Abstract
Brain temperature appears to be an important factor affecting motor activity, but it is not known to what extent brain temperature increases during prolonged exercise in humans. Cerebral heat exchange was therefore evaluated in seven males during exercise with and without hyperthermia. Middle cerebral artery mean blood velocity (MCA Vmean) was continuously monitored while global cerebral blood flow (CBF) and cerebral energy turnover were determined at the end of the two exercise trials in three subjects. The arterial to venous temperature difference across the brain (v-aDtemp) was determined via thermocouples placed in the internal jugular vein and in the aorta. The jugular venous blood temperature was always higher than that of the arterial blood, demonstrating that heat was released via the CBF during the normothermic as well as the hyperthermic exercise condition. However, heat removal via the jugular venous blood was 30 ± 6 % lower during hyperthermia compared to the control trial. The reduced heat removal from the brain was mainly a result of a 20 ± 6 % lower CBF (22 ± 9 % reduction in MCA Vmean), because the v-aDtemp was not significantly different in the hyperthermic (0.20 ± 0.05 °C) compared to the control trial (0.22 ± 0.05 °C). During hyperthermia, the impaired heat removal via the blood was combined with a 7 ± 2 % higher heat production in the brain and heat was consequently stored in the brain at a rate of 0.20 ± 0.06 J g−1 min−1. The present results indicate that the average brain temperature is at least 0.2 °C higher than that of the body core during exercise with or without hyperthermia.
Exercise endurance is markedly reduced by hyperthermia (Gonzalez-Alonso et al. 1999; Nybo et al. 2001), and the idea that central fatigue is involved in the aetiology of hyperthermia-induced fatigue is supported by the observation that exercise-induced hyperthermia reduces the voluntary activation of motorneurons during a sustained maximal muscle contraction (Nybo & Nielsen, 2001a). The reduced work capacity may relate to more than one factor, but exhaustion during prolonged exercise in the heat seems to coincide with the attainment of a critical internal temperature (Nielsen et al. 1993; Fuller et al. 1998; Gonzalez-Alonso et al. 1999; Walters et al. 2000), and experiments with goats indicate that brain temperature is a dominant factor affecting motor activity (Caputa et al. 1986). Brain temperature changes in humans during exercise with hyperthermia and the influence of active head cooling have been a matter of great interest and controversy (Cabanac & Caputa 1979; Brengelmann 1993; Cabanac 1993). The evaluation of selective brain cooling has so far been based on measurements carried out in post-operative neurological patients at rest (Shiraki et al. 1988; Mellergård 1992; Mariak et al. 1999) or on temperature differences between the body core and the tympanic membrane during exercise with or without active cooling of the head (Cabanac & Caputa 1979; Brinnel et al. 1987; Cabanac et al. 1987; Nagasaka et al. 1998). The cerebral thermodynamic response during exercise has never been directly investigated and it is not known to what extent brain temperature increases when humans exercise in the heat.
The temperature of the brain is determined by the balance between heat produced by cerebral energy turnover and the heat that is removed, primarily by the cerebral blood flow (CBF). Convection of heat between tissue and capillaries is considered to be very high (Pennes, 1948) and the temperature of the cerebral venous blood is expected to equilibrate with that of the cerebral tissue (Yablonskiy et al. 2000). Accordingly, the thermodynamic response of the human brain can be evaluated by measuring the internal jugular venous to arterial blood temperature difference (v-aDtemp) together with determinations of CBF and cerebral energy turnover. At rest a stable brain temperature is established with a v-aDtemp of ≈0.3 °C (Shevelev 1998; Yablonskiy et al. 2000), but the balance between the cerebral heat production and its removal could be disturbed during exercise and especially so when heat stress contributes to elevate the temperature of the arterial blood. It should also be considered that exercise in the heat is associated with an elevated cerebral oxygen consumption at the same time as the global CBF is reduced (Nybo & Nielsen, 2001b; Nybo et al. 2002), and that the lower cerebral perfusion could hamper the removal of metabolic heat from the brain. On the other hand, it has been proposed that a reduced CBF during exercise with hyperthermia may be advantageous, since it could contribute to protecting the brain against an excessive heat gain from the body core (Cabanac, 1993). Therefore, the aim of this study was to evaluate the thermodynamic response of the brain during prolonged exercise with and without hyperthermia.
Methods
The seven males who participated in the study had a mean age of 26 ± 2 years (± s.d.), a height of 182 ± 5 cm, a weight of 75 ± 5 kg and a maximal oxygen uptake (O2,max) of 4.6 ± 0.4 l min−1. All participants gave written informed consent to participate in the study, which was approved by the Ethical Committee of Copenhagen and Frederiksberg (KF 01-135/00) and all protocols were performed according to the Declaration of Helsinki.
Experimental design
The subjects completed two exercise bouts on a cycle ergometer (Monark 829E, Sweden) at 174 ± 12 W with a cadence of 85 ± 3 r.p.m. corresponding to ≈ 50 % of peak oxygen uptake. In one trial, exercise was carried out in a thermoneutral environment (20 °C, control), while a hyperthermic exercise condition was established in the other trial by dressing the subjects in a water impermeable suit composed of rain clothes and rubber gloves (hyperthermia). The two exercise trials were separated by 1 h of recovery and the treatment order was randomly assigned and counterbalanced across subjects.
Subjects arrived at the laboratory approximately 1 h before the start of the experiment. An oesophageal thermocouple was inserted and skin temperature thermocouples, an ultra sound transcranial Doppler probe and a heart rate (HR) monitor were attached to the subject. The subject then rested on a couch while catheters were inserted into the brachial artery of the non-dominant arm and into the bulb of the right internal jugular vein. Thermocouples to record blood temperature were inserted through the catheters and finally, a tympanic thermocouple was inserted as described by Brinnel & Cabanac (1989). Baseline values of all body temperatures were obtained after 5 min of seated rest on the ergometer.
During exercise and 30 min into the recovery period of the hyperthermic trial, the middle cerebral artery mean blood velocity (MCA Vmean) was monitored beat by beat, while the oesophageal, skin, tympanic and blood temperatures were recorded every fifth minute. In addition, the global CBF was measured at the end of the trials in three of the subjects. Head cooling was applied from 35-40 min of exercise in the hyperthermic trial by facial fanning (4-5 m s−1), and by continuously spraying a thin mist of 20 °C water to the face and to the top of the head. A rating of perceived exertion (RPE; Borg 1975) was expressed every 5 min during the exercise trials.
Cerebral blood flow
Global CBF was measured with the Kety-Schmidt technique in the desaturation mode during the last 10 min of the normo- and hyperthermic trials as previously described (Madsen et al. 1993; Nybo et al. 2002). In short, 133Xe dissolved in saline was infused intravenously for 30 min, and 133Xe activity (expressed in counts min−1 gram−1) were determined in arterial and jugular venous blood samples at equilibrium and 0.5, 1, 2, 3, 4, 6, 8 and 10 min after the stop of infusion. Global CBF in ml (100 g)−1 min−1 was calculated by the height-over-area method (i.e. CBF equals the jugular venous activity at equilibrium minus the activity 10 min after the stop of infusion divided by the integrated area under the venous-arterial curve during this period of time) as prescribed by Kety & Schmidt (1948), and the cerebral metabolic rates of oxygen and lactate were calculated using Fick's principle. However, concomitant blood temperature measurements and frequent blood sampling, as required for a Kety-Schmidt determination of CBF, was technically difficult and the Kety-Schmidt technique was only applied in three of the subjects. Furthermore, it is not possible to make frequent CBF determinations with the Kety-Schmidt technique, since 30 min of 133Xe infusion is required before each CBF measurement. Hence, the cerebral circulation was also evaluated with transcranial Doppler ultrasound (Transcan, EME, Überlingen, Germany). The proximal segment of the middle cerebral artery was insonated at a depth of 45-50 mm from the temporal bone depending on the best signal-to-noise ratio (Aaslid et al. 1982). The probe was secured with a customised headband and the position was maintained throughout the examination. Doppler ultrasound has the advantage of providing a continuous real-time recording, and hyperthermia-induced reductions in CBF during exercise are reflected in a lower MCA Vmean (Nybo & Nielsen 2001b; Nybo et al. 2002). On the other hand, transcranial Doppler ultrasound measures a regional blood velocity rather than flow and global CBF cannot be estimated with the Doppler technique. However, the combination of MCA Vmean determinations and global CBF measurements may allow for an evaluation of the time course of the changes in the cerebral circulation as well as a determination of whole-brain values for energy turnover and blood flow. The cerebral metabolic rate of oxygen (CMRoxygen) and cerebral lactate release were calculated by multiplying global CBF with the arteriovenous differences. Arterial and jugular venous blood values for blood gases and metabolites were determined on an ABL 700 apparatus (Radiometer, Copenhagen, Denmark).
Temperature measurements
The oesophageal temperature (Toes) was recorded with a thermocouple (model MOV-A, Ellab, Denmark) inserted through the nasal passage to a distance equal to one-quarter of the subject's standing height. Another thermocouple (model MAC-07170-A, Ellab) was advanced through the jugular catheter to assess the temperature of the cerebral venous blood. Placement of this thermocouple at the base of the cranium corresponding to the jugular bulb was verified by X-ray in two of the subjects. The arterial blood temperature was registered by inserting a similar thermocouple in the arterial catheter and by advancing the thermocouple 45 cm. The tip of the probe was thereby placed in, or close to, the aortic arch as verified by X-ray in two subjects. Skin temperatures at the forehead, cheek and top of the head were measured with a MHC-40050-A thermocouple (Ellab), and mean head skin temperature was calculated as an average of the three skin temperatures. All thermocouples were connected to a recorder (CTF 9008 precision thermometer, Ellab) interfaced to a computer and registered with an accuracy of 0.01 °C.
Cerebral heat transfer
The following calculations were performed on the data from the three subjects in whom global CBF was determined. Cerebral heat production per gram of brain tissue per minute was calculated as described by Yablonskiy et al. (2000) on the basis of the cerebral metabolic rate of oxygen consumption. In addition, heat production from anaerobic metabolism was estimated on the basis of the cerebral release of lactate. Heat removal via the jugular venous blood was the product of CBF, the temperature difference between jugular venous and arterial blood, and the specific heat capacity of blood (3.6 J ml−1 (°C)−1; at a haematocrit of 45 %). The temperature of the venous blood was assumed to equilibrate with the average tissue temperature (Pennes, 1948; Yablonskiy et al. 2000) and the rate of cerebral heat storage was calculated on the basis of changes in jugular blood temperature and a cerebral heat capacity of 3.64 J g−1 (°C)−1 (Shevelev, 1998).
Statistical analysis
One and two way (time-by-trial) repeated measures analysis of variance (ANOVA) was performed to evaluate differences between and within trials. Following a significant F test, pairwise differences were identified using Tukey's honestly significance (HSD) post hoc procedure. The significance level was set at P < 0.05 and data are presented as means ± s.d. unless otherwise indicated. Statistical comparisons were not performed on data sets where n < 5, i.e. the CBF and cerebral heat transfer data.
Results
The oesophageal, arterial and jugular venous blood temperatures increased progressively during the hyperthermic trial and the tympanic temperature tended to follow the internal temperatures; except during the period with active head cooling (Fig. 1). The jugular venous blood temperature was always the highest of the measured temperatures and it reached a peak value of 39.5 ± 0.2 °C at the end of the hyperthermic trial. In the control trial, the oesophageal, arterial and jugular venous blood temperatures increased by ≈1 °C during the first 15 min of exercise and then stabilised for the remainder of the exercise period.
Figure 1. Oesophageal (▵), tympanic (□), arterial (•) and jugular venous (♦) temperature responses during cycling.
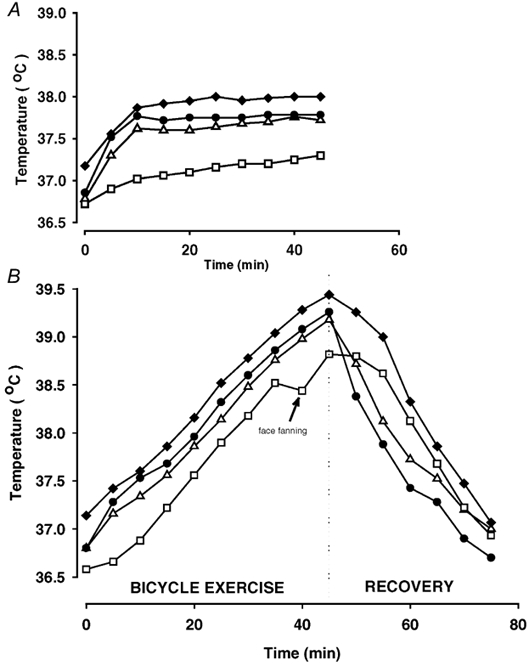
Results obtained with a normal core temperature response (A, control trial) and during a similar exercise bout with progressive hyperthermia (B). Values are means of 7 subjects. Standard deviations are omitted for simplicity, but the s.d. values of all temperatures were in the range of 0.1-0.3 °C.
The v-aDtemp was 0.33 ± 0.06 °C at rest, but during the first 10 min of exercise the arterial temperature increased at a faster rate than the jugular temperature and the v-aDtemp was therefore narrowed to ≈0.1 °C in both trials. From 15 min to the end of exercise, the v-aDtemp stabilised at 0.22 ± 0.05 °C in the control trial and at a similar level of 0.20 ± 0.05 °C in the hyperthermic trial.
The arterial blood temperature decreased rapidly during the first 10 min of recovery from the hyperthermic trial (0.15 °C min−1), while the rate of reduction in jugular venous temperature was more modest (0.05 °C min−1). The v-aDtemp was therefore increased to 0.90 ± 0.16 °C during the 10 min following the hyperthermic trial.
Cerebral blood flow and MCA Vmean
MCA Vmean increased at the onset of exercise in both trials and remained elevated throughout the control trial. In contrast, during the hyperthermic trial, MCA Vmean gradually decreased and it was 22 ± 9 % lower than in the control trial at the end of exercise (P < 0.05; Fig. 2). The MCA Vmean remained lower during the first 15 min of the recovery period in hyperthermia compared to control. Similarly, the global CBF was reduced by 20 ± 6 % at the end of the hyperthermic trial compared to control (0.41 ± 0.08 vs. 0.51 ± 0.08ml g−1 min−1.
Figure 2. Middle cerebral artery mean blood velocity (MCA Vmean) during exercise with and without hyperthermia and during the subsequent recovery period.
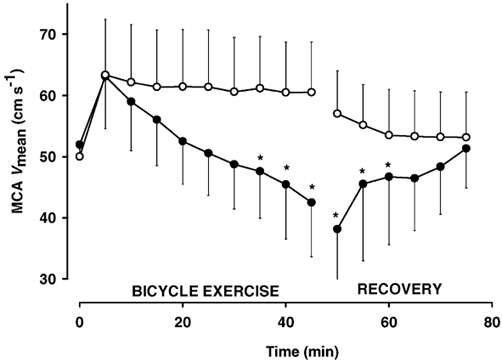
•, hyperthermia; ○, control. Values are means ± s.d. of 6 subjects. * Significantly lower than control.
Cerebral heat exchange
The rate of cerebral heat production was slightly higher at the end of the hyperthermic trial (0.69 ± 0.12 J g−1 min−1) compared to the control trial (0.65 ± 0.11 J g−1 min−1) as a result of an increase in the CMRoxygen from 3.2 ± 0.4 in the control trial to 3.4 ± 0.4ml (100 g)−1 min−1 during hyperthermia. The cerebral release of lactate was similar across trials (0.02 ± 0.01 μmol g−1 min−1). On the other hand, cerebral heat removal via the jugular venous blood was lower during hyperthermia (0.36 ± 0.09 J g−1 min−1) than during the control trial (0.50 ± 0.10 J g−1 min−1). Consequently, during the last 15 min of the hyperthermic trial, heat was stored in the brain at a rate of 0.20 ± 0.06 J g−1 min−1, while there was no storage of heat in the brain during the same period of time in the control trial (Fig. 3). In both exercise trials there appeared to be a heat loss of 0.14 ± 0.03 J g−1 min−1 via other mechanisms than by convective heat removal by the jugular venous blood (see the legend for Fig. 3 for further explanation). The cerebral metabolic and thermodynamic differences between the hyperthermic and the control trial were consistent across the subjects.
Figure 3. Rate of cerebral heat production, heat removal via the jugular venous blood and heat storage during the last 15 min of the control and hyperthermic exercise trials.
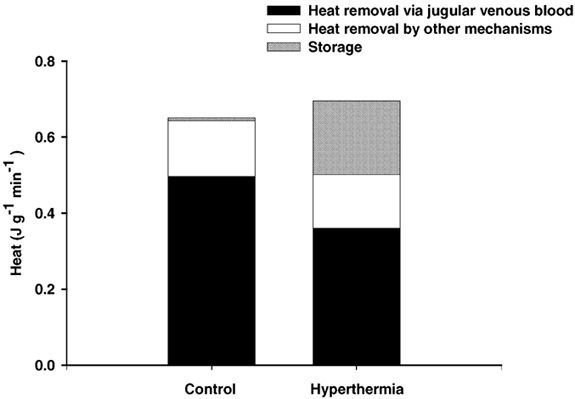
The rate of cerebral heat production, which is represented by the total height of the bars, was calculated on the basis of the Kety-Schmidt-determined values for cerebral oxygen uptake and lactate release, while heat removal via the jugular venous blood was determined on the basis of the global CBF and the arterial to internal jugular venous temperature difference. Storage is the average rate of heat storage from 30- 45 min of exercise, as determined from the change in cerebral venous blood temperature, and ‘heat removal via other mechanisms’ (than convective removal via the jugular venous blood) is the difference between the rate of cerebral heat production and the computed rate of storage in the brain summed with the rate of heat removal by the jugular venous blood. The values represent means of three subjects.
Active head cooling
Facial fanning reduced the mean head skin temperature from 37.3 ± 0.2 to 32.8 ± 0.2 °C (P < 0.001) and at the end of the cooling period, it was similar to the corresponding control trial value of 33.2 ± 0.2 °C. Concurrently with the reduction in head skin temperature there was a small lowering of the tympanic membrane temperature by 0.1 ± 0.0 °C (P < 0.05). In contrast, both the arterial and jugular venous blood temperatures increased during the cooling period and the v-aDtemp remained unchanged at 0.20 ± 0.05 °C.
All subjects reported improved thermal comfort in response to the facial fanning, but active head cooling failed to lower the RPE score. Rather, RPE increased from 16 ± 1 to 17 ± 1 units during the period with active head cooling (P < 0.05).
Discussion
The jugular venous blood temperature was stable from 15 to 45 min in the control trial indicating that cerebral heat balance is established during prolonged exercise in a thermoneutral environment. The jugular venous to arterial temperature difference was narrowed during the first 10 min of exercise, resulting in storage of heat in the brain, but a new balance between cerebral heat release and heat production was then developed. In contrast, heat was stored in the brain throughout the hyperthermic trial and this was primarily related to diminished heat removal via the venous blood, but the increased rate of cerebral heat production also contributed. It has been suggested that when the temperature of the trunk increases, the arterial blood, instead of cooling the brain, may place it in jeopardy (Brinnel et al. 1987; Cabanac, 1993). However, the arterial blood temperature was always lower than the jugular venous blood temperature indicating that heat was removed from the brain throughout the hyperthermic trial. However heat removal via the jugular venous blood was reduced by ≈30 % in the hyperthermic compared to the control trial. This reduction in heat removal from the brain was primarily a result of the lower CBF, because the v-aDtemp was not significantly different in the two trials. The impaired heat removal via the circulation combined with the 7 % increase in metabolic heat production resulted in an estimated net heat storage of ≈0.2 J (g cerebral tissue)−1 min−1 during the last 15 min of the hyperthermic trial. The increased cerebral metabolic heat production was, consistent with our previous findings, due to an accelerated aerobic energy turnover without alterations in the cerebral release of lactate, and it appears that a Q10 of ≈1.5 will explain the 7 % increase in cerebral energy turnover (Nybo et al. 2002).
The lower CBF in response to hyperthermia is primarily related to a hyperventilation-induced lowering of the arterial carbon dioxide pressure (Nybo & Nielsen 2001b). If CBF was not reduced during the hyperthermic trial or restored via hypoventilation, heat removal by the blood would be expected to increase and consequently lower the rate of cerebral heat storage. However, the temperature gradient between the incoming arterial blood and the cerebral tissue would then be narrowed which would reduce the ability of the blood to remove heat from the brain. Therefore, cerebral heat storage is an inevitable consequence of body core hyperthermia, and restoration of CBF will not protect the brain against hyperthermia. On the other hand, heat removal from the brain is highly dependent on convective heat removal by the cerebral circulation, and further reductions in CBF would be expected to increase the rate of cerebral heat storage.
Selective brain cooling
The jugular venous blood temperature was in all subjects and at all times higher than the oesophageal and aortic arch temperatures. Thus, under all the investigated conditions the brain must have been warmer than the body core. Cabanac (1993) suggested that selective brain cooling does not take place during normothermia but becomes relevant during hyperthermia. However, no indications of selective cooling of brain during the hyperthermic exercise bout were observed in the present study although the subjects reached quite high body temperatures - the highest individual core temperature was 40.1 °C with a corresponding jugular blood temperature of 40.4 °C. During the period with active cooling of the head, the jugular venous blood remained ≈0.2 °C warmer than the arterial blood and the thermodynamic response of the brain temperature did not seem to be affected by a 5 °C drop in head skin temperature. This finding is in accordance with the quantitative modelling results by Nelson & Nunneley (1998) and it supports the arguments against brain cooling during face fanning obtained with auditory evoked potentials (Jessen & Kuhnen, 1992; Nielsen & Jessen, 1992) and proton MRI spectroscopy (Corbett & Laptook, 1998). The head cooling we applied may have been less aggressive than previously used procedures (Cabanac et al. 1987; Nielsen & Jessen, 1992; Desruelle & Candas, 2000), but the active cooling did restore the mean head skin temperature to a normothermic level and the tympanic membrane temperature was, in agreement with previous findings, slightly reduced (Cabanac et al. 1987; Nielsen & Jessen, 1992). The tympanic temperature tended to follow the internal temperatures when no fanning was applied during the hyperthermic trial, but the deviation between tympanic and jugular venous blood temperature during the period with facial fanning indicates that the average brain temperature and the tympanic temperature are independent in humans (Shiraki et al. 1988).
A substantial amount of heat may be released from the surface of the head and from the upper respiratory tract during exercise (Hanson, 1974; Rasch et al. 1991), and we have observed that the temperature in the tissue adjacent to the internal carotid artery may be 1-2 °C lower than the aortic blood temperature during exercise with or without hyperthermia, whereas aortic blood temperature and the tissue temperature adjacent to the internal carotid artery were similar at rest (L. Nybo & N. Secher, unpublished observations). Therefore the basis for cooling of the arterial blood on its passage from the heart to the brain exists (see also Rubenstein et al. 1960) and especially so during exercise when the pulmonary ventilation is increased (Hanson, 1974; Rasch et al. 1991). However, the transit time in the carotid artery is relatively short and the blood temperature will not equilibrate with that of the surrounding tissue (Crezee & Lagendijk, 1992). Also, calculations based on the present results indicate that the blood temperature is lowered by less than 0.1 °C on its passage from the body core to the brain. Thus, heat removal via the jugular venous blood would account for all of the cerebral heat loss if the temperature of the arterial blood reaching the brain was 0.09 °C lower than the temperature in the aortic arch. If some of the unaccounted-for cerebral heat loss (see Fig. 3) is explained by this pre-cooling of arterial blood during the passage in the carotid artery, then it may be considered as a sort of brain cooling mechanism. However, the unaccounted-for cerebral heat loss was similar in the control and hyperthermic trials and it is therefore unlikely that the pre-cooling of arterial blood is a mechanism selectively used during hyperthermic exercise conditions. Furthermore, according to Fig. 3, a maximum of 20 % of the cerebral heat production could be removed via this mechanism and the present results indicate that the pre-cooling of arterial blood is far from sufficient to establish a brain temperature which is lower than that of the body core.
The improved thermal comfort in response to facial fanning seems to be explained by altered afferent input from the cooled skin and not by reductions in brain temperature. Thus, facial fanning reduced only the external head temperatures, while it failed to lower the temperature of the cerebral venous blood. The lowering of the head skin temperatures was not associated with a reduction in RPE, and the degree of exertion during exercise in the heat did not appear to be directly related to the perception of thermal comfort. Rather, the subjects’ rating of perceived exertion seemed to increase in parallel with the jugular and arterial blood temperatures.
Cerebral thermodynamics during recovery from hyperthermia
The marked increase in the v-aDtemp immediately after the termination of exercise forms the basis for a large heat release from the brain. However, the cerebral venous blood temperature only decreased by 0.05 °C min−1 during the first 10 min of the recovery, whereas the drop in core temperature was much more pronounced. This indicates that the brain has a relatively slow recovery response from hyperthermia, which may be related to a high cerebral heat production combined with a low perfusion of the brain. The cerebral metabolic rate is probably increased for as long as the temperature is elevated, reflecting the Q10 effect (Nybo et al. 2002), and the MCA Vmean recordings indicate that large parts of the brain have a low perfusion early in the recovery period. A faster dissipation of heat from the brain could be expected if CBF, in addition to whole-body cooling, was increased, e.g. by CO2 inhalation or via voluntary hypoventilation. Such a procedure may even have therapeutic relevance in heat stroke patients, where the length and the magnitude of the hyperthermic period is critical for the course and prognosis of the heat stroke (Shani et al. 2001). We therefore suggest that a rapid restoration of CBF under such conditions may lower the risk of heat-induced damage of cerebral tissue.
Methodological considerations
Heat loss by other mechanisms than convective removal via the jugular venous blood was calculated to account for ≈20 % of the total cerebral energy turnover in both the hyperthermic and normothermic exercise trial, consistent with the calculations presented by Shevelev (1998). This unaccounted-for loss of heat may include thermal conductance through the skull and it is also possible that some electrical energy may leave the brain through the skull, as electrical signals (EEG) can be detected on the outside of the head. Also heat loss via the blood could be underestimated due to methodological problems. Although, the Kety-Schmidt technique is regarded as the standard method for determination of global CBF (Lassen, 1985) it may estimate jugular venous blood flow rather than global CBF (Ide & Secher, 2000). A fraction of the CBF leaves the brain via other pathways than the jugular vein (Shenkin et al. 1948), consequently heat transfer via the circulation may be larger than what was measured as heat removal via the internal jugular venous blood.
Furthermore, heat removal via the jugular venous blood may also have been slightly underestimated. A crucial assumption for the calculations of cerebral heat exchange is that the blood in the internal jugular vein represents cerebral venous blood. Although the thermocouple was positioned in the bulb of the internal jugular vein, a slight (≈2 %) admixture of extracranial origin may be expected (Shenkin et al. 1948). Contamination by cool extracranial blood will result in an underestimation of the cerebral v-aDtemp and consequently also underestimate heat removal via the jugular venous blood. However, the finding that the jugular venous to arterial temperature difference was unaffected by a 5 °C reduction in mean head skin temperature indicates that the influence from admixture of extracranial blood is negligible.
Assuming that the global CBF and the cerebral oxygen uptake were similar at rest and during the control trial (Scheinberg et al. 1953; Madsen et al. 1993), it appears that ≈95 % of the cerebral heat production is removed via the jugular venous blood at rest (v-aDtemp + 0.33 °C), whereas heat removal via the jugular venous blood only accounts for ≈75 % of the cerebral loss of heat during the normothermic exercise bout. This inconsistency between rest and exercise could very well relate to an underestimation of the cerebral v-aDtemp during exercise, because the arterial blood, as already discussed, may be cooled on its passage from the aortic arch to the brain when the pulmonary ventilation is increased.
The present determinations of jugular venous blood temperature and cerebral heat exchange reveal that cerebral heat balance is achieved after ≈15 min of exercise in a thermoneutral environment. In contrast, cerebral heat production is increased, heat removal via the jugular venous blood is reduced and heat is continuously stored in the brain during hyperthermic exercise. The finding that the cerebral venous blood temperature, both at rest and during exercise with or without hyperthermia, was higher than that of the arterial blood indicates that the brain had a higher temperature than the body core. The arterial blood may be cooled on its passage from the heart to the brain and such pre-cooling of the arterial blood will benefit heat removal from the brain. However it appears that the arterial blood temperature is lowered by less than 0.1 °C and that is not sufficient to create a lowering of the brain temperature below that of the aortic blood. This picture was unaltered by facial fanning and it appears that hyperthermic humans, in contrast to some animal species, are unable to establish a brain temperature, which is lower than the trunk temperature.
References
- Aaslid R, Markwalder T-M, Nornes H. Noninvasive trancranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. Journal of Neurosurgery. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Bangsbo J. Quantification of anaerobic energy production during intense exercise. Medicine and Science in Sports and Exercise. 1998;30:47–52. doi: 10.1097/00005768-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Borg G. Simple rating for estimation of perceived exertion. In: Borg G, editor. Physical Work and Effort. New York: Pergamon; 1975. pp. 39–46. [Google Scholar]
- Brengelmann GL. Specialized brain cooling in humans. FASEB Journal. 1993;7:1148–1153. doi: 10.1096/fasebj.7.12.8375613. [DOI] [PubMed] [Google Scholar]
- Brinnel H, Nagasaka T, Cabanac M. Enhanced brain protection during passive hyperthermia in humans. European Journal of Applied Physiology. 1987;56:540–545. doi: 10.1007/BF00635367. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Selective brain cooling in humans: ‘fancy’ or fact. FASEB Journal. 1993;7:1143–1146. doi: 10.1096/fasebj.7.12.8375612. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Germain M, Brinnel H. Tympanic temperatures during hemiface cooling. European Journal of Applied Physiology. 1987;56:534–539. doi: 10.1007/BF00635366. [DOI] [PubMed] [Google Scholar]
- Caputa M, Feistkorn G, Jessen C. Effect of brain and trunk temperatures on exercise performance in goats. Pflügers Archiv. 1986;406:184–189. doi: 10.1007/BF00586681. [DOI] [PubMed] [Google Scholar]
- Corbett RJ, Laptook AR. Failure of localized head cooling to reduce brain temperature in adult humans. Neuroreport. 1998;9:2721–2725. doi: 10.1097/00001756-199808240-00007. [DOI] [PubMed] [Google Scholar]
- Crezee J, Lagendijk JJW. Temperature uniformity during hyperthermia: the impact of large vessels. Physics in Medicine and Biology. 1992;37:1321–1337. doi: 10.1088/0031-9155/37/6/009. [DOI] [PubMed] [Google Scholar]
- Desruelle AV, Candas V. Thermoregulatory effects of three different types of head cooling in humans during a mild hyperthermia. European Journal of Applied Physiology. 2000;81:33–39. doi: 10.1007/PL00013794. [DOI] [PubMed] [Google Scholar]
- Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. Journal of Applied Physiology. 1998;84:877–883. doi: 10.1152/jappl.1998.84.3.877. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen S, Jensen F, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Journal of Applied Physiology. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Hanson RDG. Respiratory heat loss at increased core temperature. Journal of Applied Physiology. 1974;37:103–107. doi: 10.1152/jappl.1974.37.1.103. [DOI] [PubMed] [Google Scholar]
- Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Progress in Neurobiology. 2000;61:397–414. doi: 10.1016/s0301-0082(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Jessen C, Kuhnen G. No evidence for brain stem cooling during face fanning in humans. Journal of Applied Physiology. 1992;72:664–669. doi: 10.1152/jappl.1992.72.2.664. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. Journal of Clinical Investigation. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. Normal average value of CBF in young adults is 50 ml/100 g/min. Journal of Cerebral Blood Flow and Metabolism. 1985;5:347–349. doi: 10.1038/jcbfm.1985.48. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiødtz G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. Journal of Applied Physiology. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245. [DOI] [PubMed] [Google Scholar]
- Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. Journal of Applied Physiology. 1999;87:1609–1613. doi: 10.1152/jappl.1999.87.5.1609. [DOI] [PubMed] [Google Scholar]
- Mellergård P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery. 1992;31:671–677. doi: 10.1227/00006123-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Nagasaka T, Brinnel H, Hales JR, Ogawa T. Selective brain cooling in hyperthermia: the mechanisms and medical implications. Medical Hypotheses. 1998;50:203–211. doi: 10.1016/s0306-9877(98)90019-6. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Nunneley SA. Brain temperature and limits on transcranial cooling in humans: quantitative modelling results. European Journal of Applied Physiology Occupational Physiology. 1998;78:353–359. doi: 10.1007/s004210050431. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales JRS, Strange NJ, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. Journal of Physiology. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Jessen C. Evidence against brain stem cooling by face fanning in severely hyperthermic humans. Pflügers Archiv. 1992;422:168–172. doi: 10.1007/BF00370416. [DOI] [PubMed] [Google Scholar]
- Nybo L, Jensen T, Nielsen B, Gonzalez-Alonso J. Effects of marked hyperthermia with and without dehydration on VO2 kinetics during intense exercise. Journal of Applied Physiology. 2001;90:1057–1064. doi: 10.1152/jappl.2001.90.3.1057. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. Journal of Applied Physiology. 2001a;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. Journal of Physiology. 2001b;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. Journal of Applied Physiology. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. Journal of Applied Physiology. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- Rasch W, Samson P, Cote J, Cabanac M. Heat loss from the human head during exercise. Journal of Applied Physiololy. 1991;71:590–595. doi: 10.1152/jappl.1991.71.2.590. [DOI] [PubMed] [Google Scholar]
- Rubenstein E, Meub DW, Eldridge F. Common carotid blood temperature. Journal of Applied Physiology. 1960;15:603–604. doi: 10.1152/jappl.1960.15.4.603. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Blackburn LI, Saslaw M, Rich M, Baum G. Cerebral circulation and metabolism in pulmonary emphysemia and fibrosis with observations on the effects of mild exercise. Journal of Clinical Investigation. 1953;32:720–728. doi: 10.1172/JCI102786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani Y, Heled Y, Epstein Y, Shapiro Y, Moran DS. The effect of rapid cooling on the course and prognosis of exertional heat stroke - two case reports. Proceedings of the International Thermal Symposium. 2001;32:143. [Google Scholar]
- Shenkin HA, Harmel MH, Kety SS. Dynamic anatomy of the cerebral circulation. Archives of Neurological Psychiatry. 1948;60:240–252. doi: 10.1001/archneurpsyc.1948.02310030021002. [DOI] [PubMed] [Google Scholar]
- Shevelev IA. Functional imaging of the brain by infrared radiation (thermoencephaloscopy) Progress in Neurobiology. 1998;56:267–305. doi: 10.1016/s0301-0082(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Shiraki K, Sagawa S, Tajima F, Yokota A, Hashimoto M, Brengelmann GL. Independence of brain and tympanic temperatures in an unanesthetized human. Journal of Applied Physiology. 1988;65:482–486. doi: 10.1152/jappl.1988.65.1.482. [DOI] [PubMed] [Google Scholar]
- Walters TJ, Ryan KL, Tate LM, Mason PA. Exercise in the heat is limited by a critical internal temperature. Journal of Applied Physiology. 2000;89:799–806. doi: 10.1152/jappl.2000.89.2.799. [DOI] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proceedings of the National Academy of Sciences of the USA. 2000;97:7603–7608. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]


