Abstract
The regulation of K+ channels by O2 levels is a key link between hypoxia and neurotransmitter release in neuroendocrine cells. Here, we examined the effects of hypoxia on K+ channels in the immortalised v-myc, adrenal-derived HNK1+ (MAH) cell line. MAH cells possess a K+ conductance that is sensitive to Cd2+, iberiotoxin and apamin, and which is inhibited by ≈24 % when exposed to a hypoxic perfusate (O2 tension 20 mmHg). This conductance was attributed to high-conductance Ca2+-activated K+ (BK) and small-conductance Ca2+-activated K+ (SK) channels, which are major contributors to the O2-sensitive K+ conductance in adrenomedullary chromaffin cells. Under low [Ca2+]i conditions that prevented activation of Ca2+-dependent K+ conductances, a rapidly activating and slowly inactivating K+ conductance, sensitive to both TEA and 4-aminopyridine (4-AP), but insensitive to 100 nm charybdotoxin (CTX), was identified. This current was also reduced (by ≈25 %) when exposed to hypoxia. The hypoxia-sensitive component of this current was greatly attenuated by 10 mm 4-AP, but was only slightly reduced by 10 mm TEA. This suggests the presence of delayed-rectifier O2-sensitive channels comprising homomultimeric Kv1.5 or heteromultimeric Kv1.5/Kv1.2 channel subunits. The presence of both Kv1.5 and Kv1.2 α-subunits was confirmed using immunocytochemical techniques. We also demonstrated that these K+ channel subunits are present in neonatal rat adrenomedullary chromaffin cells in situ. These data indicate that MAH cells possess O2-sensitive K+ channels with characteristics similar to those observed previously in isolated chromaffin cells, and therefore provide an excellent model for examining the cellular mechanisms of O2 sensing in adrenomedullary chromaffin cells.
The regulation of ion channels by O2 tension (PO2) was first demonstrated in chemoreceptive carotid body type I cells, where K+ channels were inhibited by hypoxia (Lopez-Barneo et al. 1988). The inhibitory effects of hypoxia on K+ channels have since been demonstrated in a variety of cell types (Peers, 1997; Lopez-Barneo et al. 2001). In neurosecretory cells such as the carotid body glomus cell, inhibition of K+ channel activity is thought to underlie the cell membrane depolarisation (Lopez-Barneo et al. 1988; Hescheler et al. 1989; Peers, 1990) that leads to Ca2+ entry and neurotransmitter release (Urena et al. 1994).
In recent years, the use of continuous cell lines as a replacement for primary cell cultures from hypoxia-sensitive neurosecretory tissues has become widespread in studies examining the O2 sensitivity of ion channels. The use of cell lines is advantageous since they are easy to maintain, provide an unlimited source of cells and can be readily manipulated to delineate the molecular mechanisms of O2 sensing. This approach has been beneficial in studies examining the link between the putative O2 sensor and the ion channel itself. For instance, the phaeochromocytoma (PC12) cell line, which is derived from rat adrenal tissue, has been developed as a model system for examining the effects of hypoxia on both gene expression and plasmalemmal K+ channels (Czyzyk-Krezeska et al. 1994; Zhu et al. 1996; Beitner-Johnson et al. 1997; Conforti & Millhorn, 1997; Conforti et al. 2000). However, there are discrepancies between the specific types of ion channels expressed and regulated by hypoxia in PC12 cells and their native adrenomedullary chromaffin (AMC) cell counterparts. For instance, Conforti et al. (2000) demonstrated the inhibition of a Shaker-type K+ channel containing the Kv1.2 α-subunit in response to acute hypoxia, whilst a high-conductance Ca2+-activated K+ channel (BK) was augmented by this stimulus in these cells (Conforti & Millhorn, 1997). This contrasts with the findings of Thompson & Nurse (1998), who demonstrated in isolated rat AMC cells a major role for inhibition of the BK channel in the hypoxic response, and also those of Keating et al. (2001), who demonstrated a role for inhibition of a small-conductance (SK) channel in mediating hypoxia-evoked secretion from the sheep adrenal medulla. Moreover, recent studies have demonstrated a lack of homogeneity between different batches of PC12 cells (Green et al. 2001; Shoji-Kasai et al. 2001), limiting their use as a model neurosecretory cell line.
The v-myc, adrenal-derived HNK1+ (MAH) cell line is an immortalised line derived from embryonic rat AMC cells (Birren & Anderson, 1990). Initially developed to investigate the development of sympathoadrenal progenitor cells into mature chromaffin cells and sympathetic neurons, this line shares phenotypic and morphological characteristics with its non-immortalised counterparts (Birren & Anderson, 1990). In the study presented here, we have examined the suitability of MAH cells as a surrogate cell line for investigating the hypoxic regulation of K+ channels in rat AMC cells. We demonstrate that MAH cells, like their native counterparts, exhibit a Ca2+-dependent K+ conductance that is sensitive to Cd2+ and iberiotoxin (IbTx), and which is inhibited by hypoxia. We further demonstrate that MAH cells possess an outwardly rectifying acutely O2-sensitive K+ current that is sensitive to 4-aminopyridine (4-AP), but only slightly TEA sensitive, and charybdotoxin (CTX)- and Cd2+-insensitive. The Kv1.5 and Kv1.2 α-subunits that are likely to be responsible for this O2-sensitive channel are also present in tissue sections of rat adrenal medulla. We propose that the use of MAH cells will be useful in delineating the mechanisms involved in the regulation of known O2-sensitive K+ channel types in AMC cells.
Methods
Cell culture
All experiments were carried out on rat adrenomedullary chromaffin-derived MAH cells (Birren & Anderson, 1990), which were a kind gift from Dr David Anderson. Cells were grown in modified L-15/CO2 medium supplemented with 0.6 % glucose, 1 % penicillin/streptomycin, 10 % fetal bovine serum and 5 μm dexamethasone (Hawrot & Patterson, 1979). All cultures were grown in a Forma Scientific automatic CO2 incubator in a humidified atmosphere of 95 % air/5 % CO2 at 37 °C. Cultures were fed every 1-2 days and passaged every 3-6 days. To passage cells, culture medium was removed and 0.25 % trypsin was added to detach cells from the culture substrate. The resultant suspension was centrifuged at 12 000 rpm for 3 min, the supernatant discarded and the pellet resuspended in fresh medium. Cells were then plated onto central wells of modified culture dishes (Nurse, 1990), which had been previously coated with poly-d-lysine and laminin to promote cell adhesion.
Electrophysiology
Dishes were transferred to a continually perfused (approximately 4 ml min−1) recording chamber mounted on the stage of an Axiovert S100 microscope (Zeiss). Whole-cell patch-clamp recordings (Hamill et al. 1981) were made using patch electrodes of resistance 4-7 MΩ (when filled with intracellular solution). Patch electrodes were fabricated from 1.5/0.75 mm o.d./i.d. borosilicate glass (World Precision Instruments) using a P-97 Brown-Flaming horizontal electrode puller (Sutter Instruments) and were routinely fire-polished. Electrodes were filled with a solution of composition (mm): NaCl 5, KCl 135, Hepes 10, EGTA 11, CaCl2 1 and Na2ATP 2 (pH 7.2 with KOH). When making perforated-patch recordings, EGTA and Na2ATP were omitted from the pipette solution and nystatin was included at a concentration of 500 μg ml−1. Cells were perfused with a solution composed of (mm): NaCl 135, KCl 5, MgCl2 1.2, Hepes 5, CaCl2 2.5 and d-glucose 10 (pH 7.4 with NaOH). When using symmetrical K+ solutions (equimolar K+ on either side of the membrane), cells were perfused with a similar solution containing 135 mm KCl and 5 mmNaCl. All experiments were performed at room temperature (21-24 °C).
In time-series studies, cells were voltage clamped at -60 mV, and currents evoked by step depolarisations to the indicated test potential for 100 ms at a frequency of 0.1 Hz. Current-voltage (I-V) relationships were obtained by depolarising cells to varying test potentials between -70 and +70 mV every 10 s. In some experiments, the membrane potential was ramped between -80 mV and +60 mV over a period of 1 s from a holding potential of -60 mV. Steady-state activation was studied by measuring the peak amplitude of the tail current upon repolarisation to -40 mV following 100 ms prepulses to different test potentials (between -80 and +50 mV in 10 mV increments). To examine steady-state inactivation, cells were held at various test potentials (between -80 and +40 mV in 10 mV increments) for 10 s prior to a 100 ms depolarising step to +40 mV, from which current amplitudes were obtained. Currents were normally filtered at 5 kHz, digitised at 10 kHz and stored on computer for later analysis. When analysing activation kinetics, currents were filtered at 10 kHz and digitised at 20 kHz to provide adequate data points for accurate fitting. Capacitative transients were minimised by analogue means. Analyses and voltage protocols were performed using an EPC 9 amplifier (HEKA) with integrated AD/DA converter and ITC-16 interface, and Pulse software (HEKA). Steady-state currents were measured as the average current between 90 and 99 ms of the voltage step. All data analysis and fitting of exponential curves to currents was carried out using Pulsefit software (HEKA). Graphing and fitting of curves to graphical data was performed using Origin (Microcal). Results are expressed as means ± s.e.m., and statistical comparisons were made using paired or unpaired Student's t tests, as appropriate.
Current-clamp (I = 0 pA) recordings were made using the same amplifier, and solutions were identical to those described above for the voltage-clamp experiments examining K+ currents. Voltage output was filtered at 500 Hz and digitised at 1 kHz.
The tubing used was gas impermeable (Tygon). Bath hypoxia was produced by bubbling the extracellular perfusate with 100 % N2 for at least 30 min prior to cell perfusion. Bubbling produced no shift in the pH. The degree of bath hypoxia was measured using a needle electrode (Diamond General Development, Ann Arbor, MI, USA). The time course of the change in PO2 in the recording chamber was consistent between recordings and was always stable within 15-30 s of exchanging solution.
Drug solutions were prepared by dissolving in the extracellular perfusate to the required concentration, and pH was readjusted as necessary. TEA and 4-AP were obtained from Sigma. IbTx, apamin and CTX were obtained from Alomone Labs, Israel. When using these toxins, 0.1 % BSA was included in all solutions to prevent binding to the perfusion reservoirs and tubing.
Immunofluorescence
MAH cells were plated as described above. After a brief wash in pre-warmed PBS, cells were fixed at -20 °C in 95 % methanol-5 % acetic acid for 1 h. Cells were washed (3 × 5 min each) in PBS before overnight incubation at 4 °C in the primary antiserum. Primary antibodies (all used at 1:50 dilution) were rabbit polyclonal antisera raised against (1) residues 513-602 of mouse Kv1.5, (2) residues 417-499 of rat Kv1.2 or (3) residues 1098-1196 of mouse Slo (BK). All antibodies were obtained from Alomone Labs. In control experiments, the primary antibody was pre-adsorbed overnight at 4 °C with a fusion protein corresponding to the antigen in a 3:1 ratio, as per the manufacturer's instructions. The secondary antibody (1:500 dilution) was a Cy3-conjugated goat anti-rabbit IgG (Jackson Laboratories). Samples were covered with Vectashield Mounting Medium (Vector Laboratories) before being viewed with the aid of a BIORAD Microradiance 2000 Confocal Microscope equipped with a helium/neon (543 nm) laser. Lasersharp software was used for image acquisition. Digitised images of positive immunofluorescence were taken following excitation of the fluorochrome.
To examine the localisation of Kv1.2 and Kv1.5 in the adrenal medulla, adrenal glands were isolated from 1- to 2-day-old Wistar rat pups (Thompson et al. 1997) and sectioned as described previously (Prasad et al. 2001). This procedure conforms to the standards set out by the Canadian Council for Animal Care (CCAC). Sections were immunostained as described above except that the secondary antibody was Alexa488-conjugated goat anti-rabbit IgG (Molecular Probes). Immunofluorescence was viewed with a Zeiss upright phase-contrast microscope equipped with epi-illumination and a fluorescein filter set.
Results
Presence of Ca2+-activated K+ conductances in MAH cells
During recordings with the perforated-patch configuration to preserve cytoplasmic integrity, step depolarisations from a holding potential of -60 mV to levels > -20 mV evoked sustained outward currents in MAH cells; (Fig. 1A). In most cases, I-V relationships displayed a shoulder at more positive test potentials, suggesting the presence of a Ca2+-activated K+ channel component of whole-cell current. Total K+ current was inhibited by 200 μm Cd2+ (mean inhibition 25.0 ± 4.1 %, n = 14), an indirect blocker of Ca2+-activated K+ currents (Fig. 1B, left). Currents were also inhibited by 100 nm IbTx (mean inhibition 21.3 ± 6.6 %, n = 5), a selective, direct blocker of the BK channel (Gribkoff et al. 1996; Fig. 1B, centre) and by 100 nm apamin (mean inhibition 9.7 ± 4.8 %, n = 6), a selective and direct blocker of the small-conductance Ca2+-activated K+ (SK) channel (Blatz & Magleby, 1986; Fig. 1B, right).
Figure 1. Ca2+-dependent K+ currents in adrenal-derived HNK1+ (MAH) cells.
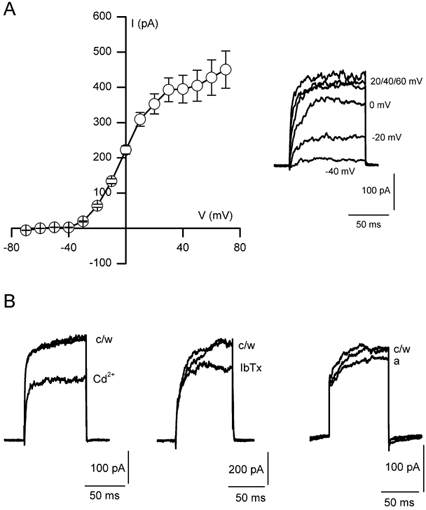
A, mean (± s.e.m.) current-voltage (I-V) relationship obtained using the perforated-patch voltage-clamp technique. Currents were evoked by 100 ms step depolarisations to a range of test potentials between -70 and +70 mV (10 mV increments; holding potential -60 mV) at a frequency of 0.1 Hz. Each point shows the mean (n = 19) current amplitude evoked by the step depolarisation. At more positive potentials, a marked shoulder in the I-V curve can be seen, indicative of the presence of a Ca2+-dependent K+ current. Inset, individual current traces from a typical cell, following depolarising steps to the test potentials indicated. B, individual traces evoked by single step depolarisations to +30 mV obtained under control conditions (c), following exposure to either 200 μm Cd2+ (Cd), 100 nm iberiotoxin (IbTx) or 100 nm apamin (a), and following washout (w).
Low [Ca2+]i conditions show that MAH cells express delayed-rectifier K+ channels
To examine other components of the total K+ current in MAH cells, whole-cell recordings were made in cells dialysed with a pipette solution which clamped [Ca2+]i at ≈10 nm, a concentration insufficient to allow the activation of Ca2+-dependent K+ conductances (Barrett et al. 1982). Under these conditions, step depolarisations from a holding potential of -60 mV to levels positive to ≈-30 mV evoked sustained currents (Fig. 2A), which were insensitive to 200 μm Cd2+ (Fig. 2B), demonstrating the lack of a Ca2+-activated component of the outward K+ current in MAH cells with low free [Ca2+]i.
Figure 2. Delayed-rectifier K+ currents in MAH cells identified under low free [Ca2+]i conditions.
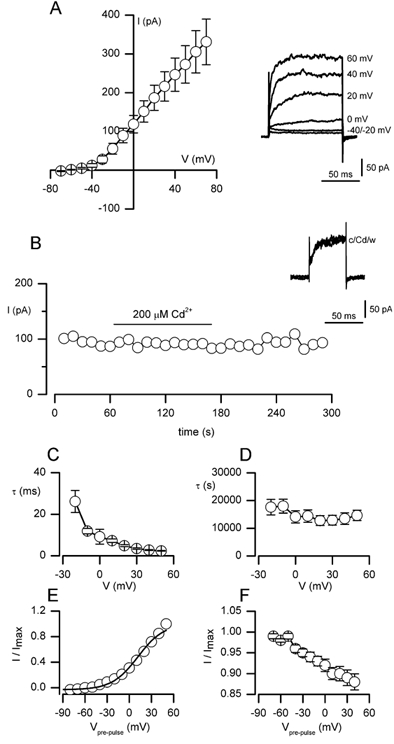
A, mean (± s.e.m.) I-V relationship obtained using the conventional whole-cell patch-clamp technique with [Ca2+]i clamped at ≈10 nm. Currents were evoked by 100 ms step depolarisations to a range of test potentials between -70 and +70 mV (10 mV increments; holding potential -60 mV) at a frequency of 0.1 Hz. Each point shows the mean (n = 14) current amplitude evoked by the step depolarisation. Inset, individual current traces from a typical cell following depolarising steps to the test potentials indicated. B, example time-series recording (typical of six such recordings) showing sustained outward current amplitudes observed when dialysed MAH cells were step depolarised to +30 mV for 100 ms (holding potential -60 mV) at a frequency of 0.1 Hz. The horizontal bar indicates the period of bath application of 200 μm Cd2+. Inset, individual current traces taken from the time series, before (c), during (Cd) and after (w) the bath application of Cd2+. C and D, time constants (τ) for activation (C) and inactivation (D) are plotted as a function of the test potential (V). Time constants were obtained by fitting activating and inactivating sections of current traces with a single exponential function. Data were averaged from 12 cells examined. E, steady-state activation is plotted as a function of the pre-pulse potential (Vpre-pulse). Data are means from 12 cells examined (error bars lie within data points and have been removed for clarity). The smooth curve represents a Boltzmann fit to activation data (see text). F, steady-state inactivation plotted as a function of Vpre-pulse. Channels failed to inactivate even at the highest test potentials examined, precluding fitting of the curve. Data are mean ± s.e.m. from 12 cells examined. Data in both E and F were normalised to the maximum evoked current. See Methods for details of voltage protocols and fitting procedures.
Kinetic analysis of the delayed-rectifier currents seen under low [Ca2+]i conditions to eliminate Ca2+-dependent K+ currents is presented in Fig. 2C-F. The time courses of activation and inactivation were well-fitted with a single exponential function. The time constants (tau, τ) for activation and inactivation are plotted as a function of the test potential in Fig. 2C and D, respectively. Activation was rapid (e.g. 3.5 ± 0.4 ms at +30 mV) and voltage-dependent, decreasing from 26.2 ± 5.3 ms at -20 mV to 2.36 ± 0.16 ms at +50 mV. The rate of inactivation was extremely slow (see Fig. 2D). Steady-state activation and inactivation are also plotted as a function of the test potential in Fig. 2E and F, respectively. The steady-state activation curve was fitted with a Boltzmann equation of the form:
where k represents the slope factor and V1/2 the voltage of half-maximal activation. In Fig. 2E, the steady-state activation curve shows a threshold for activation of around -40 mV, and the Boltzmann fit to the curve gave V1/2 = 11.6 mV and k = 17.4. For steady-state inactivation, even when prepulsing cells for long periods (> 10 s), inactivation was incomplete (data not shown) and therefore V1/2 could not be calculated. However, the small degree of inactivation seen was somewhat voltage dependent.
Pharmacological analysis of the delayed-rectifier currents showed they were reduced in a concentration-dependent manner in the presence of either TEA (0.01-300 mm; EC50 ≈1 mm; Fig. 3A and B) or 4-AP (0.01-30 mm; Fig. 3C and D) in the extracellular perfusate. Higher concentrations of 4-AP caused a loss of seal integrity in all cells examined.
Figure 3. Concentration-dependent inhibition of Ca2+-independent, delayed-rectifier K+ currents in MAH cells by TEA and 4-aminopyridine (4-AP).
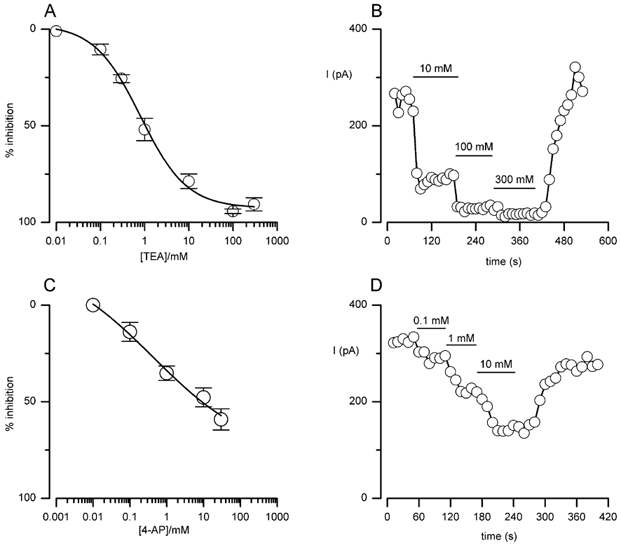
A, concentration-response curve for the inhibitory effect of TEA (0.01-300 mm) on delayed-rectifier K+ channel current. Each point shows the mean (± s.e.m.) inhibition taken from between four and six cells at each concentration. Data points were fitted with a sigmoidal curve. B, example time-series recording showing the inhibitory effects of different concentrations of TEA in a single cell. Each point shows the sustained current amplitude observed when repeatedly step depolarising to +30 mV for 100 ms (holding potential -60 mV) at a frequency of 0.1 Hz. Horizontal bars indicate the periods of bath application of 10, 100 and 300 mm TEA. C, as in A, except the curve shows the concentration-dependent inhibition due to 4-AP (0.01-30 mm). D, as in B, except the horizontal bars show the periods of bath application of 0.1, 1 and 10 mm 4-AP.
Effects of hypoxia on Ca2+-independent, delayed-rectifier K+ currents in MAH cells
During recordings from MAH cells without active Ca2+-dependent K+ currents (i.e. dialysed with a low free Ca2+ pipette solution), exposure to hypoxia (PO2, 20 mmHg) caused a rapid and reversible reduction of the outward K+ current (Fig. 4A). In seven cells examined, hypoxia caused a reduction in mean outward current density (obtained by dividing the current observed during step depolarisations to +30 mV by the whole-cell capacitance) from 51.2 ± 6.9 pA pF−1 under control conditions to 38.7 ± 6.2 pA pF−1 under hypoxic conditions (P < 0.05, paired t test). Outward current density returned to 51.4 ± 7.4 pA pF−1 upon return to normoxia. Under symmetrical K+ conditions (135 mm K+ on either side of the membrane), the O2-sensitive current (obtained by subtracting the current values evoked when cells were ramp depolarised in hypoxia from those obtained in normoxia) showed outward rectification and reversed near 0 mV, as expected of K+-selective channels (Fig. 4B).
Figure 4. Effect of hypoxia on K+ currents and membrane potential in MAH cells.
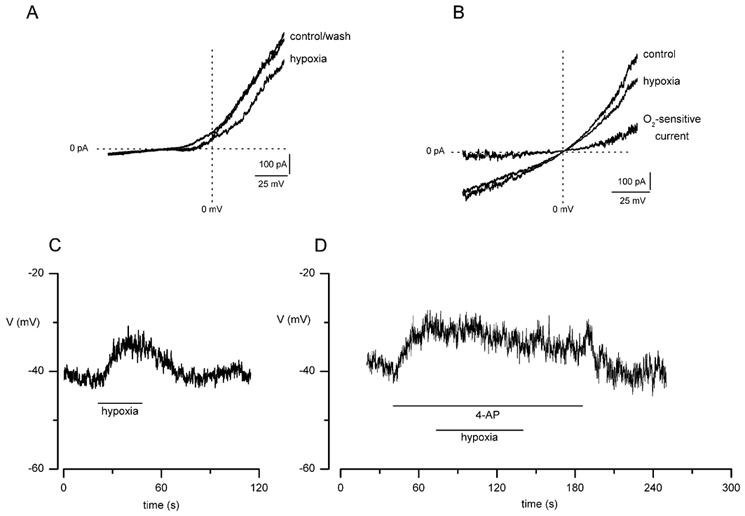
A, example ramp I-V relationships (typical of seven recordings) obtained from a single MAH cell before, during and after exposure to a hypoxic perfusate (O2 tension, PO2, 20 mmHg) using asymmetrical K+ (135 mm inside:5 mm outside) solutions in cells dialysed with a low free [Ca2+] pipette solution. Currents were evoked by a 1 s ramp depolarisation between -80 and +60 mV. B, example ramp I-V relationships using the same voltage protocol as in A, but obtained under symmetrical K+ (135 mm on either side of membrane) conditions. The magnitude of the O2-sensitive ‘difference’ current, obtained by subtracting current amplitudes in hypoxia from those obtained during normoxia, is indicated; note the reversal potential at 0 mV, as expected of a K+-selective channel. C, typical current-clamp (I = 0) recording obtained from a single MAH cell showing the depolarising effect of hypoxia on the cell membrane. The period of exposure to hypoxia (PO2 20 mmHg) is indicated by the horizontal bar. D, as in C except that the cell was exposed to 10 mm 4-AP prior to and during the exposure to hypoxia, as indicated by the horizontal bars; note that hypoxia had no additional effect on the depolarisation induced by 4-AP.
Under low [Ca2+]i conditions, the magnitude of the O2-sensitive K+ current (IKvO2) was significantly reduced from 7.7 ± 1.8 pA pF−1 under control conditions to 0.9 ± 0.7 pA pF−1 in the presence of 10 mm 4-AP (n = 6; P < 0.01, paired t test; see Fig. 5A and B), demonstrating the high sensitivity of IKvO2 to 4-AP. In contrast, in the presence of 10 mm TEA, IKvO2 was reduced slightly from 7.2 ± 2.3 pA pF−1 under control conditions to 4.4 ± 0.9 pA pF−1 in the presence of TEA, but this effect was not significant (n = 7; P > 0.05, paired t test; see Fig. 5C and D). These data suggest that the hypoxia-sensitive, delayed-rectifier K+ current is sensitive to 4-AP and relatively insensitive to TEA. These pharmacological properties of IKvO2 in MAH cells bear a strong resemblance to the properties of Kv1.5 homo/heteromultimeric delayed-rectifier channels described recently in pulmonary arterial smooth muscle cells (Smirnov et al. 2002). We further examined the effects of 4-AP on cell membrane potential and the response to hypoxia. Under current-clamp conditions (I = 0 pA) and with a physiological K+ distribution, hypoxia caused a marked membrane depolarisation in MAH cells (Fig. 4C). In six cells examined, membrane potential was reduced from -31.3 ± 3.1 mV under control conditions to -21.1 ± 3.4 mV in hypoxia (P < 0.05, paired t test). In addition, 10 mm 4-AP depolarised the cell membrane, and in the continued presence of 4-AP, the depolarising effect of hypoxia was abolished (n = 7; Fig. 4D).
Figure 5. The O2-sensitive delayed-rectifier K+ current in MAH cells is sensitive to 4-AP and relatively insensitive to TEA.
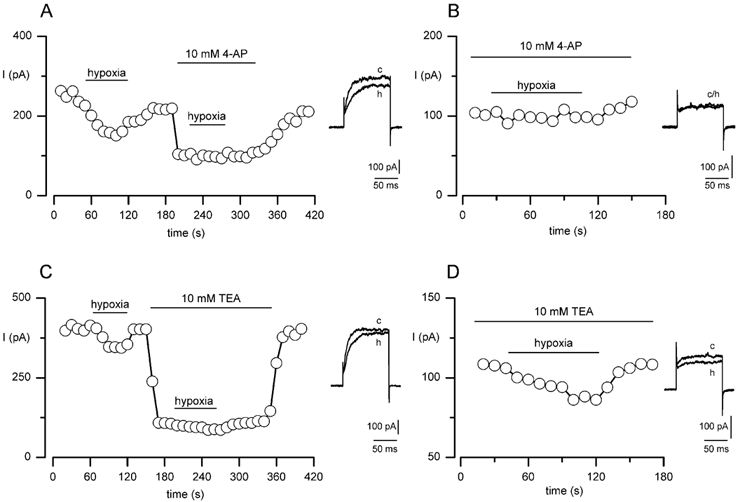
A, example time-series recording in which a single MAH cell was exposed to hypoxia (PO2 20 mmHg) under low [Ca2+]i conditions, prior to and during exposure to 10 mm 4-AP. Each point shows the sustained delayed-rectifier K+ current amplitude observed when the cell was repeatedly step depolarised to +30 mV for 100 ms (holding potential -60 mV) at a frequency of 0.1 Hz. Horizontal bars indicate the periods of exposure to hypoxia and 4-AP. Inset, individual traces taken from the time-series before (c) and during (h) exposure to hypoxia in the absence of 4-AP. B, taken from the same time-series recording as in A, except the portion of the time-series recorded during exposure to 10 mm 4-AP is shown on expanded time base to show more clearly that the effect of hypoxia was abolished in the presence of 4-AP. Inset, individual traces taken from the time-series before (c) and during (h) exposure to hypoxia in the presence of 4-AP. C, as in A, except that the cell was exposed to hypoxia prior to and during exposure to 10 mm TEA. Horizontal bars indicate the periods of exposure to hypoxia and TEA. Inset, individual traces taken from the time-series before (c) and during (h) exposure to hypoxia in the absence of TEA. D, as in B, except that the expanded time-series recording shows the effect of hypoxia in the presence of TEA on an expanded time base. Inset, individual traces taken from the time-series before (c) and during (h) exposure to hypoxia in the presence of TEA.
The pharmacological profile of IKvO2 suggests the presence of O2-sensitive delayed-rectifier Kv1.5-subunit-containing K+ channel complexes in MAH cells (Smirnov et al. 2002). The Kv1.5 subunit has previously been proposed to form heteromeric O2-sensitive K+ channels in combination with other subunits such as Kv1.2 (Hulme et al. 1999; Conforti et al. 2000; Kerr et al. 2001), which are also present in MAH cells (see below). To examine this, perforated-patch recordings were made in which cells were exposed to CTX in the presence of 200 μm Cd2+ to inhibit the activity of Ca2+-dependent K+ channels. The residual delayed-rectifier K+ current was unaffected by 100 nm CTX (n = 9), indicating it was not carried by homomultimeric Kv1.2 channels (Russell et al. 1994). The functionality of CTX was demonstrated by its ability to inhibit the Ca2+-dependent K+ current in the absence of Cd2+ (Fig. 6B). Furthermore, when blocking the Ca2+-dependent K+ current with 100 nm CTX, hypoxia still inhibited channel activity (Fig. 6C).
Figure 6. The delayed-rectifier, O2-sensitive K+ current in MAH cells is insensitive to charybdotoxin (CTX).
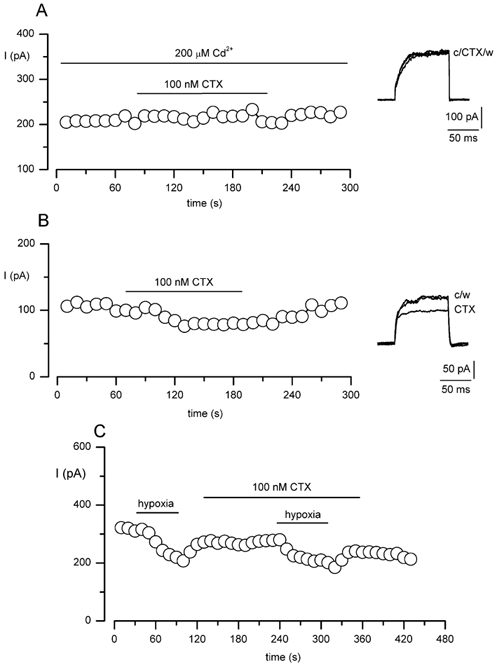
A, example time-series recording measuring the delayed-rectifier component of the K+ current in MAH cells and demonstrating the lack of effect of 100 nm CTX. Each point shows the sustained current amplitude observed when the cell was repeatedly step depolarised to +30 mV for 100 ms (holding potential -60 mV) at a frequency of 0.1 Hz, in the constant presence of 200 μm Cd2+. The lower horizontal bar indicates the period of exposure to CTX. The functionality of CTX was verified by examining the effects of the toxin on the Ca2+-dependent K+ current in MAH cells under normal [Ca2+]i conditions and in the absence of Cd2+ (B). In the presence of CTX and Cd2+, currents were inhibited by hypoxia (C), further demonstrating that the delayed-rectifier O2-sensitive K+ current in MAH cells is CTX insensitive.
Effects of hypoxia on Ca2+-dependent K+ currents in MAH cells
During perforated-patch recordings, a Ca2+-dependent K+ conductance was observed in MAH cells (see Fig. 1B). To determine the O2-sensitivity of this conductance, the effects of hypoxia were studied in the presence of 10 mm 4-AP to inhibit the O2-sensitive delayed-rectifier component. Under these conditions, hypoxia caused a 23.8 ± 2.4 % (n = 7) reduction in K+ current amplitudes (Fig. 7A). Furthermore, in the presence of 200 μm Cd2+, this O2-sensitive, 4-AP-insensitive K+ current could be completely abolished (n = 5; Fig. 7B). These data demonstrate that, similar to previous reports in isolated rat and sheep adrenal chromaffin cells (Thompson & Nurse, 1998; Keating et al. 2001), MAH cells possess a K+ current that has a Ca2+-dependent component which is inhibited by hypoxia.
Figure 7. O2 sensitivity of Ca2+-dependent K+ current in MAH cells.
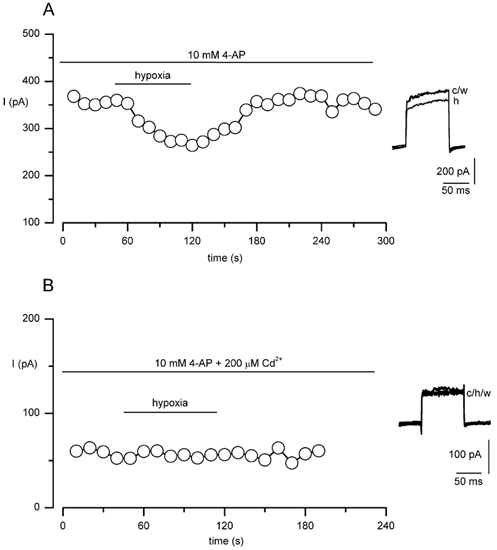
A, example time-series plot (typical of seven such recordings) demonstrating the inhibitory effect of hypoxia on the Ca2+-dependent K+ current in MAH cells, after block of the O2-sensitive delayed-rectifier K+ current with 10 mm 4-AP. Each point shows the sustained current amplitude observed when the cell was repeatedly step depolarised to +30 mV for 100 ms (holding potential -60 mV) at a frequency of 0.1 Hz. The horizontal bar indicates the period of exposure to hypoxia. B, as in A, except that the recording was made in the combined presence of 10 mm 4-AP and 200 μm Cd2+. This cell was previously demonstrated to be responsive to hypoxia (not shown).
Identification of K+ channels in MAH cells
The electrophysiological and pharmacological data suggest the presence of two hypoxia-sensitive K+ channels in MAH cells. One of them appears to be a delayed-rectifier channel consisting of either Kv1.5 α-subunits alone or in combination with Kv1.2 subunits to produce a heteromultimeric Kv1.2/Kv1.5 complex (Smirnov et al. 2002). To explore this possibility, immunocytochemical techniques were employed to validate whether or not these α-subunits were present in MAH cells. As shown in Fig. 8A, MAH cells were positively stained with specific antibodies raised against epitopes on each of the two Kv α-subunits. This staining was abolished when the primary antibody was pre-adsorbed with a fusion protein corresponding to the antigen, or in the absence of the primary antibody (not shown). Similar techniques were also employed to demonstrate the presence of the other major O2-sensitive K+ (BK) channel in MAH cells. (Fig. 8A, right). To examine whether or not the Kv1.2 and Kv1.5 α-subunits are present in native chromaffin cells of the rat adrenal medulla, sections of the adrenal gland were subjected to similar staining procedures as described for MAH cells. Typical results are shown in Fig. 8B, where the presence of both α-subunits was clearly identified in chromaffin cells of the medullary region of the gland.
Figure 8. Immunocytochemical evidence for the presence of O2-sensitive K+ channels in MAH cells and adrenal chromaffin cells in situ.
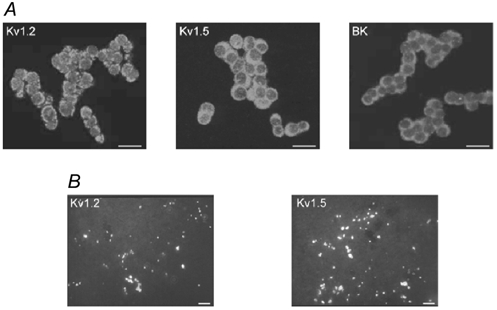
A, confocal images showing the presence of the α-subunits of Kv1.2 and Kv1.5, and the high-conductance Ca2+-activated K+ (BK) channel, in MAH cells. Cells were immunostained with specific antibodies against the indicated channel, and visualised by Cy3 fluorescence coupled to the secondary antibody. Scale bars represent 20 μm. B, micrographs showing the presence of the α-subunits of Kv1.2 and Kv1, 5, in sections of adrenal glands obtained from 1- to 2-day-old rat pups. Sections were immunostained with specific antibodies against the indicated channel and visualised by Alexa488 fluorescence coupled to the secondary antibody; positive immunostaining for these subunits was confined to chromaffin cells. Scale bars represent 50 μm.
Discussion
The use of cell lines as surrogates for examining mechanisms of O2 sensing by native cells has become widespread. For example, the PC12 cell line has been used extensively to investigate the mechanisms of hypoxic inhibition of K+ channels and subsequent neurotransmitter release, with efforts to correlate these events with those occurring in native neurosecretory cells. The benefits of this approach are multiple, and include the relative ease and lower cost of maintaining cell lines compared to preparing primary cell cultures. Furthermore, this strategy lends itself more readily to molecular approaches investigating the channel subtypes and molecular mechanisms involved in O2 sensing. Recently however, the homogeneity of cell lines such as PC12 has been called into question, and this may hinder the interpretation of data obtained in this model (Green et al. 2001; Shoji-Kasai et al. 2001).
MAH cells are an immortalised cell line derived from isolated rat AMC cells (Birren & Anderson, 1990). Since these cells were derived from AMC cells, which possess O2-sensitive K+ channels and mediate hypoxia-induced elevations in circulating catecholamine levels around birth (Thompson et al. 1997; Thompson & Nurse, 1998; Keating et al. 2001), we investigated the O2 sensitivity of ion channels in MAH cells to determine whether or not they provide a suitable substitute for primary isolated AMC cells. During perforated-patch-clamp recordings from these cells, we identified currents that were sensitive to 200 μm Cd2+, 100 nm IbTx and 100 nm apamin. This pharmacological profile demonstrates the presence of multiple Ca2+-activated K+ channels, of types previously reported to be responsible for mediating hypoxia-induced depolarisation and catecholamine release from perinatal chromaffin cells in both rat (Thompson et al. 1997; Thompson & Nurse, 1998; see also Lee et al. 2000) and sheep (Keating et al. 2001) adrenal medulla. The O2 sensitivity of this Ca2+-dependent current was demonstrated in experiments where the O2-sensitive delayed-rectifier component of total current was inhibited using 4-AP.
The presence of the BK channel, inhibition of which initiates depolarisation and catecholamine release in native chromaffin cells (authors’ unpublished observations), was confirmed in MAH cells using immunofluorescence techniques. These data show that MAH cells may serve as an attractive model for examining the effects of hypoxia on channels previously reported to be O2-sensitive in native chromaffin cells (authors’ unpublished observations). The presence of an SK current in these immortalised cells will facilitate further studies aimed at addressing the role of SK channels in mediating the release of catecholamines from the adrenal gland during hypoxia, as has been previously proposed in both rat and sheep (Lee et al. 2000; Keating et al. 2001).
To examine the role of other O2-sensitive K+ conductances that have been proposed to play a role in the membrane depolarisation due to hypoxia (e.g. a Ca2+-insensitive delayed-rectifier K+ channel: Rychkov et al. 1998; Thompson & Nurse, 1998), cells were dialysed with a pipette solution that clamped [Ca2+]i at low levels (≈10 nm) to inhibit Ca2+-dependent K+ channels (Barrett et al. 1982). Loss of the Ca2+-dependent component was confirmed due to the loss of sensitivity of the outward current to 200 μm Cd2+. This allowed the identification in MAH cells of an outwardly rectifying K+ conductance. This conductance was insensitive to CTX, a blocker of a specific Kv channel (Kv1.2) previously demonstrated to contribute to the O2-sensitive K+ current in PC12 cells (Conforti et al. 2000).
With low free [Ca2+]i conditions (to eliminate the Ca2+-dependent component of the whole-cell K+ current), during the bath application of a submaximal concentration of 4-AP (10 mm) the response to acute hypoxia was virtually abolished. This effect of 4-AP was observed under both voltage- and current-clamp conditions. In contrast, in the presence of a submaximal concentration of TEA (10 mm) the magnitude of the hypoxic response was only slightly reduced, but the effect was not significant. This pharmacological characterisation suggests the presence of a delayed-rectifier, O2-sensitive K+ current (IKvO2) in MAH cells that is open at the resting membrane potential, which is comprised of homo/heteromultimeric Kv1.5 channel complexes, and which may contribute to hypoxic depolarisation in these cells. This IKvO2 bears a strong similarity to a component of the delayed-rectifier K+ current in rat pulmonary arterial myocytes (Smirnov et al. 2002), which the authors propose underlie the O2 sensitivity of these vessels (Hu et al. 1996). In expression systems, homomultimeric Kv1.5 channel complexes are sensitive to TEA only at very high concentrations (Coetzee et al. 1999). While there are no data concerning the effects of TEA on homomultimeric Kv1.5 channels in native cells, the slight TEA sensitivity of IKvO2 may preclude such channel complexes as the O2-sensitive component of K+ current in MAH cells. In other cell types, such as rabbit systemic vascular myocytes, the presence of other Kv channel α-subunits (e.g. Kv1.2) in heteromultimeric O2-sensitive K+ channels has also been demonstrated (Hulme et al. 1999). The presence of both Kv1.2 and Kv1.5 α-subunits in MAH cells was confirmed in the present study with the aid of immunofluorescence techniques. In contrast to PC12 cells (Conforti et al. 2000), homomultimeric Kv1.2 channels appear not to be the active O2-sensitive K+ channels in MAH cells, since the delayed-rectifier channels were hypoxia-sensitive but CTX-insensitive (Russell et al. 1994). Since both α-subunits are present in MAH cells, the relative sensitivities of the hypoxia-sensitive K+ current to 4-AP, TEA and CTX suggest a Kv1.5/Kv1.2 heteromultimeric complex as the O2-sensitive K+ current in MAH cells. Confirmation of this point awaits a characterisation of the sensitivity of such heteromultimers to TEA in either native cells or expression systems. The presence of both of these K+ channel subunits in neonatal rat AMC cells was also confirmed by immunohistochemical staining of sections through the rat adrenal gland. We therefore propose that a heteromultimeric Kv1.5/Kv1.2 complex forms the basis of the O2-sensitive delayed-rectifier K+ current in the adrenal medulla, although this hypothesis also requires validation.
One mechanism proposed to explain hypoxia-mediated neurotransmitter release from neurosecretory cells involves the hypoxic inhibition of K+ channels, leading to membrane depolarisation and Ca2+ influx through voltage-gated Ca2+ channels (Peers, 1990; Urena et al. 1994; Thompson et al. 1997; Thompson & Nurse, 1998; Lopez-Barneo et al. 2001). In MAH cells, hypoxia reduced several K+ conductances, and this is thought to underlie the hypoxic membrane depolarisation seen in these studies. Previous studies (e.g. Vandenbergh et al. 1991), and our unpublished observations, have demonstrated the presence of catecholamines in MAH cells, although further studies are required to examine whether hypoxia causes secretion from these cells, as has been reported previously in AMC cells, using carbon-fibre amperometric techniques (Mojet et al. 1997; Thompson & Nurse, 2000). A physiological role for the release of catecholamines from AMC cells has been proposed to assist in maintaining fetal blood flow and circulation (West et al. 1953). It was later confirmed that noradrenaline, originating from the AMC cells and the extrachromaffin cells of Zuckerkandl, can redistribute fetal blood flow to the brain, heart and adrenal gland at the expense of other organs (Campbell et al. 1967). The possibility of a role for AMC cells in mediating this process was suggested due to previous work by Comline & Silver (1961) when they demonstrated a reduction in catecholamine content of the fetal lamb adrenal gland during hypoxia. Furthermore, in human fetuses experiencing asphyxia, a depletion in catecholamine content of the adrenal medulla is suggestive of a role of embryonic AMC cells in O2 sensing (Lagercrantz & Bistoletti, 1977). In previous electrophysiological studies (Thompson & Nurse, 2000), we demonstrated that embryonic (day 17) AMC cells are O2-sensitive, in that they depolarise in response to an acute hypoxic stimulus. This depolarisation occurs in the absence of detectable expression of the BK channel, and inhibition of other K+ channel subtypes is thought to provide the link between hypoxia and catecholamine release at this embryonic stage. The data presented here suggest that delayed-rectifier Kv channels underlie the hypoxia-induced depolarisation observed in the absence of a Ca2+-dependent component of the K+ current at the embryonic stage, and also contribute to the O2-sensitive K+ current in postnatal rat and sheep AMC cells (Thompson & Nurse, 1998; Rychkov et al. 1998; Keating et al. 2001).
To summarise, in these studies we have examined the O2 sensitivity of K+ currents in an immortalised cell line derived from AMC cells. The presence of O2-sensitive, Ca2+-dependent K+ currents in MAH cells demonstrates their similarity with isolated AMC cells. Further pharmacological and biophysical dissection of K+ currents in MAH cells allowed the identification of O2-sensitive delayed-rectifier channels, which we demonstrated to be expressed in the native cells. We propose the use of MAH cells as a model system for investigating the molecular mechanisms underlying the hypoxic inhibition of K+ channels and neurotransmitter release.
Acknowledgments
This work was supported by a Wellcome Trust Prize International Travelling Fellowship (Ref: 06154) to I. M. F., and by grants from the Heart and Stroke Foundation of Ontario (no. T-4641) to C. A. N. and the Natural Sciences and Engineering Research Council to C. A. N. and L. C. D. In addition, I. S. was supported by a John D. Schultz Science Student Scholarship from the Heart and Stroke Foundation of Ontario.
References
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. Journal of Physiology. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Shull GE, Dedman JR, Millhorn DE. Regulation of gene expression by hypoxia: a molecular approach. Respiration Physiology. 1997;110:87–97. doi: 10.1016/s0034-5687(97)00075-3. [DOI] [PubMed] [Google Scholar]
- Birren SJ, Anderson DJ. A v-myc-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990;4:189–201. doi: 10.1016/0896-6273(90)90094-v. [DOI] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Campbell AGM, Dawes GS, Fishman AP. Regional distribution of blood flow in the mature fetal lamb. Circulation Research. 1967;21:229–239. doi: 10.1161/01.res.21.2.229. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, De Miera E V-S, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy of Science. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Comline RS, Silver M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. Journal of Physiology. 1961;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Bodi I, Nisbet JW, Millhorn DE. O2-sensitive K+ channels: role of the Kv1. 2 α-subunit in mediating the hypoxic response. Journal of Physiology. 2000;524:783–793. doi: 10.1111/j.1469-7793.2000.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Millhorn DE. Selective inhibition of a slow-inactivating voltage-dependent K+ channel in rat PC12 cells by hypoxia. Journal of Physiology. 1997;502:293–305. doi: 10.1111/j.1469-7793.1997.293bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in phaeochromocytoma (PC12) cells. Journal of Biological Chemistry. 1994;269:760–764. [PubMed] [Google Scholar]
- Green KN, Taylor SC, Smith IF, Peers C. Differential coupling of voltage-gated Ca2+ channels to catecholamine secretion from separate PC12 cell batches. Neuroscience Letters. 2001;301:13–16. doi: 10.1016/s0304-3940(01)01594-4. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, McKay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Molecular Pharmacology. 1996;50:206–217. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawrot E, Patterson PH. Long-term culture of dissociated sympathetic neurons. Methods in Enzymology. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Delpiano MA, Acker H, Pietruschka F. Ionic currents on type I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Research. 1989;486:79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Hu Q, Tristani-Firouzi M, Archer SL. Voltage-gated potassium channel Kv1. 5 antibody suppresses the rise in cytosolic calcium caused by 4-aminopyridine and hypoxia in pulmonary vascular smooth muscle cells. Circulation. 1996;94:I411. [Google Scholar]
- Hulme JT, Coppock EA, Felipe A, Martens JR, Tankum MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circulation Research. 1999;85:487–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- Keating DJ, Rychkov GY, Roberts ML. Oxygen sensitivity in the sheep adrenal medulla: role of SK channels. American Journal of Physiology. 2001;281:C1434–1441. doi: 10.1152/ajpcell.2001.281.5.C1434. [DOI] [PubMed] [Google Scholar]
- Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen KT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1. 2-Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circulation Research. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatric Research. 1977;11:889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim W, Eun S, Kim SK, Kim J. Inhibition of apamin-sensitive K+ current by hypoxia in adult rat adrenal chromaffin cells. Pflügers Archiv. 2000;439:700–704. doi: 10.1007/s004249900228. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body-K+ current modulated by Po2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanisms of oxygen sensing. Annual Reviews in Physiology. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Mojet MH, Mills E, Duchen MR. Hypoxia-induced catecholamine secretion in isolated newborn rat adrenal chromaffin cells is mimicked by inhibition of mitochondrial respiration. Journal of Physiology. 1997;504:175–189. doi: 10.1111/j.1469-7793.1997.175bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA. Carbonic anhydrase and neuronal enzymes in cultured glomus cells of the carotid body of the rat. Cell and Tissue Research. 1990;261:65–71. doi: 10.1007/BF00329439. [DOI] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells-selective effect on the Ca2+ activated K+ current. Neuroscience Letters. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C. Oxygen-sensitive ion channels. Trends in Pharmacological Sciences. 1997;18:405–408. doi: 10.1016/s0165-6147(97)01120-6. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. Journal of Physiology. 2001;537:67–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SN, Overturf KE, Horowitz B. Heteromultimeric formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. American Journal of Physiology. 1994;267:C1729–1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Rychkov GY, Adams MB, McMillen IC, Roberts ML. Oxygen-sensing mechanisms are present in the chromaffin cells of the sheep adrenal medulla before birth. Journal of Physiology. 1998;509:887–893. doi: 10.1111/j.1469-7793.1998.887bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kasai Y, Morishima M, Kuwahara R, Kondo S, Itakura M, Takahashi M. Establishment of variant PC12 subclones deficient in stimulation-secretion coupling. Biochimica et Biophysica Acta. 2001;1499:180–190. doi: 10.1016/s0167-4889(00)00103-8. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. Journal of Physiology. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. Journal of Physiology. 1997;498:503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Nurse CA. Anoxia differentially modulates multiple K+ currents and depolarizes neonatal rat adrenal chromaffin cells. Journal of Physiology. 1998;512:421–434. doi: 10.1111/j.1469-7793.1998.421be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Nurse CA. O2-chemosensitivity in developing rat adrenal chromaffin cells. Advances in Experimental and Medical Biology. 2000;475:601–609. doi: 10.1007/0-306-46825-5_58. [DOI] [PubMed] [Google Scholar]
- Urena J, Fernandez-Chacon R, Benot AR, De Toledo GA, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Mori N, Anderson DJ. Co-expression of multiple neurotransmitter enzyme genes in normal and immortalized sympathoadrenal progenitor cells. Developmental Biology. 1991;148:10–22. doi: 10.1016/0012-1606(91)90313-r. [DOI] [PubMed] [Google Scholar]
- West GB, Shepherd DM, Hunter RB. The function of the organs of Zuckerkandl. Clinical Scientist. 1953;12:317–325. [PubMed] [Google Scholar]
- Zhu WH, Conforti L, Czyzyk-Krzeska MF, Millhorn DE. Membrane depolarization in PC-12 cells during hypoxia is regulated by an O2-sensitive K+ current. American Journal of Physiology. 1996;271:C658–665. doi: 10.1152/ajpcell.1996.271.2.C658. [DOI] [PubMed] [Google Scholar]


