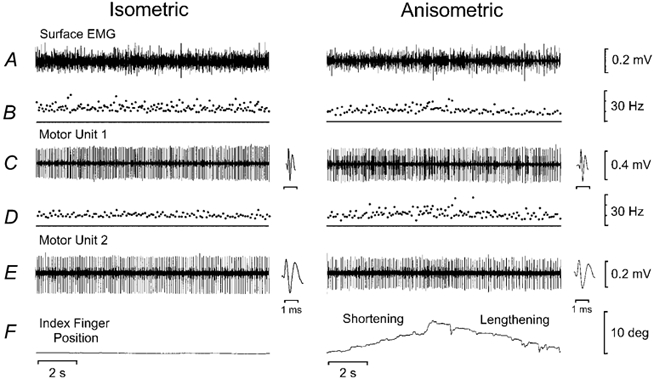
Recordings from two motor units during postural (position hold) and anisometric (shortening and lengthening) contractions. A, surface EMG for the first dorsal interosseus muscle. B, instantaneous discharge rate for motor unit 1. Mean discharge rate was 13.4 Hz for the postural contraction, 11.1 Hz for the shortening contractions and 9.6 Hz for the lengthening contractions. Mean coefficient of variation (CV) of discharge rate was 20.8 % for the postural contraction, 24.1 % for the shortening contractions and 25.3 % for the lengthening contractions. C, recording of motor unit 1 with insets showing the motor unit waveforms. D, instantaneous discharge rate for motor unit 2. Mean discharge rate was 10.8 Hz for the postural contraction, 10.5 Hz for the shortening contractions and 8.5 Hz for the lengthening contractions. Mean CV of discharge rate was 22.3 % for the postural contraction, 20.9 % for the shortening contractions and 20.2 % for the lengthening contractions. E, recording of motor unit 2 with insets showing the motor unit waveforms. F, position of the index finger with the baseline indicating 5 deg of abduction.
Motor unit synchronisation and coherence data are shown for the same subject during postural, shortening and lengthening contractions of the first dorsal interosseus (Fig. 2). A significant central peak, which is indicative of motor unit synchronisation, can be observed in each of the cross-correlograms (Fig. 2A-C). It is evident from the size of the central peak that the strength of motor unit synchronisation was greatest during the lengthening contractions. The synchrony index CIS indicated that the strength of motor unit synchronisation was 1.36 impulses s−1 during the postural contraction, 1.66 impulses s−1 during the shortening contractions and 2.39 impulses s−1 during the lengthening contractions. The synchrony index E was 0.12 impulses trigger−1 for the postural contraction, 0.10 impulses trigger−1 for the shortening contractions and 0.33 impulses trigger−1 for the lengthening contractions. The width of the central synchronous peak was narrower during the shortening contractions (13 ms) compared with the postural (21 ms) and lengthening (27 ms) contractions.
Figure 2. Examples of cross-correlation and coherence analysis from 1 motor unit pair during the three tasks.
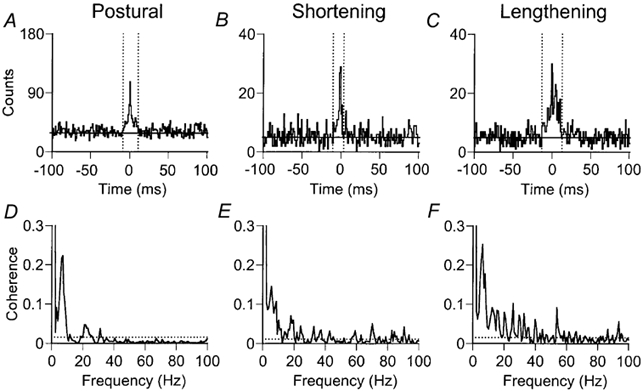
Cross-correlograms (A-C) and motor unit coherence (D-F) from the two motor units shown in Fig. 1. Data were obtained from the same pair of motor units during postural (A and D), shortening (B and E) and lengthening (C and F) contractions. A-C, the significant central peak in each correlogram indicates the strength of motor unit synchronisation. The horizontal line represents the mean number of counts outside the central peak. The dotted vertical lines denote the width of the central synchronous peak as determined from the cusum. For this subject, the strength of motor unit synchronisation was greatest during the lengthening contractions, although the width of the central synchronous peak was narrowest during shortening contractions. D-F, although significant coherence is observed at low frequencies (0-10 Hz) for all three tasks, the peak amplitude of the coherence was weaker in the shortening contractions (0.15 at 5.4 Hz) compared with the postural (0.22 at 7.0 Hz) and lengthening (0.25 at 6.2 Hz) contractions. The incidence of significant coherence across the spectrum was greater for shortening and lengthening contractions. The dotted horizontal line represents the 95 % confidence interval.
Two observations can be made from the coherence data in this subject (Fig. 2D-F). First, the strength of coherence was weaker at low (2-10 Hz) frequencies during the shortening contractions compared with the postural and lengthening contractions. The peak coherence within this frequency band was 0.22 at 7.0 Hz for the postural contraction, 0.15 at 5.4 Hz for the shortening contractions and 0.25 at 6.2 Hz for the lengthening contractions. Second, the incidence of significant coherence for the total range of frequencies (0-100 Hz) was greater for the shortening and lengthening contractions compared with the postural contraction. From 129 frequency bins (0.78 Hz resolution), there were 23 (18 %) significant frequencies for the postural contraction, 62 (48 %) for the shortening contractions and 69 (54 %) for the lengthening contractions. At frequencies above 10 Hz, there were 11/117 (9 %) significant frequencies for the postural contraction, 50/117 (43 %) significant frequencies for the shortening contractions and 57/117 (49 %) significant frequencies for the lengthening contractions.
Motor unit synchronisation during postural and anisometric contractions
Motor unit recordings were obtained from 23 motor unit pairs in 14 subjects during postural, shortening and lengthening contractions. The geometric mean discharge rate was greatest (Scheffé‘s F test) during shortening contractions (12.5 ± 0.4 Hz) compared with postural (10.2 ± 0.3 Hz, P < 0.001) and lengthening (10.4 ± 0.3 Hz, P < 0.001) contractions. The geometric mean of the coefficient of variation for discharge rate was greater for the lengthening contractions (20.9 ± 0.8 %), although this just failed to reach statistical significance (Scheffé‘s F test) compared with the postural (18.4 ± 1.0 %, P + 0.05) and shortening (18.6 ± 1.0, P + 0.08) contractions. The strength of motor unit synchronisation was greater during lengthening compared with shortening contractions in 19/23 motor unit pairs for the index CIS and 18/23 motor unit pairs for the index E (Fig. 3). Furthermore, the width of the central synchronous peak was narrower during shortening contractions compared with postural and lengthening contractions (Fig. 3C).
Figure 3. Motor unit synchronisation during postural, shortening and lengthening contractions.
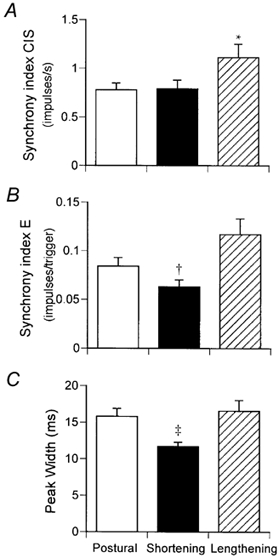
The mean (± s.e.m.) strength of motor unit synchronisation based on the index CIS (A) and index E (B) and the width of the central synchronous peak (C) in 23 motor unit pairs during the different contractions. * P < 0.05 compared with postural and shortening contractions. † P < 0.05 compared with postural contractions and P < 0.01 compared with lengthening contractions. ‡ P < 0.05 compared with lengthening contractions.
The associations between the discharge characteristics and motor unit synchronisation differed for the two indices and the contraction types (Fig. 4). There was no association between the geometric mean discharge rate and synchronisation index CIS (Fig. 4A), although a weak negative association was observed between the synchronisation index E and geometric mean discharge rate (Fig. 4C; r2 + 0.09, P < 0.01). Furthermore, a significant negative correlation (data not shown) was observed between the difference in discharge rate of the two motor units in the pair and the synchronisation index (CIS: r2 + 0.09, P < 0.05; E: r2 + 0.06, P < 0.05). The strongest association was observed between the synchrony index CIS and the difference in the discharge rate of the motor units during lengthening contractions (r2 + 0.17, P + 0.05). The geometric mean of the coefficient of variation for discharge rate of each motor unit pair was significantly related to the synchronisation index CIS for all motor units (continuous line in Fig. 4B; r2 + 0.12, P < 0.01) and for lengthening contractions (dashed line in Fig. 4B; r2 + 0.35, P < 0.01). Similarly, the coefficient of variation for discharge rate was associated with the synchronisation index E for all motor units (Fig. 4D; r2 + 0.10, P < 0.01), although the correlation just failed to reach statistical significance for the lengthening contractions (r2 + 0.16, P + 0.06). These data indicate that the strength of motor unit synchronisation was greater when the discharge rate was more variable, and this was particularly evident during lengthening contractions.
Figure 4. Relations between motor unit synchronisation and discharge characteristics.
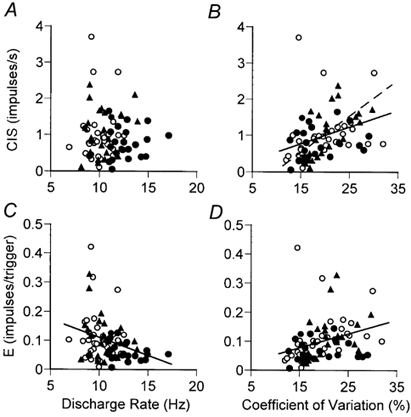
The synchrony index CIS (A and B) and synchrony index E (C and D) are plotted against the geometric mean discharge rate (A and C) and the geometric mean of the coefficient of variation for discharge rate (B and D) of the motor unit pairs contributing to the cross-correlogram during postural (○), shortening (•), and lengthening (▴) contractions. Linear regression revealed a weak negative correlation between the synchrony index E and motor unit discharge rate for all motor units (fitted line in C; r2 + 0.09, P < 0.01), whereas an association was not observed between the synchrony index CIS and discharge rate (A). There was a positive relation between the synchrony index CIS and coefficient of variation for discharge rate (B) for all motor units (continuous line in B; r2 + 0.12, P < 0.01) and for lengthening contractions (dashed line in B; r2 + 0.35, P < 0.01). Similarly, there was a positive relation between the synchrony index E and the coefficient of variation for discharge rate for all motor units (fitted line in D; r2 + 0.10, P < 0.01).
Motor unit coherence during postural and anisometric contractions
Coherence analysis was performed on the same 23 motor unit pairs used for cross-correlation analysis during postural, shortening and lengthening contractions. The typical pattern of coherence during the postural contraction comprised an increased incidence of significant coherence between 2-12 Hz and 16-32 Hz (Fig. 5A). Significant values of coherence were detected for 22/23 (97 %) motor unit pairs in both the 2-12 Hz and 16-32 Hz frequency bands in the postural contractions. The greatest incidence of significant coherence in the lower frequency band occurred at 6 Hz (19/23, 86 %), whereas in the higher frequency band it occurred at 22 Hz (16/23, 70 %). The greatest incidence of significant coherence in the low-frequency band during the shortening contractions (Fig. 5B) occurred at 2 Hz (21/23, 91 %), whereas in the high-frequency band it occurred at 19 Hz (15/23, 65 %). The greatest incidence of coherence in the low-frequency band during the lengthening contractions occurred at 2, 5 and 8 Hz (all 19/23, 83 %), whereas in the high-frequency band it occurred at 26 Hz (18/23, 78 %). The greatest difference in the incidence of coherence between postural and anisometric contractions occurred at higher frequencies (Fig. 5D-F). For example, the greatest difference in the incidence of coherence between postural and shortening contractions occurred at 2, 47 and 57 Hz (Fig. 5D), with 43 % (10/23) more motor unit pairs exhibiting significant coherence at these frequencies during shortening contractions. The largest difference between postural and lengthening contractions occurred at 50 Hz (Fig. 5E), with 52 % (12/23) more motor unit pairs exhibiting significant coherence at this frequency during the lengthening contractions. In contrast, the incidence of significant coherence was similar for the shortening and lengthening contractions (Fig. 5F); the greatest difference occurred at 5 Hz with 35 % (8/23) more motor unit pairs showing a greater incidence of significant coherence during lengthening contractions.
Figure 5. The incidence of significant coherence during postural, shortening and lengthening contractions.
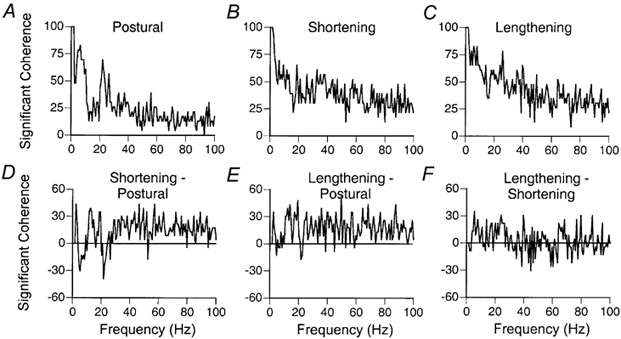
A-C, the percentage of all motor unit pairs showing significant coherence as a function of frequency during postural, shortening and lengthening contractions. D-F, the difference in the incidence of significant coherence between shortening and postural contractions, lengthening and postural contractions, and lengthening and shortening contractions. There was a greater incidence of significant coherence for the shortening and lengthening contractions compared with postural contractions.
When the strength of coherence was plotted as a function of frequency (Fig. 6A), a prominent peak in the 2-12 Hz range was evident for the postural and lengthening contractions, but not for the shortening contractions. Based on the z-score distributions (Fig. 6B), there were significant differences (Scheffé‘s F test) between postural and shortening contractions at 2 Hz (P + 0.01), 6 Hz (P + 0.04), 7 Hz (P + 0.01), 8 Hz (P + 0.01) and 12 Hz (P + 0.01) and between shortening and lengthening contractions at 5 Hz (P + 0.03) and 8 Hz (P + 0.02). There were no significant differences in the strength of coherence between postural and lengthening contractions at these frequencies. Furthermore, no consistent differences between the contractions were observed at any of the higher frequencies.
Figure 6. The strength of coherence during postural, shortening and lengthening contractions.
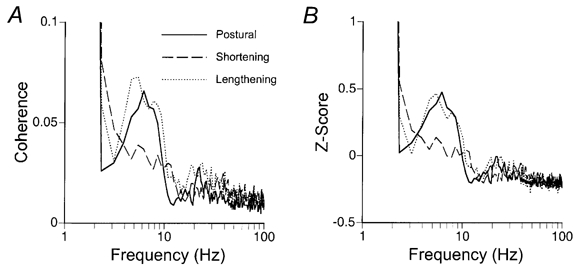
Mean (A) and normalized (B) strength of coherence as a function of frequency for 23 motor unit pairs recorded during postural (continuous line), shortening (dashed line) and lengthening (dotted line) contractions. The data were plotted on a log-linear scale to emphasise the differences in coherence within the low- (2-12 Hz) frequency band. The strength of coherence was reduced in the shortening contractions compared with the postural and lengthening contractions between 5 and 8 Hz. There was no difference in the strength of coherence between the different contractions at higher frequencies.
Motor unit synchronisation and coherence
To examine the relation between motor unit synchronisation and coherence, the strength of motor unit synchronisation (indices CIS and E) was compared with the maximum coherence observed within the 2-12 Hz and 16-32 Hz frequency bands for each pair of motor units recorded during postural, shortening and lengthening contractions. For all tasks combined (data not shown), a significant positive relation was observed between motor unit synchronisation (indices CIS and E) and the maximum coherence observed in both the 2-12 Hz and 16-32 Hz bands. The strongest relation was observed between the synchrony index E and the maximum coherence in the 2-12 Hz frequency band (r2 + 0.29, P < 0.001), whereas the weakest association occurred between the synchrony index E and the maximum coherence in the 16-32 Hz band (r2 + 0.20, P < 0.001). The relation was intermediate for the synchrony index CIS and maximum coherence in the 2-12 Hz band (r2 + 0.23, P < 0.001) and the 16-32 Hz band (r2 + 0.22, P < 0.001).
For the individual tasks, the strongest associations were observed for the shortening contractions, intermediate for the lengthening contractions and least for the postural contractions (Fig. 7). Furthermore, the strongest associations were observed for the maximum coherence in the 2-12 Hz band. For example, significant associations were observed for the shortening contractions between the synchrony index CIS and maximum coherence in both frequency bands (2-12 Hz, r2 + 0.20, P < 0.05, Fig. 7A; 16-32 Hz, r2 + 0.22, P < 0.05, Fig. 7C) and for the synchrony index E and coherence in both frequency bands (2-12 Hz, r2 + 0.45, P < 0.001, Fig. 7E; 16-32 Hz, r2 + 0.36, P < 0.01, Fig. 7G). However, significant associations for the lengthening contractions were only observed for the maximum coherence in the 2-12 Hz frequency band and the synchrony index CIS (Fig. 7B, r2 + 0.22, P < 0.05) and index E (Fig. 7F, r2 + 0.30, P < 0.01). No significant associations were observed between motor unit synchronisation and coherence for the postural contractions (data not shown).
Figure 7. Relation between motor unit synchronisation and coherence during shortening and lengthening contractions.
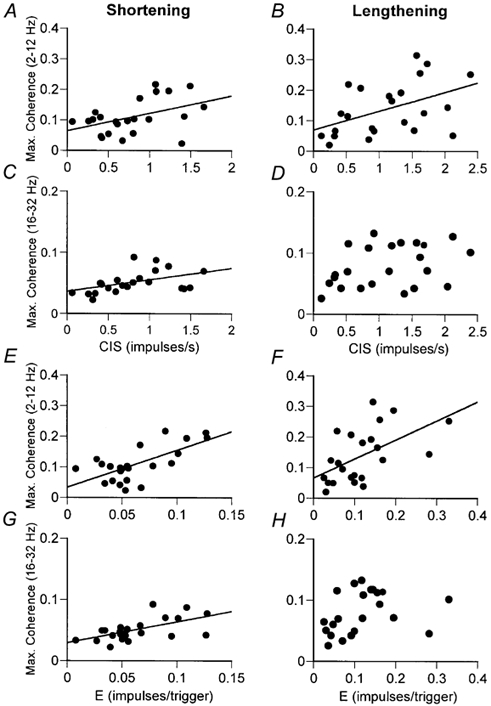
The synchrony index CIS (A-D) and index E (E-H) are plotted against the maximum value of coherence in the 2-12 Hz (A and B; E and F) and 16-32 Hz (C and D; G and H) frequency bands for each motor unit pair during shortening and lengthening contractions. The significant linear relations are for motor units from shortening contractions in the 2-12 Hz and 16-32 Hz frequency range (r2 values of 0.20-0.45), and lengthening contractions in the 2-12 Hz range only (r2 values of 0.22-0.30). For all tasks (data not shown), the strongest relations were observed between the synchrony index E and maximum coherence in the 2-12 Hz frequency band.
Discussion
Common input to motoneurones was characterised by recording the discharge times for pairs of motor units while subjects performed postural, shortening and lengthening contractions with the first dorsal interosseus muscle against a constant load applied to the index finger. The major findings were that the strength of motor unit synchronisation was greatest during lengthening contractions, the width of the central peak of the cross-correlation histogram was narrowest for shortening contractions and the strength of motor unit coherence in the low-frequency band (2-12 Hz) was less during shortening contractions compared with postural and lengthening contractions. These data provide evidence of differences in the activation strategies that are used by the nervous system to control postural, shortening and lengthening contractions of a hand muscle in young adults.
Motor unit synchronisation
Only one previous study has examined the strength of synchronisation between pairs of motor units during anisometric contractions. Kakuda et al. (1999) recorded the activity of 36 pairs of motor units from the extensor carpi radialis muscle while subjects performed slow (5 deg s−1) wrist extension and flexion movements. They found that the strength and duration of motor unit synchronisation measured from a cross-intensity function was similar for position holding and an extension movement of the wrist performed against a servo-controlled torque motor. In contrast, we found that the strength of motor unit synchronisation differed for postural, shortening and lengthening contractions when the hand muscle contracted against an inertial load (Fig. 3). The frequency of synchronous discharges was greatest during lengthening contractions, whereas the probability of synchronous discharge was less during shortening contractions.
Because the size of the peak in the cross-correlation histogram reflects the amount of common input received by the motoneurones (Sears & Stagg, 1976; Nordstrom et al. 1992; Schmied et al. 1994; Farmer et al. 1997), relative changes in the size of the peak indicate variation in the activation of the pair of motor units. Accordingly, the greater peaks observed during lengthening contractions compared with postural contractions provide direct evidence that the common input received by the motoneurones differs for these tasks in a hand muscle. The increased strength of motor unit synchronisation observed during lengthening contractions could occur through any combination of the following mechanisms: (a) an increase in the number of common inputs; (b) an increase in the discharge frequency of the common inputs; or (c) an increase in amplitude of the excitatory postsynaptic potentials delivered by the common inputs (Sears & Stagg, 1976; Kirkwood & Sears, 1978; Nordstrom et al. 1992). The converse could explain the decline in the probability of synchronous discharges (index E) during the shortening contractions.
The width of the central peak in the cross-correlation histogram is often used to distinguish between direct common input onto motoneurones and indirect common input that involves an interposed neurone. Because direct common input is more likely to evoke action potentials that occur within a few milliseconds of each other, it produces a narrow peak in the cross-correlation histogram and contrasts with the broader peak due to indirect common input (Sears & Stagg, 1976; Kirkwood, 1979; Kirkwood & Sears, 1991). Consequently, the width of the peak provides an indication of the relative contribution of direct and indirect common inputs to the motoneurones. We found that the width of the central synchronous peak was similar for postural and lengthening contractions, but was ≈4 ms narrower for shortening contractions (Fig. 3). This result suggests a greater relative contribution of direct common inputs to the motoneurones during shortening contractions compared with postural and lengthening contractions. Alternatively, the narrower peak observed during shortening contractions could represent a greater relative contribution from common excitatory inputs compared with common inhibitory inputs, as common inhibitory inputs appear to broaden the width of the central synchronous peak (Türker & Powers, 2001). Nonetheless, when combined with the increased strength of common input during lengthening contractions, these data suggest that the activation strategy differs for anisometric and postural contractions.
Several observations in humans are consistent with the view that the branched common input to motoneurones arises from supraspinal sources, at least for low-force isometric contractions. For example, the amount of motor unit synchronisation during voluntary contractions is similar in healthy subjects and in patients who have lost sensory feedback (Baker et al. 1988; Farmer et al. 1993a; Schmied et al. 1995). In contrast, motor unit synchronisation is abolished after stroke (Datta et al. 1991; Farmer et al. 1993b) or spinal cord lesions (Davey et al. 1990; Smith et al. 1999), and is absent in patients suffering from primary lateral sclerosis or amyotrophic lateral sclerosis (Schmied et al. 1995, 1999). However, the source of the common input during anisometric contractions may be influenced by the increased reliance on sensory feedback during changes in muscle length, especially during lengthening contractions (Burke et al. 1978; Schieber & Thach, 1985). The divergence of inputs from spindle afferents onto motoneurones and interneurones (Harrison & Taylor, 1981), for example, may provide substantial direct and indirect common input, and be responsible for the enhanced motor unit synchronisation observed during lengthening contractions. Alternatively, it is possible that the lower strength and narrower width during shortening contractions is a result of reduced feedback due to unloading of the muscle spindles as the muscle shortens to lift the required load.
Two commonly used indices (CIS and E) were used to quantify the strength of motor unit synchronisation in the present study. The synchrony index CIS is a measure of the frequency of common inputs, whereas index E represents the probability of extra synchronous discharges. The synchronisation index CIS has been shown to be independent of motor unit discharge rate during voluntary contractions in humans (Nordstrom et al. 1992). However, Türker & Powers (2002) have recently reported that the index CIS is positively correlated with discharge rate in repetitively discharging rat hypoglossal motoneurones in response to common input of 1-2 mV excitatory postsynaptic potentials. They suggested that the synchrony index E provided a more appropriate measure of motor unit synchronisation because it was physiologically independent of discharge rate over a wide range of values. In contrast, we found a weak negative correlation between the index E and discharge rate in pairs of tonically active human motor units recorded in the first dorsal interosseus muscle (Fig. 4C). In the same pairs of motor units, the synchronisation index CIS was not associated with the discharge rate of the contributing motor units (Fig. 4A). Our results suggest that the index CIS is appropriate to quantify the strength of synchronisation in pairs of motor units that are activated during weak voluntary contractions in humans.
Both synchronisation indices indicated that the strength of motor unit synchronisation was greater when the discharge rate of the motor units was more variable, and this was particularly evident during lengthening contractions (Fig. 4B). Although the effect of discharge rate variability has been reported previously for low-force isometric contractions (Nordstrom et al. 1992; Schmied et al. 1994, 2000; Semmler et al. 2000), the mechanism underlying this relation and its significance remain unclear. Because variability in the discharge rate of motor units is related to the fluctuations in membrane potential of the motoneurone about its mean trajectory, an increase in discharge rate variability might be caused by the branched-input delivery of large-amplitude, excitatory postsynaptic potentials, which would also increase motor unit synchronisation. However, the association between synchrony and discharge variability is usually weak statistically, suggesting that it may be of minimal physiological significance.
Motor unit coherence
Motor unit coherence is a frequency domain measure of the strength of common oscillatory input to the motoneurones. Such measurements in single motor units have established that motoneurones receive common rhythmic inputs in the frequency bands 1-12 and 16-32 Hz during low-force isometric contractions of intrinsic hand muscles (Farmer et al. 1993a). The oscillation in the 16-32 Hz frequency band is abolished by stroke (Farmer et al. 1993b) and appears to involve circuits within the motor cortex (Conway et al. 1995; Salenius et al. 1997; Donoghue et al. 1998; Halliday et al. 1998). Although the genesis and function of the cortical rhythms are unknown, recent reports provide strong evidence that oscillators within the cerebral cortex or brain stem play an important role in the correlated discharge of motoneurones during voluntary isometric contractions. This line of evidence is based on observations of coherent oscillations in humans between the contralateral sensorimotor cortex and muscle (Conway et al. 1995; Salenius et al. 1997; Halliday et al. 1998; Feige et al. 2000) and between active areas of the cortex during voluntary muscle contractions (Gerloff et al. 1998; Andres et al. 1999). The cortical oscillations have been observed at frequencies of 10 Hz (Pfurtscheller & Neuper, 1992; Stancak et al. 1997), 20-30 Hz (Toro et al. 1994; Baker et al. 1999) and 40 Hz (Brown, 1996). Under some circumstances, oscillations in motor output can be observed at all of these frequencies (McAuley et al. 1997).
Numerous reports suggest that the amplitude of the oscillations recorded over the cerebral cortex varies with the task that is performed. It appears that the 20-30 Hz oscillations recorded over the motor cortex in the monkey are most prominent during maintained posture and are reduced during movement (Sanes & Donoghue, 1993; Baker et al. 1999). Similarly, coherence between the electroencephalogram (EEG) and the magnetoencephalogram (MEG) in this frequency range is greater just before movement compared with during the movement (Toro et al. 1994; Manganotti et al. 1998). In contrast, the ≈20 Hz coherence in the EEG between different motor areas increases during movement (Leocani et al. 1997) and the ≈20 Hz oscillations in the sensorimotor cortex are present during fine manipulative movements with the hand (Murthy & Fetz, 1996a,b). These results suggest that the appearance of cortical oscillations depends on the details of the task that is performed.
Oscillations have also been observed in the motor output. For example, Vallbo & Wessberg (1993) found discontinuities in acceleration at a frequency of ≈10 Hz during slow finger movements and proposed that this was due to pulsatile control of the agonist and antagonist muscles. Subsequently, Wessberg & Kakuda (1999) demonstrated that the fluctuations in acceleration were not due to the discharge rate of motor units but were rather a consequence of modulation of discharge rate at this frequency. Kakuda et al. (1999) extended this notion by demonstrating common modulation of motor unit discharge at 6-12 Hz during slow flexion and extension movements of the wrist. They also found, however, that this rhythmic activity was markedly reduced during a position-holding task.
Although we have confirmed the existence of low-frequency (2-12 Hz) coherence between motor units, this was present during the maintained postural task and the lengthening contraction but not the shortening contractions (Fig. 6). Given that clear peaks in coherence have been observed in over 80 % of motor unit pairs during shortening contractions in a previous study (Kakuda et al. 1999), it is likely that unique components of the experimental protocol are responsible for this result. For example, Kakuda et al. (1999) used contractions of the extensor carpi radialis muscle against a constant load provided by a servo-controlled torque motor, compared with the lifting and lowering of a constant inertial load attached to the index finger in the present study. Although both studies involved position-tracking tasks, our subjects had to pay attention to confining the movement to the abduction-adduction plane, whereas the subjects in the study of Kakuda et al. were fixed to a manipulandum that limited movement to the flexion-extension plane. Our task, therefore, may have required more attention, which is known to influence the amount of motor unit synchronisation (Schmied et al. 2000). In the present study, we have shown that the neural activation strategy includes a low-frequency (2-12 Hz) common input to motor units during postural and lengthening contractions, but not shortening contractions of a hand muscle in young adults.
In addition to the strength of motor unit coherence, the incidence of significant coherence (Fig. 5) was altered during movement compared with postural contractions. The incidence of significant coherence indicates the frequency spectrum of common inputs that are shared by the motoneurones. As shown previously (Farmer et al. 1993a), postural contractions are associated with an increased incidence of significant coherence at 2-12 Hz and 16-32 Hz (Fig. 5A), indicating that motoneurones receive common oscillatory input within these discrete frequency ranges during low-force postural contractions. During shortening and lengthening contractions, however, there is an increased incidence of coherence at all frequencies except those at 2-12 Hz and 16-32 Hz (Fig. 5D and E). This suggests that movements involve a more variable oscillatory drive to the motoneurones, which is biased towards higher frequencies and superimposed onto the discrete oscillatory inputs present during postural contractions. These findings are analogous to those of other investigators (Sanes & Donoghue, 1993; Baker et al. 1997, 1999), where discrete coherence in the 20-30 Hz frequency range became less prominent during movements compared with isometric contractions. Furthermore, there was an increased strength of low-frequency coherence (≈2 Hz) during movements (particularly shortening contractions) compared with postural contractions, which probably reflects an increased common modulation of motor units at 2 Hz or less during changes in muscle length. It is possible that multiple oscillators originating from different cortical areas (see Baker et al. 1999) could be responsible for these task-related differences in coherence observed between postural contractions and movements.
Motor unit discharge, synchronisation and coherence
When motoneurones discharge action potentials coincidentally during repetitive activity, the membrane potentials must reach the threshold for action potential generation at similar times. This requires the concurrent delivery of synaptic currents to the motoneurones. Although this can occur by chance, the systematic occurrence of correlated discharges requires at least one of three mechanisms (Sears & Stagg, 1976; Kirkwood, 1979; Farmer, 1998): (a) branched input from a common source (motor unit synchronisation); (b) modulation of independent synaptic input by a common oscillator (motor unit coherence); or (c) modulation of branched synaptic input by a common oscillator (synchronisation and coherence). A significant correlation between the strength of motor unit synchronisation and coherence in the same motor unit pairs would indicate a significant contribution of branched oscillatory common inputs (mechanism (c)) to motor unit synchronisation. The absence of a correlation between motor unit synchronisation and coherence suggests that synchronous discharges could arise from either branched common inputs that are not oscillatory (mechanism (a)) or independent inputs that are oscillatory (mechanism (b)). We examined these possibilities by comparing the strength of motor unit synchronisation and coherence from the same pairs of motor units during postural, shortening and lengthening contractions.
Although the presence of common oscillatory input has been implicated in the production of motor unit synchronisation (Marsden et al. 1999; and see Farmer, 1998), this has only been directly compared on a few occasions (Farmer et al. 1993a; Halliday et al. 1999; Kilner et al. 2002). For example, Farmer et al. (1993a) were the first to show that there was an association between the maximal motor unit coherence and the magnitude of synchronisation in the same motor unit pairs (their Fig. 3; 1-12 Hz, r + 0.41; 16-32 Hz, r + 0.67) during low-force isometric contractions. Given that the high-frequency (16-32 Hz) oscillation and the central peak in the cross-correlation histogram are abolished by stroke (Farmer et al. 1993a, b), this has widely been accepted as evidence that the corticospinal pathway contributes to both of these phenomena. More recently, Kilner et al. (2002) confirmed that the strength of motor unit synchronisation was associated (their Fig. 7; r2 + 0.34) with the mean coherence in the 15-30 Hz frequency band during the hold phase of a precision grip with first dorsal interosseus. In contrast, we found the strongest associations between the strength of motor unit synchronisation and coherence to occur during movement, especially shortening contractions (Fig. 7). Furthermore, these associations were most prominent when the strength of synchronisation was compared with the maximal coherence observed in the 2-12 Hz frequency band. The data indicate that up to 45 % (index E, Fig. 7E) of the variation in motor unit synchronisation during shortening contractions could be explained by common oscillations in branched inputs that occurred at low frequencies (2-12 Hz), and up to 36 % (index E, Fig. 7G) could be explained by common oscillations in branched inputs that occurred at high frequencies (16-32 Hz). These contributions were less for lengthening contractions (up to 30 % for 2-12 Hz only, Fig. 7F), and insignificant for maintained postural tasks. Conversely, the majority of the variation in motor unit synchronisation during maintained postural tasks could be explained by non-oscillatory activity in branched common inputs or independent oscillatory inputs to the motoneurones.
Control strategies for shortening and lengthening contractions
We have examined the timing of motor unit discharges during postural, shortening and lengthening contractions with the first dorsal interosseus to infer details about the input received by the motoneurones. The results revealed an altered activation of motor units during both shortening and lengthening contractions of the first dorsal interosseus muscle compared with a maintained postural task. For the shortening contractions, there was reduced presynaptic synchronisation (revealed by a narrower synchronous peak), a decrease in the probability of extra synchronous discharges, and a reduction in low-frequency (2-12 Hz) coherence. The major difference for control of shortening contractions compared with postural and lengthening contractions is the reduction in muscle spindle feedback as the muscle shortens, which is a potential source of presynaptic synchronisation and 2-12 Hz coherence, both of which were observed during the postural and lengthening contractions. For the lengthening contractions, there was an increase in common input to motoneurones (motor unit synchronisation) where there is likely to be enhanced feedback from muscle spindles compared with postural and shortening contractions (Burke et al. 1978; Schieber & Thach, 1985). If this increased contribution from muscle spindles during lengthening contractions is delivered through large-amplitude postsynaptic potentials, this would give rise to more motoneurone membrane noise, increased discharge rate variability, and increased motor unit synchronisation, which was observed experimentally. The influence of these altered neural activation strategies on the control of motor output during shortening and lengthening contractions remains to be determined.
Acknowledgments
This research was supported by National Institutes of Health grants AG09000 and NS42734 awarded to Roger Enoka. Kurt Kornatz was supported by a National Institutes of Health Minority Supplement Award (PA-99-104).
References
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain. 1999;122:855–870. doi: 10.1093/brain/122.5.855. [DOI] [PubMed] [Google Scholar]
- Baker JR, Bremner FD, Cole JD, Stephens JA. Short-term synchronization of intrinsic hand muscle motor units in a ‘deafferented’ man. Journal of Physiology. 1988;396:155P. [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Experimental Brain Research. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. Journal of Physiology. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Muscle sound during human wrist movements. Journal of Physiology. 1996;494P:68P. [Google Scholar]
- Burke D, Hagbarth K-E, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. Journal of Physiology. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. Journal of Physiology. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Farmer SF, Stephens JA. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. Journal of Physiology. 1991;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contraction in man. Journal of Physiology. 1990;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Friedland C, Short DJ. Motor unit discharge characteristics and short term synchrony in paraplegic humans. Journal of Neurology, Neurosurgery and Psychiatry. 1990;53:764–769. doi: 10.1136/jnnp.53.9.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos G, Gaál G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. Journal of Neurophysiology. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalography and Clinical Neurophysiology. 1978;45:302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Farmer SF. Rhythmicity, synchronization and binding in human and primate motor systems. Journal of Physiology. 1998;509:3–14. doi: 10.1111/j.1469-7793.1998.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. Journal of Physiology. 1993a;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. Journal of Neuroscience Methods. 1997;74:175–187. doi: 10.1016/s0165-0270(97)02248-6. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. Journal of Physiology. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Aertsen A, Kristeva-Fiege R. Dynamic synchronization between multiple cortical motor areas and muscle activity in phasic voluntary movements. Journal of Neurophysiology. 2000;84:2622–2629. doi: 10.1152/jn.2000.84.5.2622. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain. 1998;121:1513–1531. doi: 10.1093/brain/121.8.1513. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neuroscience Letters. 1998;241:1–4. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Load-independent contributions from motor-unit synchronization to human physiological tremor. Journal of Neurophysiology. 1999;82:664–675. doi: 10.1152/jn.1999.82.2.664. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Hansen S, Christensen LOD, Petersen NT, Nielsen JB. Synchronization of lower limb motor unit activity during walking in human subjects. Journal of Neurophysiology. 2001;86:1266–1276. doi: 10.1152/jn.2001.86.3.1266. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Taylor A. Individual excitatory postsynaptic potentials due to muscle spindle Ia afferents in cat triceps surae motoneurones. Journal of Physiology. 1981;312:455–470. doi: 10.1113/jphysiol.1981.sp013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N, Nagaoka M, Wessberg J. Common modulation of motor unit pairs during slow wrist movement in man. Journal of Physiology. 1999;520:929–940. doi: 10.1111/j.1469-7793.1999.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans. Journal of Physiology. 2002;541:937–948. doi: 10.1113/jphysiol.2001.013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. On the use and interpretation of cross-correlation measurements in the mammalian central nervous system. Journal of Neuroscience Methods. 1979;1:107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. Journal of Physiology. 1978;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Cross-correlation analyses of motoneurone inputs in a coordinated motor act. In: Krüger J, editor. Neuronal Connectivity. Berlin: Springer-Verlag; 1991. pp. 225–248. [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle and Nerve. 2000;23:600–612. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallet M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalography and Clinical Neurophysiology. 1997;104:199–206. doi: 10.1016/s0168-5597(96)96051-7. [DOI] [PubMed] [Google Scholar]
- McAuley JH, Rothwell JC, Marsden CD. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Experimental Brain Research. 1997;114:525–541. doi: 10.1007/pl00005662. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallet M. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalography and Clinical Neurophysiology. 1998;109:50–62. doi: 10.1016/s0924-980x(97)00074-x. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Farmer SF, Halliday DM, Rosenberg JR, Brown P. The unilateral and bilateral control of motor unit pairs in the first dorsal interosseous and paraspinal muscles in man. Journal of Physiology. 1999;521:553–564. doi: 10.1111/j.1469-7793.1999.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalography and Clinical Neurophysiology. 1975;38:245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. Journal of Neurophysiology. 1996a;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurones during local field potential oscillations in sensorimotor cortex of awake monkeys. Journal of Neurophysiology. 1996b;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. Journal of Physiology. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. NeuroReport. 1992;3:1057–1060. doi: 10.1097/00001756-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Progress in Biophysics and Molecular Biology. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. Journal of Neurophysiology. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proceedings of the National Academy of Sciences of the USA. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Thach WT. Trained slow tracking. II. Biderectional discharge patterns of cerebellar nuclear, motor cortex, and spindle afferent neurons. Journal of Neurophysiology. 1985;54:1228–1270. doi: 10.1152/jn.1985.54.5.1228. [DOI] [PubMed] [Google Scholar]
- Schmied A, Pagni S, Sturm H, Vedel J-P. Selective enhancement of motoneurone short-term synchrony during an attention demanding task. Experimental Brain Research. 2000;133:377–390. doi: 10.1007/s002210000421. [DOI] [PubMed] [Google Scholar]
- Schmied A, Pouget J, Vedel J-P. Electromechanical coupling and synchronous firing of single wrist extensor motor units in sporadic amyotrophic lateral sclerosis. Clinical Neurophysiology. 1999;110:960–977. doi: 10.1016/s1388-2457(99)00032-2. [DOI] [PubMed] [Google Scholar]
- Schmied A, Vedel J-P, Pagni S. Human spinal lateralization assessed from motoneurone synchronization: dependence on handedness and motor unit type. Journal of Physiology. 1994;480:369–387. doi: 10.1113/jphysiol.1994.sp020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmied A, Vedel J-P, Pouget J, Forget R, Lamarre Y, Paillard J. Changes in motoneurone connectivity evaluated from neuronal synchronization analysis. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York: Plenum; 1995. pp. 469–477. [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. Journal of Physiology. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG. Motor unit synchronization and neuromuscular performance. Exercise and Sport Sciences Reviews. 2002;30:8–12. doi: 10.1097/00003677-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Enoka RM. Neural contributions to changes in muscle strength. In: Zatsiorsky VM, editor. Biomechanics in Sport: The Scientific Basis of Performance. Oxford: Blackwell Science Ltd; 2000. pp. 3–20. [Google Scholar]
- Semmler JG, Kornatz KW, Miles TS, Enoka RM. Low frequency (2–12 Hz) motor unit coherence differs between shortening and lengthening contractions of a hand muscle. Society for Neuroscience Abstracts. 2002;28:665. [Google Scholar]
- Semmler JG, Kutzscher DV, Zhou S, Enoka RM. Motor unit synchronization is enhanced during slow shortening and lengthening contractions of the first dorsal interosseus muscle. Society for Neuroscience Abstracts. 2000;26:463. [Google Scholar]
- Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Experimental Brain Research. 1998;119:27–38. doi: 10.1007/s002210050316. [DOI] [PubMed] [Google Scholar]
- Smith HC, Davey NJ, Savic G, Maskill DW, Ellaway PH, Frankel HL. Motor unit discharge characteristics during voluntary contraction in patients with incomplete spinal cord injury. Experimental Physiology. 1999;84:1151–1160. [PubMed] [Google Scholar]
- Stancak A, Riml A, Pfurtscheller G. The effects of external load on movement-related changes of the sensorimotor EEG rhythms. Electroencephalography and Clinical Neurophysiology. 1997;102:495–504. doi: 10.1016/s0013-4694(96)96623-0. [DOI] [PubMed] [Google Scholar]
- Toro C, Friehs G, Ojakangas C, Maxwell R, Gates JR, Gumnit RJ, Ebner TJ. 8–12 Hz rhythmic oscillations in human motor cortex during two-dimensional arm movements: evidence for representation of kinematic parameters. Electroencephalography and Clinical Neurophysiology. 1994;93:390–403. doi: 10.1016/0168-5597(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Effects of common excitatory and inhibitory inputs on motoneuron synchronization. Journal of Neurophysiology. 2001;86:2807–2822. doi: 10.1152/jn.2001.86.6.2807. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. The effects of common input characteristics and discharge rate on synchronization in rat hypoglossal motoneurones. Journal of Physiology. 2002;541:254–260. doi: 10.1113/jphysiol.2001.013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbo Å B, Wessberg J. Organization of motor output in slow finger movements in man. Journal of Physiology. 1993;469:673–691. doi: 10.1113/jphysiol.1993.sp019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Kakuda H. Single motor unit activity in relation to pulsatile motor output in human finger movements. Journal of Physiology. 1999;517:273–285. doi: 10.1111/j.1469-7793.1999.0273z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


