Abstract
Xanthine oxidoreductase (XOR), a key enzyme of purine metabolism, has been implicated in the secretion of the milk fat droplet in lactating mammary epithelial cells, possibly through structural interactions with other milk fat globule proteins including butyrophilin (Btn) and adipophilin (ADPH). To help determine the mechanism by which XOR is regulated, we examined the expression and localization of XOR in the non-secretory states of late pregnancy and induced involution compared with the state of active secretion. XOR mRNA levels started to increase at mid-pregnancy, turned sharply upwards at the onset of lactation and decreased rapidly with forced involution, indicating transcriptional control of the enzyme level by differentiation and secretory function. During pregnancy and involution the enzyme was diffusely distributed in the cytoplasm, but moved rapidly to the apical membrane of the cells when secretion was activated, where it colocalized with both Btn and ADPH, similar to the situation in the milk fat globule itself. Size-exclusion chromatography of solubilized milk fat globule membrane proteins showed that XOR formed a sulphydryl-bond-dependent complex with Btn and ADPH in the milk fat globule membrane. XOR returned to a diffuse cytoplasmic localization shortly after induced involution, while Btn remained localized to the apical membrane, suggesting that localization of XOR is not dependent on the presence of Btn in the apical membrane. Our findings indicate that the expression and membrane association of XOR in the mammary gland are tightly regulated by secretory activity, and suggest that the apical membrane association of XOR regulates the coupling of lipid droplets to the apical plasma membrane during milk lipid secretion.
Xanthine oxidoreductase (XOR) is a highly conserved, ubiquitously expressed protein whose principal physiological function is generally understood to be the regulation of purine degradation by catalysing the oxidation of hypoxanthine and xanthine to uric acid (Stryer, 1988). Although there is extensive biochemical and genetic data to support this function, there is increasing evidence that XOR may play a structural role in milk lipid secretion. It has been known for nearly a century that milk is one of the richest sources of XOR (Bray, 1975; Hille & Nishino, 1995). The enzyme in milk is associated with milk fat globules (MFGs) and is a major component of the MFG membrane (Mather, 1987; Keenan & Patton, 1995). XOR is also found at high concentrations in the lactating alveolar cell, comprising 1-2 % of the soluble protein in lactating glands from cattle and mice (Keenan & Patton, 1995; McManaman et al. 1999). Moreover, it has been shown that the expression of XOR mRNA is elevated in alveolar epithelial cells during pregnancy (Hayden et al. 1991; Kurosaki et al. 1996), and the protein has been immunolocalized to the apical plasma membrane of these cells in lactating glands (Jarasch et al. 1981). The finding that the human milk enzyme exists primarily in a demolybdo form that is markedly reduced in its ability to catalyse xanthine oxidation (Abadeh et al. 1992; Godber et al. 1997) raises the possibility that its mammary function may not correspond entirely to its traditional enzymatic role in purine degradation.
An alternative role for XOR in the secretion of milk lipids has been proposed by Mather & Keenan (1998). Milk lipid globules are secreted as structures that are enveloped in apical plasma membrane through as yet unknown processes. Electron microscope and biochemical analyses have shown that the triglyceride core of these structures is separated from the membrane bilayer by a proteinaceous coat that is thought to be composed primarily of XOR, the cytoplasmic lipid-droplet-associated protein adipophilin (ADPH) and the cytoplasmic domain of the integral plasma membrane protein butyrophilin (Btn) (Keenan & Patton, 1995; Mather & Keenan, 1998). Potential interactions between XOR and Btn are indicated by the finding that a recombinant glutathione-S-transferase fusion protein containing the cytoplasmic C-terminal (A271-P524) domain of mouse Btn bound XOR in soluble extracts of cultured mammary epithelial cells (Ishii et al. 1995). These observations suggest that the plasma membrane localization of XOR involves interactions with Btn, and have led to the proposal that a Btn-XOR complex interacts with ADPH, or other proteins, on the surface of lipid droplets to mediate their association with the apical plasma membrane during lipid secretion (Mather & Keenan, 1998). At present there is no direct evidence to support this mechanism; however, if it is correct then the expression and apical membrane localization of XOR should correlate with the lactational status of the mammary gland, and complexes between XOR and Btn and ADPH should be present at the apical plasma membrane during lactation. To test this hypothesis and further clarify the potential involvement of XOR in lipid secretion, we have characterized XOR expression and localization during secretory differentiation and in response to physiological alterations in the secretory status of the mammary gland during lactation, and we have investigated interactions between XOR, Btn and ADPH in lactating mammary tissue and in isolated MFGs.
It has been shown that secretory differentiation comprises an early (initiation) and late (activation) phase, also referred to as lactogenesis-1 and −2, respectively (Hartmann, 1973; Fleet et al. 1975, Neville et al. 2002). The initiation phase begins around mid-pregnancy and is associated with increased expression of some milk proteins (such as β-casein) and enzymes involved in lipid synthesis (Neville et al. 2002). Some epithelial cells are sufficiently differentiated by this stage to secrete small quantities of milk-specific components. However, generalized secretion is held in check by high circulating levels of progesterone (Kuhn, 1977). The fall in progesterone, which occurs at around parturition in rodents, leads to the concerted amplification of the production of milk proteins (Hobbs et al. 1982; Neville et al. 2002) and biosynthetic enzymes (Baldwin & Yang, 1974), closure of tight junctions (Nguyen et al. 2001) and ultrastructural changes associated with copious milk secretion (Hollman, 1974). The synthetic and secretory capacities of the mammary gland are regulated by both systemic hormones and local factors (Wilde & Hurley, 1996), and preventing milk removal (milk stasis) for prolonged periods induces a coordinated multiphase involution process involving decreased gene expression, induction of apoptosis and regression of alveolar structures (Strange et al. 1992; Lund et al. 1996; Li et al. 1997; Marti et al. 1997). As yet it is unknown if secretory activity is required to maintain the expression and membrane localization of XOR. However, determining how XOR expression and localization are affected by changes in secretory status, and correlating these effects with the cellular and physiological changes known to occur at the onset and end of lactation, are necessary steps in understanding its potential physiological functions and identifying factors that regulate its properties.
Our studies demonstrate that both the expression and apical membrane localization of XOR are tightly linked to secretory activity. Furthermore, we demonstrate that the apical membrane localization of XOR occurs selectively at sites that contain Btn and ADPH, and provide biochemical evidence of a thiol-dependent complex between XOR and these proteins. These data provide strong evidence that the levels of expression and the apical membrane localization of XOR are crucial properties of secreting mammary epithelial cells and advance the hypothesis that the membrane association of XOR regulates coupling of cytoplasmic lipid droplets to the apical plasma membrane during lipid secretion.
Methods
Materials
RT-PCR oligonucleotide primers were synthesized by the Department of Biochemistry and Molecular Biology at the University of New Mexico. The fluorescent nucleotide tags used for these primers were obtained from Perkin Elmer Applied Biosystems (Foster City, CA, USA). Anti-ADPH and anti-Btn antibodies were kindly provided by Professors Thomas Keenan (Virginia Polytechnic Institute) and Ian Mather (University of Maryland), respectively.
Experimental manipulation of animals and tissue preparation
CD-1 mice (Charles River) were maintained as a breeding colony at the Animal Resource Center of the University of Colorado Health Sciences Center and housed individually. Pregnancy was timed by the observation of vaginal plugs after mating. Milk stasis was induced by cauterizing the nipples of one of the inguinal (fourth) glands of lactating mice (L15) under Avertin (6.7 mg kg−1, i.p.) anaesthesia. The remaining nipples were left intact. After full recovery (5-10 min) the animals were returned to their litters and observed to verify uninterrupted nursing from the unablated nipples. Paired ablated and unablated inguinal glands were harvested at 18 and 42 h after nipple ablation. Animals were killed by cervical dislocation after anaesthetization with Avertin as described above. Mammary tissue was frozen in liquid nitrogen for RNA analysis or fixed and processed for immunofluorescence microscopy as described below. Mouse milk was obtained by aspiration at day 12 of lactation. Fresh unpasteurized bovine milk was obtained from a local dairy. All animal procedures were approved by the Institutional Animal Care and Use Committee of the UCHSC.
Isolation and quantification of mRNA
Total RNA was extracted in Trizol (Life Technologies) according to the manufacturer's instructions. Random primed reverse transcription was carried out on 30 ng of RNA in 0.5 μl of Maloney leukaemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA), 0.5 μl RNasin (Promega, San Luis Obispo, CA, USA), 1 μl Random Hexamers (Perkin Elmer Applied Biosystems), 3 μl of dNTP mix (10 mm), 3 μl of 10 × buffer containing 15 mm MgCl2 and 7 μl of 0.1 % diethylene pyrocarbonate. Reactions were incubated at 23 °C for 10 min and at 42 °C for 30 min. The enzyme was denatured at 94 °C for 5 min. The PCRs were initiated by mixing 2 μl of RT reaction medium, 2 μl of each primer (10 μm), 2 μl of dNTP mix (10 mm), 5 μl of 10 × buffer containing 15 mm MgCl2, 36.5 μl of water and 0.5 μl of AmpliTaq DNA Polymerase (Perkin Elmer Applied Biosystems). A 416 base pairs (bp) region of mouse XOR cDNA was amplified using primers (5′-3′) TET-GGC CAG TCG AGC CAA GCT G and CCA TCC CAC CAG GGG CGT C. A 218 bp region of mouse α-lactalbumin cDNA was amplified using primers (5′-3′) HEX-CCT TTC AAG CCA CAG AGC TT and CGA CTC GGG GAA CTC ACT AC. A 299 bp region of mouse β-casein cDNA was amplified using primers (5′-3′) HEX-GGG ACA GCT GCA GGC AGA G and GAC TGG CAA GGC TGG GG. A 246 bp region of mouse whey acidic protein cDNA was amplified using primers (5′-3′) HEX-AAA GCT GGC TTC TGC CCT TG and CCA CGA GTG AAG GGT CCT GC. A 350 bp region of mouse β-actin cDNA was amplified using primers (5′-3′) 6-FAM-AGC AGC CGT GGC CAT CTC TTG CTC GAA GTC and AAC CGC GAG AAG ATG ACC CAG ATC ATG TTT. Samples were prepared for loading onto the Applied Biosystems 310 Genetic Analyser by mixing 12 μl of formamide, 1 μl of TAMARA size standard (Perkin Elmer Applied Biosystems) and 2 μl of PCR product. The size and amount of PCR product was calculated using GeneScan software (Perkin Elmer Applied Biosystems). Control experiments were performed to optimize signal linearity. S1 nuclease protection assays were performed on total RNA extracted according to Chirgwin et al. (1979). Oligonucleotide probes for mouse XOR (5′-GGTGAACTGATCCACACA AGCGTTTCG GATCTTCTCCGGAGTGGCGGGGACA CA-3′) and mouse glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (5′-GGG CCATCCACAGTCTTCTGGGTGGCAGTGATGGCATGGCATG GACTGTGACACC-3′) were obtained from Bethesda Research Laboratories and purified by gel electrophoresis and 32P-labelled by reaction with 32P-ATP and polynucleotide kinase (Ausubel et al. 1994). Hybridization reactions were performed by incubating 1 μl of probe with 50 μg of total RNA in 1 m sodium chloride, 0.30 mm EDTA, 0.150 mm Hepes, pH 7.5 for 10 min at 70 °C and hybridizing at 65 °C overnight. S1 nuclease solutions (300 U S1 nuclease in 0.28 m sodium chloride, 4.5 mm zinc sulphate, 50 mm sodium acetate, pH 4.5 containing 0.02 μg ml−1 sperm whale DNA) were added and allowed to incubate at 37 °C for 30 min. The reaction was stopped with EDTA. RNA was precipitated in ethanol and analysed by electrophoresis in 8 % acrylamide gels containing urea (Ausbel et al. 1994). The gels were dried and exposed to film to detect specific reaction products. Protected reaction products were excised and the amount of radioactivity in each band was quantified by Cerenkov counting in a Beckman scintillation counter.
Immunofluorescence microscopy
Tissue sections were derived from both frozen paraformaldehyde (PFA)-fixed, tissues and from formalin-fixed, paraffin-embedded, tissues. Similar results were obtained with either method of tissue preparation. Frozen tissue sections were prepared by sequentially perfusing Avertin-anaesthetized animals with ice-cold Krebs ringer solution, 2 % neutral-buffered PFA and 4 % PFA, by intracardiac injection. Mammary glands were then removed, frozen in liquid nitrogen cooled in isopentane and stored at −70 °C prior to cryostat sectioning. Cryostat sections (10 μm) were transferred to coverslips, fixed briefly in Ringer solution containing 3 % PFA and 3 % sucrose, washed in phosphate-buffered saline (PBS), permeabilized in PBS containing 0.5 % Triton X-100 and blocked with 5 % goat serum. Paraffin-embedded sections were prepared by overnight incubation of freshly dissected mammary tissue in neutral-buffered formalin prior to embedding in paraffin at the UCHSC Pathology Core Facility. Microtome sections (8 μm) were mounted on glass slides, deparaffinized and hydrated, subjected to antigen retrieval (Vector Laboratories) according the manufacturer's instructions, permeabilized in 0.2 % glycine in PBS and blocked with 5 % normal goat serum and 0.1 % (w/v) Saponin in PBS. Mouse MFGs were prepared for immunostaining by drying drops of fresh milk (diluted 1:8 with 2 % PFA) on coverslips, permeabilizing them with 0.5 % Triton X-100 in PBS and blocking with 20 mg ml−1 BSA in PBS overnight at 4 °C. Tissue sections, or milk droplets, were immunolabelled with anti-XOR antibodies for 1 h at room temperature, rinsed in PBS and then incubated with secondary antibody solution (1:150 dilution of Cy3- (or fluorescein isothiocyanate, FITC)-labelled donkey anti-rabbit IgG antibody; 0.03 μg ml−1 4′,6-diamidino-2-phenylindol (DAPI) in PBS) for 45 min, rinsed with PBS and then mounted.
For colocalization studies, paraffin sections were incubated with primary antibody no. 1, washed with PBS and incubated with Cy3-labelled donkey anti-rabbit IgG containing 0.5 μg ml−1 DAPI for 45 min as described above. The sections were then incubated with unlabelled goat anti-rabbit overnight at 4 °C, washed with PBS, incubated with primary antibody no. 2 for 1 h at room temperature, washed with PBS, incubated with FITC-labelled donkey anti-rabbit IgG at room temperature for 45 min, washed with PBS and mounted for light microscopy. Control experiments were performed: (1) in some sections it was verified that preincubation (prior to incubation with the primary and then fluorescently labelled secondary antibodies) with unlabelled donkey anti-rabbit IgG for 45 min at room temperature resulted in failure to detect the antigen and (2) the order of addition of the two primary antibodies (nos 1 and 2) was changed to verify that this had no effect on the colocalization results. Antibodies against purified mouse XOR (McManaman et al. 1999), amino acids 1-26 of mouse ADPH (Heid et al. 1996) and amino acids 504-524 of mouse Btn (Ogg et al. 1996) were generated in rabbits. The specificity of the antibodies was verified by demonstrating that they only react with their cognate antigen in Western blots of extracts of mouse MFG membranes. The identities of the XOR, Btn and ADPH bands in MFG membranes were verified by N-terminal amino acid sequencing after transfer to polyvinylidene fluoride membranes and staining with Coomassie blue (Matsudaira, 1997).
Immunofluorescence images were captured on a Nikon Diaphot fluorescent microscope equipped with a Cooke SensiCam CCD camera (Tonawand, NY, USA) using Slidebook software (Intelligent Imaging Innovations, Denver, CO, USA). All images were digitally deconvolved using the No Neighbors algorithm (Slidebook), converted to TIF files and processed with Photoshop software (Adobe Systems, Mountain View, CA, USA). The extent of colocalization of XOR with ADPH and Btn at the apical membrane was quantified on digitally deconvolved images using masking functions in Slidebook. Masks of the apical membrane staining areas for each protein were defined manually, and mask overlap was calculated using Slidebook.
Apoptosis assay
The relative degree of apoptosis was estimated on paraffin-embedded sections using the in situ cell death (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling; TUNEL) assay procedure (Roche, Indianapolis, IN, USA), according to the manufacturer's instructions.
Preparation and analysis of MFG membrane proteins
MFGs were isolated from fresh bovine milk according to Patton & Huston (1986). MFGs were diluted with 10 volumes of 0.05 m Tris, 0.15 mNaCl, pH 7.5 (Tris-buffered saline; TBS) and churned in a Waring blender at low speed. Curds were isolated by straining through cheesecloth and MFG membranes were pelleted by centrifugation at 90 000 g for 60 min at 4 °C. The supernatant and lipid were removed and membranes were washed by subjecting them to three rounds of centrifugation and resuspension in 10 volumes of TBS as described above. MFG membrane samples (100-200 μg) were extracted by mixing in three volumes of 0.05 m Tris, 1 % Triton X-100, pH 7.5 for 2 h at 4 °C and insoluble material was removed by centrifugation at 90 000 g for 1 h at 4 °C. HPLC-size-exclusion chromatography (HPLC-SEC) of Triton-X100-solubilized MFG membranes was performed on a Beckman System Gold Instrument using a 7.8 mm × 600 mm SEC-3000 column (Phenomenex) equilibrated in PBS, pH 7.5 using a 100 μl loop for sample injection. Chromatography was performed at room temperature at a flow rate of 1 ml min−1. The elution of protein was monitored at 220 nm using an online UV detector. Fractions (300 μl) were collected using an electronically activated fraction collector (Gilson, FC 203B) that was synchronously integrated to sample injection. Fractions (20 μl) were assayed for XOR activity using dichlorophenol-indophenol as a substrate (McManaman et al. 1999). Selected fractions (25 μl) were analysed by SDS-PAGE on 10 % acrylamide gels using silver staining (Wray et al. 1981) to detect protein bands. The identities of the XOR, Btn and ADPH bands were determined by N-terminal sequence analysis, as described above.
Results
XOR mRNA expression during alveolar cell differentiation
To assess fully the developmental context of changes in mammary XOR between pregnancy and lactation we examined the relative steady-state XOR mRNA levels at closely spaced intervals starting at day 7 post coitus (DPC 7). XOR mRNA was quantified by RT-PCR, normalized to β-actin mRNA levels, and by S1 nuclease protection analysis, normalized to total RNA (Fig. 1). Both forms of analysis revealed a biphasic increase in XOR mRNA during pregnancy, with an initial increase occurring between DPC 12 and DPC 14, resulting in a 6.7 ± 1.5-fold increase in XOR mRNA over levels in virgin animals. XOR mRNA remained relatively constant between DPC 14 and DPC 18, but then increased 3.2 ± 0.8-fold between DPC 18 and postpartum day 2 (DPC 21). Mammary XOR mRNA levels remained reasonably constant during lactation, but began to decline as pups were weaned (DPC 40-48; Fig. 1, inset). In contrast to the changes observed in mammary tissue, XOR mRNA did not change significantly in liver tissue during pregnancy or lactation (Fig. 1, inset). We compared the mRNA expression time course profile of XOR with those of β-casein, whey acidic protein (WAP) and α-lactalbumin. Previous studies have shown that β-casein mRNA expression is induced around mid-pregnancy in association with the initial phase of secretory differentiation, whereas WAP and α-lactalbumin mRNA expression are induced towards the end of pregnancy (Robinson et al. 1995) in conjunction with secretory activation. Our data show that the initial increase in XOR mRNA occurs with a time course similar to the initial increase in β-casein mRNA, increasing again during secretory activation in conjunction with increases in WAP and α-lactalbumin mRNA expression. These results are consistent with those of previous studies that showed increases in XOR protein and mRNA during pregnancy (Kurosaki et al. 1996; McManaman et al. 1999) and suggest that XOR levels in the mammary gland are regulated primarily at the transcriptional level, involving factors associated with both the initiation and activation phases of secretory differentiation.
Figure 1. Steady-state levels of xanthine oxidoreductase (XOR) and milk protein mRNA in the mammary gland during pregnancy and lactation.
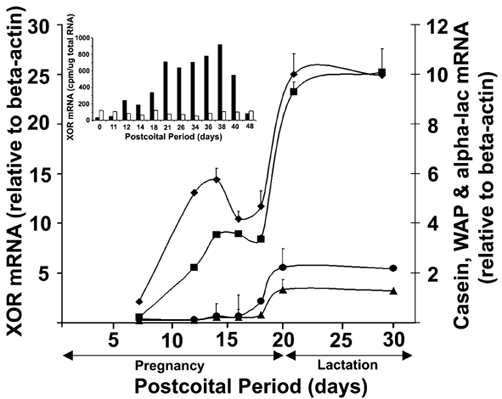
The average steady-state levels of XOR mRNA (▪), β-casein (♦), whey acidic protein (WAP; •) and α-lactalbumin (alpha-lac, ▴) in mammary tissue are shown for the indicated days post coitus. RNA levels were determined by RT-PCR using the primer pairs described in Methods and normalized to those of β-actin. The points show the mean ± s.e.m. for 3-4 animals. The error bars for some points lie within the symbols. The inset shows the change in the average steady-state levels of XOR in the mammary gland (closed bars) and liver (open bars) during pregnancy, lactation and involution, as determined by S1 nuclease protection. RNA values are averages of duplicates from different animals expressed as the amount of radioactivity (cpm) associated with protected bands after normalization to 25 μg of total RNA. Individual values were within 10 % of the averages. Parturition in our colony occurs between days 19 and 20 post coitus.
XOR localization during secretory activation
It has been demonstrated that there is an association between XOR and the apical plasma membrane of alveolar epithelial cells in lactating mammary glands (Jarasch et al. 1981). To determine if this membrane association is related to secretory differentiation we investigated the localization of XOR in the alveoli of mammary glands of late pregnant mice at just prior to birth (DPC 19), in postpartum mice just after delivery (lactation day 2; L2) and in fully lactating mice (L10). As shown in Fig. 2 (a-c), there are marked changes in the morphology of alveoli at these periods. At late pregnancy the cells contain large lipid droplets that all but obliterate the lumen. At parturition there is a marked increase in alveolar size and a loss of the large lipid droplets in the cytoplasm of epithelial cells as the gland undergoes secretory activation. At L10 the lumina have further expanded and lipid droplets in the process of being secreted can be seen at the apical surface (arrows, Fig. 2c). XOR was distributed diffusely within the cytoplasm of alveolar epithelial cells in DPC19 glands, and no XOR staining was detected at the apical surface (Fig. 2d). At L2, XOR was detected in both the cytoplasm and at the apical surface (arrowheads) of alveolar epithelial cells. Staining was also detected on MFGs in the lumina in some sections; however, detection of these structures was variable due to the loss of milk during fixation. Low-power (× 20) images of L10 glands show XOR within the cytoplasm and at the apical surfaces (arrowheads), and intense staining was observed in association with lipid droplets at the apical surface and in the lumina (arrow). Higher-power (× 100) images (in the insets) show that XOR localized to specific regions of the apical membrane in lactating glands (Fig. 2d and e) and occasionally could be seen surrounding cytoplasmic lipid droplets that appeared to be in the process of being secreted (Fig. 2f). These findings indicate that the membrane localization of XOR is associated with the transition of alveolar epithelial cells into a secretory phenotype.
Figure 2. Lobuloalveolar morphology and XOR localization in alveolar epithelial cells from pregnant and lactating mice.
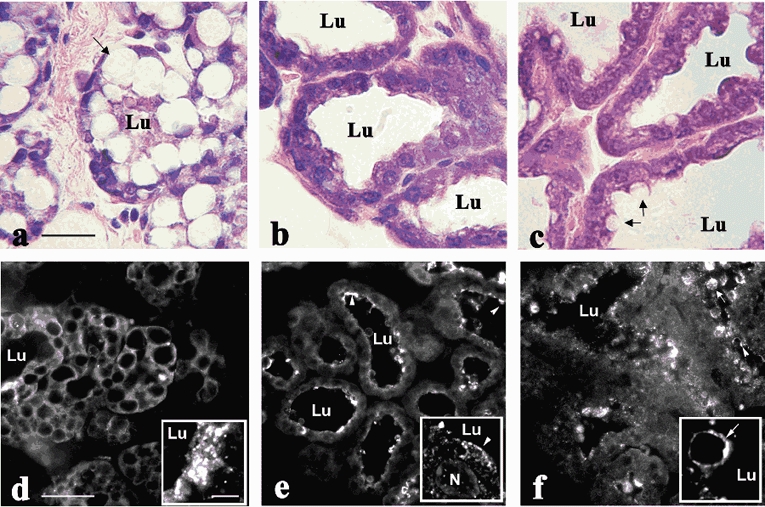
Haematoxylin/eosin staining (a-c; scale bar + 25 μm) of mammary gland sections from mice at day 19 of pregnancy (a), day 1 of lactation (b) and day 10 of lactation (c). The presence of large intracellular lipid droplets (arrow, a), the small lumen (Lu) size and the lack of luminal material indicate the absence of significant secretory activity at this period. The significant increase in luminal size and the loss of large intracellular lipid droplets in glands at day 1 of lactation indicate active secretion (b). At day 10 of lactation, lipid droplets in the process of being secreted can be seen at the apical membrane (arrows, c). Immunolocalization of XOR (d-f; low power images, scale bar + 50 μm) in perfusion-fixed mammary glands from mice at day 19 of pregnancy (d), day 1 of lactation (e) and day 10 of lactation (f). Insets in d-f show higher power images of XOR localization (scale bar + 1 μm). Arrowheads indicate apical membrane association of XOR, arrows show XOR labelling of secreted lipid droplets. The arrow in the inset in panel f indicates XOR on the surface of a lipid droplet in the process of being secreted.
To examine the time course of this process in more detail we used ovariectomy to produce a fall in progesterone and initiate secretory activation (Nguyen et al. 2001). Figure 3 shows XOR immunofluorescence and corresponding Hoffman contrast images in alveoli from animals 4, 12 and 20 h after ovariectomy on DPC 17. At 4 h post-ovariectomy, XOR staining was diffusely localized to the cytoplasm (Fig. 3a), but by 12 h post-ovariectomy (Fig. 3b) XOR staining was detected at the apical membrane (arrowheads), and this membrane association persisted at 20 h post-ovariectomy (Fig. 3c). These results suggest that the membrane association of XOR is induced during secretory activation by the fall in progesterone at parturition.
Figure 3. Effect of ovariectomy on XOR localization.
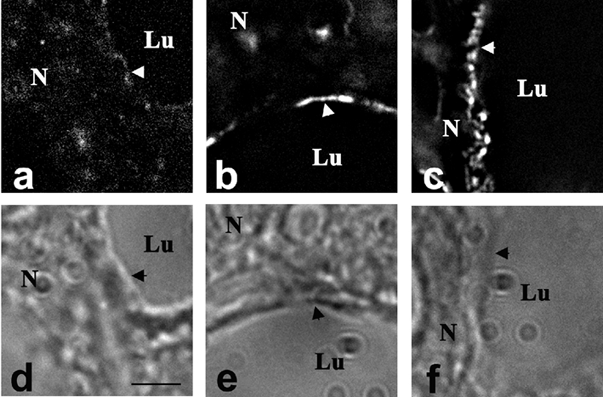
Immunofluorescence staining of XOR in alveolar epithelial cells at 4 h (a), 12 h (b) and 20 h (c) after ovariectomy of mice at day 17 of pregnancy. Panels d-e show the corresponding Hoffman contrast images of a-c. Arrowheads indicate the apical membrane. Scale bar + 5 μm.
Effects of inhibiting milk secretion on XOR mRNA expression and localization
Blocking milk removal causes a rapid decline in the rate of milk secretion and induces mammary gland involution (Strange et al. 1992; Lund et al. 1996; Li et al. 1997; Marti et al. 1997). To determine if changes in the secretory status of the lactating mammary gland affect XOR mRNA expression or localization, we investigated the effects of inducing milk stasis by unilateral nipple ablation of animals at L15. Nipples on the contralateral side were left intact and pups were observed suckling from these glands. Using this approach the unablated glands from the same animal serve as controls and there is no decline in the levels of systemic hormones, as is the case with forced weaning models of milk stasis (Feng et al. 1995). Figure 4A shows that 18 h after nipple ablation there was significantly less XOR mRNA in the ablated glands (51 ± 4 % of control) and the levels continued to decline at 42 h (13 ± 4 % of control). In contrast, as reported previously (Strange et al. 1992; Lund et al. 1996), there was only a modest decline in the levels of β-casein mRNA (71 ± 6 % of control) in ablated glands at 18 or 42 h post-ablation (Fig. 4A) and no change in G3PDH mRNA (data not shown). Figure 4B shows that 24 h after nipple ablation XOR was still associated with the apical membrane in functioning, unablated glands (Fig. 4B, a), and could be seen associated with lipid droplets that were in the process of being secreted (arrow). However, in ablated glands (Fig. 4B b) XOR was diffusely localized to the cytoplasm. Together the results shown in Fig. 3 and Fig. 4B suggest that the expression and membrane localization of XOR are dynamic processes that are narrowly dependent on the secretory activity of alveolar epithelial cells.
Figure 4. Effects of milk stasis on XOR expression and localization.
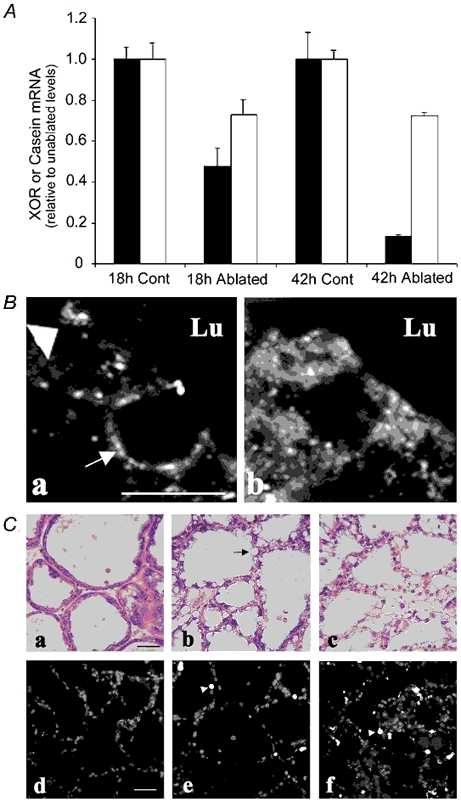
A, relative steady-state mRNA levels of XOR (▪) and β-casein (□) in mammary glands from paired unablated (control) and nipple-ablated (ablated) animals at 18 and 42 h after nipple ablation on day 15 of lactation. RNA levels determined by RT-PCR are shown as the percentage of the respective control values at each time point. B, immunolocalization of XOR in alveolar epithelial cells (a and b, scale bar + 2 μm) from unablated mammary glands (a) and nipple-ablated mammary glands 18 h after ablation on day 15 of lactation (b). The large arrowhead in a indicates XOR at the apical membrane, and the arrow in a shows XOR staining around a lipid droplet in the process of being secreted into the lumen. Note the absence of XOR at the apical membrane in ablated tissue. C, histological and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling (Tunel) analysis of mammary tissue after nipple ablation. Panels a-c show haematoxylin/eosin staining of mammary glands from unablated animals (a), animals nipple ablated for 24 h (b) and 48 h (c). Scale bar + 20 μm. Panels d-e show Tunel staining in similar sections from unablated glands (d) and glands 24 h (e) and 48 h (f) after nipple ablation. The arrowheads indicate Tunel-positive nuclei. Scale bar + 40 μm.
Table 1.
Quantification of XOR colocalization with adipophillin (ADPH) and butyrophilin (Btn) at the apical plasma membrane
| Apical membrane area; mean ± s.e.m.(μm2) | Percentage overlap | |
|---|---|---|
| XOR and ADPH colocalization | ||
| XOR | 4.40 ± 0.44 | 73% |
| ADPH | 4.65 ± 0.47 | 68% |
| Overlap | 3.10 ± 0.27 | — |
| XOR and Btn colocalization | ||
| XOR | 5.86 ± 0.85 | 76% |
| Btn | 11.08 ± 2.0 | 50% |
| Overlap | 4.39 ± 0.51 | — |
The overlap between XOR and ADPH immunofluorscence and XOR and Btn immunofluorescence at the apical plasma membrane of alveolar epithelial cell was quantified in mammary glands at day 10 of laction. The results are shown as the average (± s.e.m.) statining are (μm2) per field for each proteing, the area of staining overlap and the percent overlap (overlap area/total apical membrane staining area of each protein – 100). Areas were calculated using the Masking Algrothm of Slidebook and represent averages of 15separate fields from two animals.
Milk stasis is known to induce alveolar epithelial apoptosis and tissue remodelling as part of the involution process (Strange et al. 1992; Lund et al. 1996; Li et al. 1997; Marti et al. 1997). To determine if the decline in XOR mRNA or its loss from the apical surface is associated with apoptosis or disruption of lobuloalveolar structures, sections from normal and nipple-ablated mammary glands were stained for apoptotic nuclei using the Tunel assay and analysed histologically (Fig. 4C). Twenty-four hours after nipple ablation, large lipid droplets (arrow, Fig. 4C b) accumulated in alveolar epithelial cells, suggesting impairment of milk lipid secretion. However, general alveolar morphology remained similar to that of unablated glands (Fig. 4C a) and Tunel analysis showed only a limited number of scattered apoptotic nuclei (Fig. 4C d). Forty-eight hours after nipple ablation, alveolar morphology was beginning to show evidence of disruption (Fig. 4C c) and there was a marked increase in apoptotic nuclei (Fig. 4C f), in agreement with the mammary involution time course shown previously for BALB/C mice (Lund et al. 1996). Nevertheless, significant portions of individual alveoli remained non-apoptotic. Thus, the initial decline in XOR mRNA and the loss of XOR from the apical surface are associated with the effects of milk stasis on milk secretion, independent of alveolar epithelial cell apoptosis.
XOR colocalizes with Btn and ADPH at the apical plasma membrane
If a complex between XOR and Btn at the apical plasma membrane and ADPH on surface of cytoplasmic lipid droplets functions in the secretion of milk lipids (Mather & Keenan, 1998), XOR should colocalize with these proteins at the apical membrane. Figure 5 shows that regions on the apical surface of alveolar epithelial cells that contain XOR staining (Fig. 5c and d) also stain for Btn and ADPH (Fig. 5e and f) and that within these regions the XOR staining significantly overlaps with Btn and ADPH staining (merged, Fig. 5a and b). Quantification of the degree of staining overlap showed that the areas of overlap of XOR immunostaining with Btn and ADPH immunostaining corresponded to 76 % and 73 %, respectively, of the total apical membrane staining area of XOR. Those areas of XOR staining not directly overlapping with Btn or ADPH staining were close to regions that did overlap; we did not observe XOR staining at sites distant from regions of overlap. Similarly, the overlap between XOR and ADPH immunostaining at the apical surface corresponded to 68 % of the total ADPH staining at the apical membrane, and ADPH was only detected in the regions near the regions of overlap. Thus, XOR appears to be found at the apical membrane only within regions that also contain Btn and/or ADPH, and within these regions it exhibits a high degree of overlap with each of these proteins. On the other hand, the overlap between XOR and Btn staining represented only 50 % of the total Btn staining at the apical membrane, and significant amounts of Btn staining were observed at sites distant from those that contained some degree of XOR staining (data not shown). Thus it seems likely that Btn associates with the apical membrane independently of the presence of XOR or ADPH. If these interactions are important in milk lipid droplet secretion, as has been hypothesized (Mather & Keenan, 1998), it is likely that they persist in the milk fat droplet, a proposition that was tested next.
Figure 5. Colocalization of XOR, butyrophilin (Btn) and adipophilin (ADPH) in lactating mouse mammary tissue.
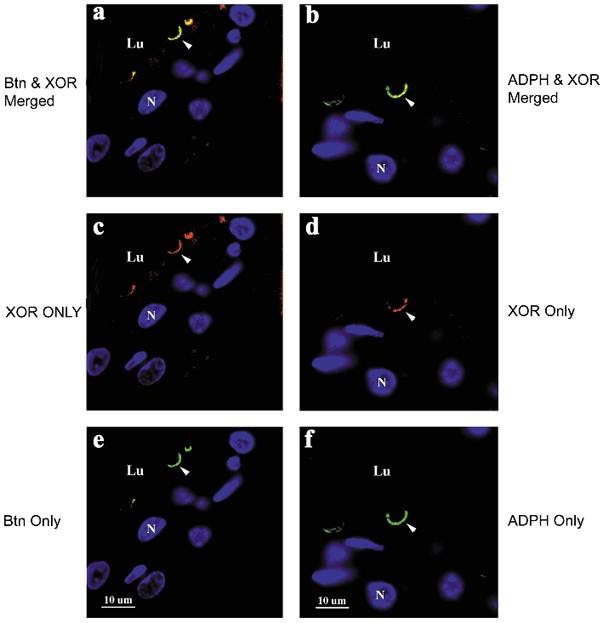
XOR was separately immunolocalized with Btn or ADPH in paraffin-embedded mammary tissue from mice at day 10 of lactation. XOR immunostaining was detected with cy3-labelled secondary antibodies (red), and Btn and ADPH immunostaining were detected with fluorescein isothiocyanate (FITC; green)-labelled secondary antibodies, as described in Methods. a, merged image of XOR and Btn staining; c, XOR only staining; e, Btn only staining; b, merged image of XOR and ADPH staining; d, XOR only staining; f, ADPH only staining. Nuclei (N) and luminal regions (Lu) are indicated. Scale bar + 10 μm.
Interactions of XOR with Btn and ADPH on MFG membranes
Figure 6A shows that XOR, Btn and ADPH colocalized on fat globules isolated from mouse milk and that membranes isolated from these droplets are enriched in these proteins (Fig. 6B). To determine if XOR exists as a complex with Btn and ADPH, isolated MFG membranes were extracted with Triton X-100 and the resultant solution was analysed by HPLC-SEC. We found that 50-70 % of the total XOR activity associated with isolated MFG membranes could be extracted by incubation in 1 % Triton X-100 (data not shown). Figure 6C a shows that the extracted XOR enzymatic activity eluted as two peaks from the HPLC-SEC. The first peak (I) eluted in the void volume (> 600 kDa), along with a major peak of UV-absorbing material. The second peak (II) eluted with the same retention time as purified native mouse mammary gland XOR (data not shown). The protein compositions of each peak, determined by SDS-PAGE in conjunction with silver staining, are shown in the inset. The void volume peak (peak I) contained a limited number of bands and was enriched in bands that were identified as XOR, Btn and ADPH by microsequence analysis (data not shown). Peak II, by contrast, was composed primarily of XOR. These results suggest that significant amounts of MFG membrane-associated XOR exists as a Triton X-100 stable complex with Btn and ADPH. To further characterize the nature of this complex, peak I was isolated, reduced with 5 mm DTT and reanalysed by HPLC-SEC. Figure 6C b shows that following disulphide bond reduction nearly all of the XOR activity eluted in peak II. SDS-PAGE and silver stain analysis of the proteins in peaks I and II showed that some Btn and ADPH were still found in peak I, but most of the XOR protein was now in peak II. In contrast, if peak I was subjected again to chromatography without prior thiol reduction, all of the XOR activity remained in peak I (data not shown). Together these results indicate that the association of XOR with apical membranes involves, at least in part, thiol-dependent interactions with Btn and/or ADPH.
Figure 6. Colocalization of XOR, Btn and ADPH on milk lipid globule membranes.
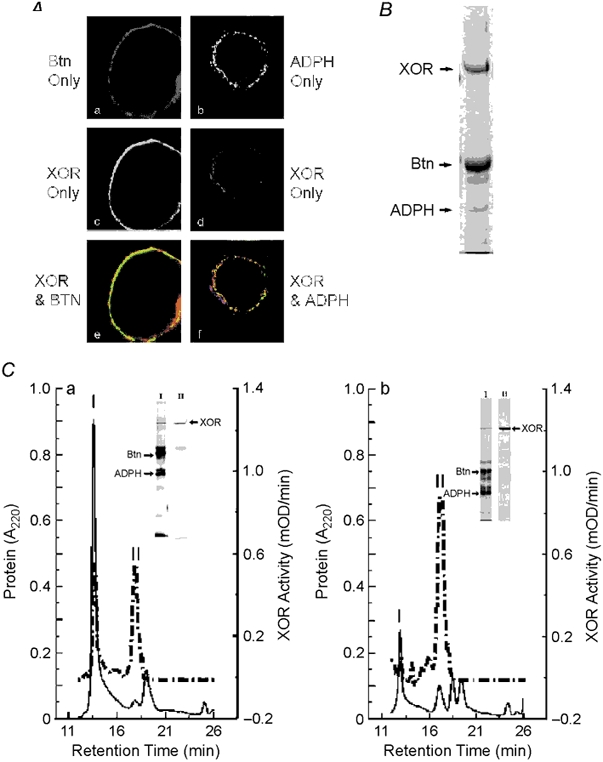
A, XOR was separately immunolocalized with Btn or ADPH in paraformaldehyde-fixed mouse milk. XOR was detected with FITC-labelled antibodies (green) and Btn and ADPH were detected with cy3-labelled antibodies (red) as described. a, Btn only staining; b, XOR only staining; c, merged image of XOR and Btn staining; d, ADPH only staining; e, XOR only staining; f, merged image of XOR and ADPH staining. Note that XOR is largely localized internal to Btn and that all ADPH staining appears to overlap with XOR, as shown by the yellow areas. B, Coomassie-blue-stained gel of mouse milk lipid droplet membrane proteins separated by SDS-PAGE. XOR, Btn and ADPH bands are indicated; their identities were verified by N-terminal microsequence analysis. C, size-exclusion chromatography (SEC) analysis of interactions between XOR, Btn and ADPH isolated from milk lipid globule membranes. Panel a shows the elution properties of XOR enzymatic activity (dashed line) and 220 nm absorbing material (continuous line) following SEC of Triton-X-100-solubilized milk lipid globule membrane proteins. The insets show silver-stained gels of proteins in peaks I and II separated by SDS-PAGE. The identities of XOR, Btn and ADPH bands were confirmed by N-terminal microsequence analysis and are indicated. XOR bands in peaks I and II are indicated by the arrow at the right of the peak II lane. Panel b shows the elution properties of XOR, Btn and ADPH after incubating peak I with 5 mm DTT. XOR activity and A220 nm absorbance are indicated by the dashed and continuous lines, respectively. Silver-stained gels of the proteins in peaks I and II are shown in the insets and the positions of XOR, Btn and ADPH are indicated as above.
Inhibiting milk secretion does not affect the apical membrane localization of Btn
The association of XOR with Btn at the apical membrane raises the possibility that the loss of XOR from the apical plasma membrane following milk stasis was due to a loss of Btn. To test this possibility we immunolocalized Btn in sections of control and nipple-ablated mammary glands. Figure 7 shows that Btn remained localized to the apical surface of alveolar epithelial cells 24 h after nipple ablation, at a time when XOR had totally redistributed to the cytoplasm.
Figure 7. Effect of milk stasis on Btn localization.
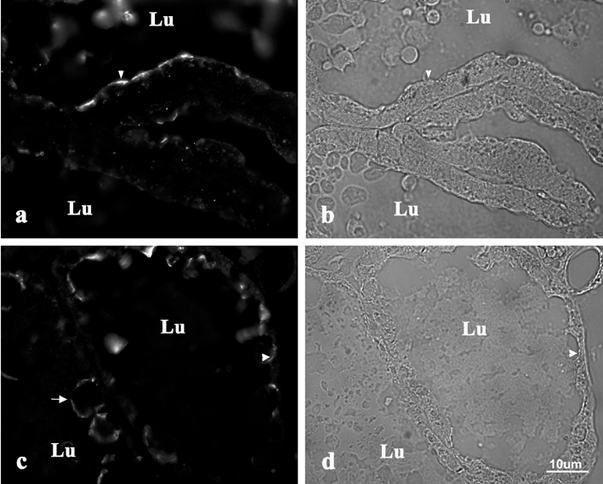
Immunolocalization of Btn in alveolar epithelial cells (× 60) from unablated mammary glands (a) and nipple-ablated mammary glands (c) 24 h after ablation on day 15 of lactation. Panels b and d are corresponding Hoffman contrast images of a and c, respectively. Arrowheads indicate the apical surfaces, the arrow in c shows Btn staining around milk fat globules in the lumen (Lu).
Discussion
Our results show that XOR is regulated developmentally at two levels, mRNA and protein localization. The increase in XOR mRNA in differentiating mammary tissue follows a biphasic time course that corresponds temporally with the initiation and activation phases of secretory differentiation (Houdebin et al. 1985; Neville et al. 2002). Previous studies have demonstrated distinct temporal patterns of milk protein gene expression that correlate with the different phases of secretory differentiation. Some genes, such as that for β-casein, are activated early, while others, such as those for α-lactalbumin and WAP, are activated in late pregnancy or at parturition (Robinson et al. 1995). From the PCR results, the mRNA levels of XOR and β-casein appear to increase with similar time courses and suggest that, like the β-casein gene, activation of the XOR gene is an early event in secretory differentiation of the mammary gland. At present, the factors that regulate the early phase of secretory differentiation are poorly understood. Progesterone and prolactin (or placental lactogen) are required for alveolar development during pregnancy (Neville et al. 2002) and prolactin has been shown to increase the expression of several milk proteins, including XOR, in cell culture models (Kurosaki et al. 1996; McManaman et al. 2000). However, it is unclear to what extent prolactin-induced gene expression is responsible for the initial increase in XOR expression between 8 and 12 days of pregnancy, as there is significant proliferation of alveolar epithelial cells in mouse mammary tissue during this period (Borst & Mahoney, 1982). Moreover, in situ analysis of β-casein expression in mouse mammary tissue at mid-pregnancy showed significant heterogeneity in the population of alveolar epithelial cells expressing β-casein mRNA (Robinson et al. 1995). Thus, the early increase in XOR mRNA is likely to reflect both a cell-specific gene activation as well as an increase in the population of alveolar epithelial cells. The increased expression of XOR mRNA during the activation phase of secretory differentiation, in contrast, is likely to reflect increased cellular levels of XOR mRNA, as alveolar epithelial cell proliferation is markedly decreased (Borst & Mahoney, 1982) and in situ studies indicate that there is a relatively homogeneous expression of milk protein genes in alveolar cells at this time (Robinson et al. 1995). The factors regulating XOR expression at parturition are currently under investigation; however, progesterone has been shown to repress β-casein expression during pregnancy (Rosen et al. 1978; Nishikawa et al. 1994), and the fall in progesterone levels at parturition is associated with increased β-casein expression (Hobbs et al. 1982). Therefore, the finding that XOR mRNA levels increase at parturition raises the possibility that progesterone also participates in the regulation of XOR expression in the mammary gland.
Continuous milk secretion appears to be required to maintain elevated expression of XOR in lactating mammary tissue, as evidenced by observations that XOR mRNA levels fall rapidly when milk secretion is inhibited. Although inhibiting milk secretion is known to induce apoptosis of alveolar epithelial cells and tissue remodelling associated with mammary gland involution (Strange et al. 1992; Lund et al. 1996; Li et al. 1997; Martie et al. 1997), it is unlikely that the decline in XOR mRNA is due to a of loss secretory cells, since the fall in XOR mRNA levels occurred prior to the appearance of significant Tunel-stained nuclei or morphological evidence of involution. Moreover, the rapid decline in XOR mRNA induced by inhibiting milk secretion does not appear to reflect a generalized inhibition of milk protein gene expression, as we found, in agreement with others (Strange et al. 1992; Lund et al. 1996), that there was only a modest decline in β-casein mRNA 42 h after inhibiting milk secretion, whereas XOR mRNA decreased by 87 %. The finding that the decline in XOR mRNA only occurred in nipple-ablated glands suggests that local factors related to milk retention, rather than systemic hormone levels, are primarily responsible for maintaining XOR expression during lactation.
The association of XOR with the apical plasma membrane of alveolar epithelial cells also appears to be dependent upon secretory activity. This conclusion is supported by the observations that: (1) the membrane association of XOR was temporally associated with the onset of lactation during normal differentiation, (2) the apical membrane association of XOR was induced prematurely by ovariectomy of pregnant animals at P17, (3) the ovariectomy-induced association of XOR with the apical membrane followed a time course similar to that of ovariectomy-induced tight-junction closure and (4) milk stasis resulted in the loss of XOR from the apical membrane, suggesting that continuous secretory activity is required to maintain its apical membrane association. Together, these findings indicate that the membrane association of XOR is linked to secretory activity and is indirectly related to the hormonal changes that take place at parturition (Neville et al. 2002).
At present the factors that control the appearance of XOR at the apical membrane are unknown. However, the selective colocalization of XOR with Btn and ADPH within discrete patches at the apical plasma membrane suggests that its membrane association does not occur at random sites or result from adventitious interactions with other membrane proteins. XOR has been reported to bind to the C-terminal region of recombinant Btn in cultured mammary epithelial cells (Ishii et al. 1995). Coupled with the demonstration that Btn is a type 1 transmembrane protein with approximately two-thirds of its sequence containing the C-terminal domain oriented towards the cytoplasm (Banghart et al. 1998), this finding suggests that the apical membrane association of XOR involves specific interactions with a C-terminal domain of Btn within the cytoplasm. However, Btn is detected at the apical plasma membrane of alveolar epithelial cells at day 18 of pregnancy (J. L. McManaman, unpublished observation) and remains at the plasma membrane following nipple-ablation. Therefore, it appears that the apical membrane expression of Btn is in itself unable to account for the membrane association of XOR, and that additional changes in the properties of these proteins and/or in the activation of auxiliary processes or membrane components at the onset of secretion are likely to be important for the association of XOR to apical membranes. The observation that significant amounts of Btn staining on the apical plasma membrane occurred at sites distant from those that also contained XOR staining further supports the idea that the association between XOR and Btn is a regulated process. Previous studies have shown that XOR constitutes a high percentage of the total soluble protein in lactating mouse mammary glands (McManaman et al. 1999); thus it is unlikely that its association with Btn is limited by its cellular concentration.
XOR is known to exist in two distinct and interconvertible enzymatic forms, a thiol reduced form (XD) and a thiol oxidized form (XO), which differ in their enzymatic properties and conformations (Waud & Ragjgopalan, 1976; Massey et al. 1989; Saito et al. 1989; McManaman & Bain, 2002). We have shown that XD is the predominant form of XOR in mammary tissue, whereas XO is the predominant form in milk (McManaman et al. 1999). Mammary tissue and MFG membranes have been shown to contain a thiol oxidase that is capable of converting XD to XO (Clare et al. 1981; McManaman et al. 1999). These observations, together with our demonstration that XOR can be isolated from MFG membranes as a DTT-sensitive complex with Btn and ADPH, raise the possibility that the association between XOR and the apical plasma membrane may be mediated by thiol-dependent processes that involve the formation of disulphide bond cross-links with Btn, ADPH or other membrane proteins, and/or conformational changes in XOR (McManaman & Bain, 2002).
The colocalization of XOR, Btn and ADPH at the apical plasma membrane, and the isolation of a stable complex of these proteins from secreted MFG membranes is the first direct evidence of specific and close interactions between these proteins. These results indicate that XOR does not associate with Btn and ADPH by coincidence, and provide support for the proposed involvement of these proteins in milk lipid secretion (Mather & Keenan, 1998). At present the exact nature of these interactions, the processes that regulate their formation and the mechanisms by which they mediate milk lipid secretion are unknown. However, based on observations of specific interactions between XOR, Btn and ADPH, and the finding that XOR undergoes a dynamic, secretory-dependent association with the apical plasma membrane, we propose that it may be the functional link that couples cytoplasmic lipid droplets to the apical plasma membrane during lipid secretion. If this proposal is correct, then depletion of XOR from the mammary gland or interference with its interactions with Btn or ADPH is predicted to impede the secretion of lipids. Studies are currently underway in our laboratory to identify functionally important interactions between XOR, Btn and ADPH and to establish the role of these proteins in milk lipid secretion.
Acknowledgments
This work was supported by NIH grants HL 45582, R37 HD19547, PO1 HD 38129, RO1 HD 15437. The authors gratefully acknowledge the excellent technical assistance of Ms Linda Hanson in these studies, and thank Dr Michael Lewis for his insightful comments on the studies and helpful suggestions on this manuscript. We would especially like to thank Drs Thomas Keenan and Ian Mather for their generous gifts of antibodies to ADPH and Btn, respectively. Microsequencing was performed at the UCHSC Cancer Center's Protein Chemistry Core, which is supported by grant CA46934 from NCI.
References
- Abadeh S, Killacky J, Beboubetra M, Harrison R. Purification and partial characterization of xanthine oxidase from human milk. Biochimica et Biophysica Acta. 1992;1117:25–32. doi: 10.1016/0304-4165(92)90157-p. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- Baldwin RL, Yang YT. Enzymatic and metabolic changes in the development of lactation. In: Larson BL, Smith VR, editors. Lactation. New York: Academic Press; 1974. pp. 349–407. [Google Scholar]
- Banghart LR, Chamberlain CW, Velarde J, Korobko IV, Ogg SL, Jack LJ, Vakharia VN, Mather IH. Butyrophilin is expressed in mammary epithelial cells from a single-sized messenger RNA as a type I membrane glycoprotein. Journal of Biological Chemistry. 1998;273:3171–4179. doi: 10.1074/jbc.273.7.4171. [DOI] [PubMed] [Google Scholar]
- Borst DW, Mahoney WB. Mouse mammary gland DNA synthesis during pregnancy. Journal of Experimental Zoology. 1982;221:245–250. doi: 10.1002/jez.1402210216. [DOI] [PubMed] [Google Scholar]
- Bray RC. Molybdenum iron-sulfur flavin hydroxylases and related enzymes. In: Boyer PD, editor. The Enzymes. 3. Vol. 12. New York: Academic Press; 1975. pp. 299–419. [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clare DA, Blakistone BA, Swaisgood HE, Horton HR. Sulfhydryl oxidase-catalyzed conversion of xanthine dehydrogenase to xanthine oxidase. Biochimica et Biophysica Acta. 1981;211:44–47. doi: 10.1016/0003-9861(81)90427-6. [DOI] [PubMed] [Google Scholar]
- Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. Journal of Cell Biology. 1995;131:1095–10103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet IR, Goode JA, Hamon MH, Laurie MS, Linzell JL, Peaker M. Secretory activity of goat mammary glands during pregnancy and the onset of lactation. Journal of Physiology. 1975;251:763–773. doi: 10.1113/jphysiol.1975.sp011120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godber B, Sanders S, Harrison R, Eisenthal R, Bray RC. ≥ 95 % of xanthine oxidase in human milk is present as the demolybdo form, lacking molybdopterin. Biochemical Society Transactions. 1997;25:519S. doi: 10.1042/bst025519s. [DOI] [PubMed] [Google Scholar]
- Hartmann PE. Changes in the composition and yield of the mammary secretion of cows during the initiation of lactation. Journal of Endocrinology. 1973;59:249–259. doi: 10.1677/joe.0.0590231. [DOI] [PubMed] [Google Scholar]
- Hayden TJ, Brennan D, Quirke K, Murphy P. Xanthine oxidase/dehydrogenase in mammary gland of mouse: relationship to mammaogenesis and lactogenesis in vivo and in vitro. Journal of Dairy Research. 1991;58:401–409. doi: 10.1017/s0022029900030004. [DOI] [PubMed] [Google Scholar]
- Heid H, Schnolzer M, Keenan TW. Adipocyte differentiation-related protein is secreted into milk as a constituent of milk lipid globule membrane. Biochemical Journal. 1996;320:1025–1030. doi: 10.1042/bj3201025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB Journal. 1995;9:995–1003. [PubMed] [Google Scholar]
- Hobbs AA, Richards DA, Kessler DJ, Rosen JM. Complex hormonal regulation of rat casein gene expression. Journal of Biological Chemistry. 1982;257:3598–3605. [PubMed] [Google Scholar]
- Hollman KH. Cytology and fine structure of the mammary gland. In: Larson BL, Smith VR, editors. Lactation. New York: Academic Press; 1974. pp. 3–95. [Google Scholar]
- Houdebine L-M, Djiane J, Dusanter-Fourt I, Martel P, Kelly PA, Devinoy E, Servely J-L. Hormonal action controlling mammary activity. Journal of Dairy Science. 1985;68:489–500. doi: 10.3168/jds.S0022-0302(85)80848-1. [DOI] [PubMed] [Google Scholar]
- Ishii T, Aoki N, Noda A, Adachi T, Nakamura R, Matsuda T. Carboxy-terminal cytoplasmic domain of mouse butyrophilin specifically associates with a 150-kDa protein of mammary epithelial cells and milk fat globule membrane. Biochimica et Biophysica Acta. 1995;1245:285–292. doi: 10.1016/0304-4165(95)00102-6. [DOI] [PubMed] [Google Scholar]
- Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Keenan TW, Patton S. The structure of milk: implications for sampling and storage. A. The milk lipid globule membrane. In: Jensen RG, editor. Handbook of Milk Composition. San Diego: Academic Press; 1995. pp. 5–49. [Google Scholar]
- Kuhn NJ. Lactogenesis: the search for trigger mechanisms in different species. In: Peaker M, editor. Comparative Aspects of Lactation. London: Academic Press; 1977. pp. 165–192. [Google Scholar]
- Kurosaki M, Zanotta S, Calzi ML, Garattini E, Terao M. Expression of xanthine oxidoreductase in mouse mammary epithelium during pregnancy and lactation: regulation of gene expression by glucocorticoids and prolactin. Biochemical Journal. 1996;319:801–810. doi: 10.1042/bj3190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu X, Robinson G, Bar-Peled U, Wagner K-U, Young WS, Hennighausen L, Furth P. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proceedings of the National Academy of Sciences of the USA. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Romer J, Doby-Thomasset N, Soderberg H, Pyke C, Bissell MJ, Dane K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL, Bain DL. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. Journal of Biological Chemistry. 2002;277:21261–21268. doi: 10.1074/jbc.M200828200. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Hanson L, Neville MC, Wright RM. Lactogenic hormones regulate xanthine oxidoreductase and beta-casein in mammary epithelial cells by distinct mechanisms. Achieves of Biochemistry and Biophysics. 2000;372:318–327. doi: 10.1006/abbi.1999.1573. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Neville MC, Wright RM. Mouse mammary gland xanthine oxidoreductase: purification, characterization and regulation. Archives of Biochemistry and Biophysics. 1999;371:308–316. doi: 10.1006/abbi.1999.1432. [DOI] [PubMed] [Google Scholar]
- Marti A, Feng Z, Altermatt HJ, Jaggi R. Milk accumulation triggers apoptosis of mammary epithelial cells. European Journal of Cell Biology. 1997;73:158–165. [PubMed] [Google Scholar]
- Massey V, Schopfer LM, Nishino T, Nishino T. Differences in protein structure of xanthine dehydrogenase and xanthine oxidase revealed by reconstitution with flavin active site probes. Journal of Biological Chemistry. 1989;264:10567–10573. [PubMed] [Google Scholar]
- Mather IH. Proteins of the milk-fat-globule membrane as markers of mammary epithelial cells and apical plasma membrane. In: Neville MC, Daniel CW, editors. The Mammary Gland: Development, Regulation and Function. New York: Plenum Press; 1987. pp. 217–268. [Google Scholar]
- Mather IH, Keenan TW. Origin and secretion of milk lipids. Journal Mammary Gland Biology and Neoplasia. 1998;3:259–273. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. Journal of Biological Chemistry. 1987;262:10035–10038. [PubMed] [Google Scholar]
- Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- Nguyen D-A, Parlow AF, Neville MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. Journal of Endocrinology. 2001;170:347–356. doi: 10.1677/joe.0.1700347. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Moore RC, Nonomura N, Oka T. Progesterone and EGF inhibit mouse mammary gland prolactin receptor and β-casein gene expression. American Journal of Physiology. 1994;267:C1467–1472. doi: 10.1152/ajpcell.1994.267.5.C1467. [DOI] [PubMed] [Google Scholar]
- Ogg SL, Komaragiri MVS, Mather IH. Structural organization and mammary specific expression of the butyrophilin gene. Mammalian Genome. 1996;7:900–905. doi: 10.1007/s003359900265. [DOI] [PubMed] [Google Scholar]
- Patton S, Huston GE. A method for isolation of milk fat globules. Lipids. 1986;21:170–174. doi: 10.1007/BF02534441. [DOI] [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighousen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- Rosen JM, O'Neal DL, McHugh JE, Comstock JP. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978;17:290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Saito T, Nishino T, Massey V. Differences in environment of FAD between NAD-dependent and O2-dependent types of rat liver xanthine dehydrogenase shown by active site probe study. Journal of Biological Chemistry. 1989;264:15930–15935. [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Stryer L. Biochemistry. 3. New York: Freeman; 1988. [Google Scholar]
- Waud WR, Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O) Archives of Biochemistry and Biophysics. 1976;172:365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Wilde CJ, Hurley WL. Animal models for the study of milk secretion. Journal of Mammary Gland Biology and Neoplasia. 1996;1:123–134. doi: 10.1007/BF02096307. [DOI] [PubMed] [Google Scholar]
- Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Analytical Biochemistry. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]


