Abstract
The pontine oral reticular nucleus, gigantocellular reticular nucleus (Gi) and dorsal paragigantocellular nucleus (DPGi) of the medulla are key elements of a brainstem-reticulospinal inhibitory system that participates in rapid eye movement (REM) sleep atonia. Our recent study has shown that excitation of these brainstem nuclei in decerebrate rats inhibits locus coeruleus cells and the midbrain locomotor region neurons related to muscle tone facilitation. In the present study we have examined the influences of electrical and chemical stimulation of Gi and DPGi inhibitory sites on the activity of neurons located in the magnocellular part of the red nucleus (RMC), a cell group that participates in both the tonic and phasic regulation of motor output. A total of 192 RMC neurons were recorded in precollicular-premammillary decerebrate rats with muscle rigidity and induced locomotion. Thirty-three RMC neurons were identified antidromically as rubrospinal (RMC-spinal) cells by stimulation of the contralateral dorsolateral funiculus at the L2 level. A total of 141 RMC neurons (88.7 %) and all RMC-spinal neurons were inhibited during electrical stimulation of Gi and DPGi inhibitory sites. This cessation of activity was correlated with bilateral muscle atonia or blockage of locomotion. Six RMC cells (3.8 %) were excited (224 ± 50 %, n = 6, minimum = 98, maximum = 410, P < 0.05) and 11 cells (7 %) gave no response to Gi and DPGi stimulation. Microinjections of kainic acid (100 μm, 0.2 μl) into Gi and DPGi inhibitory sites, previously identified by electrical stimulation, produced a short-latency (35 ± 3.5 s, n = 11) decrease of rigid hindlimb muscle tone and inhibition of all tested RMC (n = 7) and RMC-spinal (n = 5) neurons. These results, combined with our recent published data, suggest that inhibition of motor function during activation of the brainstem inhibitory system is related to both the descending inhibition of spinal motoneurons and suppression of activity in supraspinal motor facilitatory systems. These two mechanisms acting synergistically may cause generalized motor inhibition during REM sleep and cataplexy.
Inhibition of evoked locomotor activity and muscle tone in intact, anaesthetized and decerebrate animals may be elicited by electrical and chemical stimulation of the medial and dorsolateral regions of the pons, as well as the medial division of the medulla (Magoun & Rhines, 1946; Mori et al. 1978; Lai & Siegel, 1988; Mileikovskii et al. 1991; Oka et al. 1993). It is generally agreed that this inhibition is related to excitation of a descending reticulospinal system, which in turn hyperpolarizes spinal alpha motoneurons (Jankowska et al. 1968; Takakusaki et al. 1989, 1994). However, recent studies have shown that excitation of brainstem inhibitory regions also produces a marked reduction of activity in some supraspinal motor systems, which participate in facilitation of muscle tone and locomotion (Iwakiri et al. 1995; Mileikovskii et al. 2000; Mileykovskiy et al. 2000). We propose that excitation of brainstem inhibitory structures evokes generalized suppression in the brainstem motor facilitatory systems, and this suppression acts synergistically with inhibition produced by the descending inhibitory reticulospinal system. The red nucleus (RN) is a prominent structure in the motor system of mammals and consists of posterior magnocellular (RMC) and anterior parvocellular parts. RMC neurons form the rubrospinal tract and participate in the production of skilled forelimb movements (Martin & Ghez, 1991; Martin et al. 1993; Jarratt & Hyland, 1999; van Kan & McCurdy, 2001), regulation of locomotion (Orlovsky 1972; Arshavsky et al. 1988; Ruigrok et al. 1996; Rho et al. 1999; Muir & Whishaw, 2000) and coordination of the motor responses to pain (Matsumoto & Walker, 1991; Horn et al. 1998).
The medial parts of the gigantocellular reticular nucleus (Gi) and dorsal paragigantocellular reticular nucleus (DPGi) are the main elements of the reticulospinal inhibitory system responsible for the hyperpolarization of spinal alpha motoneurons (Takakusaki et al. 1989; 1994; Chase & Morales, 1990). In the present study we have used precollicular-premammillary decerebrate rats to determine the responses of unidentified RMC and RMC-spinal neurons, which are related to hindlimb muscle activity, to electrical and chemical stimulation of Gi and DPGi sites that suppress muscle tone and block locomotion.
Methods
Surgery
We used a precollicular-premammillary preparation to study the influence of medullary inhibitory site stimulation upon the discharge patterns of the RN neurons that control both the phasic parameters of movement and static torque. All procedures were approved by the Animal Studies Committee of the Sepulveda VA Medical Center/UCLA, in accordance with US Public Health Service guidelines. Animals were anaesthetized with halothane (2.5 % halothane plus oxygen) followed by ketamine HCl (Ketalar, 70 mg kg−1) for cannulation of the trachea and decerebration. Two holes (diameter 1 mm) were drilled over the skull above the RMC at 6 mm posterior to bregma and 0.7 mm lateral to the midline for insertion of unit recording electrodes. One hole (diameter 2.0 mm) was drilled above the Gi and DPGi at 11.0 mm posterior to bregma for the insertion of stimulating electrodes or injection cannulae. The coordinates of all structures were based on the atlas of Paxinos & Watson (1997).
Two rectangular holes were cut in each parietal bone in preparation for decerebration. The transverse anterior and posterior borders of these holes were located 1 and 5 mm posterior to bregma, respectively, with the longitudinal sagittal border 0.2 mm from the midline. Precollicular-premammillary decerebration was performed 3.5-4.0 mm posterior to bregma with a stainless steel spatula, taking care not to injure the sagittal vein. The forebrain was completely removed and excess fluid was aspirated by syringe and absorbed with Gelfoam. A cotton tampon moistened with saline and 2 % lidocaine was then inserted into the skull in place of the forebrain. The level of transection was verified by histological analysis and plotted according to the rat brain atlas (Paxinos & Watson, 1997).
Three insulated stainless steel wires (diameter 62 μm) were implanted into the spinal cord to stimulate the right and left dorsolateral funiculus at the L2 level. Wires were fixed with a specially designed clamp and dental acrylic. Pressure points on the head were infiltrated with lidocaine (4 %). Anaesthesia was then discontinued. Eighteen Wistar rats (250-300 g) were operated on, of which eight showed bilateral muscle rigidity after precollicular-premammillary decerebration, with EMG amplitudes ranging from 50 to 400 μV. Five rats responded with 30-180 s of repetitive stepping behaviour after exteroceptive stimulation (pinching or touching of the back, tail or hindlimbs).
Stimulation and recording
Electrical stimulation of Gi and DPGi sites (0.2 ms; 50 Hz; 30-250 μA, continuous stimulation 10-15 s) via a bipolar stimulating electrode (stainless steel, 100 μm, tip separation 80-100 μm) was used to produce muscle atonia and locomotor and withdrawal reflex suppression. To select Gi and DPGi inhibitory sites, electrical stimulation was performed during electrode insertion in 0.3 mm steps. Bipolar stimulating electrodes were used to reduce electrical artefacts during unit recording. Stimulation artefacts were determined to have a duration of less than 1.4 ms. Electrical stimuli were delivered with the aid of a S88 stimulator (Grass Instrument) and Grass SIU5 stimulus isolation unit. Extracellular unit recordings were performed using tungsten monopolar microelectrodes (A-M Systems, Carlborg, WA, USA). The indifferent electrode was placed in the frontal cranial bone. Spikes were amplified with a model 1700A-M Systems amplifier. Identification of RMC neurons was based on electrophysiological characteristics, as reported previously (Eccles et al. 1975; Gibson et al. 1985; van Kan & McCurdy 2001): (1) action potentials of large amplitude (0.4-2 mV), (2) a first spike wave with a long duration (0.5-1.0 ms) and (3) background activity that was modulated during movements of contralateral body parts. RMC cells were determined to be rubrospinal (RMC-spinal) neurons related to hindlimb muscle activity, if units met the following criteria: (1) stimulation of the contralateral dorsolateral funiculus at the L2 level (0.2 ms, 50-300 μA, trains of three pulses at a frequency of > 200 Hz) produced antidromic spikes at an invariant latency of < ± 0.2 ms and (2) collision of antidromic spikes with spontaneous spikes could be demonstrated.
EMG electrodes (stainless steel wires, 100 μm) were implanted bilaterally into the semimembranosus muscles (Sm), tibialis anterior muscles (Ta), sartorius muscles (Srt), and gastrocnemius muscles (Gc). EMG activity was amplified using a Grass polygraph (model 78D). Unit pulses and EMG were recorded on a personal computer using the 1401 plus interface and Spike 2 program (Cambridge Electronic Design, Cambridge, UK). The rate of digitization was 372 Hz for the EMG and 21 kHz for unit activity.
Microinjections of kainic acid
To exclude the possibility that excitation of axons of passage was responsible for the effects of Gi and DPGi stimulation, microinjections of kainic acid (KA) (100 μm, 0.2 μl) were performed into Gi inhibitory sites (n = 11). These sites were previously identified by electrical stimulation (0.2 ms, 50-200 μA, 50 Hz, continuous 10-15 s) via tungsten microelectrodes (A-M Systems, Carlborg, WA, USA) as points producing bilateral hindlimb muscle atonia. KA was dissolved in 0.9 % saline to obtain the required concentration. Control saline microinjections (n = 5) into Gi and DPGi inhibitory sites were carried out in two rats. A 1 μl Hamilton microsyringe and injecting cannulae with an outside tip diameter of 0.2 mm were used for all microinjections.
Histology
Cathodal current (30-50 μA, 10-30 s) was passed through the microelectrodes at the end of each recording track. Rats were deeply anaesthetized with pentobarbital (70 mg kg−1, i.p.) and perfused transcardially with 0.01 m PBS, pH 7.4, followed by 4 % paraformaldehyde in 0.1 M PBS. Brains were removed and cut into 60 μm sections. The location of recorded neurons was determined by using the track made by the microelectrode, the depth of the marking lesion and the depth measurements on the microdrive. The electrode tracks and marking lesions were visualized with the aid of a Nikon microscope and plotted with a Neurolucida interface according to the rat brain atlas (Paxinos & Watson, 1997). Cannula tracks were visualized using a Leica MZ6 microscope.
Data analysis
Unit firing rates were analysed using the Wilcoxon matched-pairs test. The average unit firing rates were calculated for 10 s before and during Gi and DPGi electrical or chemical stimulation in animals with muscle rigidity and for 0.2 s in the 10 swing and stance phases of the locomotor cycle. The latency of muscle tone suppression was measured from the start of electrical stimulation or microinjection to a 50 % decrease in EMG amplitude compared with baseline. The duration of suppression was calculated from the time of onset of a decrease in muscle tone to return of the tone to 50 % of the baseline level. The latencies and durations of muscle tone suppression were averaged for right and left hindlimbs for each electrical stimulation and microinjection. All values are given as the mean ± s.e.m.
Results
Electrophysiological characteristics of RMC and RMC-spinal neurons
A total of 159 RMC and 33 RMC-spinal neurons were recorded in eight rats with bilateral muscle rigidity and five rats that showed stepping in response to exteroceptive stimulation. The duration of the first spike wave in RMC and RMC-spinal neurons was 0.66 ± 0.01 ms (n = 192, minimum (min) = 0.51 ms, maximum (max) = 1.0 ms). Amplitudes of RMC spikes ranged between 0.4 mV and 1.0 mV. RMC-spinal neurons that send projections to lumbar levels were located mainly in the ventrolateral part of the RMC (Fig. 1 and Fig. 5) and responded antidromically upon stimulation of the contralateral dorsolateral funiculus at the L2 level with a latency ranging from 2.2 ms to 6.2 ms and a mean of 3.6 ± 0.2 ms (n = 33).
Figure 1. Coronal views of the rat midbrain showing the location of recorded magnocellular red nucleus (RMC) and rubrospinal RMC (RMC-spinal) neurons.
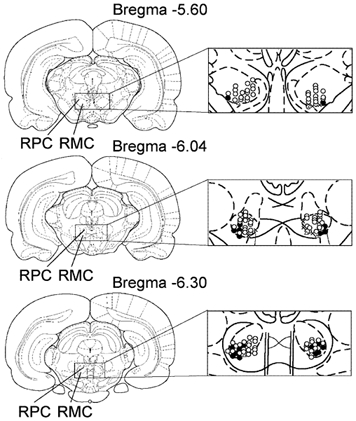
Open and black circles indicate RMC and RMC-spinal neurons, respectively, which were inhibited by Gi and DPGi stimulation, producing bilateral muscle atonia. Triangles indicate RMC neurons that were excited by Gi and DPGi stimulation. Crosses indicate RMC neurons that were unaffected by Gi and DPGi stimulation. RPC, parvocellular part of the red nucleus.
Figure 5. Coronal views of the rat midbrain showing the location of recorded RMC and RMC-spinal neurons.
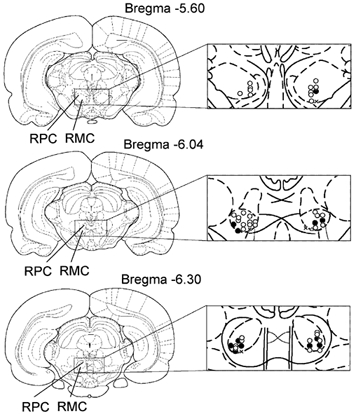
Open and black circles indicate RMC and RMC-spinal neurons, respectively, inhibited by Gi and DPGi stimulation, suppressing locomotion and the withdrawal reflex. Triangles indicate RMC neurons that were excited by Gi and DPGi stimulation. Crosses indicate RMC neurons that were unaffected by Gi and DPGi stimulation.
Animals with rigid muscle tone
RMC-spinal neurons
The firing rates of 21 RMC-spinal neurons (Fig. 1) were analysed during electrical stimulation (0.2 ms, 50 Hz, 50-250 μA) of Gi and DPGi sites producing bilateral muscle atonia in rats with muscle rigidity (Fig. 2). RMC-spinal neurons had an average firing rate of 28 ± 3 spikes s−1 (n = 21, min = 5 spikes s−1, max = 63 spikes s−1) during muscle rigidity. Electrical stimulation of Gi and DPGi inhibitory sites produced bilateral suppression of muscle tone and inhibition of all RMC-spinal neurons (Fig. 3A). Sixteen RMC-spinal neurons were completely inhibited and the discharge of five cells was reduced by 79.2 ± 5.0 % compared with the baseline (n = 5, min = 63 %, max = 92 %, P < 0.05). Inhibition of RMC-spinal neurons preceded the reduction in muscle tone during Gi and DPGi stimulation by an average of 1.5 ± 0.2 s (n = 21, min = 0.3 s, max = 3.5 s) and resumption of the baseline firing rate in these cells preceded the recovery of muscle tone by an average of 1.6 ± 0.2 s (n = 21, min = 0.5 s, max = 3.8 s).
Figure 2. Location of gigantocellular reticular nucleus (Gi) and dorsal paragigantocellular nucleus (DPGi) sites that produced bilateral muscle atonia, blockage of locomotion and the withdrawal reflex.
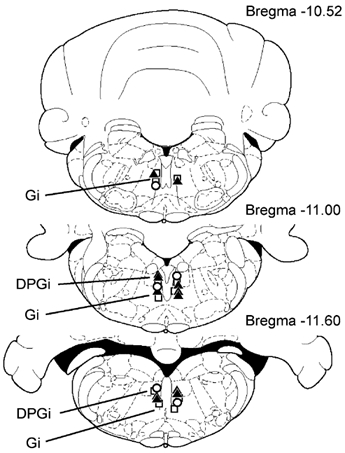
Squares and triangles indicate Gi and DPGi sites that produced bilateral muscle atonia during electrical stimulation (< 100 μA) and microinjections of kainic acid (100 μm, 0.2 μl), respectively. Circles indicate Gi and DPGi sites that suppressed locomotion and the withdrawal reflex.
Figure 3. RMC-spinal neuron inhibition (A) and RMC neuron excitation (B) during Gi electrical stimulation inducing bilateral muscle atonia.
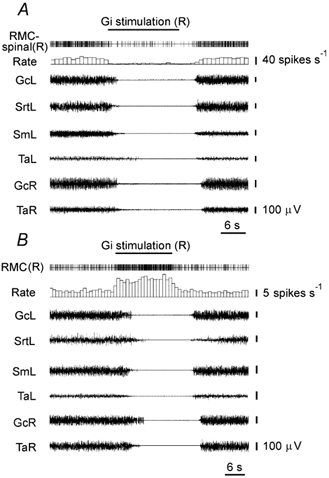
RMC-spinal(R), RMC-spinal neuron (right side); RMC(R), RMC neuron (right side); GcL, GcR, EMG of gastrocnemius muscle, left side and right side, respectively; SmL, EMG of the semimembranosus muscle (left side); SrtL, EMG of sartorius muscle (left side); TaL, TaR, EMG of the tibialis anterior muscle, left side and right side, respectively. Stimulating currents were < 100 μA.
RMC neurons
The activity of 117 RMC neurons (Fig. 1) was analysed during stimulation (50-180 μA) of Gi and DPGi sites producing bilateral hindlimb muscle atonia. The RMC cells could be divided into three groups based on their responses. The first group (n = 104) consisted of RMC neurons that were depressed by Gi and DPGi stimulation. Forty-two RMC neurons ceased discharge completely and the firing rate of 62 cells was reduced by 68.7 ± 2.2 % during stimulation (n = 62, min = 37 %, max = 93 %, P < 0.0001). Reduction of activity in these neurons during Gi and DPGi stimulation preceded muscle tone suppression by an average of 2.4 ± 0.2 s (n = 104, min = 0.3 s, max = 8.7 s) and firing rate resumption preceded the muscle tone recovery after stimulation by an average of 3.1 ± 0.3 s (n = 104, min = 0.1 s, max = 13.5 s). The average firing rate of RMC neurons in this group was 24.9 ± 1.4 spikes s−1 (n = 104, min = 6 spikes s−1, max = 62 spikes s−1) during muscle rigidity. Neurons in the second group (n = 5) were excited by stimulation of Gi and DPGi inhibitory sites (Fig. 3B). These RMC cells had an average firing rate of 3.7 ± 0.5 spikes s−1 during muscle rigidity (n = 5, min = 2.5 spikes s−1, max = 5 spikes s−1). The discharge rate of these cells increased by an average of 249 ± 54 % (n = 5, min = 98 %, max = 410 %, P < 0.05) during Gi and DPGi stimulation compared with the baseline. RMC neurons in the third group (n = 8) did not respond to stimulation of Gi and DPGi inhibitory sites. These cells had an average firing frequency of 16.5 ± 2.6 spikes s−1 during muscle rigidity (n = 8, min = 7 spikes s−1, max = 25 spikes s−1).
KA microinjections into the Gi and DPGi
The firing rates of seven RMC and five RMC-spinal neurons were analysed during KA microinjections (n = 12) into Gi and DPGi inhibitory sites previously identified with electrical stimulation (Fig. 2). All microinjections produced a bilateral short-latency decrease of rigid hindlimb muscle tone and reduction of discharges in both RMC and RMC-spinal neurons (Fig. 4). The latency of muscle tone suppression after KA microinjections ranged from 18 to 56 s, with a mean of 35.8 ± 3.5 s (n = 12). The duration of muscle tone suppression ranged from 192 s to 615 s, with a mean of 407 ± 46 s. Three RMC and two RMC-spinal cells were inhibited completely, and six cells exhibited a 75 ± 3 % reduction in firing rate after KA microinjections into Gi and DPGi inhibitory sites (min = 65 %, max = 82 %, n = 6, P < 0.05).
Figure 4. Inhibition of a RMC-spinal neuron after KA microinjection in the Gi site producing bilateral muscle atonia.
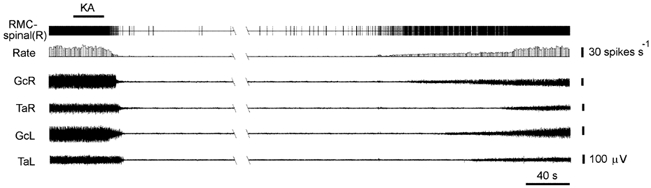
The top horizontal left bar indicates the time and duration of the KA injection.
Animals with induced locomotion
RMC-spinal neurons
Twelve spontaneously active RMC-spinal neurons (Fig. 5) were recorded during baseline resting conditions, induced locomotion and motor inhibition evoked by Gi and DPGi stimulation. During the resting condition, RMC-spinal neurons discharged with an average frequency of 13 ± 2 spikes s−1 (n = 12, min = 5 spikes s−1, max = 27 spikes s−1). When animals started locomotion, 10 RMC-spinal neurons showed rhythmic activity related to contralateral hindlimb stepping. The maximal activity of nine RMC-spinal neurons (66 ± 11 spikes s−1, n = 9, min = 32 spikes s−1, max = 125 spikes s−1) correlated with swing phases and decreased in stance phases (3.6 ± 0.7 spikes s−1, n = 9, min = 1 spike s−1, max = 7 spikes s−1). The firing rate of one RMC-spinal neuron increased (to about 45 spikes s−1) during stance phases and two showed only a tonic increase of firing rate (20-30 spikes s−1) during locomotion. Electrical stimulation of Gi and DPGi inhibitory sites (50-120 μA) inhibited stepping and decreased the activity of all RMC-spinal neurons (Fig. 6A). The firing rate of nine RMC-spinal neurons was completely inhibited and the discharge of three cells was reduced by 64-82 % compared with the baseline during resting conditions. After Gi and DPGi stimulation, RMC-spinal neurons recovered their baseline firing rates and following of locomotion-related rhythmic activity.
Figure 6. Inhibition of RMC-spinal and RMC neurons during Gi stimulation producing blockage of locomotion and the withdrawal reflex induced by hindlimb pinch.
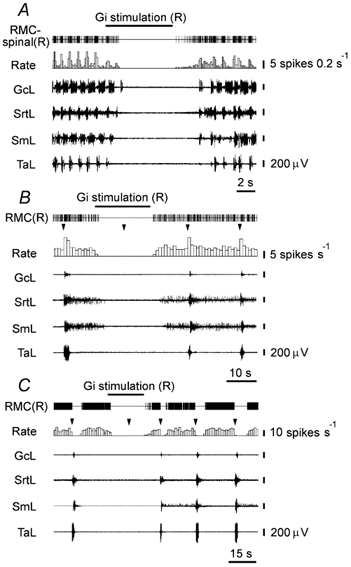
A, inhibition of the RMC-spinal neuron modulated by locomotor rhythm. B, C, inhibition of the RMC neurons suppressed and excited by a pinch; triangles indicate the time of pinch.
RMC neurons
The activity of 42 RMC neurons (Fig. 5) was additionally analysed during Gi and DPGi stimulation, which blocked the withdrawal hindlimb reflex evoked by contralateral hindlimb pinches. A total of 34 RMC neurons had a tonic discharge pattern with an average frequency of 8.4 ± 0.8 spikes s−1 (n = 34, min = 2 spikes s−1, max = 22 spikes s−1) and eight RMC cells showed spontaneous burst activity with an average burst duration of 4.1 ± 0.3 s (n = 8, min = 3 s, max = 5 s) and period between bursts 2.3 ± 0.3 s (n = 8, min = 2 s, max = 4 s) during the rest period. The maximal frequency of firing in bursts ranged from 8 to 48 spikes s−1 with a mean of 21 ± 4 spikes s−1 (n = 8).
All RMC neurons could be divided into one of three groups based on their responses. The first group (n = 9) consisted of RMC neurons whose firing rate increased by an average of 253 ± 33 % during the hindlimb withdrawal reflex compared with the baseline (min = 80 %, max = 367 %, P < 0.01). Neurons in the second group (n = 14) exhibited a firing rate increase (150 ± 20 %, min = 53 %, max = 275 %, P < 0.01; 0.8 ± 0.1 s, min = 0.3 s, max = 1.2 s) followed by complete inhibition for 3.8 ± 0.5 s (min = 1 s, max = 7 s). RMC cells in the third group (n = 19) were completely inhibited for 3.6 ± 0.5 s after the pinch was applied (min = 1 s, max = 8 s).
Gi and DPGi stimulation produced withdrawal reflex suppression and profoundly inhibited the activity of RMC neurons in all three groups. In the first group, four neurons were inhibited completely (Fig. 6B) and the firing rates of four neurons were decreased by 50-75 % compared with baseline. One RMC neuron in the first group did not respond to Gi stimulation. In the second group, six neurons ceased their discharge and eight neurons reduced their firing rate by an average of 72 ± 4 % (min = 55 %, max = 82 %, P < 0.03). In the third group, seven neurons were inhibited completely (Fig. 6C) and nine neurons exhibited a reduction in their discharge rate by an average of 74 ± 3 % (min = 60 %, max = 85 %, P < 0.01). Two RMC neurons in the third group did not respond to Gi stimulation and the firing rate of one neuron was increased by 100 %.
Discussion
Our results show that electrical and chemical stimulation of Gi and DPGi sites that suppress muscle tone and locomotion reduced the activity of RMC and RMC-spinal neurons, which contribute to both the control of phasic parameters of movement and static torque (Mewes & Cheney, 1994). This reduction may be related to both partial disfacilitation (Tsukahara et al. 1965) and the direct inhibition of rubral cells (Ralston & Milroy, 1992). Disfacilitation in rubral neurons might be a result of inhibition of transmission in ascending somatosensory pathways during the excitation of brainstem inhibitory regions (Kirzon & Kaplan 1978; Katayama et al. 1984) and a decrease in excitatory influences from external sources. Suppression of adrenergic and serotonergic systems during excitation of brainstem inhibitory sites (Wang et al. 1976; Taek, 1991; Mileykovskiy et al. 2000; Lai et al. 2001) or rapid eye movement (REM) sleep (Hobson et al. 1975; Fornal et al. 1985; Jacobs, 1986) might disfacilitate rubral neurons. However, microiontophoretic applications of serotonin and noradrenaline induce a significant level of depression in the majority of RN cells (Licata et al. 1998; Ciranna et al. 2000). Glutamate and acetylcholine also are not likely to be involved in the process of disfacilitation of rubral neurons since REM sleep is accompanied by increased release of these neurotransmitters (Kodama et al. 1992, 1998), which produce cell excitation in the RN (Marshall et al. 1980; Nieoullon et al. 1988; Kinney, 1995; Licata et al. 1998). On the other hand, direct inhibition of rubral neurons may be related to the activation of a local interneuron network in the RN, as well as distant inhibitory influences. Electron microscopy studies have demonstrated the presence of GABA immunoreactive cells that form symmetrical (inhibitory) synapses on RMC-spinal neurons, and GABAergic terminals from extranuclear sources (Ralston & Milroy, 1992). Intracellular recording has demonstrated that electrical stimulation of the dorsolateral mesencephalic reticular formation (DLMRF), including the deep mesencephalic nucleus, elicits monosynaptic hyperpolarizing postsynaptic potentials in about 95 % of rubral neurons. These hyperpolarizing potentials were blocked reversibly by bicuculline, a GABAA receptor antagonist. Histochemical and electron microscopy studies have also demonstrated the existence of direct GABAergic projections from the DLMRF to RN neurons (Fu et al. 1996). Since neurons of the DLMRF receive excitatory inputs from medullary inhibitory sites and participate in the inhibition of muscle tone in anaesthetized and decerebrate animals (Sinnamon et al. 1987; Mileikovskii et al. 1991; Mileykovskiy et al. 2002), this midbrain region might be involved in the inhibition of rubral neurons during Gi stimulation. The Gi also sends projections to the pontine oral reticular nucleus (PnO), which is involved in the induction of REM sleep muscle atonia (Henley & Morrison, 1974; Shammah-Lagnado et al. 1987; Chase & Morales, 1990; Lai & Siegel, 1990; Siegel et al. 1992). Electrical stimulation of the PnO evokes GABA and glycine release in the vicinity of the locus coeruleus and inhibition of noradrenergic neurons in this nucleus (Mileykovskiy et al. 2000). The PnO, like the DLMRF, receives excitatory inputs from medullary inhibitory sites (Mileikovskii et al. 1991), therefore inhibition of RN cells during Gi stimulation might also result from excitation of this pontine nucleus.
It is worth noting that the reduction in RN neuronal activity during medullary stimulation and firing resumption in the poststimulation period preceded muscle tone suppression and recovery in our study. This indicates that the alteration in RN firing was not a result of the change of afferent inflow from the muscle groups (Cheney et al. 1988; Mewes & Cheney, 1994; Van Kan & McCurdy, 2001).
Since brainstem transection at the pontomedullary junction as well as pontine lidocaine injections attenuate the muscle atonia induced by medullary stimulation (Siegel et al. 1983; Kohyama et al. 1998), we propose that the inhibition of rostral brainstem structures, in particular the midbrain locomotor region, locus coeruleus (Mileykovskiy et al. 2000), and RN, may contribute to muscle atonia. Electrical stimulation of the RN evokes EMG facilitatory responses in forelimb and hindlimb flexor muscles (Rho et al. 1999). About 70 % of neurons located in the magnocellular part of the RN participate in the dynamic control of movement, and 27 % of these cells have a tonic component of discharge, which contributes to static torque (Mewes & Cheney, 1994). These data suggest that cessation of the activity of RN cells during stimulation of the medial medulla reduces activity in circuits related to both the phasic and tonic regulation of muscle activity. Moreover, cessation of the activity in RN neurons may influence the integrative functions of the cerebellar nuclei participating in the regulation of postural muscle tone and motor control (Keifer & Houk, 1994; Keifer 1996; Pananceau et al. 1996; Jiang et al. 2002).
Terminals of corticorubral axons originating from the ipsilateral primary and supplementary motor cortex are mainly mapped in the parvocellular part of the RN, and only area 4 of the cortex sends a small direct projection to the RMC (Burman et al. 2000). Removing the motor cortex does not significantly change the cell discharges associated with finger movements in the denervated RMC (Houk et al. 1988). On the other hand, the RMC receives dense projections from the interpositus nucleus (Daniel et al. 1987, 1988). It was postulated that the cerebellorubral circuit, including the cerebellum, RN and reticular formation, acts as a positive loop that generates motor commands and conveys them to the spinal cord via the rubrospinal pathways (Orlovsky, 1972; Amassian & Batson, 1988; Arshavsky et al. 1988; Keifer & Houk, 1994). We propose that stimulation of the medial medulla may suppress transmission in this loop, which provides a dynamic cerebellar control of motor activity and regulation of the somatosensory information received from the dorsal column nuclei of the spinal cord (Fanardzhian & Sarkisian, 1984; Taepavarapruk et al. 2002).
The Gi and PnO are important components of the brainstem-reticulospinal inhibitory system, which participates in REM sleep atonia (Takakusaki et al. 1989; 1994; Chase & Morales, 1990; Morales et al. 1999; Hajnik et al. 2000). We propose that PnO and Gi cell groups, when activated during REM sleep, evoke the suppression of activity in excitatory brainstem motor systems in addition to descending inhibition of spinal motoneurons, and this coordinated activity underlies muscle atonia during REM sleep and motor regulation in waking. RN unit recording across the sleep-waking cycle revealed that these cells decreased their activity in transition from waking to non-REM sleep and during tonic periods of REM sleep (Gassel et al. 1965; Harper & Jacobs, 1972). During the phasic events of REM sleep, the RN unit firing rate is significantly increased compared with quiet waking and other sleep stages. On the other hand, Jacobs et al. (1970) reported that some RN cells had higher firing rates in tonic periods of REM than in non-REM sleep. Unfortunately, recorded RN cells were not identified as rubrospinal neurons, therefore they might be elements of the GABAergic apparatus (Vuillon-Cacciuttolo et al. 1984; Ralston & Milroy, 1992) activated during REM sleep (Nitz & Siegel, 1997a, b). Alternatively, inhibitory influences from brainstem inhibitory sites might be partially masked by excitatory flows from structures of ascending activating reticular systems excited during REM sleep (Siegel et al. 1977; Shiromani et al. 1988; Cornwall et al. 1990; Steriade et al. 1990; Quattrochi et al. 1998). An increase in RN unit firing rate during REM sleep after lesioning of the brainstem inhibitory sites might demonstrate these inhibitory influences.
It is suggested that the reduction in the activity of mesopontine neurons and the reduction in eye movements during cataplexy compared with both REM sleep and wakefulness is related to active inhibition of the central motor systems during this state in narcoleptic dogs (Siegel et al. 1992). This hypothesis is supported by data showing that cataplexy is accompanied by excitation of a specialized subpopulation of cells in the ventromedial medulla (Siegel et al. 1991), a region that is responsible for REM sleep atonia (Sakai 1980; Lai & Siegel, 1988; Schenkel & Siegel, 1989). Moreover, cessation of the activity of locus coeruleus neurons in cataplexy (Wu et al. 1999) and during the electrical stimulation of medullary inhibitory sites (Mileikovskiy et al. 2000) also demonstrates the active nature of muscle tone suppression during cataplectic attacks. Thus, we propose that the activity of RMC-spinal neurons, like activity of locus coeruleus and mesopontine reticular cells, might be reduced in cataplexy, and this reduction is related to activation of the medullary neurons responsible for muscle atonia.
The current results together with our recent published data suggest that excitation of the pontine and medullary inhibitory regions evokes a generalized suppression of activity in brainstem facilitatory motor systems in addition to direct inhibition of spinal motoneurons. Moreover, brainstem inhibitory structures may block the descending excitatory influences addressed to spinal motor centres from rostral motor structures, and thus promote the spinal motoneuronal inhibition evoked by the reticulospinal inhibitory system (Mileikovskii et al. 2000). Abnormal function of these brainstem inhibitory mechanisms or disruption of their coordinated activity may contribute to human motor disorders such as cataplexy, restless legs syndrome, dystonia and spastic cerebral palsy.
Acknowledgments
This work was supported by USPHS grants HL41370, HL60296 and the Medical Research Service of the Department of Veterans Affairs.
References
- Amassian VE, Batson D. Long loop participation of red nucleus in contact placing in the adult cat with facilitation by tactile input at the spinal level. Behavioral Brain Research. 1988;28:225–232. doi: 10.1016/0166-4328(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Orlovsky GN, Perret C. Activity of rubrospinal neurons during locomotion and scratching in the cat. Behavioral Brain Research. 1988;28:193–199. doi: 10.1016/0166-4328(88)90096-4. [DOI] [PubMed] [Google Scholar]
- Burman K, Darian-Smith C, Darian-Smith I. Macaque red nucleus: origins of spinal and olivary projections and terminations of cortical inputs. Journal of Comparative Neurology. 2000;423:179–196. doi: 10.1002/1096-9861(20000724)423:2<179::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cirrana L, Licata F, Li Volsi G, Santangelo F. Neurotransmitter-mediated control of neuronal firing in the red nucleus of the rat: reciprocal modulation between noradrenaline and GABA. Experimental Neurology. 2000;163:253–263. doi: 10.1006/exnr.2000.7377. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. The atonia and myoclonia of active (REM). sleep. Annual Review of Psychology. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Mewes K, Fetz EE. Encoding of motor parameters by corticomotoneuronal (CM) and rubromotoneuronal (RM). cells producing postspike facilitation of forelimb muscles in the behaving monkey. Behavioral Brain Research. 1988;28:181–191. doi: 10.1016/0166-4328(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Research Bulletin. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Daniel H, Angaut P, Batini C, Billard JM. Topographic organization of the interpositorubral connections in the rat. A WGA-HRP study. Behavioral Brain Research. 1988;28:69–70. doi: 10.1016/0166-4328(88)90078-2. [DOI] [PubMed] [Google Scholar]
- Daniel H, Billard JM, Angaut P, Batini C. The interposito-rubrospinal system. Anatomical tracing of a motor control pathway in the rat. Neuroscience Research. 1987;5:87–112. doi: 10.1016/0168-0102(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Scheid P, Taboricova H. Response of red nucleus neurons to antidromic and synaptic activation. Journal of Neurophysiology. 1975;38:947–964. doi: 10.1152/jn.1975.38.4.947. [DOI] [PubMed] [Google Scholar]
- Fanardzhian VV, Sarkisian DS. Neuronal mechanisms of red nucleus interaction with brain stem structures. Neirofiziologiia. 1984;16:665–678. [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Experimental Neurology. 1985;88:590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- Fu YS, Tseng GF, Yin HS. Extrinsic inhibitory innervation to rubral neurons in rat brain-stem slices. Experimental Neurology. 1996;137:142–150. doi: 10.1006/exnr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Gassel MM, Marchiafava PL, Pompeiano O. Activity of red nucleus during deep desynchronized sleep in unrestrained cats. Archives Italiennes de Biologie (Pisa) 1965;103:369–396. [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. Journal of Physiology. 1985;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, Siegel JM. Atonia related regions in the rodent pons and medulla. Journal of Neurophysiology. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Jacobs BL. Red nucleus neuronal activity during sleep and wakefulness. Sleep Research. 1972;1:20. [Google Scholar]
- Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiologiae Experimentalis (Warszawa) 1974;34:215–232. [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wizynski PV. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Horn KM, Hamm TM, Gibson AR. Red nucleus stimulation inhibits within the inferior olive. Journal of Neurophysiology. 1998;80:3127–3136. doi: 10.1152/jn.1998.80.6.3127. [DOI] [PubMed] [Google Scholar]
- Houk JC, Gibson AR, Harvey CF, Kennedy PR, Van Kan PL. Activity of primate magnocellular red nucleus related to hand and finger movements. Behavioral Brain Research. 1988;28:201–206. doi: 10.1016/0166-4328(88)90097-6. [DOI] [PubMed] [Google Scholar]
- Iwakiri H, Oka T, Takakusaki K, Mori S. Stimulus effects of the medial pontine reticular formation and the mesencephalic locomotor region upon medullary reticulospinal neurons in acute decerebrate cats. Neuroscience Research. 1995;23:47–53. [PubMed] [Google Scholar]
- Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Progress in Neurobiology. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Harper RM, McGinty DJ. Neural coding of motivational level during sleep. Physiology and Behavior. 1970;5:1139–1143. doi: 10.1016/0031-9384(70)90202-7. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lunberg A, Pompeiano O. Inhibitory effect evoked through ventral reticulospinal pathways. Archives Italiennes de Biologie (Pisa) 1968;106:124–140. [PubMed] [Google Scholar]
- Jarratt H, Hyland B. Neuronal activity in rat red nucleus during forelimb reach-to-grasp movements. Neuroscience. 1999;88:629–642. doi: 10.1016/s0306-4522(98)00227-9. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Alheid GF, Nunzi MG, Houk JC. Cerebellar input to magnocellular neurons in the red nucleus of the mouse: synaptic analysis in horizontal brain slices incorporating cerebello-rubral pathways. Neuroscience. 2002;110:105–21. doi: 10.1016/s0306-4522(01)00544-9. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Dewitt DS, Becher DP, Hayes RL. Behavioral evidence for cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Research. 1984;296:241–262. doi: 10.1016/0006-8993(84)90062-3. [DOI] [PubMed] [Google Scholar]
- Keifer J. Effects of red nucleus inactivation on burst discharge in turtle cerebellum in vitro: evidence for positive feedback. Journal of Neurophysiology. 1996;76:2200–2210. doi: 10.1152/jn.1996.76.4.2200. [DOI] [PubMed] [Google Scholar]
- Keifer J, Houk JC. Motor function of the cerebellorubrospinal system. Physiological Reviews. 1994;74:509–542. doi: 10.1152/physrev.1994.74.3.509. [DOI] [PubMed] [Google Scholar]
- Kinney GG. Peripheral nicotine administration increases rubral firing rates in the urethane-anesthetized rat. Neuroscience Letters. 1995;198:1–4. doi: 10.1016/0304-3940(95)11945-s. [DOI] [PubMed] [Google Scholar]
- Kirzon MV, Kaplan A Ya. Depression of evoked potentials in rat thalamic ventro-basal complex and somatosensory cortex after reticular stimulation. Neuroscience and Behavioral Physiology. 1978;9:204–210. doi: 10.1007/BF01182618. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhancement of acetylcholine release during REM sleep in the caudomedial medulla as measured by in vivo microdialysis. Brain Research. 1992;580:348–350. doi: 10.1016/0006-8993(92)90967-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Research. 1998;780:176–179. [PubMed] [Google Scholar]
- Kohyama J, Lai YY, Siegel JM. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep. 1998;21:695–699. doi: 10.1093/sleep/21.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. Journal of Neuroscience. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Medullary regions mediating atonia. Journal of Neuroscience. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. Journal of Neuroscience. 1990;10:2727–2734. doi: 10.1523/JNEUROSCI.10-08-02727.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata F, Li Volsi G, Ciranna L, Maugeri G, Santangelo F. 5-Hydroxytryptamine modifies neuronal responses to glutamate in the red nucleus of the rat. Experimental Brain Research. 1998;118:61–70. doi: 10.1007/s002210050255. [DOI] [PubMed] [Google Scholar]
- Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. Journal of Neurophysiology. 1946;9:165–171. doi: 10.1152/jn.1946.9.3.165. [DOI] [PubMed] [Google Scholar]
- Marshall KC, Flumerfelt BA, Gwyn DG. Acetylcholinesterase activity and acetylcholine effects in the cerebello-rubro-thalamic pathway of the cat. Brain Research. 1980;190:493–504. doi: 10.1016/0006-8993(80)90291-7. [DOI] [PubMed] [Google Scholar]
- Martin JH, Cooper SE, Ghez C. Differential effects of local inactivation within motor cortex and red nucleus on performance of an elbow task in the cat. Experimental Brain Research. 1993;94:418–428. doi: 10.1007/BF00230200. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Task-related coding of stimulus and response in cat red nucleus. Experimental Brain Research. 1991;85:373–388. doi: 10.1007/BF00229415. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Walker JM. Inhibition of rubral neurons by noxious and non-noxious pressure. Brain Research. 1991;556:78–84. doi: 10.1016/0006-8993(91)90549-b. [DOI] [PubMed] [Google Scholar]
- Mewes K, Cheney PD. Primate rubrospinal cells: parametric relations and contribution to wrist movement. Journal of Neurophysiology. 1994;72:14–30. doi: 10.1152/jn.1994.72.1.14. [DOI] [PubMed] [Google Scholar]
- Mileikovskii BY, Kiyashchenko LI, Titkov ES. Inhibition of medullary reticulospinal neurons by excitation of the dorsolateral parts of the pons, which block movement and muscle tone in rats. Neuroscience and Behavioral Physiology. 2000;30:475–479. doi: 10.1007/BF02463103. [DOI] [PubMed] [Google Scholar]
- Mileikovskii BY, Verevkina SK, Nozdrachev AD. Central neurophysiological mechanisms of the regulation of inhibition. Neuroscience and Behavioral Physiology. 1991;21:263–268. doi: 10.1007/BF01191667. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai YY, Siegel JM. Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. Journal of Neuroscience. 2000;20:8551–8558. doi: 10.1523/JNEUROSCI.20-22-08551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Muscle tone facilitation and inhibition after orexin-A (hypocretin-1). microinjections into the medial medulla. Journal of Neurophysiology. 2002;87:2480–2489. doi: 10.1152/jn.2002.87.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR, Sampogna S, Yamuy J, Chase MH. c-fos expression in brainstem premotor interneurons during cholinergically induced active sleep in the cat. Journal of Neuroscience. 1999;19:9508–9518. doi: 10.1523/JNEUROSCI.19-21-09508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M. Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. Journal of Neurophysiology. 1978;41:1580–1591. doi: 10.1152/jn.1978.41.6.1580. [DOI] [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Red nucleus lesions impair overground locomotion in rats: a kinetic analysis. European Journal of Neuroscience. 2000;12:1113–1122. doi: 10.1046/j.1460-9568.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Vuillon-Cacciuttolo G, Dusticier N, Kerkerian L, Andre D, Bosler O. Putative neurotransmitters in the red nucleus and their involvement in postlesion adaptive mechanisms. Behavioral Brain Research. 1988;28:163–174. doi: 10.1016/0166-4328(88)90093-9. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. American Journal of Physiology. 1997a;273:R451–455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997b;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Iwakiri H, Mori S. Pontine-induced generalized suppression of postural muscle tone in a reflexively standing acute decerebrate cat. Neuroscience Research. 1993;17:127–140. doi: 10.1016/0168-0102(93)90090-d. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of rubrospinal neurons during locomotion. Brain Research. 1972;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Quattrochi J, Datta S, Hobson JA. Cholinergic and non-cholinergic afferents of the caudolateral parabrachial nucleus: a role in the long-term enhancement of rapid eye movement sleep. Neuroscience. 1998;83:1123–1136. doi: 10.1016/s0306-4522(97)00471-5. [DOI] [PubMed] [Google Scholar]
- Pananceau M, Rispal-Padel L, Meftah EM. Synaptic plasticity of the interpositorubral pathway functionally related to: forelimb flexion movements. Journal of Neurophysiology. 1996;75:2542–2561. doi: 10.1152/jn.1996.75.6.2542. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Ralston DD, Milroy AM. Inhibitory synaptic input to identified rubrospinal neurons in Macaca fascicularis: an electron microscopic study using a combined immuno-GABA-gold technique and the retrograde transport of WGA-HRP. Journal of Comparative Neurology. 1992;320:97–109. doi: 10.1002/cne.903200107. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on locomotor pattern and timing in the intact cat: a comparison with the motor cortex. Journal of Neurophysiology. 1999;81:2297–2315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH, Van Der Burg H, Sabel-Goedknegt E. Locomotion coincides with c-Fos expression in related areas of inferior olive and cerebellar nuclei in the rat. Neuroscience Letters. 1996;214:119–122. doi: 10.1016/0304-3940(96)12898-6. [DOI] [PubMed] [Google Scholar]
- Sakai K. Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In: Hobson JA, Brazier MA, editors. The Reticular Formation Revised. New York: Raven; 1980. pp. 427–447. [Google Scholar]
- Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neuroscience Letters. 1989;98:159–165. doi: 10.1016/0304-3940(89)90503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Negrao N, Silva BA, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat. Neuroscience. 1987;20:961–989. doi: 10.1016/0306-4522(87)90256-9. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC. Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep. 1988;11:1–16. doi: 10.1093/sleep/11.1.1. [DOI] [PubMed] [Google Scholar]
- Siegel JM, McGinty DJ, Breedlove SM. Sleep and waking activity of pontine gigantocellular field neurons. Experimental Neurology. 1977;56:553–573. doi: 10.1016/0014-4886(77)90321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, Lufkin R. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. Journal of Neuroscience. 1992;12:1640–1646. doi: 10.1523/JNEUROSCI.12-05-01640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer H, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science. 1991;262:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Research. 1983;268:344–348. doi: 10.1016/0006-8993(83)90501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnamon HM, Ginzburg RN, Kurose GA. Midbrain stimulation in the anesthetized rat: direct locomotor effects and modulation of locomotion produced by hypothalamic stimulation. Neuroscience. 1987;20:695–707. doi: 10.1016/0306-4522(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. Journal of Neuroscience. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taek OH. Effects of stimulation of locus coeruleus-parabrachial areas on activity of neurons in the nucleus raphe magnus. Seoul University Journal of Pharmacological Science (Korea) 1991;16:20–33. [Google Scholar]
- Taepavarapruk N, McErlane SA, Soja PJ. State-related inhibition by GABA and glycine of transmission in Clarke's column. Journal of Neuroscience. 2002;22:5777–5788. doi: 10.1523/JNEUROSCI.22-13-05777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Experimental Brain Research. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shimoda N, Matsuyama K, Mori S. Discharge properties of medullary reticulospinal neurons during changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Experimental Brain Research. 1994;99:361–374. doi: 10.1007/BF00228973. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Toyama K, Kosaka K, Udo M. Disfacilitation’ of red nucleus neurones. Experientia. 1965;21:544–545. doi: 10.1007/BF02138988. [DOI] [PubMed] [Google Scholar]
- Van Kan PL, McCurdy ML. Role of primate magnocellular red nucleus neurons in controlling hand preshaping during reaching to grasp. Journal of Neurophysiology. 2001;85:1461–1478. doi: 10.1152/jn.2001.85.4.1461. [DOI] [PubMed] [Google Scholar]
- Vuillon-Cacciuttolo G, Bosler O, Nieoullon A. GABA neurons in the cat red nucleus: a biochemical and immunohistochemical demonstration. Neuroscience Letters. 1984;52:129–134. doi: 10.1016/0304-3940(84)90362-8. [DOI] [PubMed] [Google Scholar]
- Wang RY, Gallager DW, Aghajanian GK. Stimulation of pontine reticular formation suppresses firing of serotonergic neurones in the dorsal raphe. Nature. 1976;264:365–368. doi: 10.1038/264365a0. [DOI] [PubMed] [Google Scholar]
- Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


