Abstract
Light has been shown to modulate NMDA receptor function. In this study, we have performed experiments aimed at elucidating the putative site of action of light within the receptor structure. Whole-cell recordings were performed in Chinese hamster ovary cells expressing various combinations of NMDA receptor subunits. Although there was no apparent difference in the actions of light between wild-type NR1-NR2A and NR1-NR2B subunit configurations, the light enhancement of NMDA-induced currents was either completely abolished or substantially diminished in the redox site mutants NR1a (C744A, C798A)-NR2B and NR1a (C744A, C798A)-NR2A. Further studies demonstrated that chemical reduction of NR1a-NR2B NMDA receptors decreased its sensitivity to light. In addition, sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES), used to irreversibly bind free sulfhydryl groups and inactivate the redox site, abolished the effects of light on wild-type receptors. In contrast, no free sulfhydryls were available for MTSES following light stimulation, suggesting that light itself could not reduce the redox modulatory site. Our results suggest that a functionally intact, oxidized redox site is necessary for light-induced potentiation. Hence, light and redox modulation of the NMDA receptor may share a common intramolecular pathway for altering the function of this ion channel.
NMDA receptor function has been intimately linked to many critical processes in the brain, as the activity of this ion channel is not only responsible for changes in synaptic efficacy, but also for mediating excitotoxic neuronal cell death (Ozawa et al. 1998). The NMDA receptor channel is gated by the co-agonists glutamate and glycine and its function is modulated by a number of endogenous agents such as protons, zinc, polyamines and intracellular Ca2+ (Dingledine et al. 1999).
Physical factors, such as heat, mechanical stretch and light have also been shown to dramatically influence NMDA-induced currents (Ascher et al. 1988; Paoletti & Ascher, 1994; Chung & Kuyucak, 1995; Casado & Ascher, 1998; Leszkiewicz et al. 2000a). Previously, we have demonstrated that a brief (600 ms to 2 s) light pulse containing UV and visible wavelengths reversibly potentiates NMDA receptor-mediated responses in cultured central neurones (Leszkiewicz et al. 2000a). Furthermore, wavelengths and intensities of light that typically reach the retina also enhanced NMDA-induced currents, suggesting a potential physiological role for this type of modulation. In addition to our results with NMDA receptors, our laboratory and others have demonstrated that GABAA receptor-mediated responses are also potentiated by light (Leszkiewicz et al. 2000b, 2002; Chang et al. 2001). Middendorf and colleagues (2000) have also reported that cyclic nucleotide-gated channel (CNG) currents are attenuated following exposure to UV light. Although these studies have confirmed that light modulates the function of certain ion channels, the potential light-sensitive moieties within these receptors remain undefined.
We have previously established that the glutamate and glycine binding sites, as well as the proton-sensitive site of the NMDA receptor are unaffected by light (Leszkiewicz et al. 2000a). Thus, we began to search for other NMDA receptor moieties that could be responsible for the light enhancement of agonist-induced currents. A substantial number of different modulatory proteins and ion channels contain PAS domains (named for repeat sequences in Drosophila proteins, Period clock protein, Aryl hydrocarbon receptor nuclear translocator, and Single-minded protein), consensus sequences responsible for conferring redox modulation, and in some cases, light sensitivity (reviewed by Taylor & Zhulin, 1999). While we were unable to locate any PAS domain sequences within any of the NMDA receptor subunits, it is possible that other analogous light- and redox-sensitive sites exist in proteins. Since, NMDA-induced currents are modified following exposure to redox agents (Aizenman et al. 1989; Tang & Aizenman, 1993), we hypothesized that the redox modulatory site may be linked in some way to the actions of light. Here, we describe experiments that demonstrate that a functionally intact, oxidized redox site is required for the actions of light at the NMDA receptor.
Methods
Tissue culture
Chinese hamster ovary (CHO) cells were transfected as previously described (Boeckman & Aizenman, 1996; Brimecombe et al. 1997). Briefly, cells were grown in Ham's F-12 nutrient medium with 10 % fetal bovine serum and 1 mm glutamine, and were passaged 2-3 times per week for a total of no more than 30 times. Functional receptors were expressed by transfecting the cDNAs of wild-type NR1a or NR1b, or the redox-insensitive mutants NR1a (C744A, C798A) or NR1b (C765A, C819A), along with either NR2A or NR2B; green fluorescent protein (GFP) was used as a positive transfection marker (Brimecombe et al. 1997). The transfection protocol consisted of a 4 h incubation at 37 °C in serum-free medium containing a total of 1.4 μg of cDNA (0.34 μg NR1, 1.01 μg NR2 and 0.05 μg GFP) and Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA). Following the incubation, the medium was exchanged for serum-containing medium including 300 μm ketamine to prevent receptor-mediated cell death (Boeckman & Aizenman, 1996). Cells were replated onto coverslips at 24 h post transfection; electrophysiological recordings typically started 24 h later.
Electrophysiological recordings
Electrophysiological recordings were conducted using previously described methods (Tang & Aizenman, 1993). The extracellular recording solution contained (mm): NaCl, 150; KCl, 2.8; CaCl2, 1.0; Hepes, 10; glycine, 0.01; pH adjusted to 7.2 with 0.3 m NaOH. The intracellular (pipette) solution contained (mm): CsF, 140; EGTA, 10; CaCl2, 1.0; pH adjusted to 7.2 with CsOH. Whole-cell measurements were performed under voltage clamp with 2 MΩ electrodes. Data were filtered at 0.5-1 kHz and digitized at 1-2 kHz (Axopatch 1C Amplifier and DigiData 1200 Interface; Axon Instruments, Union City, CA, USA). NMDA, glycine, dithiothreitol (DTT) and 5,5′-dithio-bis-(2-nitro-benzoic acid) (DTNB) were dissolved in external solution and applied to cells using a multi-barrel fast perfusion system (Warner Instruments, Hamden, CT, USA). Sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES; Toronto Research Chemicals, Toronto, Canada) was dissolved in DMSO, and then diluted in external solution to a final concentration of 10 mm. All drugs and chemicals were obtained from Sigma-Aldrich Corp. (St Louis, MO, USA) unless otherwise noted.
Light stimuli
Light was directed on to cells as previously described (Leszkiewicz et al. 2000a; Kandler et al. 1998, 1999). Light from a 100 W mercury lamp (Oriel Corporation, Stratford, CT, USA) was focused through an electronic shutter (Vincent Associates, Rochester, NY, USA) and guided on to the cells via a 50 μm (i.d.) fused silica light fibre. In order to decrease the exposure of the cells to UV wavelengths, a 280 nm long-pass filter (Oriel Corporation) was employed in all of the experiments.
Results
Light modulation of recombinant NMDA receptors
We tested the effects of light on NR1a-NR2A and NR1a-NR2B receptors transiently expressed in CHO cells. NMDA receptor-mediated responses were measured both before and 5 s after a brief pulse of light that illuminated the entire cell (1 s, > 280 nm; Fig. 1A). The ‘light sensitivity’ of the NMDA receptor was defined as the ratio of post-flash to pre-flash maximum current amplitudes (I*/I; Leszkiewicz et al. 2000a). NMDA-induced currents mediated by wild-type NR1a-NR2A and NR1a-NR2B receptors were potentiated approximately 100 % in response to the brief light pulse, as reflected by an I*/I ratio near 2 (Fig. 1B). We also tested wild-type NR1b subunits paired with NR2A and NR2B, and found no difference between the light sensitivities of these receptors when compared to NR1a-containing receptors (data not shown).
Figure 1. The redox site on the NR1 subunit of the NMDA receptor is required for light sensitivity.
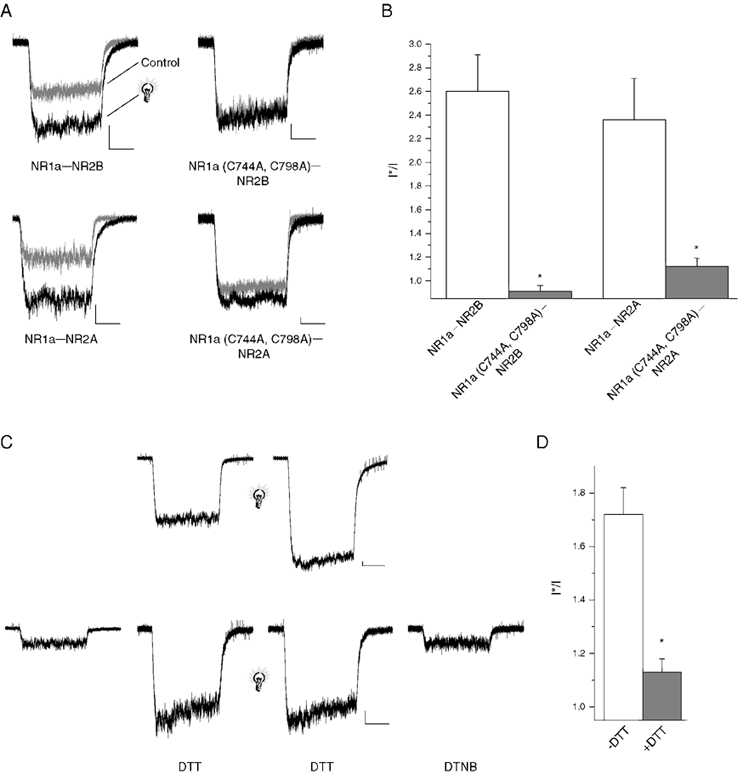
A, whole-cell NMDA (10 μm)-induced currents from receptors NMDA receptors transiently expressed in CHO cells (membrane potential: −60 mV in this and all subsequent figures). Sample traces represent currents before (grey trace) and shortly after (5 s; black trace) a brief light pulse (2 s, > 280 nm). Similar effects were seen in 5-12 cells for each subunit configuration. Scale bars: 100 pA and 1 s. B, peak response amplitudes for responses such as those seen in A were measured and a post-flash to pre-flash (I*/I) ratio was calculated (* P < 0.05, significantly different from wild-type control, Student's two tailed t test; n = 5–12 cells). C, top, NMDA-elicited responses from NR1a-NR2B receptors both before and after a brief flash of light (light bulb; 2 s, > 280 nm) Scale bars: 500 pA and 500 ms. C, bottom, NMDA-mediated responses from NR1a-NR2B receptors before and after 8 min of 5 mm DTT incubation. Chemically reduced receptors were then exposed to brief flash (2 s, > 280 nm) followed by chemical oxidation with 500 μm DTNB (2 min). Scale bars: 100 pA and 1 s. D, I*/I ratios demonstrating a significant decrease in light sensitivity following chemical reduction (* P < 0.05, significantly different from ‘-DTT’ control, Student's two tailed t test, n = 5–7 cells).
Our earlier studies demonstrated that the glutamate and glycine binding sites of the NMDA receptor, as well as the proton-sensitive site, were unaffected by light (Leszkiewicz et al. 2000a). As some proteins containing PAS domains have redox sites that may be structurally related to light-sensitive sites (Taylor & Zhulin, 1999), we tested the effects of light on the redox-insensitive NR1a (C744A, C798A)-NR2B subunit combination (Sullivan et al. 1994; Brimecombe et al. 1999). Whole-cell NR1a (C744A, C798A)-NR2B-mediated responses were completely unaffected by light (1 s, > 280 nm; Fig. 1A, bottom right). The light sensitivity, or lack thereof, for this subunit combination was substantially different from the wild-type NR1a subunit (Fig. 1B). The NR1a (C744A, C798A)-NR2A receptor, which still retains some redox sensitivity (Brimecombe et al. 1999), was only partially sensitive to light (Fig. 1A, top right). Nonetheless, the I*/I ratio of this mutant receptor configuration was still drastically different from the wild-type receptor (Fig. 1B). A similar decreased sensitivity to light was seen when the NR1a (C744A, C798A) subunit was replaced with the NR1b subunit containing point mutations of the equivalent cysteines C765A and C819A (data not shown). These data strongly suggest that the presence of an intact redox site in NR1 is necessary for the potentiation of NMDA receptor-mediated responses following a brief pulse of light.
Redox state of the NMDA receptor influences light sensitivity
After establishing that the presence of a redox site in NR1 is necessary for light-induced potentiation, we determined whether changes in the redox state alter the light sensitivity of wild-type receptors. The light sensitivity of receptors oxidized with 500 μm DTNB was similar to receptors unexposed to DTNB (data not shown). This result is not surprising since NMDA receptors in our culture conditions tend to be mostly oxidized at the onset of recording (Tang & Aizenman, 1993). NMDA receptors in another group of cells were reduced following exposure to 5 mm DTT for 5-8 min. The reduced state of the NMDA receptor substantially attenuated their light sensitivity (Fig. 1C and D). These results suggest that chemical reduction and light-induced potentiation of the NMDA receptor may be mediated via the same mechanism.
Inactivation of the redox site by MTSES alters light sensitivity of the NMDAR
MTSES and related compounds irreversibly bind free sulfhydryl groups of proteins and have been useful in cysteine scanning mutagenesis studies (Akabas et al. 1992; Kuner et al. 1996) and redox modulation experiments (Wang et al. 1997). We tested the effects of light following the irreversible alteration of sulfhydryl groups within the NMDA receptors. Receptors were first reduced with 5 mm DTT for 5-8 min followed by exposure to 10 mm MTSES for 1 min. DTT only (-MTSES) and DTT-MTSES (+MTSES)-treated cells were exposed to 500 μm DTNB for 2 min to confirm the permanent inactivation of the redox site on the NMDA receptor. Previous results from our laboratory have similarly demonstrated the inactivation of the redox site on the NMDA receptor with the alkylating agent N-ethylmalemide (Tang & Aizenman, 1993). Inactivation of the redox site was quantified as the degree of oxidation with DTNB following potentiation with DTT (% oxidation; Fig. 2B). MTSES-treated cells were substantially less sensitive to DTNB oxidation than their untreated counterparts (Fig. 2B, top). Since MTSES treatment of NMDA receptors could in fact largely abolish redox modulation, we then evaluated whether MTSES inactivation of the redox site altered the light sensitivity of the receptor. The light sensitivities of chemically reduced MTSES-treated and untreated NMDA receptors were tested by exposure to a brief pulse of light (2 s, > 280 nm; Fig. 2A). Following re-oxidation with DTNB, we observed that the light-induced potentiation of MTSES-treated receptor responses was significantly less than in non-MTSES-treated cells (Fig. 2B, bottom). These results indicated that chemically inactivating the redox site decreases the light sensitivity of the receptor, a result akin to using cysteine mutated NMDA receptors.
Figure 2. MTSES inactivates redox modulation following chemical reduction but not light-induced potentiation.
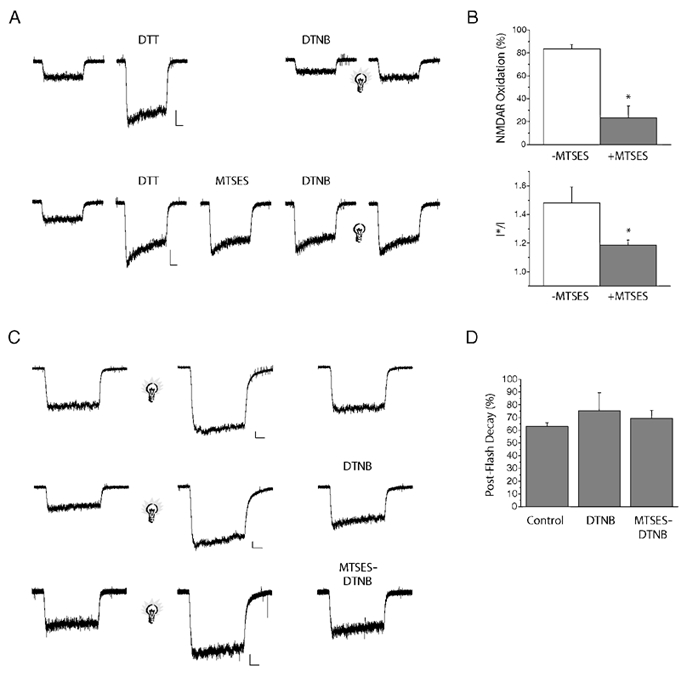
A, top, sample trace showing whole-cell NR1a-NR2B receptor-mediated responses (10 μm NMDA) following potentiation with DTT, oxidation with DTNB, and a brief pulse of light (light bulb; 2 s, > 280 nm). A, bottom, redox sites were inactivated by exposure to 10 mm MTSES (+MTSES) following chemical reduction (DTT). Receptors were then oxidized (500 μm DTNB) and exposed to light (2 s, > 280 nm). Scale bars: 500 pA and 500 ms. B, top, quantification of the amount of oxidation following exposure to DTT in the presence and absence of 10 mm MTSES (% oxidation was calculated as (IDTT/IDTNB) × 100; * P < 0.05, significantly different from - MTSES control, Student's two tailed t test, n = 5–6 cells). B, bottom, I*/I ratio of receptor responses after incubation with DTT in the presence and absence of MTS compound (* P < 0.05, significantly different from - MTSES control, Student's two tailed t test, n = 5–6 cells). C, sample traces of NMDA-elicited responses both before and after a light flash (light bulb; 2 s, > 280 nm). Cells were then exposed to control external solution, 500 μm DTNB, or MTS reagents followed by 500 μm DTNB. Scale bars: 250 pA and 500 ms. D, quantification of the amount of decay 3 min after a brief flash of light, in the presence of control external solution, DTNB or MTS reagents followed by DTNB (P > 0.05, no significant difference between control external and the DTNB and MTS-DTNB-treated groups, a one-way ANOVA, n = 4–6 cells).
After establishing that MTSES irreversibly inactivates the redox site on the NR1 subunit, we investigated whether light could directly reduce the redox site. For this purpose, DTNB-oxidized receptors were exposed to a brief flash of light (2 s, > 280 nm) and then immediately incubated with 10 mm MTSES. After incubation with MTSES for 1 min, the cells were oxidized again with DTNB for 2 min. to test for the inactivation of the redox site (Fig. 2C). Cells treated with MTSES after the light pulse were oxidized to the same degree as untreated cells, indicating that NMDA receptors had not been reduced by the light flash (Fig. 2D). MTSES had access to light-enhanced receptors as the currents remained potentiated by 42 ± 5 % above baseline 1 min after the light flash (mean ± s.e.m.; n = 3, data not shown). These results suggest that even though an intact functional redox site is necessary for the effects of light to be manifested, light itself does not appear to alter the redox state of the receptor.
Discussion
The results presented in this study demonstrate that light sensitivity of the NMDA receptor is essentially abolished following the mutation of cysteines 744 and 798 on the NR1a subunit or cysteines 765 and 819 on the NR1b subunit. The presence of additional redox sites on the NR2A subunit may be responsible for the retention of the light effect following mutations in the redox site on the NR1 subunit. We have also shown that the chemical reduction of the NMDA receptor attenuated the effects of light on agonist-induced currents. Additional experiments demonstrated that MTSES inactivation of redox modulation also decreased the effects of light. As such, we conclude that the light sensitivity of the NMDAR is critically dependent on the presence of a functionally intact, oxidized redox site on the NR1 subunit.
Two other research groups have described the UV modification of ion channels in vitro. Chang et al. (2001), described the light-induced potentiation of GABAA receptors, while Middendorf et al. (2000) demonstrated the attenuation of CNG channel currents by UV light. Interestingly, it has also been shown that both the GABAA receptor and the CNG channels can be modulated via redox agents (Broillet & Firestein, 1996; Amato et al. 1999). GABAA-mediated responses can be potentiated following chemical reduction with DTT, and subsequently attenuated with DTNB (Amato et al. 1999). The inclusion of the γ2S subunit not only eliminated the redox sensitivity of the GABAA receptor, but also abolished the enhancement in the maximum current amplitudes induced in these receptors by a long-duration, high-intensity UV pulse of light (Amato et al. 1999; Chang et al. 2001). Interestingly, the CNG channel is activated following exposure to DTNB and inactivated following exposure to DTT (Broillet & Firestein, 1996). As such, the effects of light mimic the effects of chemical reduction; both NMDA and GABAA receptor-mediated responses are potentiated by DTT and light while CNG channels are inhibited by DTT and light. Together, these observations seem to functionally link the redox and light sensitivity of these ligand-gated channels.
Although we were unable to demonstrate it here, it is conceivable that the redox site on the NR1 subunit of the NMDA receptor may still be directly reduced following a brief flash of light. Previous investigations have demonstrated that light inactivation of proteins in vitro can be mediated by both aromatic and non-aromatic residues (Vladimirov et al. 1970). A number of studies have also shown that disulfide bonds can be hydrolysed with UV light (review by Creed, 1984), and the presence of aromatic amino acids such as tryptophan leads to a 2- to 4-fold greater effect of light on the inactivation of nearby cysteine residues (Risi et al. 1967). Further, Middendorf et al. (2000) demonstrated that the combination of the tryptophan and cysteine absorbance spectra best fitted the action spectrum of light modulation of the CNG channel. Hence, the possibility exists that MTSES did not have access to a sufficient number of free sulfhydryls following light exposure for us to observe a noticeable effect. Since potentiation following chemical reduction is approximately 6-fold greater than light-induced potentiation, it is feasible that a smaller number of disulfide bonds are reduced following the light pulse. This, in combination with the spontaneous reversal of the light effect, may lead to the presence of fewer free sulfhydryls following a light pulse, and a failure of MTSES to effectively inactivate the redox site. However, given the lack of data, we must conclude at this point that light does not hydrolyse the disulfide bond and may modulate the NMDA receptor via a different transduction mechanism that requires an oxidized redox state.
In summary, these results suggest that the presence of an intact functional redox site is necessary for the light-induced potentiation of the NMDA receptor. Additionally, we have demonstrated that the effects of light are attenuated following the chemical reduction of the NMDA receptor. Collectively, these results show that light and redox modulation of the NMDA receptor may induce equivalent allosteric changes that lead to alterations in the function of this ion channel.
Acknowledgments
We thank S. Du and K. Hartnett for technical assistance, G. A. Herin, S. Pal and Drs M. Cascio, J. Horn, J. Johnson and K. Kandler for helpful discussions throughout the course of these investigations. This work was supported by NIH grant NS29365 (E.A.), and by an American Heart Association grant (D.N.L.).
References
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Amato A, Connolly CN, Moss SJ, Smart TG. Modulation of neuronal and recombinant GABAA receptors by redox reagents. Journal of Physiology. 1999;517:35–50. doi: 10.1111/j.1469-7793.1999.0035z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Bregestovski P, Nowak L. N-Methyl-d-aspartate-activated channels of mouse central neurones in magnesium-free solutions. Journal of Physiology. 1988;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-d-aspartate receptor subunits. Journal of Pharmacology and Experimental Therapeutics. 1996;279:515–523. [PubMed] [Google Scholar]
- Brimecombe JC, Boeckman FA, Aizenman E. Functional consequences of NR2 subunit composition in single recombinant N-methyl-D-aspartate receptors. Proceedings of the National Academy of Sciences of the USA. 1997;94:11019–11024. doi: 10.1073/pnas.94.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimecombe JC, Potthoff WK, Aizenman E. A critical role of the N-methyl-D-aspartate (NMDA) receptor subunit (NR) 2A in the expression of redox sensitivity of NR1/NR2A recombinant NMDA receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;291:785–792. [PubMed] [Google Scholar]
- Broillet MC, Firestein S. Direct activation of the olfactory cyclic nucleotide-gated channel through modification of sulfhydryl groups by NO compounds. Neuron. 1996;16:377–385. doi: 10.1016/s0896-6273(00)80055-0. [DOI] [PubMed] [Google Scholar]
- Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. Journal of Physiology. 1998;513:317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Xie Y, Weiss DS. Positive allosteric modulation by ultraviolet irradiation on GABAA, but not GABAC, receptors expressed in Xenopus oocytes. Journal of Physiology. 2001;536:471–478. doi: 10.1111/j.1469-7793.2001.0471c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Kuyucak S. Changes in the kinetics and conductance of N-methyl-D-aspartate (NMDA)-receptor activated single channels with temperature. Neuroscience Letters. 1995;187:181–184. doi: 10.1016/0304-3940(95)11369-8. [DOI] [PubMed] [Google Scholar]
- Creed D. The photophysics and photochemistry of the near-UV asorbing amino acids - III. Cystine and its simple derivatives. Photochemistry and Photobiology. 1984;39:577–583. [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacology Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Kandler K, Givens R, Katz LC. Photostimulation with caged glutamate. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: A Laboratory manual. NY, USA: Cold Spring Harbor Laboratory Press; 1999. pp. 27.1–27.9. [Google Scholar]
- Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nature Neuroscience. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Karlin A, Seeburg PH, Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Leszkiewicz DN, Kandler K, Aizenman E. Enhancement of NMDA receptor-mediated currents by light in rat neurones in vitro. Journal of Physiology. 2000a;524:365–374. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszkiewicz DN, Kandler K, Aizenman E. Enhancement of GABA-mediated currents by light. Society for Neuroscience Abstracts. 2000b;00:000. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszkiewicz DN, McLaughlin BA, Aizenman E. Protein kinases and light: unlikely partners in a receptor localization puzzle. Physiology and Behavior. 2002 doi: 10.1016/s0031-9384(02)00911-3. in the Press. [DOI] [PubMed] [Google Scholar]
- Middendorf TR, Aldrich RW, Baylor DA. Modification of cyclic nucleotide-gated ion channels by ultraviolet light. Journal of General Physiology. 2000;116:227–252. doi: 10.1085/jgp.116.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progress in Neurobiology. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Risi S, Dose K, Rathinasamy TK, Augenstein L. The effect of environment on cystine disruption by ultraviolet light. Photochemistry and Photobiology. 1967;6:423–436. doi: 10.1111/j.1751-1097.1967.tb08889.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Tang LH, Aizenman E. The modulation of N-methyl-d-aspartate receptors by redox and alkylating reagents in rat cortical neurones in vitro. Journal of Physiology. 1993;465:303–323. doi: 10.1113/jphysiol.1993.sp019678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential and light. Microbiology and Molecular Biology Reviews. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov YA, Roshchupkin DI, Fesenko EE. Photochemical reactions in amino acid residues and inactivation of enzymes during U. V.-irradiation. Photochemistry and Photobiology. 1970;11:227–246. doi: 10.1111/j.1751-1097.1970.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Nara M, Wang YX, Kotlikoff MI. Redox regulation of large conductance Ca(2+)-activated K+ channels in smooth muscle cells. Journal of General Physiology. 1997;110:35–44. doi: 10.1085/jgp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


