Abstract
The obese gene product, leptin is an important circulating satiety factor that regulates energy balance via its actions in the hypothalamus. However, leptin receptors are also expressed in brain regions not directly associated with energy homeostasis, such as the hippocampus. Here, leptin inhibits hippocampal neurones via activation of large conductance Ca2+-activated K+ (BK) channels, a process that may be important in regulating neuronal excitability. We now show that leptin receptor labelling is expressed on somata, dendrites and axons, and is also concentrated at synapses in hippocampal cultures. In functional studies, leptin potently and reversibly reduces epileptiform-like activity evoked in lean, but not leptin-resistant Zucker fa/fa rats. Furthermore, leptin also depresses enhanced Ca2+ levels evoked following Mg2+ removal in hippocampal cultures. The ability of leptin to modulate this activity requires activation of BK, but not KATP, channels as the effects of leptin were mimicked by the BK channel activator NS-1619, and inhibited by the BK channel inhibitors, iberiotoxin and charybdotoxin. The signalling mechanisms underlying this process involve stimulation of phosphoinositide 3-kinase (PI 3-kinase), but not mitogen-activated protein kinase (MAPK), as two structurally unrelated inhibitors of PI 3-kinase, LY294002 and wortmannin, blocked the actions of leptin. These data indicate that leptin, via PI 3-kinase-driven activation of BK channels, elicits a novel mechanism for controlling neuronal excitability. As uncontrolled excitability in the hippocampus is one underlying cause of temporal lobe epilepsy, this novel action of leptin could provide an alternative therapeutic target in the management of epilepsy.
The obese gene product leptin is an important circulating, satiety factor that regulates energy balance via activation of the hypothalamic form of the leptin receptor (Ob-Rb; Jacob et al. 1997); an action that has been attributed to inhibition of hypothalamic neurones via ATP-sensitive K+ (KATP) channel activation (Spanswick et al. 1997). However, leptin receptor immunoreactivity (Hakansson et al. 1998) and mRNA (Mercer et al. 1996) are also expressed in areas of the CNS not directly associated with energy homeostasis, suggesting that leptin has additional functions in these brain regions. Leptin itself crosses the blood-brain barrier and may be released locally in the CNS (Morash et al. 1999).
The leptin receptor is a member of the class I cytokine receptor superfamily (Tartaglia et al. 1995) that signals via association with janus tyrosine kinases (JAKs). Several pathways are activated by JAKs including insulin receptor substrate (IRS) proteins (Myers & White, 1996), and phosphoinositide 3-kinase (PI 3-kinase) is one protein activated downstream of IRS-1 (Shepherd et al. 1998). Indeed, leptin signals via PI 3-kinase in insulinoma cells (Harvey et al. 2000 b), muscle cells (Berti et al. 1997) and hepatocytes (Zhao et al. 2000). The main function of PI 3-kinase is to convert phosphatidylinositol bisphosphate (PtdIns(4,5)P2) into phosphatidylinositol trisphosphate (PtdIns(3,4,5)P3; Shepherd et al. 1998). Signalling cascades activated downstream of PI 3-kinase that utilise PtdIns(3,4,5)P3 as a second messenger include mitogen-activated protein kinase (MAPK), stress-activated protein kinase 2 (SAPK2) and protein kinase B. Indeed, activation of MAPK has also been implicated as a signalling intermediate for leptin (Takahashi et al. 1997; Tanabe et al. 1997). Hippocampal neurones also express high levels of IRS-1, PI 3-kinase (Folli et al. 1994) and MAPK (Fiore et al. 1993). Indeed, leptin modulates NMDA receptor function in the hippocampus via a PI 3-kinase- and MAPK-dependent process (Shanley et al. 2001).
We have shown recently that leptin inhibits hippocampal neurones via activation of large conductance Ca2+-activated K+ (BK) channels (Shanley et al. 2002). Neuronal BK channel activity is highly dependent on the levels of intracellular Ca2+ ([Ca2+]i) at any given voltage (Latorre, 1989). BK channels are activated during an action potential when the membrane potential depolarises and [Ca2+]i rises, and are critical in determining action potential firing rates as well as burst firing patterns. As leptin activates BK channels in hippocampal neurones (Shanley et al. 2002), we hypothesised that leptin, via BK channel stimulation, could modulate aberrant synaptic activity in hippocampal neurones. In this study we show, using hippocampal slices and cultured neurones, that leptin inhibits epileptiform-like activity via PI 3-kinase-driven BK channel activation. This process represents a novel mechanism for controlling hippocampal excitability. Some of these data have been published previously in abstract form (Shanley et al. 2000).
Methods
Materials
Recombinant human leptin (Sigma, St Louis, MO, USA) prepared in 0.01-0.02 % bovine serum albumin as a carrier was used in all experiments. LY 294002, wortmannin, (Calbiochem, La Jolla, CA, USA); tetrodotoxin, PD 98059 (Tocris Cookson, Baldwin, MO, USA); NS-1619 (Biomol); nifedipine, D-APV, diazoxide, glipizide (Sigma); and iberiotoxin and charybdotoxin (Alomone Labs, Israel) were all obtained commercially.
Cell culture
Cultures of hippocampal neurones were prepared using standard procedures as described previously (Irving & Collingridge, 1998), but were maintained in serum replacement medium (SR2, Sigma). In brief, rat pups 1-3 days old were killed by cervical dislocation and hippocampi removed. The hippocampi were washed in standard Hepes-buffered saline (HBS) comprising (mm): NaCl 135; KCl 5; CaCl2 1; MgCl2 1; Hepes 10; d-glucose 25; at pH 7.4. The hippocampi were then treated with a mixture of protease type XIV and type X (both at 0.5 mg ml−1; Sigma) for 25 min at room temperature. Dissociated cells were plated onto sterile culture dishes, pretreated with poly-l-lysine (20 μg ml−1 for 1-2 h). Cultures were maintained in a humidified atmosphere of 5 % CO2 at 37 °C for up to 2 weeks.
Immunocytochemistry
A goat polyclonal antibody directed against the C-terminal domain of the leptin receptor (Santa Cruz Biotechnology; Hakansson et al. 1998) was used. All immunocytochemical procedures were carried out in HBS. Prior to labelling, hippocampal cultures were fixed with 4 % paraformaldehyde and permeabilised with 0.1 % triton X-100. Cells were then exposed to 10 % blocking milk for 15 min. Hippocampal cultures were incubated with the leptin receptor antibody overnight at 4 °C. Immunostaining was visualised by the addition of an Alexa 488-conjugated donkey anti-goat secondary antibody (Molecular Probes). In dual labelling experiments, monoclonal markers for MAP2, GAP43 and synapsin 1 (all from Sigma) were visualised with a Cy3-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch). In the absence of primary antibody, no labelling was observed following incubation with any of the secondary antibodies. In control experiments, leptin receptor immunoreactivity was blocked by prior incubation of primary antibody with control peptide (200 μg ml−1). A laser scanning confocal imaging system (Bio-Rad Microradiance or Zeiss LSM 510) was used for image acquistion. Laser lines of 488 and 543 nm were used to excite Alexa 488 and Cy3, respectively. With the Bio-Rad system, sequential images were obtained (Kalman average of 4-8 individual scans) and the two parent images were merged using Lasersharp software. With the Zeiss confocal microscope, dual-labelling images were obtained in multi-tracking mode using a 15 s scan speed.
Ca2+ imaging techniques
A conventional digital epifluorescence imaging system (12 bit; Perkin-Elmer, Emeryville, CA, USA) mounted on an Olympus BX50WI microscope (×40 objective) was used to measure changes in [Ca2+]i. Cells were incubated with the Ca2+-sensitive dye fura-2 AM (6 μm; 40-60 min; room temperature). Dye loading and subsequent experiments were performed in Hepes-buffered saline (mm): NaCl 135, Hepes 10, KCl 5, CaCl2 1, glycine 0.01 and d-glucose 25 at pH 7.4, with or without added MgCl2 (1 mm), at room temperature (22-25 °C). Ratiometric images (350/380 nm excitation; 510 nm emission) were collected at 2 s intervals and data are expressed as changes in fluorescence ratio.
Compounds were applied directly to the perfusate. Data were derived from the somata of individual hippocampal neurones (6-14 days in culture), identified by their morphological and functional characteristics (Rae et al. 2000). Exposure of cultures to Mg2+-free Hepes buffered saline resulted in an increase in intracellular Ca2+ levels associated with the appearance of spontaneous Ca2+ oscillations. The effects of leptin and all the other agents on this activity were quantified by measuring the mean fluorescence ratio over a 2-3 min period immediately prior to leptin and/or agent addition and for a similar time period in the presence of leptin and/or agent. All n values represent data (number of cells) obtained from a minimum of three different cultures prepared from different rats.
Electrophysiological recordings
Rat hippocampal slices (400 μm) were prepared from 3-4-week- old Zucker rats using standard techniques and perfused with artificial cerebrospinal fluid (aCSF), bubbled with 95 % O2 and 5 % CO2 and containing (mm): NaCl 124, KCl 3, NaHCO3 26, NaHPO4 1.25, CaCl2 2, MgSO4 1 and d-glucose 10. Epileptogenic conditions were obtained by maintaining hippocampal slices in aCSF with no added Mg2+. Extracellular recording pipettes (5-15 MΩ) filled with aCSF were positioned in stratum pyramidale of CA1 and stimuli were delivered every 2 min via a stimulating electrode placed in the stratum radiatum. Stimulation intensity was set to two times the intensity to yield maximum population spikes (range, 40-90 V for 0.2 ms duration). Such stimulation resulted in the generation of spontaneous ‘interictal’ epileptiform activity, following perfusion of Mg2+-free media for 2-3 h (Kohling et al. 2000). Epileptiform events were continuously monitored on a chart recorder and the frequency of interictal activity was analysed off line. Experiments were performed at room temperature (22-25 °C) and the CA3 region was left intact to increase the levels of spontaneous activity. Extracellular field recordings were made using an Axopatch 200B amplifier; signals were filtered at 2 kHz and stored on a computer.
Analysis
All data are expressed as means ± s.e.m. and statistical analyses were performed using Student's unpaired t test, for comparison of means or ANOVA (analysis of variance) for comparisons between multiple groups. P < 0.05 was considered significant.
Results
Characterisation of the spontaneous Ca2+ transients induced by Mg2+-free conditions
Epileptiform events are associated with elevations in intracellular Ca2+ levels (Badea et al. 2001). In cultured hippocampal neurones, low or zero Mg2+ conditions result in phasic elevations in [Ca2+]i associated with the induction of recurrent bursts of synaptic activity (Abele et al. 1990; McLeod et al. 1998). This process, which requires NMDA receptor activation, action potential propagation and the influx of Ca2+ via voltage-gated Ca2+ channels, resembles seizure-like activity observed in brain slices following Mg2+ removal (Abele et al. 1990; McLeod et al. 1998; Delorenzo et al. 1998). Perfusion of hippocampal cultures with glycine-containing, Mg2+-free medium resulted in an increase in [Ca2+]i that was subsequently followed by the generation of spontaneous Ca2+ oscillations (Fig. 1A). In order to determine if the properties of these oscillations were consistent with those described previously (Abele et al. 1990; Shen et al. 1996; McLeod et al. 1998), the effects of inhibitors of NMDA receptors, Na+ channels and voltage-gated Ca2+ channels (VGCC) were examined. Application of the selective Na+ channel blocker, tetrodotoxin (TTX; 500 nm) abolished the mean, global enhancement in Ca2+ levels associated with the oscillations (n = 84; Fig. 1B and C). Furthermore, addition of the competitive NMDA receptor antagonist, d(-)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 μm) or the L-type VGCC blocker, nifedipine (10 μm) reduced the Ca2+ levels by 70.5 ± 3.9 % (n = 80) and 36.7 ± 9.3 % (n = 28), respectively (Fig. 1B and C). Thus, these data indicate that the characteristics of the spontaneous Ca2+ transients in the present study parallel those reported previously as they are dependent on NMDA receptor activation, influx of Ca2+ via VGCCs and action potential propagation.
Figure 1. Exposure of hippocampal cultures to Mg2+-free conditions induces spontaneous Ca2+ oscillations.
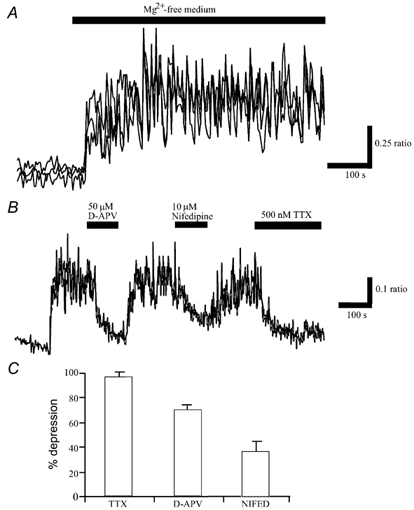
A, perfusion of hippocampal cultures with Mg2+-free Hepes-buffered saline resulted in a rise in Ca2+ levels that was accompanied by the appearance of synchronised oscillations in Ca2+. Traces are derived from the somata of individual neurones within a field of cells. B, following generation of the spontaneous Ca2+ transients, application of TTX (500 nm) for the time indicated resulted in complete inhibition of the enhanced Ca2+ load associated with Mg2+ removal. Application of the NMDA receptor antagonist, D-APV (50 μm) or VGCC blocker, nifedipine (10 μm), attenuated the spontaneous activity in a reversible manner. C, pooled data illustrating the relative depressions of mean Ca2+ load in Mg2+-free medium following the addition of TTX (1 μm; n = 84), D-APV (50 μm; n = 80) or nifedipine (10 μm; n = 28). Thus, these synaptically driven Ca2+ transients required glutamatergic synaptic transmission as well as the influx of Ca2+ via VGCCs.
Leptin inhibits Ca2+ levels in Mg2+-free medium via activation of BK, but not KATP, channels
Application of leptin (0.1-30 nm; 5 min) rapidly (within 1-2 min) reduced the enhancement in global Ca2+ levels following 10-20 min exposure to Mg2+-free medium by 62.6 ± 2.4 % (n = 416; Fig. 2A), in a readily reversible manner. This action of leptin was reproducible as subsequent re-application of leptin, 15-20 min later induced a comparable response (n = 225; Fig. 2B and C). This protocol was therefore used to investigate the cellular mechanisms underlying leptin action.
Figure 2. Leptin inhibits the enhanced Ca2+ levels evoked following Mg2+ removal.
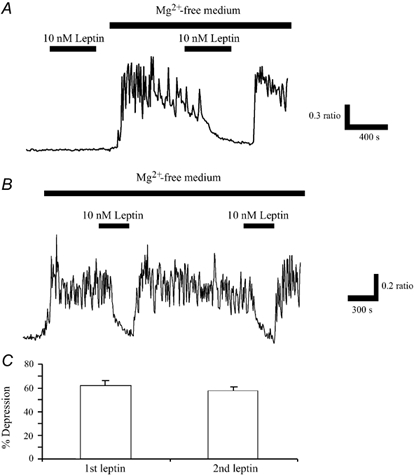
A, in control conditions (Mg2+-containing solution), application of leptin (10 nm) for the time indicated by the bar, had no effect the basal levels of Ca2+. However, following perfusion of Mg2+-free medium, which itself resulted in the generation of spontaneous Ca2+ oscillations, addition of leptin (10 nm) caused a rapid inhibition of the Ca2+ levels. This action of leptin was readily reversed on washout. B, the ability of leptin to inhibit this enhanced Ca2+ load was readily reproducible as subsequent application of leptin (10 nm), 20-30 min after the first exposure, resulted in a comparable response. C, pooled data illustrating that the relative depressions of mean Ca2+ load in Mg2+-free medium induced by sequential applications of leptin do not differ significantly.
Previous studies have shown that potassium channel activators, such as diazoxide, can modulate aberrant synaptic activity, evoked following Mg2+ removal (Abele & Miller, 1990). Furthermore, leptin activates KATP channels in insulin-secreting cells (Harvey et al. 1997; Keiffer et al. 1997) and GR hypothalamic neurones (Spanswick et al. 1997). Thus, in the next series of experiments the role of KATP channels in leptin action was examined. In agreement with previous studies (Abele & Miller, 1990), the KATP channel opener, diazoxide mimicked the actions of leptin, such that addition of diazoxide (200 μm) inhibited the enhanced Ca2+ levels by 53.7 ± 2.8 % (n = 57; Fig. 3A). This action of diazoxide was evident within 1-2 min of application and reversed on washout. Application of the selective KATP channel inhibitor, glybenclamide (1-10 μm) had no effect on the mean Ca2+ levels per se, such that the mean ratio values obtained in the absence and presence of glybenclamide were 1.07 ± 0.05 and 1.07 ± 0.04, respectively (n = 92; P > 0.05; Fig. 3B). Furthermore, exposure of the cultures to glybenclamide (1 μm) for at least 20 min prior to the addition of leptin (10 nm), had little or no effect on the ability of leptin to inhibit the enhanced Ca2+ levels associated with exposure to Mg2+-free medium (Fig. 3B and F). Thus, in the presence of glybenclamide, leptin (10 nm) lowered the enhanced Ca2+ levels by 38 ± 3.3 % compared with 44 ± 3.5 % in its absence (n = 57; P > 0.05). Similarly, addition of another KATP channel inhibitor, glipizide (1 μm) had no effect on the ability of leptin to modulate the mean Ca2+ load in Mg2+-free medium (Fig. 3F). Thus, leptin lowered Ca2+ levels by 39.0 ± 1.7 and 37.2 ± 2.8 % in the absence and presence of 1 μm glipizide, respectively (n = 51; P > 0.05), indicating that a process independent of KATP channel activation underlies leptin action.
Figure 3. Inhibition of Ca2+ levels by leptin involves BK, but not KATP, channel activation.
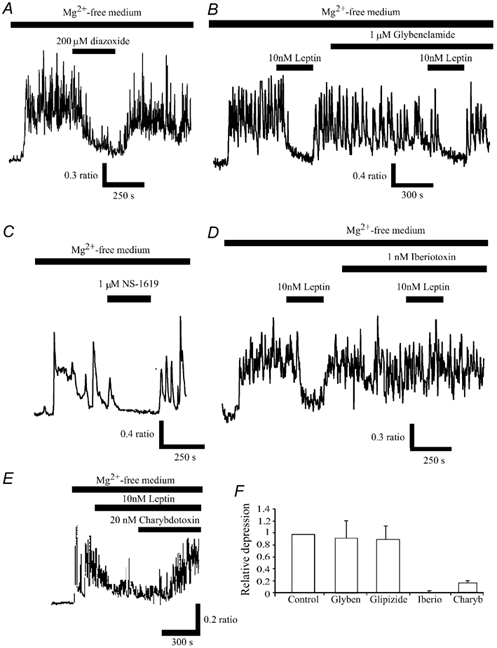
A, following perfusion of Mg2+-free medium and the generation of the spontaneous Ca2+ oscillations, addition of the KATP channel opener, diazoxide (200 μm) reversibly depressed the mean enhancement of Ca2+ levels following Mg2+ removal. C, similarly, the BK channel activator, NS-1619 also depressed Ca2+ levels in a readily reversible manner. B and D, the KATP channel inhibitor, glybenclamide did not affect the leptin-modulation of Ca2+ levels, whereas the selective BK channel inhibitor, iberiotoxin completely blocked the actions of leptin. E, leptin-induced depression of the spontaneous Ca2+ transients was readily reversed by the addition of charybdotoxin (20 nm). F, pooled data illustrating the relative depressions of mean Ca2+ load in Mg2+-free medium by leptin in control conditions, and in the presence of glybenclamide (1 μm), glipizide (1 μm), iberiotoxin (1 nm) or charybdotoxin (20 nm).
We have shown previously that leptin inhibits hippocampal neurones via activation of BK channels (Shanley et al. 2002). In the next series of experiments we examined whether BK channel activation is also involved in the actions of leptin in hippocampal cultures. Application of the BK channel activator, NS-1619 (1 μm; 5 min; Olesen et al. 1994) mimicked the actions of leptin, causing 50 ± 6.4 % inhibition of the mean Ca2+ load in Mg2+-free medium (n = 31; Fig. 3C). Application of the BK channel inhibitor, iberiotoxin (1 nm; Galvez et al. 1990) itself had no effect on Ca2+ levels per se, with mean ratio values of 0.92 ± 0.07 and 0.90 ± 0.08, in the absence and presence of iberiotoxin, respectively (n = 42; P > 0.05). However, the ability of leptin to modulate intracellular Ca2+ levels was completely blocked by prior incubation with this agent (n = 42; Fig. 3D and F). Thus, the mean depressions induced by leptin in the absence and presence of 1 nm iberiotoxin were 45 ± 2.3 and 2.4 ± 1.8 %, respectively (n = 42; P < 0.05). Similarly, incubation with charybdotoxin (20 nm) significantly reduced the leptin-induced inhibition of Ca2+ levels (Fig. 3F), such that the mean depressions induced by leptin (10 nm) were 47.7 ± 3.6 and 9.9 ± 6.5 %, in the absence and presence of charybdotoxin, respectively (n = 48; P < 0.05). Furthermore, application of charybdotoxin (20-50 nm) 10-12 min after the addition of leptin, rapidly reversed the leptin-induced depression of the enhanced Ca2+ levels in Mg2+-free medium (n = 65; Fig. 3E), such that the mean depression induced by leptin was reduced from 69.7 ± 5.3 % (10 min after exposure to leptin) to 26.2 ± 7.3 %, 13-15 min after exposure to 20 nm charybdotoxin (n = 65; P < 0.05). Together these data indicate that leptin inhibition of spontaneous, synaptically driven Ca2+ transients in cultured hippocampal neurones involves activation of BK, but not KATP, channels.
Leptin inhibits Ca2+ levels via a PI 3-kinase, but not by a MAPK, driven process
There is growing evidence that PI 3-kinase is a key component of leptin receptor signalling pathways in insulinoma cells (Harvey et al. 2000 b), cardiac cells (Berti et al. 1997) and hepatocytes (Zhao et al. 2000). A PI 3-kinase-dependent process also underlies leptin-induced activation of KATP channels in hypothalamic neurones (Mirshamsi & Ashford, 2001) and leptin stimulation of BK channels in CA1 hippocampal neurones (Shanley et al. 2002). Thus, we next determined whether activation of this enzyme plays a role in leptin action. Application of the PI 3-kinase inhibitors, LY 294002 (10 μm; n = 108; Vlahos et al. 1994) or wortmannin (10-50 nm; n = 77; Powis et al. 1994) for 25 min had no effects on the mean Ca2+ levels in Mg2+-free medium (Fig. 4A and B). Thus, the mean ratio values obtained in control conditions were 0.57 ± 0.05 (LY294002) and 0.98 ± 0.09 (wortmannin), whereas following incubation with the PI 3-kinase inhibitors for at least 15 min, the ratio values were 0.56 ± 0.06 (n = 108; LY294002; P > 0.05) and 0.93 ± 0.08 (n = 77; wortmannin; P > 0.05). However, both agents substantially reduced the effects of leptin (Fig. 4C). Thus, the mean depressions induced by leptin (10 nm) in the presence of LY 294002 (10 μm) or wortmannin (10 nm) were 3.3 ± 2.2 % (n = 38; P < 0.05) and 5.5 ± 1.7 % (n = 67; P < 0.05), respectively (Figure 4C).
Figure 4. Leptin inhibition of Ca2+ levels requires stimulation of PI 3-kinase.
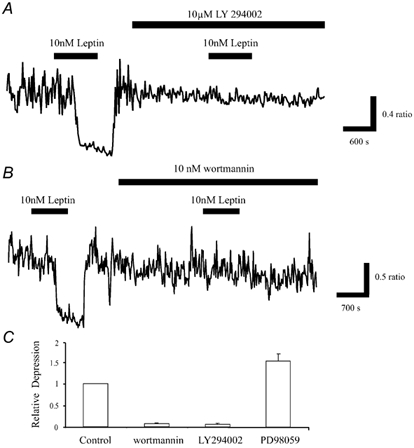
A, application of leptin (10 nm) for the time indicated caused reversible inhibition of the enhanced Ca2+ load in Mg2+-free medium. Exposure of the neurones to the PI 3-kinase inhibitor, LY 294002 (10 μm) for at least 15 min had no effect on the enhanced Ca2+ levels. However, subsequent addition of leptin in the continued presence of LY 294002 failed to inhibit Ca2+ levels. B, similarly, wortmannin (10 nm), another PI 3-kinase inhibitor had no effect on the enhanced Ca2+ levels, but it did significantly reduce the effects of leptin. C, pooled data illustrating the effects of leptin on the mean Ca2+ load in Mg2+-free medium in control conditions, and in the presence of wortmannin (10 nm; n = 67), LY 294002 (10 μm; n = 38) or the inhibitor of MAPK activation, PD 98059 (10 μm; n = 51).
Activation of MAPK has also been implicated as a signalling intermediate for leptin in various cell types (Takahashi et al. 1997; Tanabe et al. 1997), actions sensitive to PD 98059, an inhibitor of MAPK activation (Alessi et al. 1995). Leptin facilitation of NMDA receptor-mediated responses also requires activation of MAPK (Shanley et al. 2001). Thus, the effects of PD 98059 on the ability of leptin to lower Ca2+ levels were examined. Application of PD 98059 (10 μm) for 15-20 min caused little or no effect on the Ca2+ levels in Mg2+-free medium, with mean ratio values of 0.70 ± 0.08 and 0.69 ± 0.09 obtained in the absence and presence of PD 98059, respectively (n = 100; P > 0.05). However, subsequent addition of leptin (10 nm) in the continued presence of PD 98059 resulted in 62.2 ± 5.3 % inhibition of the mean Ca2+ load compared with 47.8 ± 5.1 % inhibition in control conditions (n = 60; P < 0.01; Fig. 4C). Thus these data indicate not only that activation of MAPK pathways are not essential for leptin action, but also that the ability of leptin to modulate the mean Ca2+ load is enhanced following blockade of MAPK activation.
Leptin reduces epileptiform-like activity in hippocampal slices
As BK channels are particularly suited for controlling neuronal excitability, and epileptiform burst-like activity can be modulated by BK channel activity (Alger & Williamson, 1988; Golding et al. 1999; Shao et al. 1999), we next determined if leptin was also able to modulate epileptiform-like activity evoked in hippocampal slices. Furthermore, in order to address if the actions of leptin are due to activation of the leptin receptor, we compared the effects of leptin in hippocampal slices from control lean and obese Zucker fa/fa rats. In this rodent model of obesity, the fa mutation gives rise to a leptin-resistant state (Ahima & Flier, 2000). In these experiments, slices were bathed in Mg2+-free solution for at least 3 h prior to recording. In slices from Zucker lean rats, the frequency of interictal activity was significantly lower than in age-matched Zucker obese fa/fa rats. Thus, the mean frequency of interictal events in lean and fa/fa rats was 0.037 ± 0.003 Hz (n = 4) and 0.101 ± 0.031 Hz (n = 4), respectively (P < 0.05), suggesting that animals with leptin receptor signalling deficiency show increased neuronal excitability.
In slices from lean animals, application of leptin (50 nm) inhibited the mean frequency of interictal events by 67 % from 0.037 ± 0.003 to 0.012 ± 0.003 Hz (8 min after addition of leptin; n = 4; P < 0.05: Fig. 5A and B). This action of leptin readily reversed on washout, with recovery to 0.039 ± 0.004 Hz (10-15 min later; n = 4). In order to address the locus of leptin action, the effects of leptin on evoked field excitatory postsynaptic potentials (fEPSPs) were monitored simultaneously. In 4 slices, leptin induced a small but significant depression of the slope and amplitude of evoked fEPSPs (n = 4; Fig. 5E and F). Thus, application of leptin (50 nm) caused 17.8 ± 3.1 and 18.8 ± 2.8 % depression in the fEPSP slope and amplitude, respectively (n = 4; P < 0.05). Leptin also lowered the level of excitability by reducing the number of population spikes evoked by single shock stimulation (Fig. 5E).
Figure 5. Leptin inhibits interictal activity in Zucker lean, but not obese fa/fa rats.
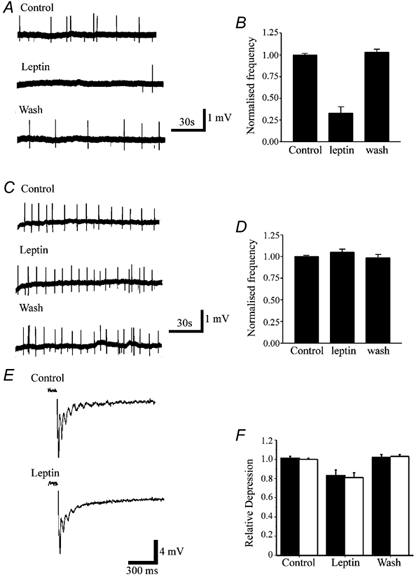
A, sample traces of extracellularly recorded spontaneous interictal events evoked in a hippocampal slice obtained from a Zucker lean rat. Leptin (50 nm) reduced the frequency of interictal activity in a readily reversible manner. B, plot of the pooled data illustrating the normalised interictal frequency in control conditions, in the presence of leptin (50 nm) and following washout (n = 4). C, sample traces of extracellularly recorded hippocampal epileptiform interictal activity in slices from obese Zucker fa/fa rats. Addition of leptin (50 nm) failed to affect the frequency of interictal events. D, pooled data illustrating the normalised frequency of interictal events in slices from fa/fa rats, in control conditions, in the presence of leptin and following washout (n = 4). E, sample records of evoked fEPSPs obtained in control conditions and following exposure to 50 nm leptin. Note that leptin not only reduced the slope and peak amplitude of the fEPSP, but it also reduced the number of population spikes. F, pooled data illustrating the relative depressions of the slope (▪) and amplitude (□) of fEPSPs evoked in control conditions, following exposure to leptin (50 nm) and on washout.
In order to address whether leptin receptor activation was required for leptin action, the effects of leptin were also investigated in slices obtained from Zucker fa/fa rats. Addition of leptin (50-100 nm; for up to 20 min) had no effect on interictal activity evoked in slices from age-matched obese Zucker fa/fa rats (n = 4; Fig. 5C and D), such that the mean frequency of interictal events was 0.10 ± 0.04 Hz in control conditions and 0.11 ± 0.06 Hz following 15 min incubation with leptin (n = 4; P > 0.05). Together these data indicate not only that animals with leptin resistance display enhanced neuronal excitability, but also that leptin, via leptin receptor activation, is a potent modulator of epileptiform-like activity.
Functional localisation of leptin receptors in cultured hippocampal neurones
Previous studies have shown that leptin receptor immunoreactivity (Hakansson et al. 1998) and mRNA (Mercer et al. 1996) are widely expressed throughout the hippocampal formation. In this study, the functional localisation of leptin receptors was assessed in cultured hippocampal neurones using dual labelling immunocytochemical techniques. Leptin receptor labelling was present on cultured hippocampal neurones (Fig. 6). In young cultures (6-8 days) labelling was primarily associated with the somato-dendritic region and putative growth cones (axonal and dendritic). However, in older cultures punctate labelling was also detected along fine processes (Fig. 6). At somata, leptin receptor immunoreactivity was associated with the plasma membrane. In some somata bright, punctate leptin receptor labelling was also observed within the cytoplasm (Fig. 6), which is likely to relate to intracellular sites of leptin receptor synthesis and metabolism. Comparison of leptin receptor immunoreactivity with monoclonal antibody markers for axons (GAP-43) and soma/dendrites (MAP2) demonstrated leptin receptor expression within these different neuronal compartments (Fig. 6). Dual labelling with an antibody against synapsin 1 indicated that the clusters of leptin receptor immunolabelling on fine processes were associated with synaptic terminals (Fig. 6). This is likely to reflect expression at presynaptic terminals as leptin receptor labelling in fine processes is associated with GAP-43, and follows the path of fibres expressing synapsin 1 (Irving et al. 2000).
Figure 6. Leptin receptor localisation on cultured hippocampal neurones.
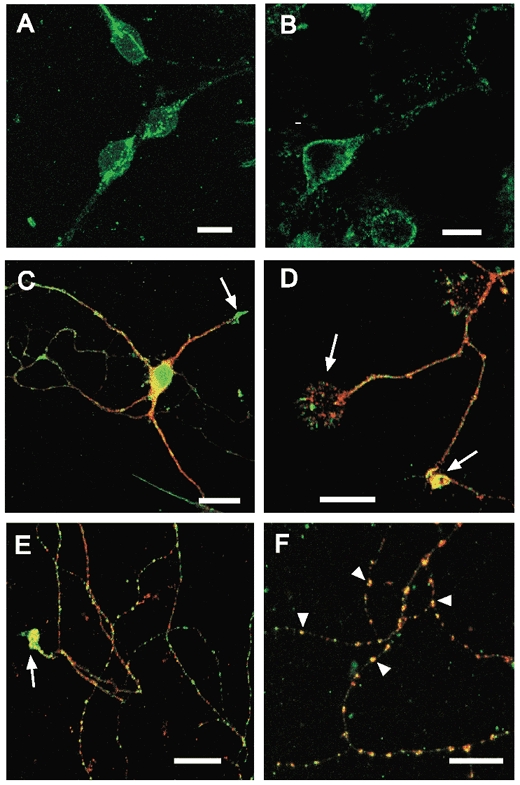
A and B, leptin receptor immunoreactivity on neuronal somata, illustrating punctate labelling within the cytoplasm (A) and labelling associated with the plasma membrane (B). A is a Z-projection of a series of confocal images taken at 1 μm intervals, B is a single confocal section. Primary antibody labelling was visualised with an Alexa 488-conjugated donkey anti-goat secondary antibody (green). C, D, E and F, merged, single plane confocal images comparing leptin receptor labelling with monoclonal markers (red; Cy3) for soma/dendrites (MAP2; C), axons (GAP43, D) or synaptic terminals (synapsin 1; E and F). The yellow areas correspond to points of overlap. In these images, the confocal gain and/or laser intensity was increased to facilitate the visualisation of fine processes. Leptin receptor immunoreactivity was associated with synaptic markers in older cultures (9-14 days, E and F). In the zoomed image (F), arrowheads show examples of co-localisation with synapsin 1 at individual puncta. Strong leptin receptor immunoreactivity was also associated with dendritic (C) and axonal (D and E) growth cones (arrows). Scale bars are 10 μm (A, B, D, E and F) and 20 μm (C).
Discussion
It is well established that the obese gene product leptin plays a major role in regulating energy balance via its actions in the hypothalamus (Elmquist et al. 1999). This process may be attributable, at least in part, to inhibition of GR hypothalamic neurones via stimulation of KATP channels (Spanswick et al. 1997). However, there is growing evidence that this hormone can have other functions. Indeed, leptin is thought to play an essential role in both immune (Fantuzzi & Faggioni, 2000) and reproductive function (Caprio et al. 2001), is an important factor controlling bone formation (Takeda & Karsenty 2001) and may modulate synaptic plasticity (Shanley et al. 2001). In this study, we provide further evidence of a role for leptin in the CNS that is unrelated to hypothalamic control of energy balance. Using immunocytochemical approaches, we demonstrate that leptin receptor immunoreactivity is present on hippocampal neurones. The pattern of labelling is consistent with the expression of functional receptors on soma and proximal dendrites and also at presynaptic terminals. We show that leptin inhibits epileptiform-like synaptic activity in hippocampal neurones, via BK channel activation, indicating that activation of this leptin receptor-driven signalling is a novel pathway for modulating hippocampal excitability.
Previous studies have indicated that leptin receptor immunoreactivity (Hakansson et al. 1998) as well as leptin receptor mRNA (Mercer et al. 1996), are expressed in hippocampal slices. The present study indicates that leptin receptor labelling is localised throughout cultured hippocampal neurones, with immunoreactivity present on somata, dendrites and axons, and is also concentrated at points of synaptic contact and at growth cones. Thus, leptin receptors are well positioned to regulate neuronal excitability in hippocampal neurones. Our data indicate that leptin reduced the frequency of interictal activity induced in the CA1 region of hippocampal slices, in a readily reversible manner. Furthermore, hippocampal slices from Zucker fa/fa rats exposed to Mg2+-free conditions displayed an increased frequency of interictal activity relative to lean controls, suggesting that animals with leptin receptor signalling deficiencies have an increased degree of neuronal excitability.
Leptin also lowered the enhanced Ca2+ levels evoked following Mg2+ removal in hippocampal cultures, but had no effect on the basal levels of Ca2+. The ability of leptin to modulate this activity in hippocampal neurones parallels its reported actions on spontaneous Ca2+ oscillatory activity evoked in hypothalamic neurones (Glaum et al. 1996). Consistent with previous studies (Abele & Miller, 1990), application of the KATP channel opener, diazoxide inhibited the mean Ca2+ load in a readily reversible manner. Similarly, addition of the BK channel activator, NS-1619 inhibited this activity. However, the modulatory actions of leptin were prevented by the BK channel inhibitors, iberiotoxin and charybdotoxin, but not by the KATP channel blocker glybenclamide or glipizide, indicating that leptin receptor-driven activation of BK, but not KATP, channels underlies this process.
In hippocampal pyramidal neurones, postsynaptic BK channels are activated during an action potential by membrane depolarisation, together with a rise in [Ca2+]i. The resulting potassium current is largely responsible for repolarisation of action potentials and the generation of the fast after-hyperpolarisation (Storm, 1987; Lancaster & Nicoll, 1987), which in turn delays recovery of the membrane potential to firing threshold. Thus, postsynaptic BK channel activity is vital in regulating the level of neuronal excitability, which determines action potential firing rate and burst firing patterns. Indeed, a role for BK channels in regulating epileptiform-like activity has been suggested previously (Alger & Williams, 1988; Golding et al. 1999; Shao et al. 1999). Recent evidence also indicates that presynaptic BK channels may influence synaptic transmission at CA1 synapses, but only under conditions of enhanced excitability, for example induced by the K+ channel blocker, 4-AP (Hu et al. 2001). Thus, the role of BK channels in hippocampal function is greatly influenced by their subcellular location.
In the present study, leptin receptor immunoreactivity was detected both at presynaptic terminals and somato-dendritic regions. Previous studies have shown that BK channels have a similar localisation (Hu et al. 2001), suggesting that leptin could theoretically influence hippocampal hyperexcitability, via BK channel stimulation, at either location. In hippocampal slices, leptin markedly inhibited epileptiform-like activity, but only exerted a small depressant effect on evoked field potentials (in Mg2+-free medium). Our previous studies have indicated that at the cellular level, leptin hyperpolarises CA1 pyramidal neurones via stimulation of postsynaptic BK channels (Shanley et al. 2002), thus moving the membrane potential of these neurones away from their firing threshold. We have also reported recently that leptin induces a small reduction in the amplitude of evoked EPSCs (Shanley et al. 2001), an action that is independent of any change in input resistance. Thus, it is likely that both pre- and post-synaptic actions of leptin on CA1 pyramidal neurones act in concert to inhibit unregulated excitability in the hippocampus. However, given the proportionately greater effect of leptin on spontaneous interictal activity in comparison with evoked synaptic responses, the postsynaptic actions of leptin are likely to be the more important mechanism underlying the effects of leptin on epileptiform-like events. The mechanisms underlying leptin modulation of EPSCs is unclear at this stage. Studies using blockers of BK channels suggest that they do not regulate transmitter release under basal (low frequency) conditions (Hu et al. 2001). However, the present work suggests that the effects of leptin involve activation of BK channels, whose actions may be manifest at the presynaptic terminal under more physiological conditions.
A noteworthy observation in the present study concerns the lack of effect of BK channel blockers on the enhanced Ca2+ levels in Mg2+-free medium. This activity has been shown to reflect synchronous burst firing within populations of cultured neurones leading to somatic depolarisation and an influx of intracellular Ca2+ (Shen et al. 1996). Given that BK channels modulate bursting, it might be expected that BK channel inhibitors would enhance this activity. The lack of effect of iberiotoxin and charybdotoxin on the aberrant synaptic activity may reflect the rapidly desensitising nature of BK channels, such that their activity declines during repetitive firing (Shao et al. 1999). Thus, the sustained bursting induced in Mg2+-free medium may no longer be influenced by ‘conventional’ action potential-dependent activation of BK channels. However, leptin, which activates BK channels via a Ca2+-independent mechanism (Shanley et al. 2002), is still effective.
There is growing evidence that PI 3-kinase is a key component of leptin receptor signalling in peripheral cells including insulin-secreting cells (Harvey et al. 2000b), myotubes (Berti et al. 1997) and hepatocytes (Zhao et al. 2000). Recent evidence also indicates that PI 3-kinase activation is required for leptin-induced facilitation of NMDA receptor function in hippocampal neurones (Shanley et al. 2001) and activation of hypothalamic KATP channels by leptin (Mirshamsi & Ashford, 2001). Furthermore, leptin stimulation of BK channels in hippocampal neurones involves activation of a PI 3-kinase-driven process (Shanley et al. 2002). Similarly, in the present study the ability of leptin to modulate the Ca2+ activity in hippocampal cultures required stimulation of PI 3-kinase. Activation of MAPK by leptin has also been reported in a variety of peripheral cell types (Takahashi et al. 1997: Tanabe et al. 1997) and leptin-induced enhancement of NMDA responses in hippocampal neurones involves MAPK activation (Shanley et al. 2001). In contrast, in the present study the effects of leptin were not attenuated by PD 98059, indicating that leptin inhibition of the raised Ca2+ levels in Mg2+-free medium involves activation of a MAPK-independent pathway. The present data does however indicate that the ability of leptin to modulate this enhanced Ca2+ load was significantly enhanced in the presence of PD 98059, suggesting that leptin receptor-driven MAPK activation possibly acts as a negative feedback mechanism to regulate the actions of leptin.
One function of PI 3-kinase is to promote the conversion of PtdIns(4,5)P2 into PtdIns(3,4,5)P3 (Shepherd et al. 1998). There is also growing evidence that inositol phospholipids closely associate with the actin cytoskeleton, where they can modulate a variety of proteins (see Janmey, 1998 for review). Altering the integrity of cytoskeletal networks can also modulate the activity of a variety of ion channels. Indeed, leptin activation of KATP channels in insulinoma cells involves PI 3-kinase-driven disruption of the actin cytoskeleton (Harvey et al. 2000a). Activation of other cytokine or growth factor receptors can also lead to cytoskeletal organisation (Goh et al. 1997), via PI 3-kinase-dependent mechanisms (Kim & Feldman, 1998). Thus, it is possible that the ability of leptin to activate BK channels in hippocampal neurones involves a change in the dynamics of the cytoskeleton. In support of this possibility, the activity of human BK channels expressed in meningioma cells is influenced by changes in cytoskeletal architecture (Kraft et al. 2000). However, further experiments are required to explore whether this mechanism underlies leptin modulation of hippocampal BK channels.
In conclusion, we have shown that the obese gene product leptin, via PI 3-kinase driven activation of BK channels, inhibits different forms of epileptiform-like activity evoked in hippocampal neurones. It is well established that reducing the levels of extracellular Mg2+ elicits epileptic discharges in brain slice preparations (Kohr & Heinemann, 1989) and intense synaptic activity in neuronal cultures (Robinson et al. 1993). The aberrant synaptic activity that develops is thought to limit the viability of the neurones and can ultimately lead to paroxysmal neuronal firing and cell death (Abele et al. 1990; Rose et al. 1990). Thus, the ability of leptin to potently inhibit the mean Ca2+ load associated with this aberrant synaptic activity suggests that leptin is a novel neuroprotective agent and that the leptin receptor-driven signalling pathway may be a useful target in the treatment of epilepsy and certain neurodegenerative disorders.
Acknowledgments
This work is supported by The Wellcome Trust (grant no. 055291) and Tenovus, Scotland. J.H. is a Wellcome R.C.D. Fellow.
References
- Abele AE, Miller RJ. Potassium channel activators abolish excitotoxicity in cultured hippocampal pyramidal neurons. Neuroscience Letters. 1990;115:195–200. doi: 10.1016/0304-3940(90)90454-h. [DOI] [PubMed] [Google Scholar]
- Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;4:413–419. doi: 10.1016/0896-6273(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Leptin. Annual Review Physiology. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cuendo A, Cohen P, Dudley DT, Saltiel AR. PD 98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. Journal of Biological Chemistry. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Alger BE, Williamson AA. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarisation in rat hippocampus. Journal of Physiology. 1988;399:191–205. doi: 10.1113/jphysiol.1988.sp017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea T, Goldberg J, Mao B, Yuste R. Calcium imaging of epileptiform events with single cell resolution. Journal of Neurobiology. 2001;48:215–227. doi: 10.1002/neu.1052. [DOI] [PubMed] [Google Scholar]
- Berti L, Kellerer M, Capp E, Haring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a PI 3-kinase mediated effect. Diabetologia. 1997;40:606–609. doi: 10.1007/s001250050722. [DOI] [PubMed] [Google Scholar]
- Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends in Endocrinological Metabolism. 2001;12:65–72. doi: 10.1016/s1043-2760(00)00352-0. [DOI] [PubMed] [Google Scholar]
- Delorenzo RJ, Pal S, Sombati S. Prolonged activation of N-methyl-d-aspartate receptor-Ca2+-transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proceedings National Academy Sciences of the USA. 1998;24:14482–14487. doi: 10.1073/pnas.95.24.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation and hematopoiesis. Journal of Leukocyte Biology. 2000;68:437–446. [PubMed] [Google Scholar]
- Folli F, Bonfanti L, Renard E, Khan R, Merighi A. Insulin receptor substrate-1 (IRS-1). distribution in the central nervous system. Journal of Neuroscience. 1994;14:6412–6416. doi: 10.1523/JNEUROSCI.14-11-06412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore RS, Bayer VE, Pelech SL, Posada J, Cooper JA, Baraban JM. P42 mitogen-activated protein kinase: prominent localisation in neuronal cell bodies. Neuroscience. 1993;55:463–472. doi: 10.1016/0306-4522(93)90516-i. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garci ML. Purification and characterisation of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from the venom of the scorpion Buthus tamulus. Journal of Biological Chemistry. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Glaum SR, Hara M, Bindokas VP, Lee CC, Polansky KS, Bell GI, Miller RJ. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Molecular Pharmacology. 1996;50:230–235. [PubMed] [Google Scholar]
- Goh EL, Picher TJ, Wood TJ, Norstedt G, Graichen R, Lobie PE. Growth hormone induced reorganisation of the actin cytoskeleton is not required for STAT5- mediated transcription. Endocrinology. 1997;138:3207–3215. doi: 10.1210/endo.138.8.5298. [DOI] [PubMed] [Google Scholar]
- Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarisation are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. Journal of Neuroscience. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M-L, Brown H, Ghuilardi N, Skoda RC, Meister BJ. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. Journal of Neuroscience. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Hardy SC, Ashford ML. Leptin activation of KATP channels in rat CRI-G1 insulinoma cells involves disruption of the actin cytoskeleton. Journal of Physiology. 2000a;527:95–107. doi: 10.1111/j.1469-7793.2000.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, Ashford MLJ. Essential role of phosphoinositide 3-kinase in leptin induced KATP channel activation in the rat CRI-G1 insulinoma cell line. Journal of Biological Chemistry. 2000b;275:4660–4669. doi: 10.1074/jbc.275.7.4660. [DOI] [PubMed] [Google Scholar]
- Harvey J, McKenna F, Herson PS, Spanswick D, Ashford MLJ. Leptin activates ATP sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. Journal of Physiology. 1997;504:527–535. doi: 10.1111/j.1469-7793.1997.527bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao L-R, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Otterssen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarisation and regulation of transmitter release. Journal of Neuroscience. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL. A characterization of muscarinic receptor-mediated intracellular Ca2+ mobilization in cultured rat hippocampal neurons. Journal of Physiology. 1998;511:747–759. doi: 10.1111/j.1469-7793.1998.747bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Coutts AA, Bewick GS, Harvey J, Rae MG, Mackie K, Pertwee RG. Functional expression of cell surface cannabinoid CB1 receptors on presynaptic inhibitory terminals in cultured rat hippocampal neurons. Neuroscence. 2000;98:253–262. doi: 10.1016/s0306-4522(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Shrewin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes. 1997;46:150–152. doi: 10.2337/diab.46.1.150. [DOI] [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signalling: component localisation and mechanical coupling. Physiological Review. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Keiffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP sensitive K+ channels in pancreatic beta cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Feldman EL. Differential regulation of focal adhesion kinase and mitogen-activated protein kinase tyrosine phosphorylation during insulin like growth factor-1 mediated cytoskeletal re-organisation. Journal of Neurochemistry. 1998;71:1333–1336. doi: 10.1046/j.1471-4159.1998.71031333.x. [DOI] [PubMed] [Google Scholar]
- Kohling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. Journal of Neuroscience. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Heinemann U. Effects of NMDA antagonists on picrotoxin-, low Mg2+- and low Ca2+-induced epileptogenesis and on evoked changes in extracellular Na+ and Ca2+ concentrations in rat hippocampal slices. Epilepsy Research. 1989;4:187–200. doi: 10.1016/0920-1211(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Kraft R, Benndorf K, Patt S. Large conductance Ca2+-activated K+ channels in human meningioma cells. Journal of Membrane Biology. 2000;175:25–33. doi: 10.1007/s002320001052. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarisations in rat hippocampal neurons. Journal of Physiology. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R. Ion channel modulation by divalent cations. Acta Physiology Scandanavia. 1989;(suppl. 582):13. [PubMed] [Google Scholar]
- McLeod JR, Shen M, Kim DJ, Thayer SA. Neurotoxicity mediated by aberrant patterns of synaptic activity between rat hippocampal neurons in culture. Journal of Neurophysiology. 1998;80:2688–2698. doi: 10.1152/jn.1998.80.5.2688. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localisation of leptin receptor mRNA and the long form slice varient (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridisation. FEBS Letters. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Mirshamsi S, Ashford MLJ. PI 3-kinase mediates leptin activation of KATP channels in rat acutely dispersed hypothalamic neurones. Journal of Physiology. 2001;536.P:18P. [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Myers MG, White MF. Insulin signal transduction and the IRS proteins. Annual Review Pharmacology and Toxicology. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. European Journal of Pharmacology. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Powis G, Bonjouklion R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindley G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol 3-kinase. Cancer Research. 1994;54:2419–2423. [PubMed] [Google Scholar]
- Rae MG, Martin DJ, Collingridge GL, Irving AJ. Role of Ca2+ stores in metabotropic l-glutamate receptor-mediated supralinear Ca2+ signalling in rat hippocampal neurons. Journal of Neuroscience. 2000;20:8628–8636. doi: 10.1523/JNEUROSCI.20-23-08628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HP, Kawahara M, Jimbo Y, Torimitsu K, Kuroda Y, Kawana A. Periodic synchronised bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurones. Journal of Neurophysiology. 1993;70:1606–1616. doi: 10.1152/jn.1993.70.4.1606. [DOI] [PubMed] [Google Scholar]
- Rose K, Christine C, Choi D. Magnesium removal induces paroxysmal neuronal firing and NMDA-receptor mediated neuronal degeneration in cortical cultures. Neuroscience letters. 1990;115:313–317. doi: 10.1016/0304-3940(90)90474-n. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. Journal of Physiology. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin inhibits spontaneous Ca2+ oscillations in cultured rat hippocampal neurones. Journal of Physiology. 2000;528.P:58P. [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. Journal of Neuroscience. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. RC 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Rae MG, Ashford MLJ, Harvey J. Leptin inhibits rat hippocampal neurons via activation of large conductance calcium-activated K+ channels. Nature Neuroscience. 2002;5:299–300. doi: 10.1038/nn824. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd PR, Withers DW, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochemistry Journal. 1998;333:417–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan sd, Ashford MLJ. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarisation and a fast after-hyperpolarisation in rat hippocampal pyramidal cells. Journal of Physiology. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase dependent proliferation of C1H10T1/2 cells. Journal of Biological Chemistry. 1997;272:12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- Takeda S, Karsenty G. Central control of bone formation. Journal of Bone Minerals and Metabolism. 2001;19:195–198. doi: 10.1007/s007740170042. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Okuya S, Tanizawa T, Matsutani A, Oka Y. Leptin induces proliferation of pancreatic beta cell line MIN6 through activation of mitogen-activated protein kinase. Biochemical and Biophysical Research Communications. 1997;241:765–768. doi: 10.1006/bbrc.1997.7894. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dempski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolfe EA, Monroe CA, Tepper RT. Identification and expression cloning of a leptin receptor, Ob-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase: 2-(4-morpho linyl)-8-phenyl-4H-1-benzopyran-4one (LY 294002) Journal of Biological Chemistry. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Zhao AZ, Shinihara MM, Huang D, Shimizu M, Eldar-Finkleman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signalling that antagonises cAMP elevation by glucagon in hepatocytes. Journal of Biological Chemistry. 2000;275:11348–11354. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]


