Abstract
The influence of Ca2+-activated force, the rate of dissociation of Ca2+ from troponin C (TnC) and decreased crossbridge detachment rate on the time course of relaxation induced by flash photolysis of diazo-2 in rabbit skinned psoas fibres was investigated at 15 °C. The rate of relaxation increased as the diazo-2 chelating capacity (i.e. free [diazo-2]/free [Ca2+]) increased. At a constant diazo-2 chelating capacity, the rate of relaxation was independent of the pre-photolysis Ca2+-activated force in the range 0.3-0.8 of maximum isometric force. A TnC mutant that exhibited increased Ca2+ sensitivity caused by a decreased Ca2+ dissociation rate in solution (M82Q TnC) also increased the Ca2+ sensitivity of steady-state force and decreased the rate of relaxation in fibres by approximately twofold. In contrast, a TnC mutant with decreased Ca2+ sensitivity caused by an increased Ca2+ dissociation rate in solution (NHdel TnC) decreased the Ca2+ sensitivity of steady-state force but did not accelerate relaxation. Decreasing the rate of crossbridge kinetics by reducing intracellular inorganic phosphate concentration ([Pi]) slowed relaxation by approximately twofold and led to two phases of relaxation, a slow linear phase followed by a fast exponential phase. In fibres, M82Q TnC further slowed relaxation in low [Pi] conditions by approximately twofold, whereas NHdel TnC had no significant effect on relaxation. These results are consistent with the interpretation that the Ca2+-dissociation rate and crossbridge detachment rate are similar in fast-twitch skeletal muscle, such that decreasing either rate slows relaxation, but accelerating Ca2+ dissociation has little effect on relaxation.
Muscle relaxation occurs when Ca2+, sequestrated by the sarcoplasmic reticulum (SR) Ca2+ pump, dissociates from troponin (Tn) to deactivate the thin filaments, leading to crossbridge detachment and force decay. It is well established that the rate of Ca2+ sequestration by the SR can control relaxation kinetics. The aim of the present study was to determine the influence of Ca2+ dissociation from TnC, the decreased crossbridge detachment rate and relative force or free [Ca2+] ([Ca2+]free) on relaxation kinetics.
The dissociation of Ca2+ from Tn may be an important determinant of the rate of relaxation, but this effect has not been studied in skeletal muscle. In cardiac muscle it has been postulated as a possible mechanism for the acceleration of relaxation observed upon β-adrenergic stimulation. The β-adrenergic response is associated with phosphorylation of TnI by a cAMP-dependent kinase A (PKA) that is known to decrease the Ca2+ sensitivity and increase the rate of dissociation of Ca2+ from cardiac Tn (Robertson et al. 1982). Furthermore, Zhang et al. (1995) showed that the half-time of relaxation (t1/2) induced by photolysis of the caged Ca2+ chelator diazo-2, decreased by ≈40 % when TnI was phosphorylated with PKA in skinned porcine cardiac muscle at room temperature. However, TnI phosphorylation with PKA did not significantly affect the rate of relaxation in skinned guinea-pig trabeculae at 12 °C (Johns et al. 1997). Thus, the influence of the rate of dissociation of Ca2+ from Tn on the rate of relaxation is controversial in cardiac muscle and unknown in skeletal muscle.
The influence of Ca2+ dissociation from Tn on the rate of relaxation can be investigated by incorporating TnC mutants with varying Ca2+ dissociation rates from the N-terminal regulatory sites into the Tn complex in muscle fibres. The following recombinant chicken fast-twitch skeletal TnCs have been characterized previously: wild-type (rTnC), M82Q and NHdel (deletion of residues 1-11) TnC. In solution, compared to rTnC, M82Q TnC exhibits an increased Ca2+ affinity by decreasing the Ca2+ dissociation rate without changing the Ca2+ association rate. In contrast, NHdel TnC exhibits a decreased Ca2+ affinity mainly from an increased Ca2+ dissociation rate, with little change in the association rate (Pearlstone et al. 1992; Chandra et al. 1994; Johnson et al. 1994). The Ca2+ sensitivity of isometric force in fibres reconstituted with NHdel TnC is reduced compared to rTnC (Chandra et al. 1994; Regnier et al. 1999) and is predicted to increase with M82Q TnC. In order to investigate systematically the effects of alterations in the rate of Ca2+ dissociation from TnC on muscle relaxation, comparative analyses need to be carried out on these mutant TnCs under similar experimental conditions.
Crossbridge detachment could also be a rate-limiting step in muscle relaxation. In skeletal muscle, two direct tests were carried out to investigate the effect of the crossbridge cycling rate on relaxation when crossbridge kinetics were changed by altering intracellular inorganic phosphate concentration ([Pi]; Mulligan et al. 1999; Nencini et al. 2000). These studies produced differing results. Mulligan et al. (1999) showed that the relaxation rate induced by diazo-2 decreased with increasing [Pi] in a dose-dependent manner in frog skinned fibres. In contrast, Nencini et al. (2000) found that increasing [Pi] sped up relaxation in a dose-dependent manner in single myofibrils from rabbit psoas muscle using fast solution exchange. The inconsistency on this issue of skeletal muscle relaxation requires further investigation since the nature of the influence of [Pi] on the rate of relaxation suggests different views about the rate-limiting pathway of relaxation.
The rate of relaxation also may be modulated by the amount of Ca2+-activated force generated at the time of relaxation. The rate of relaxation induced by diazo-2 photolysis decreases with increased level of Ca2+ activation in fast-twitch skeletal muscle (Patel et al. 1998; Wahr et al. 1998). This slowing effect on the relaxation rate of high relative pre-photolysis forces or increased number of attached crossbridges could occur via positive feedback to increase thin filament activation and slow crossbridge detachment. In support of this view, when fibres were activated in the presence of NEM-S1, a strongly binding crossbridge derivative, or after phosphorylation of myosin regulatory light chain, the rate of diazo-2-induced relaxation decreased and the dependence of relaxation rate on relative force was eliminated (Patel et al. 1998). In contrast, when [Ca2+]free was rapidly lowered during maximum contraction by solution exchange in single myofibrils, the relaxation time course was essentially independent of relative force or initial [Ca2+]free (Tesi et al. 2002). These varying results may be due to methodological differences. For example, no systematic investigation of the influence of total diazo-2 concentration ([diazo-2]) on relaxation rate has been reported.
To assess directly the effects of myofibrillar factors on relaxation rate, skinned rabbit psoas fibres devoid of functional SR were induced to relax by rapidly lowering [Ca2+]free with photolysis of the caged Ca2+ chelator diazo-2 after modifying: (1) the relative Ca2+-activated force of contraction, (2) the rate of dissociation of Ca2+ from TnC, (3) crossbridge kinetics and/or (4) both the rate of dissociation of Ca2+ from TnC and crossbridge kinetics. The influence of [diazo-2] per se on muscle relaxation also was determined. Mutant TnCs (NHdel and M82Q TnC) with varying Ca2+ dissociation rates were characterized in solution and reconstituted into fibres to change the rate of dissociation of Ca2+ from TnC. The effects of altered crossbridge kinetics on relaxation were examined under conditions of lowered intracellular [Pi]. By comparing the kinetics of diazo-2-induced relaxation under these interventions with control values, the rate-limiting processes for skeletal muscle relaxation were determined.
Methods
Skinned fibre preparation and experimental apparatus
Female New Zealand white rabbits were anaesthetized with ketamine (60 mg kg−1i.m.) and then euthanized with sodium pentobarbital (150 mg kg−1i.v.) before tissue harvest. The animal use protocol for these experiments was approved by the Institutional Laboratory Animal Care and Use Committee of the Ohio State University. Bundles of psoas muscle fibres were skinned and stored at -20 °C for up to 4 weeks in glycerinating solution containing leupeptin to prevent proteolysis (Table 1). On the day of an experiment, bundles were cut into segments of ≈2 mm in length, and individual fibres were dissected from the bundles in dissecting solution (Table 1). Single fibres were then soaked in dissecting solution containing 1 % (v/v) triton X-100 for 30 min to remove the sarcolemma and residual SR. To minimize end compliance, fibres were transferred into a glycerinating solution with 1 % bovine serum albumin (BSA) to chemically fix both ends. This was done by regional microapplication of 25 % glutaraldehyde in H2O containing Coomassie blue as a visual indicator (Chase & Kushmerick, 1988). The fixed ends were then wrapped in aluminium foil T-clips in dissecting solution for attachment to hooks on the experimental apparatus. Fibres were manipulated immediately or maintained at 4 °C in dissecting solution for up to 4 h.
Table 1.
Standard bathing solutions for skinned fibre experiments
| Glycerinating (0.5 mg per 100 ml leupeptin) | Dissecting | Pre-activating | pCa9.0 | pCa4.0 | |
|---|---|---|---|---|---|
| pCa | — | — | 9.0 | 9.0 | 4.0 |
| KCl | 141.30 | 141.30 | 74.38 | 74.38 | 59.66 |
| CaCl2 | — | — | 0.017 | 0.017 | 7.25 |
| MgCl2 | 4.45 | 4.45 | 6.14 | 6.14 | 5.96 |
| ATP | 4.72 | 4.72 | 4.78 | 4.78 | 4.93 |
| CP | — | — | 14.5 | 14.5 | 14.5 |
| Imid | 10.0 | 10.0 | 20.0 | 20.0 | 20.0 |
| EGTA | 2.0 | 2.0 | 0.4 | 7.0 | 7.0 |
| HDTA | — | — | 6.6 | — | — |
| Glycerol | 50%(v/v) | — | — | — | — |
Concentration are given in millimolar. Ionic strenght, 180 mm; pH at 7.0 at 15°C. For dissecting solution: free [Mg2+] = 1 mm, [Mg ATP] = 4 mm. for activating solution: free [Mg2+] = 1 mm, [Mg. ATP] = 4.39 mm. CP, creating phosphate; Imid, imidazole; HDTA, 1,6-hexamethylenediamine-N,N,N’,N’-tetraacetic acid.
The single-fibre experimental setup that was used has been described previously (Wahr & Rall, 1997). Briefly, single fibres were mounted between two hooks by means of the T-clips in one of three ≈325 μl chambers milled in a spring-mounted stainless steel block. Small aluminium inserts were used to decrease the volume of the chambers to ≈50-100 μl in caged compound experiments. One hook was attached to a force transducer (model 400, Cambridge Technology, Cambridge, MA, USA) and the other to a stationary hook. The output of the force transducer was recorded on a digital oscilloscope (model 2090-III, Nicolet, Madison, WI, USA) for off-line analysis. Fibres were aligned and set at a resting sarcomere length of ≈2.6 μm using the first-order diffraction pattern from a He-Ne laser directed through the fibres. Temperature was maintained by circulating coolant (50 % v/v ethylene glycol) inside the aluminium block and continuously monitored by a small thermocouple attached to the stationary hook near the fibre. Experiments were performed at 15 °C.
The UV flash for the photolysis of diazo-2 was provided by a frequency-doubled ruby laser (model QSR2, Lumonics, Warwickshire, UK), which produced a 30 ns duration pulse at 347 nm with an energy of ≈300 mJ. The energy was maintained at ≈100 mJ at the level of the fibre by placing glass slides in the beam path to attenuate the laser output. The laser beam was focused onto the fibre from above by means of a reflecting prism and a cylindrical condensing lens. The fibre/laser beam alignment was adjusted by means of positioners on the stage and checked by examining the burn pattern on ZAP-IT paper (Kentek, Pittsfield, NH, USA) placed just above the fibre (Wahr & Rall, 1997). The width of the focused beam was ≈2 mm. The beam length was controlled using an adjustable mask placed over the fibre such that the T-clips were not flashed but the fibre was illuminated over its entire length.
Solutions
Standard solutions
The bathing solutions for the steady-state force experiments were prepared according to a computer program developed by R. Godt (Medical College of Georgia). A complete list of standard solutions is shown in Table 1. Three types of bathing solutions were used: (a) dissecting solution (2 mm EGTA and no added Ca2+), (b) pre-activating solution (0.4 mm EGTA, 6.6 mm 1,6-hexamethylenediamine-N,N,N‘,N‘-tetraacetic acid (HDTA) and pCa 9.0) and (c) activating solution (7 mm EGTA and various values of pCa). All of the bathing solutions contained 4-4.39 mm Mg-ATP and 1 mm free Mg2+. The Ca2+-activating solutions also contained 14.5 mm creatine phosphate (CP). Two extreme Ca2+-activating solutions were prepared from stocks: a full Ca2+-activating solution (pCa 4.0) and a relaxing solution (pCa 9.0). Intermediate Ca2+-activating solutions were then obtained from the two solutions of pCa 9.0 and pCa 4.0 according to a pCa mixing table. The ionic strength of all of the solutions was adjusted to 180 mm using mainly K2EGTA or K2HDTA and KCl. Imidazole (20 mm) was used as a buffer to maintain all of the solutions at pH 7.0 at 15 °C, which was adjusted by the addition of small amounts of 1 M KOH or HCl. The standard solutions had no added Pi. The bathing solutions were stored in a refrigerator and used within 4 weeks.
Diazo-2-containing solutions
The diazo-2 solutions for the relaxation kinetics experiments were prepared using the computer program of Fabiato (1988). The published value of the Ca2+ binding constant for diazo-2 (Kd,Ca = 2.2 μm, Adams et al. 1989) was adjusted for experimental temperature, ionic strength and pH such that it was possible to produce solutions with anticipated [Ca2+]free. This was accomplished empirically by selecting a value of Kd,Ca for diazo-2 (1.5 μm) that resulted in similar fractional force in the absence and presence of diazo-2. The Mg2+ binding constant (Kd,Mg) to diazo-2 was 5.5 mm (Adams et al. 1989). The diazo-2 solutions contained (mm): ATP 3.0, CP 14.5, imidazole 20.0, HDTA 7.0, antioxidant glutathione 10 and various concentrations of diazo-2 from 0.8 to 16. The solutions were adjusted to 180 mm ionic strength and pH 7.0 at 15 °C. Solutions contained 1 mm free Mg2+ and 2.5 mm Mg-ATP. The total [diazo-2] required to induce relaxation from a desired level of pre-photolysis force (i.e. pCa) at different diazo-2 chelating capacities (defined as [diazo-2]free/[Ca2+]free) was calculated using the Fabiato program according to the following equations.
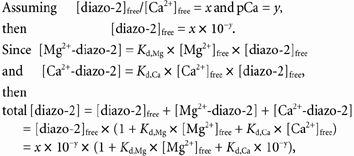 |
where x and y are the desired values of diazo-2 chelating capacity and pre-photolysis pCa, respectively. Diazo-2 solutions were prepared from stocks containing all ingredients except diazo-2 and were stored frozen in aliquots of ≈1 ml. Shortly before an experiment, these pre-solutions were thawed and the appropriate amount of diazo-2 was added to give the final solution. The final diazo-2 solutions were stored in a light-proof container in the freezer and used within 1 or 2 days to avoid any problems associated with degradation of diazo-2. Diazo-2 (tetra-potassium salt) was purchased from Molecular Probes (Eugene, OR, USA).
Low [Pi] solution: enzymatic Pi scavenger
The low [Pi] solutions for testing the effects of crossbridge kinetics on relaxation were prepared by adding an enzymatic Pi scavenger, nucleoside phosphorylase (NP) with substrate 7-methylguanosine (MEG) into the standard bathing or caged compound solutions. Under the experimental conditions, NP served as a Pi scavenger to catalyse the reaction:
strongly towards the right to reduce the level of Pi contamination (Brune et al. 1994). The unbuffered [Pi] within rabbit psoas fibres was assumed to be ≈200 μm, mainly from breakdown of Mg-ATP and CP by the fibre ATPase. With the use of this Pi scavenger, Pi contamination was reduced to less than 5 μm according to either the calculated [Pi] in actively contracting muscle fibres or measured resting [Pi] (Millar & Homsher, 1990; Pate et al. 1998; Tesi et al. 2000). Two different doses of Pi scavenger were used: (a) low [Pi] scavenger containing 1 unit ml−1 NP and 1 mm MEG for pCa 9.0 solution, and (b) a high [Pi] scavenger containing 12 units ml−1 NP and 10 mm MEG for activating solutions and caged compound solutions. NP (bacterial) and 7-MEG were purchased from Sigma.
Recombinant Tn C
Recombinant TnCs of chicken fast skeletal muscle included wild-type TnC (rTnC) and mutant TnCs that exhibited increased (M82Q TnC) or decreased (NHdel TnC, deletion of N-terminal residues 1-11) Ca2+ affinity at their regulatory sites. To introduce a spectral probe (Trp) into the regulatory N domain of TnC, F29W was also made either in single mutant F29W TnC or double mutants M82Q/F29W TnC and NHdel/F29W TnC (Pearlstone et al. 1992; Chandra et al. 1994).
Ca2+ titrations and rates of dissociation of Ca2+ from TnC in solution
Ca2+ binding to the regulatory sites of purified TnC in solution was detected by using the mutation F29W as a spectral probe. With the F29W mutation, Ca2+ produced an increase of ≈threefold in the Trp fluorescence of F29W, M82Q/F29W and NHdel/F29W TnC. Since TnC is naturally devoid of Trp and Tyr, this increase in the Trp fluorescence of these mutants can be unambiguously attributed to the newly introduced Trp. Studies have shown that the F29W mutation has minimal effect on the secondary structure and on Ca2+ or Mg2+ binding to the C domain (sites III and IV) of TnC. Neither Ca2+ affinity nor cooperativity between sites I and II is significantly affected with this mutation (Pearlstone et al. 1992). Thus, F29W served as a wild-type-like intrinsic probe for specifically monitoring Ca2+ exchange with the regulatory sites of TnC. Steady-state fluorescence measurements were performed using a Perkin-Elmer LS5 Spectrofluorimeter at 15 °C. Trp fluorescence was excited at 275 nm and monitored at 345 nm as increasing [Ca2+] was added to 1 ml of each TnC mutant (0.6 μm) in a buffer of 200 mm Mops, 90 mm KCl, 2 mm EGTA and 1 mm DTT at pH 7.0. Each titration curve represents an average of 3-5 titrations fitted with the logistic sigmoid function, as described previously (Black et al. 2000). Ca2+ dissociation rates were measured using an Applied Photophysics (Leatherhead, UK) model SX.18 MV stopped-flow instrument with a dead time of 1.4 ms at 15 °C. The samples were excited using a 150 W xenon arc source. The stopped-flow measurements were conducted by rapidly mixing mutant TnC (0.6 μm) in 10 mm Mops, 90 mm KCl, 1 mm DTT, 60 μm CaCl2 with an equal volume of 10 mm Mops, 90 mm KCl, 1 mm DTT, 5 mm EGTA, pH 7.0. Each trace represents an average of at least five traces and the data were fitted with a single exponential (variance < 6.0 × 10−4).
Isometric force versus pCa protocol
Before activation, fibres were soaked in pre-activating solution for 2 min. Fibres were then activated at various pCa values and returned to pCa 9.0 solution after force reached a steady state. This cycle of pre-activating solution, Ca2+ activation and relaxation was repeated to accumulate data over a range of pCa 7.0-4.0. Submaximal forces were measured in random order. Since maximal force (F4.0) sometimes declined slightly (< 10 %) throughout the course of experiment, submaximal activations were bracketed by a pCa 4.0 contraction and submaximal forces were then normalized as fractions of the bracketing pCa 4.0 force (F/F4.0). The relationships between force and pCa were fitted to the Hill equation: relative force F/F4.0 = 1/[1 + 10(-nH(pCa50 - pCa))], where nH is the Hill coefficient and pCa50 is the pCa at 0.5F4.0. Relationships of isometric force to pCa were determined before and after extraction/reconstitution with rTnC or mutant TnC.
TnC extraction and reconstitution
TnC extraction was performed at 20 °C using an extraction solution that contained 5 mm EDTA, 10 mm Hepes and 0.5 mm trifluoperazine dihydrochloride (TFP) at pH 7.0 (Metzger et al. 1989). Fibres were stretched to sarcomere length of 3.5 μm in pCa 9.0 solution and then soaked in the extraction solution for 2 min. Fibres were then transferred back to pCa 9.0 solution and released to a sarcomere length of 2.6 μm. Activation of fibres in pCa 4.0 was then used to assess the extent of TnC extraction. A 2 min incubation in extraction solution was adequate to reduce average pCa 4.0 force to less than 10 % of the maximal isometric force before extraction. After extraction, fibres were washed five times with pCa 9.0 solution to remove TFP. Reconstitution of TnC was accomplished at 20 °C by soaking TnC-extracted fibres in a pCa 9.0 solution containing 16.7 μm purified TnC at a resting sarcomere length of 2.6 μm. Fibres were bathed in this solution for a brief duration (20-60 s), then activated at pCa 4.0 and returned to pCa 9.0 solution with no TnC added. Fibres were cycled through this procedure until maximum force recovery was achieved. After ≈2 min incubation in rTnC or TnC mutants, force recovered to ≥ 80 % of the maximal isometric force before extraction.
Diazo-2 protocol
Relaxation was induced in skinned fibres by photolysis of the caged Ca2+ chelator diazo-2. Within a few milliseconds the Ca2+ affinity of diazo-2 increases ≈30-fold upon exposure to UV light (Adams et al. 1989; Mulligan et al. 1999). To induce relaxation, fibres were first soaked in a diazo-2-containing solution until force reached a plateau as the diazo-2 diffused into the fibres. Fibres were then transferred into an empty chamber and flashed in air and the relaxation time course recorded. In order to avoid changes in the condition of the fibres, caged compound experiments were ‘one flash’ (i.e. one diazo-2-induced relaxation per fibre). Under the conditions of these experiments, it was not possible to induce complete relaxation with photolysis of diazo-2 from a maximum isometric contraction, probably due to the relatively small increase in Ca2+ affinity upon diazo-2 photolysis. Based on control experiments with fibres containing endogenous TnC (see results), the general strategy employed in testing the effects of rTnC and TnC mutants on relaxation in standard and low [Pi] solutions was to adjust conditions so that fibres developed approximately the same submaximum pre-photolysis force and a similar final post-photolysis force. These conditions were satisfied when the pre-photolysis force was approximately 60 % of the maximum isometric force. At this force level, the rate of relaxation was not significantly correlated with either the pre- or post-photolysis force (see results). Also, since it was found that relaxation depended on the diazo-2 chelating capacity, [diazo-2]free/[Ca2+]free (see Results), relaxation was induced at similar diazo-2 chelating capacities.
The protocol to study the effects of rTnC or mutant TnCs on relaxation was: (a) determine maximum force in pCa 4.0 (F4.0), (b) determine force at pCa to be examined (F/F4.0), (c) extract TnC until the force in pCa 4.0 was < 5 % of F4.0, (d) reconstitute with rTnC or mutant TnC until the force in pCa 4.0 was > 90 % of F4.0, (e) determine the force at pCa to be examined in the presence of reconstituted TnC and (f) add diazo-2, develop a contraction and then induce relaxation by flash photolysis of diazo-2. The protocol was the same for fibres in the presence of endogenous TnC except that TnC was not extracted.
Pi scavenging protocol
To scavenge Pi, fibres were first soaked in low [Pi] pCa 9.0 solution for 10 min and then activated in a Ca2+-activating solution or a caged compound solution containing the Pi scavenger. The protocol to study the effects of low [Pi] on relaxation in the presence of rTnC or mutant TnCs was: (a) determine the maximum force in standard pCa 4.0 (F4.0), (b) determine the maximum force in low [Pi] pCa 4.0 (F4.0(low [Pi])), (c) extract TnC until the force in standard pCa 4.0 was < 5 % of F4.0, (d) reconstitute with rTnC or mutant TnC until the force in standard pCa 4.0 was > 90 % of F4.0, (e) determine the maximum force in low [Pi] pCa 4.0 in the presence of reconstituted TnC and (f) add diazo-2 in low [Pi], develop a contraction and then induce relaxation by photolysis of diazo-2.
Data analysis
Pre-photolysis and post-photolysis steady-state forces generated in diazo-2 solution were normalized to the maximal force in adjacent pCa 4.0 contraction, Fpre/F4.0 and Fpost/F4.0, respectively. The extent of relaxation (ΔF = Fpre - Fpost) induced by photolysis of diazo-2 was normalized to the pre-photolysis steady-state force, ΔF/Fpre = (Fpre - Fpost)/Fpre. The diazo-2-induced relaxation trace was resolved into two phases: a slow linear phase followed by a fast exponential phase (Fig. 1). The slow phase was not observed in standard solution (Fig. 1A) but it became obvious in the presence of low [Pi] (Fig. 1B). The fast phase was fitted by selecting points that were evenly distributed along the trace following the shoulder to a single exponential equation:
where a is the amplitude of a single exponential with rate constant kf, b is the residual and to is an estimate of the time to shoulder. The slow phase was fitted to a linear equation: F/ΔF = a + ks × t, where a is the y-axis intercept and ks is the slope of the linear phase. The data points of the slow phase were selected from 1/4 to the shoulder to exclude any artefact due to the laser pulse from the fits. The t1/2 (the time for force to decrease to 0.5ΔF) was also used as a measure of relaxation kinetics. In order to emphasize the kinetic aspects of the relaxation, the results in Figs 4, 7 and 8 are plotted as F/ΔF versus time, where Fpre/F4.0 is set to 1.0 and Fpost/F4.0 is set to zero. Results are presented as means ± s.e.m. Data were analysed using Student's t test and simple and multiple linear regressions, where r = correlation coefficient. P ≤ 0.05 was considered statistically significant.
Figure 1. Analysis of relaxation induced by photolysis of diazo-2 in skinned psoas fibres at 15 °C.
A, an example of relaxation induced in standard diazo-2 solution where F6.1pre/F4.0 = 0.44, Fpost/F4.0 = 0.04, ΔF/Fpre = 0.91, time constant of the fast phase of relaxation, kf = 32 s−1, time to shoulder (to) = 9 ms, half-relaxation time (t1/2) = 31 ms. B, an example of relaxation induced in the presence of M82Q TnC and a solution containing a low concentration of inorganic phosphate ([Pi]), where F7.0pre/F4.0(low[Pi]) = 0.58, Fpost/F4.0(low[Pi]) = 0.13, ΔF/Fpre = 0.78, time constant of the slow phase of relaxation, ks = 1.4 (F/ΔF) s−1, kf = 8 s−1, to = 100 ms, t1/2 = 185 ms.
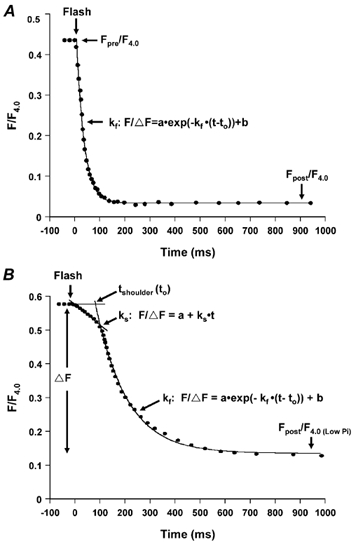
Figure 4. Examples of the effect of relative Ca2+-activated force and the total concentration of diazo-2 ([diazo-2]) on the time course of relaxation induced by photolysis of diazo-2 in skinned psoas fibres containing endogenous TnC at 15 °C in standard [Pi] solution.
To emphasize the kinetics of relaxation, force has been normalized to the extent of relaxation, ΔF. Thus Fpre/F4.0 was set to 1.0 and Fpost/F4.0 was set to 0. A, effect of relative force from 0.19 to 0.84 Fpre/F4.0 on relaxation rate at constant total [diazo-2]= 4 mm. For these examples, Fpost/F4.0 = 0.31 at pCa 5.5, 0.01 at pCa 6.1 and 0.004 at pCa 6.2. B, effect of total [diazo-2] at 2-16 mm on relaxation rate at similar relative force (≈0.8 Fpre/F4.0, pCa 5.8), where Fpost/F4.0 = 0.13 for 2 mm diazo-2, 0.15 for 9.68 mm diazo-2 and 0.38 for 16 mm diazo-2.
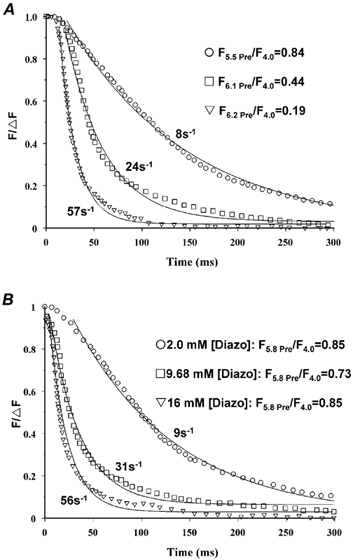
Figure 7. Diazo-2-induced relaxation at 15 °C in skinned psoas fibres with endogenous TnC, rTnC or mutant TnC in standard solution.
A, examples of relaxation in fibres reconstituted with rTnC (□) or M82Q TnC (▵). To emphasize the kinetics of relaxation, force has been normalized to the extent of relaxation, ΔF. Relaxation was induced at similar pre-photolysis force (Fpre/F4.0 = 0.48 for rTnC and 0.53 for M82Q TnC) and similar chelating capacity (2440 for rTnC and 3238 for M82Q TnC) and resulted in a similar post-photolysis force (Fpost/F4.0 = 0.08 for rTnC and 0.05 for M82Q TnC) but a slower rate of relaxation in the presence of M82Q TnC. B, mean half-relaxation time (t1/2) values are plotted as a function of relative force for fibres containing different forms of TnC. Relaxation was induced at similar chelating capacities: endogenous TnC (▪) 2880, rTnC (□) 2440, M82Q TnC (▵) 3238 and NHdel TnC (○) 3082. See Table 3 for further details.
Figure 8. Diazo-2-induced relaxation at 15 °C in skinned psoas fibres containing endogenous TnC, rTnC or mutant TnC in standard or low [Pi] solution.
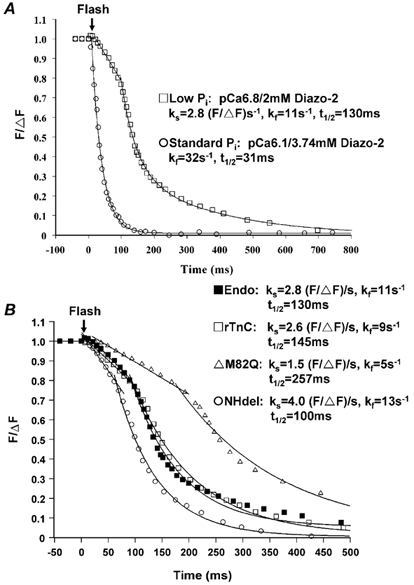
To emphasize the kinetics of relaxation, force has been normalized to the extent of relaxation, ΔF. A, examples of relaxation induced by photolysis of diazo-2 in fibres containing endogenous TnC in standard and low [Pi] solution. Standard [Pi] solution: chelating capacity of 2800, Fpre/F4.0 = 0.44 and Fpost/F4.0 = 0.04. Low [Pi] solution: chelating capacity of 9800, Fpre/F4.0 = 0.61 and Fpost/F4.0 = 0.17. B, effects of endogenous TnC, rTnC or mutant TnCs on relaxation in low [Pi] solution. Relaxation was induced at a similar pre-photolysis force (Fpre/F4.0 = 0.61 for endogenous TnC, 0.63 for rTnC, 0.58 for M82Q TnC and 0.72 for NHdel TnC) and similar diazo-2 chelating capacity (9800 for endogenous TnC, 7800 for rTnC, 10 000 for M82Q TnC and 8800 for NHdel TnC) and resulted in similar values of post-photolysis force (Fpost/F4.0 = 0.17 for endogenous TnC, 0.15 for rTnC, 0.13 for M82Q TnC and 0.16 for NHdel TnC) but dramatically different relaxation rates. See legend to Table 3 for further details.
Results
Ca2+ affinity and the rate of dissociation of Ca2+ from mutant TnC in solution
Ca2+ titrations of mutant TnCs following the increase in Trp fluorescence showed that Ca2+ bound half-maximally (pCa50) to the regulatory sites of M82Q/F29W, F29W and NHdel/F29W TnC at pCa 6.16 ± 0.003, 5.49 ± 0.01 and 5.08 ± 0.01, respectively (Fig. 2A). Thus, compared to F29W TnC, the M82Q mutation increased the Ca2+ affinity of TnC by ≈4.6-fold and the NHdel mutation decreased Ca2+ affinity of TnC by ≈2.6-fold. Also using F29W as a spectral probe, the rates of dissociation of Ca2+ from M82Q/F29W, F29W and NHdel/F29W TnC were 76 ± 2, 340 ± 5 and 515 ± 4 s−1, respectively (Fig. 2B). Thus, consistent with the differing Ca2+ affinities, compared to F29W TnC, the M82Q mutation resulted in an ≈4.5-fold decrease in the rate of Ca2+ dissociation from the regulatory sites of TnC, whereas the NHdel mutation increased the Ca2+ dissociation rate by ≈1.5-fold.
Figure 2. Changes in fluorescence of the spectral probe Trp during Ca2+ titrations (A) and Ca2+ dissociation rates (B) of the following troponin (Tn) C mutants in solution at 15 °C: F29W (□), M82Q/F29W (▵) and NHdel/F29W TnC (○).
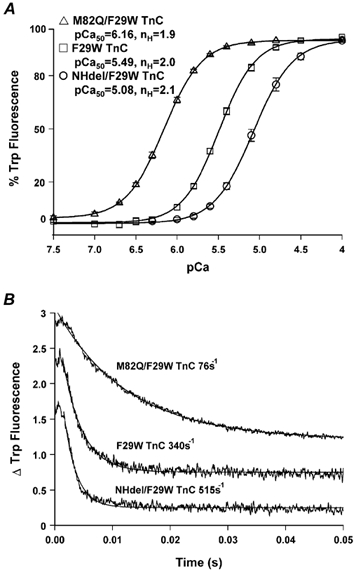
Each titration curve represents the average of 3-5 measurements fitted with the logistic sigmoid function. Each dissociation trace represents an average of at least five traces fitted with a single exponential equation. Ca2+ dissociation traces are displaced vertically for clarity. pCa50 = pCa resulting in 50 % of maximum fluorescence change and nH = Hill coefficient.
Ca2+ sensitivity of isometric force in control and mutant reconstituted skinned fibres
The force versus pCa relationships in fibres exhibited the following order of decreasing pCa50 values: M82Q/F29W TnC(6.13 ± 0.01), unextracted (endogenous TnC) (6.04 ± 0.01), rTnC (6.01 ± 0.01), F29W TnC (5.94 ± 0.02) and NHdel TnC (5.69 ± 0.02; Fig. 3). The force versus pCa relationships for unextracted (endogenous) fibres and fibres reconstituted with either rTnC or F29W TnC were not significantly different from each other (Fig. 3A). Thus the extraction/reconstitution procedures had minimal effect on Ca2+ sensitivity as well as maximal force production. In contrast, compared to fibres reconstituted with F29W TnC, M82Q/F29W TnC produced an ≈1.6-fold increase in the Ca2+ sensitivity of isometric force and NHdel TnC produced an ≈1.8-fold decrease in Ca2+ sensitivity of force (Fig. 3B). These shifts were qualitatively the same and in the case of NHdel TnC quantitatively similar to the shifts of pCa50 in the Ca2+ titrations measured in solution (Fig. 2A). These data clearly demonstrate that the M82Q or NHdel induced increase or decrease in the Ca2+ affinity of TnC in solution resulted in a corresponding increase or decrease in the Ca2+ sensitivity of isometric force in reconstituted muscle fibres, respectively.
Figure 3. Isometric force versus pCa relationships in skinned psoas fibres at 15 °C in the presence of endogenous TnC (▪, A), rTnC (•, A), F29W TnC (□, A, B), M82Q/F29W TnC (▵, B) or NHdel TnC (○, B) in standard solution.
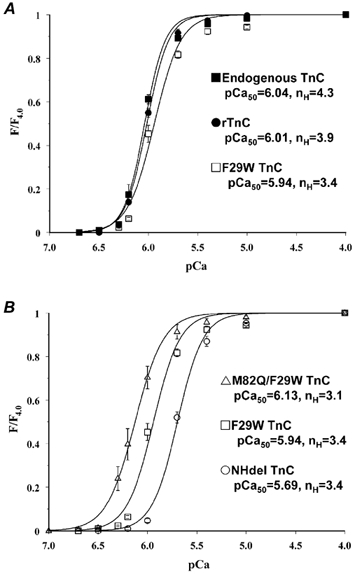
Values are means ±s.e.m. Mechanical properties were determined with endogenous TnC before extraction and after reconstitution with rTnC or mutant TnC. The force in pCa 4.0 after extraction divided by the force in pCa 4.0 before extraction in the same fibre averaged from 0.07 to 0.12. The force in pCa 4.0 after reconstitution with rTnC or mutant TnC divided by the adjacent force in pCa 4.0 before extraction averaged from 0.81 to 0.95. pCa50 = pCa at 0.5 F4.0 and nH = the Hill coefficient.
Effect of pre- and post-photolysis force and diazo-2 chelating capacity on relaxation rate
First, the effects of varying the initial Ca2+-activated force (Fpre/F4.0) on relaxation were determined at a constant total [diazo-2]. Relaxation was induced in fibres at various values of Fpre/F4.0 by varying pCa levels. Typical time courses of relaxation induced at 4 mm diazo-2 from three different values of Fpre/F4.0 are shown in Fig. 4A. Elevating [Ca2+]free increased Fpre/F4.0 and concomitantly slowed the time course of relaxation following photolysis of diazo-2. The rate constant kf decreased from a mean value of 44.8 ± 5.9 s−1 (n = 4) to 9.4 ± 0.9 s−1 (n = 5) as Fpre/F4.0 was increased from 0.1 to 0.8 by elevating [Ca2+]free. These data show that at constant total [diazo-2] (4 mm), an increase in [Ca2+]free and, as a consequence, an increase in the steady-state force at the onset of relaxation had a significant effect on the subsequent rate of relaxation.
Because the rate of relaxation could be directly dependent on the relative concentrations of diazo-2 and [Ca2+]free at the time of photolysis, a systematic study to determine the effects of total [diazo-2] on relaxation rate at different initial forces was undertaken. Examples of diazo-2-induced relaxation at similar high forces but different levels of total [diazo-2] are shown in Fig. 4B and demonstrate that at similar initial force level (≈0.8Fpre/F4.0, pCa 5.8), the time course of relaxation became progressively faster as total [diazo-2] increased from 2 to 16 mm. Similar results were found when relaxation was induced at low force level (≈0.35Fpre/F4.0, pCa 6.1). The data are summarized in Table 2. Clearly, the rate of diazo-2-induced relaxation depended on the total [diazo-2] over the range of 2-16 mm at high force (pCa 5.8) and 0.8-6 mm at low force (pCa 6.1).
Table 2.
Rates and extent of relaxation induced by diazo-2 at 15°C in skinned psoas fibres at different relative force, diazo-2 chelating capacity and total diazo-2 concentration ([diazo-2)] in the presence of endogenous troponin (Tn)C in standard solutions
| [diazo-2]free/[Ca2+]free (mm/mm) | Total [dizo-2] (mm) | Fpre/F4.0 | Fpost/F4.0 | (ΔF/Fpre) × 102 (%) | kf(s−1) | n |
|---|---|---|---|---|---|---|
| High force (pCa 5.8) | ||||||
| 579 | 2.0 | 0.82 ± 0.02 | 0.13 ± 0.02 | 85 ± 1 | 10.8 ± 1.4 | 4 |
| 2800 | 9.68 | 0.78 ± 0.03 | 0.16 ± 0.03 | 80 ± 3 | 34.6 ± 4.0 | 5 |
| 4634 | 16.0 | 0.84 ± 0.01 | 0.35 ± 0.02 | 58 ± 2 | 48.0 ± 3.0 | 4 |
| Low force (pCa 6.1) | ||||||
| 600 | 0.8 | 0.30 ± 0.02 | 0.05 ± 0.01 | 84 ± 3 | 17.0 ± 1.4 | 5 |
| 2800 | 3.74 | 0.42 ± 0.01 | 0.07 ± 0.02 | 84 ± 5 | 37.2 ± 2.5 | 5 |
| 4487 | 6.0 | 0.36 ± 0.03 | 0.05 ± 0.01 | 87 ± 2 | 42.5 ± 3.5 | 4 |
Diazo-2 chelating capacity, [diazo-2]free/[Ca2+]free, is calculated from the Fabiato program. Fpre/F4.0 is the pre-photolysis steady-state force divided by the force in an adjacent pCa 4.0 solution. Fpost/F4.0 is the final post photolysis force as a fraction of the maximum force. ΔF/Fpre is the extent of diazo-2 induced relation (ΔF =Fpre- Fpost) as a fraction of pre-photolysis force, (Fpre- Fpost)/Fpre. Kf, rate constnat of relaxation; n, number of fibres. Values given as means ± s.e.m.
To assess quantitatively the influence of the capacity of diazo-2 to chelate free Ca2+ on relaxation, the relaxation rates were plotted as a function of diazo-2 chelating capacity, defined as [diazo-2]free/[Ca2+]free, in Fig. 5. The rate of diazo-2-induced relaxation increased with diazo-2 chelating capacity. At constant diazo-2 chelating capacity, the rate of force decline following photolysis was no longer dependent on the initial force before relaxation over the range of 0.3-0.8 Fpre/F4.0. For all experiments with fibres containing endogenous TnC (n = 41), there was no significant correlation between the rate of relaxation and Fpre/F4.0 (r = 0.29, P > 0.05; Fig. 6A). In contrast, the diazo-2 chelating capacity significantly correlated with the rate of relaxation when the data was fitted with a linear (r = 0.89, P < 0.01) or exponential relationship (r = 0.88, P < 0.05), as shown in Fig. 6B. The linear correlation of rate of relaxation with the total [diazo-2] also was significant but with a lower r value (0.58) than observed for correlation with chelating capacity (0.89). Therefore, diazo-2 chelating capacity, but not pre-photolysis Ca2+-activated force, is an important controlling factor in the measurement of relaxation kinetics.
Figure 5. Rate of diazo-2-induced relaxation in skinned psoas fibres containing endogenous TnC at 15 °C as a function of diazo-2 chelating capacity, [diazo]free/[Ca2+]free, at different relative pre-photolysis forces.
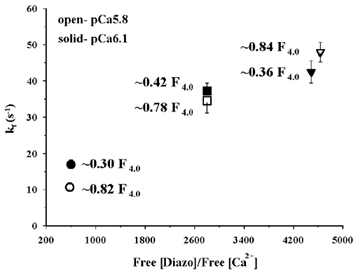
See Table 2 for further details.
Figure 6. Rate of diazo-2-induced relaxation in skinned psoas fibres containing endogenous TnC at 15 °C as a function of pre-photolysis force (Fpre/F4.0), diazo-2 chelating capacity ([diazo-2]free/[Ca2+]free) and post-photolysis force (Fpost/F4.0).
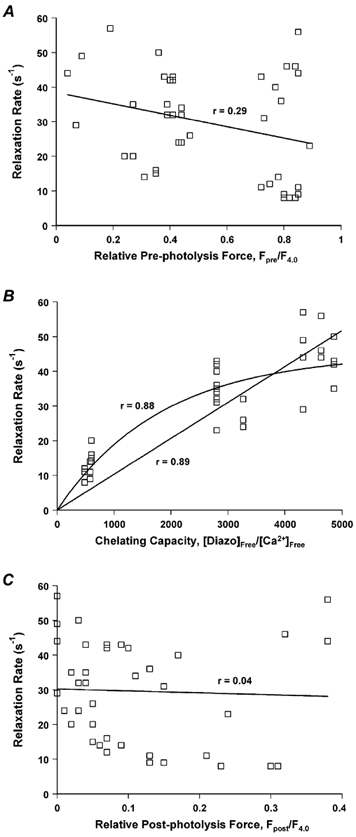
A, the relaxation rate was not significantly correlated with Fpre/F4.0. B, the relaxation rate was significantly correlated with the diazo-2 chelating capacity. Data were equally well fitted with a linear relationship, relaxation rate = 0.0103 ± 0.0004 × (chelating capacity) or with an exponential relationship, relaxation rate = 45 s−1× (1 - e−k× chelating capacity), where k = 5.4 × 10−4± 5 × 10−5. C, relaxation rate was not significantly correlated with Fpost/F4.0. Results in each panel are from the same fibres (n = 41).
Photolysis of diazo-2 did not induce complete relaxation under the conditions of these experiments. From the data given in Table 2, it is apparent that in general, when Fpre/F4.0 was between 0.3 and 0.8 the extent of relaxation (ΔF) induced by diazo-2 photolysis averaged ≈85 % of the pre-photolysis force. ΔF/Fpre did not depend upon the diazo-2 chelating capacity in the range of 580-4860. The exception to this conclusion occurred when the relative force was high (Fpre/F4.0 = 0.84) and the diazo-2 chelating capacity was also high (4634). This condition required the highest [diazo-2] of 16 mm. Under these conditions, the extent of relaxation was 60 %. This diminished extent of relaxation may be due to an inner-filter effect occurring at very high [diazo-2], which diminishes its chelating effect.
For all experiments with fibres containing endogenous TnC (n = 41), no significant correlation was found between the rate of relaxation and final, post-photolysis force, Fpost/F4.0 (r = 0.04, P > 0.05; Fig. 6C). Multiple linear regression analysis was utilized to explore further the possible relationship between the rate of relaxation and chelating capacity, Fpre/F4.0 and Fpost/F4.0. Only the chelating capacity correlated significantly with the rate of relaxation. Thus neither the pre-photolysis nor post-photolysis force influenced the measured rate constants in these experiments, but the diazo-2 chelating capacity had a significant effect on the rate of relaxation.
Effect of altered Ca2+ dissociation from TnC on relaxation rate
To examine the effects of Ca2+ dissociation from TnC on muscle relaxation, the kinetics of relaxation were measured in fibres containing endogenous TnC, rTnC or mutant TnC (Fig. 7 and Table 3). In an attempt to minimize as much variation as possible, experiments were done at nearly constant, intermediate diazo-2 chelating capacities and in a submaximum pre-photolysis force range where neither the pre- or post-photolysis force influenced the rate of relaxation. Fibres that contained endogenous TnC exhibited similar t1/2 values for relaxation at constant diazo-2 chelating capacity (≈2800) over the force range of 0.4-0.8 Fpre/F4.0. The t1/2 increased ≈twofold following reconstitution with wild-type TnC. This difference between rTnC and endogenous TnC is probably not due to the extraction/reconstitution procedure because rTnC produced a similar force versus pCa relationship (Fig. 3A) and similar maximum rate of contraction in response to flash photolysis of a caged Ca2+ (Y. Luo & J. A. Rall, unpublished observations) as observed in unextracted fibres. The difference is not due to differences in Fpre/F4.0 or Fpost/F4.0. It is possible that the difference in the diazo-2 chelating capacity between the measurements with rTnC (2440) and endogenous TnC (2800) might, at least in part, be responsible for this slowing of relaxation with rTnC. Also, there is the possibility of a species difference, since the rTnC is derived from chicken TnC and re-constituted into mammalian muscle.
Table 3.
Kinetics of relaxation induced by photolysis of diazo-2 in skinned psoas fibres that contain endogenous TnC, rTnC or mutant TnC in standard or low [Pi] solution at 15°C
| Standard solution | Low [Pi] solution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fpre/F4.0 | Fpost/F4.0 | kf(S−1) | Obs t½ (ms) | Sim t½ (ms) | n | Fpre/F4.0* | Fpost/F4.0 | ks†(S−1) | kf(s−1) | Obs t½ (ms) | Sim t½ (ms) | n | |
| RTnC | 0.51 ± 0.05 | 0.06 ± 0.02 | 16.8 ± 2.3 | 57 ± 6 | 57 | 5 | 0.55 ± 0.02 | 0.15 ± 0.02 | 3.0 ± 0.4 | 9.0 ± 0.8 | 127 ± 11 | 127 | 6 |
| M82Q | 0.54 ± 0.02 | 0.06 ± 0.01 | 8.6 ± 0.8 | 128 ± 13 | 127 | 5 | 0.63 ± 0.03 | 0.15 ± 0.01 | 1.5 ± 0.1 | 5.5 ± 0.6 | 251 ± 21 | 225 | 6 |
| NHdel | 0.66 ± 0.01 | 0.22 ± 0.02 | 9.5 ± 0.7 | 62 ± 1 | 47 | 2 | 0.64 ± 0.05 | 0.20 ± 0.03 | 3.7 ± 0.4 | 11.0 ± 1.6 | 104 ± 12 | 118 | 5 |
| ENDO | 0.42 ± 0.04 | 0.07 ± 0.02 | 37.2 ± 2.5 | 28 ± 1 | — | 5 | 0.06 ± 0.02 | 0.16 ± 0.02 | 3.3 ± 0.4 | 9.6 ± 1.0 | 128 ± 18 | — | 5 |
| ENDO | 0.78 ± 0.03 | 0.16 ± 0.03 | 34.6 ± 4.0 | 25 ± 3 | — | 5 | — | — | — | — | — | — | — |
Low Pi
F/ΔF; Obs, observed; Sim, simulated. Relaxation was induced at the following levels of diazo-2 chelating capacity ([diazo-2]free/[Ca2+]free) and [diazo]total: (1) for standard [Pi] solution: endogenous Tnc (ENDO) (2800, 3.74 m m and 9.68 m m, respectively); rTnC (2440, 4 m m, respectively); M82Q TnC (3238, 4 mm, respectively) and NHdel TnC (3082, 15 mm, respectively) and (2) for low [Pi] solution: endogenous TnC (ENDO) (9800, 2 mm, respectively), M82Q TnC (10000, 1.25 mm, respectively) and NHdel TnC (8800, 5 mm, respectively). Fpre/F4.0 and Fpost/F4.0 were determined by dividing the submaximum steady-state force before or after photolysis of diazo-2 by the force in an adjacent pCa 4.0 normal solution or solution enzymatically scavenged for Pi. ks = slope of the slow, linear phase of relaxation. kf = rate constant of fast phase of relaxation. t½ = time for force to fall to 50% of ΔF, the change in force after the flash. The force in a pCa 4.0 solution after extraction divided by the force in a pCa 4.0 solution before extraction averaged 0.04–0.07. the force in a pCa 4.0 solution after rTnC or mutant TnC constitution divided by the force in a pCa 4.0 solution before extraction averaged 0.92-1.05.n = number of fibres. Values given as mean ± s.e.m. Simulations were based on a sequential model of relaxation where kCa = rate of Ca2+ dissociation from TnC and kCB = rate of crossbridge detachment (see text for details).
Compared to rTnC, M82Q TnC fibres relaxed more slowly (Fig. 7A), which resulted in an additional ≈twofold increase in t1/2 at similar Fpre/F4.0 (0.54 versus 0.51), the same Fpost/F4.0 (0.06) and higher diazo-2 chelating capacity (3238 versus 2440). Thus, this effect is not due to differences in the relative force or the diazo-2 chelating capacity. This ≈twofold increase in t1/2 in fibres compares with the ≈4.5-fold decrease in the rate of dissociation of Ca2+ from M82Q TnC in solution (Fig. 2B). These results provide direct evidence that the rate of dissociation of Ca2+ from TnC can significantly influence the kinetics of relaxation.
However, the kinetics of relaxation were not significantly affected in fibres reconstituted with NHdel TnC compared to those with rTnC (Fig. 7B and Table 3). This result is in contrast to the observed effect of increasing rate of dissociation of Ca2+ from TnC in solution. This result may be related to some difficulties in the experiments with NHdel TnC. First, measurements with NHdel TnC were performed at very high total [diazo-2] to maintain a certain level of relative force and chelating capacity, because NHdel TnC produced a rightward shift in the force versus pCa relationship. The high [diazo-2], through an inner-filter effect, could lead to a non-uniform uptake of Ca2+ across the fibre upon photolysis of diazo-2 and possibly limits the rate of subsequent relaxation. Second, the accelerating effect of NHdel TnC on relaxation may be in the linear phase of relaxation that is not detected under these experimental conditions. Therefore, in the following experiments, attempts were made to prolong the linear phase of relaxation to test this hypothesis.
Effect of altered crossbridge kinetics on relaxation rate
Crossbridge detachment is proposed to play a role in the rate of relaxation. Lowering intracelluar [Pi] is expected to slow the rate of relaxation, presumably by decreasing the crossbridge kinetics via slowing of the Pi association step in the crossbridge cycle (Millar & Homsher, 1990; Nencini et al. 2000). Compared to the standard solution, low [Pi] increased both maximum isometric force (F4.0(lowPi)/F4.0 = 1.12 ± 0.03, n = 4) and submaximum isometric force (i.e. increased fibre Ca2+ sensitivity). Thus in the presence of low [Pi], relaxation from a given relative force was induced in diazo-2 solutions containing higher pCa levels than in the standard solution. This led to a significant increase in the diazo-2 chelating capacity in the millimolar range of total [diazo-2]. But even at higher chelating capacity (8000-10 000 versus 2400-3300 for standard [Pi] solution), low [Pi] still slowed relaxation and produced two distinct phases of relaxation, a slow linear phase followed by a fast exponential phase. As shown in the example in Fig. 8A, reducing the [Pi] produced a slow linear phase and a decreased rate of the exponential phase even though the chelating capacity was higher in low [Pi] (≈9800) than in the standard solution (2800). On average, low [Pi] decreased both slow (ks) and fast (kf) rate constants of relaxation, resulting in an increased t1/2 from 26.2 ± 1.6 ms (n = 10) to 127.6 ± 16.0 ms (n = 5; Table 3). This significant effect of low [Pi] on relaxation, compared to the small effect on the maximal contraction rate (10-15 % decrease) induced by flash photolysis of a caged Ca2+ (Y. Luo & J. A. Rall, unpublished observations), indicates that the rate of relaxation is more sensitive to the change in [Pi] than the rate of contraction.
The distinct slow and fast phases of relaxation in low [Pi] provide a good model with which to study potential relaxant factors, especially the factors that are thought to speed up the slow phase, such as an increased rate of dissociation of Ca2+ from NHdel TnC.
Effect of altered Ca2+ dissociation from TnC and crossbridge kinetics on relaxation rate
The effects of Ca2+ dissociation from TnC on relaxation kinetics were further examined in the presence of low [Pi]. Relaxation was induced in fibres containing endogenous TnC, rTnC or mutant TnC under conditions of similar Fpre/F4.0 (0.55-0.64), Fpost/F4.0 (0.15-0.2) and diazo-2 chelating capacity (8000-10 000) in low [Pi]. As shown in the examples in Fig. 8B and the average results in Table 3, low [Pi] produced two phases of relaxation: a slow linear rate ks: 3.3 ± 0.4 (F/ΔF) s−1 and a fast exponential rate kf: 9.6 ± 0.9 s−1 (n = 5) in fibres with endogenous TnC. The kinetics of relaxation were not significantly changed following reconstitution with rTnC. However, M82Q TnC further slowed relaxation with an ≈twofold decrease in the linear and exponential rates which resulted in an ≈twofold increase in t1/2 compared to rTnC. This result is almost identical to the ≈twofold increase in t1/2 with M82Q TnC observed in standard [Pi] solution. Thus when decreased Ca2+ dissociation rate (M82Q TnC) and decreased crossbridge kinetics (low [Pi]) were combined, the effect was cumulative since the average t1/2 was increased by ≈fourfold. Consistent with the insignificant effect of NHdel TnC on relaxation observed in standard [Pi] solution, after low [Pi] slowed relaxation, NHdel TnC still did not significantly speed up relaxation. Fibres containing NHdel TnC exhibited on average an ≈20 % shorter, but not significantly different, t1/2 for relaxation compared to fibres with rTnC. Thus, these results showed that decreasing the rate of Ca2+ dissociation from TnC and the crossbridge kinetics either independently or simultaneously could dramatically slow relaxation. In contrast, increasing the rate of Ca2+ dissociation from TnC did not speed up relaxation significantly even after relaxation was slowed.
Table 3 also gives a comparison of the kinetics of relaxation in the presence of mutant or rTnC under standard and low [Pi] conditions. It is apparent that low [Pi] increased the t1/2 of relaxation by ≈twofold for each exchanged TnC when compared to its own standard [Pi] control. Thus, relaxation can be slowed by slower crossbridge kinetics and/or slower Ca2+ dissociation from TnC.
These results have been simulated assuming a simple two-step sequential model for muscle relaxation where Ca2+ dissociates from TnC at a rate of kCa, which then allows myosin to dissociate from actin at a rate kCB, which causes relaxation. Using this model, in order for slower Ca2+ dissociation or slower crossbridge kinetics to both slow relaxation kCa and kCB must be similar. Interestingly, when kCa = kCB = 30 s−1, the simulated t1/2 of relaxation matched the observed results for rTnC containing fibres in standard solution (57 ms; Table 3). Based on solution studies (Fig. 2B), relaxation in mutant TnC-containing fibres was simulated by slowing kCa by fourfold (M82Q) or increasing kCa by 1.5-fold (NHdel). Furthermore, slowing kCB by fourfold simulated relaxation in low [Pi] conditions, since low [Pi] conditions slowed relaxation to a similar extent as did M82Q TnC under normal [Pi] conditions. As can be seen in Table 3, the simulated t1/2 of relaxation for the different TnC mutants and [Pi] conditions match closely the observed experimental values. Thus, in the fibre the rate of Ca2+ dissociation from TnC may be similar to the rate of crossbridge detachment under normal [Pi] conditions.
Discussion
Utilization of diazo-2 to investigate the kinetics of relaxation in skinned muscle fibres
Diazo-2 has distinct advantages in the study of rapid, non-diffusion-limited relaxation in skinned muscle fibres, but it also possesses certain disadvantages that must be recognized. Flash photolysis of diazo-2 induces an ≈30-fold increase in Ca2+ affinity within a few milliseconds. The rapid change in affinity and convenience of use in skinned fibre experiments are major advantages. The disadvantages relate mainly to the relatively small increase in affinity of diazo-2 upon photolysis and the fact that the rate of relaxation is dependent on [diazo-2] in its readily useable concentration range. Because of the relatively small increase in diazo-2 affinity upon photolysis, relaxation is incomplete and the final post-photolysis force is greater when the pre-photolysis force is greater (see Table 2). Thus it is necessary to work at submaximum forces and to demonstrate that the results are not dependent upon the specific pre- and post-photolysis forces. Also, the rate of relaxation depends upon the diazo-2 chelating capacity (Fig. 6B) and thus this parameter must be held relatively constant. It is not clear whether a saturating rate of relaxation was achieved at any value of diazo-2 chelating capacity in the present experiments (skinned psoas fibres at 15 °C) since the data in Fig. 6B could be equally well fitted with a linear or an exponential relationship. A consequence of this limitation is that the absolute rate of relaxation cannot be readily compared to results generated at other diazo-2 chelating capacities or using other techniques. Nonetheless, it is possible to deduce meaningful conclusions with regard to the effects of experimental perturbations on relaxation rate using diazo-2 when: (1) the diazo-2 chelating capacity is relatively constant and (2) pre- and post-photolysis force do not affect relaxation rate. These conditions were established in the current experiments.
Summary and implications of results
Experiments were designed to test whether relative force, rate of dissociation of Ca2+ from TnC and/or the cross-detachment rate influence the kinetics of relaxation in rabbit skinned psoas fibres. First, the effect of pre-photolysis Ca2+-activated force on relaxation rate could be eliminated by maintaining a constant diazo-2 chelating capacity. Second, at similar levels of pre- and post-photolysis force and diazo-2 chelating capacity, decreasing the Ca2+ dissociation rate from TnC with M82Q TnC produced a slower relaxation with an ≈twofold increased t1/2 compared to control under both standard [Pi] and low [Pi] conditions. But increasing the rate of Ca2+ dissociation from TnC with NHdel TnC did not significantly speed up relaxation in either standard [Pi] or low [Pi] conditions. Third, after ruling out influences of pre-photolysis Ca2+-activated force, post-photolysis force and diazo-2 chelating capacity, decreased crossbridge kinetics due to reduced intracellular [Pi] resulted in an ≈twofold increase in t1/2. Similar slowing effects due to low [Pi] on relaxation also were observed in fibres containing M82Q TnC, NHdel TnC or rTnC.
Ca2+ sequestration is presumably a rate-limiting step for relaxation in intact fibres (Hou et al. 1991). However photolysis of diazo-2 appears to take up Ca2+ considerably faster than the intact SR, as suggested by the fact that diazo-2 induced relaxation in intact fibres shortens the slow phase of relaxation and thus speeds up relaxation (Lannergren & Arner, 1992). The finding that the rate of relaxation induced by diazo-2 is dependent on diazo-2 chelating capacity indicates that the Ca2+ uptake by diazo-2 can also be rate limiting for relaxation in skinned fibres. Furthermore, the observation of distinct slow and fast phases in skinned fibre relaxation in low [Pi] conditions, as seen in intact fibres, suggests that the mechanism of relaxation is likely to be fundamentally similar for both intact and skinned fibres. Thus the kinetics of relaxation can be studied in skinned fibres with the aid of diazo-2. Using this method, Ca2+ uptake can be controlled quantitatively by the diazo-2 chelating capacity, which enables exclusive investigation of the rate-limiting effects on the relaxation of [Ca2+]freeper se, Ca2+ dissociation from TnC and crossbridge detachment without the confounding influence of Ca2+ sequestration by the SR.
Effect of Ca2+-activated force on relaxation rate
With regard to the effect of relative force or [Ca2+]free on the rate of relaxation, previous studies have reported conflicting results. The rate of relaxation induced by diazo-2 was slower when the pre-photolysis force or [Ca2+]free was increased in frog or rabbit fast-twitch skeletal muscle (Wahr & Rall, 1998; Patel et al. 1998). In contrast, the rate of relaxation was little affected when [Ca2+]free was decreased by rapid solution change in single frog or rabbit fast-twitch myofibrils (Tesi et al. 2002). The present results indicate that the pre-photolysis force or [Ca2+]free dependence of relaxation rate in the diazo-2 experiments can be eliminated by maintaining the diazo-2 chelating capacity constant. Thus, the current results are in agreement with those of Tesi et al. (2002). This finding can be readily interpreted with a two-state model (Brenner, 1988), in which the transitions between detached, non-force-generating crossbridges and strong, force-generating crossbridges are simplified as first-order reactions with attachment (fapp) and detachment (gapp) rate constants, respectively. fapp is thought to be a function of [Ca2+], but gapp is not. If skeletal muscle relaxation is dominated by gapp, then the rate of relaxation should not exhibit a strong Ca2+ dependence. In addition, in skeletal muscle, Ca2+ binding to TnC does not show a strong dependence on the attachment of cycling crossbridges (Li & Fajer; 1998; Martyn et al. 1999), although an earlier study showed that the Ca2+ affinity of skeletal TnC increased as the number of bound crossbridges increased (Guth & Potter, 1987). Consistent with these suggestions, we conclude that the rate of diazo-2-induced relaxation is independent of pre-photolysis force or [Ca2+]free at constant diazo-2 chelating capacity.
Effect of altered Ca2+ dissociation from TnC on relaxation rate
The finding that decreasing the Ca2+ dissociation rate from TnC with M82Q TnC slowed relaxation both under control conditions and when low [Pi] slowed relaxation provides the first direct evidence that Ca2+ dissociation from TnC can dramatically influence the kinetics of skeletal muscle relaxation. However, increasing the Ca2+ off-rate from TnC with NHdel did not significantly speed up relaxation in either control or after low [Pi] slowed relaxation. One possible explanation for this negative result is that the observed increase in the rate of dissociation of Ca2+ from NHdel TnC in solution was only ≈1.5-fold, whereas the decrease for M82Q TnC was substantially larger at ≈4.5-fold. Another possibility is that the Ca2+ dissociation rate from TnC and crossbridge detachment kinetics may be tuned close to each other to limit the rate of relaxation in skeletal muscle fibres. In this case, the accelerating effect on relaxation of the increased Ca2+ dissociation rate from NHdel TnC, if any, would be dampened by the rate limit of crossbridge detachment kinetics. Results from simulations of the data with a two-step sequential model of relaxation are consistent with this conclusion. However, studies have shown that cardiac TnI phosphorylation with β-adrenergic stimulation accelerates relaxation, presumably through the effect of increasing the rate of Ca2+ dissociation from TnC (Zhang et al. 1995, Fentzke et al. 1999). Thus, if this is the case, it may point out important differences in TnC Ca2+ binding between skeletal and cardiac muscle.
Effect of altered crossbridge kinetics on relaxation rate
The present finding that low [Pi] slowed relaxation is in agreement with the results of Nencini et al. (2000) but in disagreement with the results of Mulligan et al. (1999). Nencini et al. (2000) found that decreased [Pi] with the same Pi scavenger as used in the present study prolonged and slowed the linear phase but did not affect the fast exponential phase of relaxation in skinned single myofibrils from rabbit psoas muscle using fast solution exchange to rapidly lower [Ca2+]free. In contrast, Mulligan et al. (1999) showed that reducing [Pi] using a different Pi scavenger, sucrose phosphorylase + sucrose, did not significantly affect the slow phase but sped up the fast phase of relaxation induced by photolysis of diazo-2 at constant total [diazo-2] = 2 mm in frog skinned fast-twitch skeletal fibres. The discrepancy between the results obtained in the present study and those reported by Mulligan et al. (1999) may be explained by differences in experimental conditions, such as the different animals, different temperatures and different Pi scavengers used. The Michaelis constant for Pi is in the micromolar range for the purine NP, but in the millimolar range for sucrose phosphorylase (Mieyal & Abeles, 1972; Brune et al. 1994). Thus, the purine NP is able to buffer the intrafibre [Pi] generated from the fibre ATPase to submicromolar concentrations, but sucrose phosphorylase can only reduce [Pi] to the level of hundreds of micromolars. Another possible explanation may be related to the diazo-2 chelating capacity. As shown in the present study, chelating capacity is an important controlling factor in diazo-2-induced relaxation. The observation of an accelerating or slowing effect on relaxation with the Pi scavenger could be related to mismatches in the diazo-2 chelating capacity. It is possible that reducing [Pi] (to ≈100 μm) led to an increase in the diazo-2 chelating capacity at constant total [diazo-2], due to a leftward shift in the force versus pCa relationship, which would consequently cause the acceleration of relaxation as observed by Mulligan et al. (1999). However, a larger reduction in [Pi] (to ≈5 μm) might have a more dramatic effect on relaxation, which would cause a slower relaxation, as observed in this study. Since the influences of chelating capacity and relative force have been ruled out in this study, the slowing of relaxation in low [Pi] must be a real effect.
The observation of slower relaxation in low [Pi] solution has a significant implication for the possible pathway undergone by crossbridges during muscle relaxation. After diazo-2 photolysis, fibres could undergo relaxation via forward crossbridge transitions through the Pi release step, as in contracting muscle (forward path), or alternatively, the force-bearing crossbridges could relax by a reversal of the crossbridge transitions involving the Pi association step (backward path). If a forward path was predominant, then reducing [Pi], in favour of Pi release, would speed up relaxation. Otherwise, low [Pi] would be expected to slow relaxation by slowing the Pi association step, if a backward path is involved. The present observation of the slowing of relaxation in low [Pi] suggests that relaxation may undergo through a backward path in fast-twitch skeletal muscle. In conclusion, low [Pi] slows the kinetics of crossbridge detachment and in turn slows the rate of relaxation in skeletal muscle.
In summary, the rate of diazo-2-induced relaxation in rabbit skinned psoas fibres increases as the diazo-2 chelating capacity is increased, but is independent of pre- and post-photolysis Ca2+-activated force at constant diazo-2 chelating capacity. These data are consistent with the working hypothesis that the kinetics of relaxation in fast-twitch skeletal muscle can be modulated by the rate of Ca2+ dissociation from TnC and the kinetics of crossbridge detachment. More direct evidence comes from the observations that decreasing the rate of Ca2+ dissociation from TnC and the kinetics of crossbridge detachment either independently or simultaneously can dramatically slow relaxation. Thus, the present results suggest that both processes of Ca2+ dissociation from TnC and crossbridge detachment can dramatically affect the rate of relaxation in fast-twitch skeletal muscle.
Acknowledgments
This paper is dedicated to the memory of our friend and co-investigator, Dr J. David Johnson (1949-2000). We thank Eric Rennie for contributions to the early experiments. The authors also thank C. Poggesi and C. Tesi for sharing methodological details and a manuscript with us before publication. This work was supported by US NIH AR-20792 to J.A.R., The Canadian Institute of Health Research to L.B.S. and an AHA postdoctoral fellowship to J.P.D.
References
- Adams SR, Kao JPY, Tsien RY. Biological useful chelators that take up Ca2+ upon illumination. Journal of the American Chemical Society. 1989;111:7957–7968. [Google Scholar]
- Black DJ, Tikunova SB, Johnson JD, Davis JP. Acid pairs increase the N-terminal Ca2+ affinity of CaM by increasing the rate of Ca2+ association. Biochemistry. 2000;39:13831–13837. doi: 10.1021/bi001106+. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M, Hunter JL, Corrie ET, Webb MR. Direct, real time measurements of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry. 1994;33:8262–8271. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- Chandra M, Da Silva EF, Sorenson MM, Ferro JA, Pearlstone JR, Nash BE, Borgford T, Kay CM, Smillie LB. The effects of N helix deletion and mutant F29W on the Ca2+ binding and functional properties of chicken skeletal muscle troponin C. Journal of Biological Chemistry. 1994;269:14988–14994. [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophysical Journal. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentration in aqueous solutions containing multiple metals and ligands. In: Fleischer S, Fleischer B, editors. Methods in Enzymology, Biomembranes. New York: Academic Press; 1988. pp. 157pp. 378–417. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. Journal of Physiology. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth K, Potter JD. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. Journal of Biological Chemistry. 1987;262:13627–13635. [PubMed] [Google Scholar]
- Hou T, Johnson JD, Rall JA. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. Journal of Physiology. 1991;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns EC, Simnett SJ, Mulligan IP, Ashley CC. Troponin I phosphorylation does not increase the rate of relaxation following laser flash photolysis of diazo-2 in guinea-pig skinned trabeculae. Pflügers Archiv. 1997;433:842–844. doi: 10.1007/s004240050353. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Nakkula RJ, Vasulka C, Smillie LB. Modulation of Ca2+ exchange with the Ca2+-specific regulatory sites of troponin C. Journal of Biological Chemistry. 1994;269:8919–8923. [PubMed] [Google Scholar]
- Lannergren J, Arner A. Relaxation rate of intact striated muscle fibers after flash photolysis of a caged calcium chelator (diazo-2) Journal of Muscle Research and Cell Motility. 1992;13:630–634. doi: 10.1007/BF01738252. [DOI] [PubMed] [Google Scholar]
- Li HC, Fajer PG. Structural coupling of troponin C and actomyosin in muscle fibers. Biochemistry. 1998;37:6628–6635. doi: 10.1021/bi972062g. [DOI] [PubMed] [Google Scholar]
- Martyn DA, Freitag CJ, Chase PB, Gordon AM. Ca2+ and cross-bridge-induced changes in troponin C in skinned skeletal muscle fibers: effects of force inhibition. Biophysical Journal. 1999;76:1480–1493. doi: 10.1016/S0006-3495(99)77308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Journal of General Physiology. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieyal JJ, Abeles RH. Disaccharide Phosphorylases The Enzymes. Vol. 7. New York: Academic Press; 1972. pp. 515–532. [Google Scholar]
- Millar NC, Homsher E. The effects of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. Journal of Biological Chemistry. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Mulligan IP, Palmer RE, Lipscomb S, Hoskins B, Ashley CC. The effect of phosphate on the relaxation of frog skeletal muscle. Pflügers Archiv. 1999;437:393–399. doi: 10.1007/s004240050793. [DOI] [PubMed] [Google Scholar]
- Nencini S, Colomo F, Piroddi N, Tesi C, Poggesi C. The effect of phosphate on the relaxation of single myofibrils from fast and slow skeletal muscle. Biophysical Journal. 2000;78:A791. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Franks-Skiba K, Cooke R. Depletion of phosphate in active muscle fibers probes actomyosin states within the powerstroke. Biophysical Journal. 1998;74:369–380. doi: 10.1016/S0006-3495(98)77794-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Diffee GM, Huang XP, Moss RL. Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophysical Journal. 1998;74:360–368. doi: 10.1016/S0006-3495(98)77793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstone JR, Borgford T, Chandra M, Oilawa K, Kay CM, Herzberg O, Moult J, Herklotz A, Reinach FC, Smillie LB. Construction and characterization of a spectral probe mutant of troponin C: application to analyses of mutants with increased Ca2+ affinity. Biochemistry. 1992;31:6545–6553. doi: 10.1021/bi00143a026. [DOI] [PubMed] [Google Scholar]
- Regnier M, Rivera AJ, Chase PB, Smillie LB, Sorenson MM. Regulation of skeletal muscle tension redevelopment by troponin C constructs with different Ca2+ affinities. Biophysical Journal. 1999;76:2664–2672. doi: 10.1016/S0006-3495(99)77418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. Journal of Biological Chemistry. 1982;257:260–263. [PubMed] [Google Scholar]
- Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. The effects of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophysical Journal. 2000;78:3081–3092. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesi C, Piroddi N, Colomo F, Poggesi C. Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle. Biophysical Journal. 2002;83:2142–2151. doi: 10.1016/S0006-3495(02)73974-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahr PA, Johnson JD, Rall JA. Determinants of relaxation rate in skinned frog skeletal muscle fibers. American Journal of Physiology. 1998;274:C1608–1615. doi: 10.1152/ajpcell.1998.274.6.C1608. [DOI] [PubMed] [Google Scholar]
- Wahr PA, Rall JA. Role of calcium and cross bridges in determining rate of force development in frog muscle fibers. American Journal of Physiology. 1997;272:C1664–1671. doi: 10.1152/ajpcell.1997.272.5.C1664. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increased the rate of cardiac muscle relaxation. Circulation Research. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]


