Abstract
The contributions of HCO3−-dependent, DIDS-sensitive mechanisms to the maintenance of steady-state pHi, and the regulation of their activities by cAMP-dependent protein kinase (PKA), were investigated in CA1 neurons with the H+-sensitive fluorophore, BCECF. The addition of HCO3−/CO2 to neurons with ‘low’ (pHi ≤ 7.20) and ‘high’ (pHi > 7.20) initial pHi values under Hepes-buffered conditions, increased and decreased steady-state pHi, respectively. Conversely, under HCO3−/CO2-buffered conditions, DIDS caused pHi to decrease and increase in neurons with low and high initial pHi values, respectively. In the presence, but not the absence, of HCO3−, the PKA inhibitor Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS; 50 μm) evoked DIDS-sensitive increases and decreases in pHi in neurons with low and high initial pHi values, respectively. In contrast, in neurons with low initial pHi values, activation of PKA with the Sp isomer of cAMPS (Sp-cAMPS; 25 μm) elicited increases in pHi that were smaller in the presence than in the absence of HCO3−, whereas in neurons with high initial pHi values, Sp-cAMPS-evoked rises in pHi were larger in the presence than in the absence of HCO3−; the differences between the effects of Sp-cAMPS on pHi under the different buffering conditions were attenuated by DIDS. Consistent with the possibility that changes in the activities of HCO3−-dependent, DIDS-sensitive mechanisms contribute to the steady-state pHi changes evoked by the PKA modulators, in neurons with initial pHi values ≤ 7.20, Rp-cAMPS concurrently inhibited Na+-independent Cl−-HCO3− exchange and stimulated Na+-dependent Cl−-HCO3− exchange; in contrast, Sp-cAMPS concurrently stimulated Na+-independent Cl−-HCO3− exchange and inhibited Na+-dependent Cl−-HCO3− exchange. Data from a limited number of neurons with initial pHi values > 7.20 suggested that the directions of the reciprocal changes in anion exchange activities (inhibition or stimulation) evoked by Rp- and Sp-cAMPS may be opposite in cells with low vs. high resting pHi values. Taken together, the results indicate that the effects of modulating PKA activity on steady-state pHi in rat CA1 neurons under HCO3−/CO2-buffered conditions reflect not only changes in Na+-H+ exchange activity but also changes in Na+-dependent and Na+-independent Cl−-HCO3− exchange activity that, in turn, may be dependent upon the initial pHi.
Intracellular pH (pHi) is an important determinant of neuronal function and yet, compared to non-neuronal cell types, relatively little information is available concerning either the mechanisms that act to regulate pHi in mammalian central neurons or the factors that control their activities. In rat hippocampal neurons, the majority of studies have focused on the participation of Na+-H+ exchange in acid extrusion and the maintenance of steady-state pHi; these studies have also established that Na+-H+ exchange activity can be modulated by pHi, pHo, temperature, intracellular second messengers and pathophysiological events such as anoxia (Raley-Susman et al. 1991; Baxter & Church, 1996; Bevensee et al. 1996; Smith et al. 1998; Diarra et al. 1999; Sheldon & Church, 2002). By comparison, relatively little is known about the roles of HCO3−-dependent mechanisms in pHi regulation in this cell type. Although Na+-dependent and Na+-independent Cl−-HCO3− exchangers have been identified in rat CA1 neurons (Kopito et al. 1989; Raley-Susman et al. 1993; Grichtchenko et al. 2001b), and the former has been shown to contribute to DIDS-sensitive acid extrusion (Schwiening & Boron, 1994; Baxter & Church, 1996), neither their roles in the maintenance of steady-state pHi nor the possibility that their activities might be regulated by second messengers have been systematically addressed. The paucity of information available concerning both the function and the regulation of the activities of Cl−-HCO3− exchangers in mammalian central neurons is surprising, not only because they are important determinants of pHi in other nucleated cell types (Roos & Boron, 1981; Vaughan-Jones, 1986; Olsnes et al. 1987; Boyarsky et al. 1988; Cassel et al. 1988; Ganz et al. 1989; Kikeri et al. 1990; Tønnessen et al. 1990; Kramhøft et al. 1994; Strazzabosco et al. 1997; Leem et al. 1999) but also because they play important roles in morphogenesis, cytoskeletal reorganization and other cellular properties (e.g. Phillips & Baltz, 1999; Schwab, 2001). It has also been established that the activities of Cl−-HCO3− exchangers in a variety of cell types can be regulated by intracellular second messengers, with consequent effects on cellular function (e.g. Boron et al. 1978; Reuss, 1987; Vigne et al. 1988; Green et al. 1990; Ludt et al. 1991; Pucéat et al. 1998).
In the present study, we initially assessed the contribution of HCO3−-dependent, DIDS-sensitive mechanisms to the maintenance of steady-state pHi in our preparation of acutely dissociated adult rat hippocampal CA1 neurons (see Schwiening & Boron, 1994; Bevensee et al. 1996). Next, in the knowledge that activation of cAMP-dependent protein kinase (PKA) under nominally HCO3−/CO2-free, Hepes-buffered conditions increases pHi in rat CA1 neurons by stimulating Na+-H+ exchange (Smith et al. 1998), we asked whether PKA is involved in the control of pHi under physiological conditions, that is in the presence of HCO3−/CO2. In light of the results, which indicated that the modulation of PKA activity leads to HCO3−-dependent, DIDS-sensitive changes in steady-state pHi, we examined the regulation by PKA of the activities of the Na+-dependent and Na+-independent Cl−-HCO3− exchangers which contribute to acid and alkali extrusion, respectively, in rat CA1 neurons.
Portions of this work have been presented in abstract form (Brett & Church, 1998; Kelly et al. 2000).
Methods
Cell preparation
All procedures conformed to guidelines established by the Canadian Council on Animal Care and were approved by The University of British Columbia Animal Care Committee.
Acutely dissociated CA1 neurons were prepared as previously described (Smith et al. 1998). In brief, male Wistar rats (200-260 g) were anaesthetized with 3 % halothane in air and rapidly decapitated. Transverse hippocampal slices (450 μm) were cut and allowed to recover for 1 h in HCO3−/CO2-buffered saline (see below). To isolate CA1 neurons, slices were enzymatically digested for 30 min in HCO3−/CO2-buffered saline containing 1.5 mg ml−1 protease type XIV (Sigma-Aldrich Canada Ltd, Oakville, ON, Canada); the CA1 regions were then microdissected and triturated with fire-polished Pasteur pipettes of diminishing tip diameters. The triturated suspension was deposited onto a glass coverslip mounted in a perfusion chamber to form the floor of the chamber and neurons were allowed to adhere for 30 min, during which time they were loaded with fluorophore (see below).
Solutions and chemicals
The standard nominally HCO3−/CO2-free perfusion medium contained (mm): NaCl 136.5, KCl 3, CaCl2 2, NaH2PO4 1.5, MgSO4 1.5, d-glucose 17.5 and Hepes 10; and was titrated to the appropriate temperature-corrected pH with 10 m NaOH. In standard HCO3−/CO2-buffered media, Hepes was isosmotically replaced by NaCl and solutions contained either 19.5 mm (37 °C) or 29 mm (room temperature, RT; 20-22 °C) NaHCO3, by equimolar substitution for NaCl, together with the constituents listed above; pH measured in the recording chamber was 7.35 after equilibration with 5 % CO2-95 % O2. Solutions containing 20 mm NH4Cl were prepared by equimolar substitution for NaCl. For Na+-free HCO3−/CO2-buffered media, NaH2PO4 was omitted and Na+ salts were replaced with the appropriate choline salts. When external Cl− was omitted, it was replaced isosmotically with gluconate. The Na+-free, Cl−-free HCO3−/CO2-buffered medium contained (mm): choline base (aqueous) 127, choline HCO3− 19.5, d-gluconic acid 127, potassium gluconate 3, hemicalcium gluconate 4, MgSO4 1.5 and d-glucose 17.5; pH 7.35 after equilibration with 5 % CO2-95 % O2. The HCO3−/CO2 solutions employed to impose internal alkali loads contained twice the [NaHCO3] as standard HCO3−/CO2-buffered media, by isosmotic substitution for NaCl, and were equilibrated with 10 % CO2-90 % O2 (pH 7.35).
Test compounds were obtained from Sigma-Aldrich Canada Ltd with the exceptions of 2′,5′-dideoxyadenosine (DDA; Biomol Research Laboratories Inc., Plymouth Meeting, PA, USA) and the Sp- and Rp- isomers of adenosine-3′,5′-cyclic monophosphorothioate (Sp- and Rp-cAMPS, respectively; Biolog Life Science Institute, La Jolla, CA, USA). Test compounds were applied by superfusion. Stock solutions of DIDS were prepared on the day of the experiment, immediately prior to dilution with experimental media to the working concentration (200 μm).
Recording techniques
Neurons were loaded with the acetoxymethyl ester form of 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF, 2 μm for 15 min; Molecular Probes Inc., Eugene, OR, USA) and were then superfused at 2 ml min−1 for 15 min with the initial experimental solution at the appropriate experimental temperature prior to the start of an experiment. Experiments were performed at 37 °C unless otherwise noted in the text.
The dual-excitation ratio method was used to estimate pHi, employing a digital fluorescence ratio imaging system (Atto Instruments Inc., Rockville, MD, USA). Details of the methods employed have been presented previously (Baxter & Church, 1996; Smith et al. 1998; Sheldon & Church, 2002). In brief, fluorescence emissions measured at 520 nm were detected by an intensified charge-coupled device camera and collected from regions of interest placed on individual neuronal somata. Raw emission intensity data at each excitation wavelength (488 and 452 nm) were corrected for background fluorescence prior to calculation of the ratio. Ratio pairs were acquired at 1-12 s intervals. Analysis was restricted to those neurons able to retain BCECF, as judged by the stability of the fluorescence emission intensity recorded during excitation at 452 nm, throughout the course of an experiment (see Bevensee et al. 1996). To reduce photobleaching of the fluorophore and cell damage, the output of the 100 W mercury arc lamp was attenuated electronically, neutral density filters were placed in the light path and a high-speed shutter was employed to limit light exposure to periods required for data acquisition.
The one point high-[K+]/nigericin technique was employed to convert background-corrected BCECF emission intensity ratios (BI488/BI452) into pHi values (see Boyarsky et al. 1996). Parameters employed in the calculation of pHi values were derived from non-linear least squares regression fits to background-subtracted ratio vs. pH data which, in turn, were obtained in full calibration experiments (see Baxter & Church, 1996). For the seventeen full calibration experiments utilized in analysing all data, the mean values for Rn,max (the maximum obtainable value for the normalized ratio), Rn,min (the minimum obtainable value for the normalized ratio) and pKa (the -log of the dissociation constant of BCECF) were (means ± s.e.m.) 1.88 ± 0.04, 0.41 ± 0.02 and 7.16 ± 0.02, respectively. These values were not dependent on the temperature at which the calibration was conducted. To limit cross-contamination by nigericin, perfusion lines were replaced and the imaging chamber was decontaminated after each experiment by soaking in ethanol and then in 20 % Decon 75 (BDH Inc., Toronto, ON, Canada), as described by Leem et al. (1999).
Experimental procedures and data analysis
The effects of changes in perfusate composition and pharmacological treatments were examined on steady-state pHi and/or rates of pHi recovery from internal acid loads (imposed by the NH4+ prepulse technique) or alkaline loads (imposed by exposure to and subsequent removal of medium containing high concentrations of HCO3− and CO2 at a constant pHo).
As detailed in the Results, the effects of modulating PKA activity on steady-state pHi and Cl−-HCO3− exchanger activities were dependent on the initial pHi of a neuron prior to a test treatment (also see Bevensee et al. 1996). In any given series of experiments, neurons were classified as ‘low’ (pHi≤7.20) or ‘high’ (pHi > 7.20) pHi neurons on the basis of a least squares regression fit to data points relating the changes in pHi evoked on the transition from a Hepes- to a HCO3−/CO2-buffered medium at a constant pHo (7.35) to the initial pHi values under Hepes-buffered conditions (Fig. 1B; also see Schwiening & Boron, 1994; Smith et al. 1998).
Figure 1. Effects on pHi of the transition from Hepes- to HCO3−/CO2-buffered medium, and the addition of DIDS to HCO3−/CO2-buffered medium.
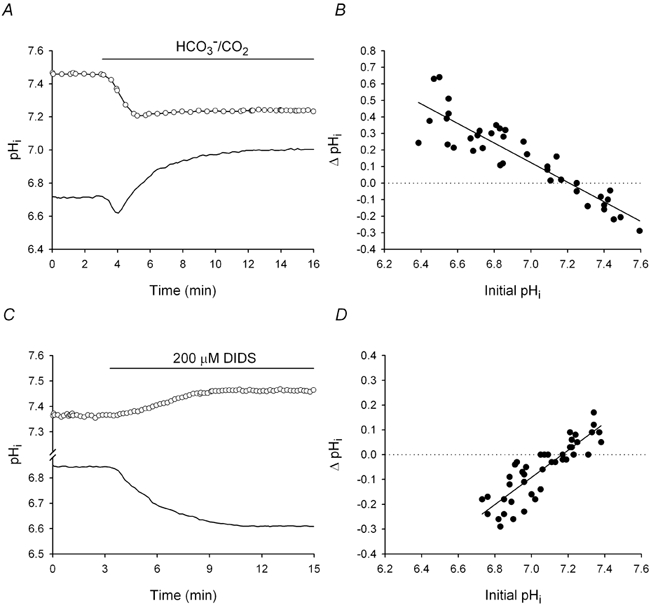
A, a neuron perfused with a Hepes-buffered medium (pH 7.35) had a low initial pHi (continuous line). Upon exposure to HCO3−/CO2-buffered medium at a constant pHo, pHi decreased transiently and then increased to a new steady-state level. In contrast, a different neuron with a high initial pHi in Hepes-buffered medium (○) exhibited an internal acidification upon exposure to HCO3−/CO2. B, the changes in pHi (ΔpHi) evoked by the addition of HCO3−/CO2 plotted against initial pHi values in Hepes-buffered medium (n = 39). A linear least squares regression fit to the data points (r2= 0.84) had a negative slope and intersected the abscissa at pHi 7.21. C, in a neuron with a low initial pHi value under HCO3−/CO2-buffered conditions at pHo 7.35 (continuous line), DIDS caused pHi to decrease to a new steady-state level. In contrast, DIDS caused pHi to rise in a different neuron with a high initial pHi (○). D, ΔpHi elicited by 200 μm DIDS applied under HCO3−/CO2-buffered conditions plotted against initial pHi values (n = 44). A linear least squares regression fit to the data points (r2= 0.75) had a similar x-intercept (pHi 7.17) but an opposite slope to the fit representing the pHi response to HCO3−/CO2 application.
In each experiment in which rates of pHi recovery were examined, two consecutive acid or alkali loads were imposed, the first being employed to calculate control rates of pHi recovery for a given neuron and the second being performed under the influence of a test treatment. The consistency of rates of pHi recovery following two consecutive acid or alkali loads imposed in the absence of a test treatment was established in control experiments (data not shown; see Smith et al. 1998; Sheldon & Church, 2002). Full details of the methods employed in analysing data obtained in acid load recovery experiments have been presented previously (Baxter & Church, 1996; Smith et al. 1998). In brief, the recovery of pHi following an NH4+ prepulse was fitted to a single exponential function and the first derivative of this function was used to determine the rate of pHi change (dpHi/dt). Instantaneous rates of pHi recovery under control and test conditions were then plotted against absolute pHi values, compared statistically at corresponding values of pHi, and the data points were fitted by weighted non-linear least squares regression (r2 ≥ 0.90 in all cases). In addition, at each corresponding absolute value of pHi, the percentage difference between the control rate of pHi recovery and the rate of pHi recovery under the influence of a test treatment was calculated and the mean of the resultant differences was employed to describe the overall effect of a test treatment on the rate of pHi recovery. Similar procedures were employed to quantify rates of pHi recovery from internal alkali loads, except that the recovery was fitted to a single exponential decay function; analysis was then identical to the procedures used to analyse rates of pHi recovery from acid loads.
Data are reported as means ± s.e.m., with the accompanying n value referring to the number of neurons from which data were obtained. Statistical comparisons were performed with Student's two-tailed t tests (paired or unpaired, as appropriate); significance was assumed at the 5 % level.
Results
Contribution of HCO3−-dependent, DIDS-sensitive mechanisms to the maintenance of pHi
Initially, we examined the effects on pHi of exposing neurons originally perfused with Hepes-buffered medium to a solution buffered with HCO3−/CO2 at a constant pHo (7.35). As previously described (Schwiening & Boron, 1994; Smith et al. 1998), switching to a HCO3−/CO2- containing medium caused pHi to increase and decrease in neurons with low (≤7.20; n = 28) and high (> 7.20; n = 11) initial pHi values, respectively, in Hepes-buffered medium (Fig. 1A and B). The increases and decreases in pHi occasioned by the addition of HCO3− in neurons with low and high pHi values, respectively, under nominally HCO3−-free conditions are consistent with the shift from a bimodal to a unimodal distribution of steady-state pHi values that occurs in rat CA1 neurons upon exposure to HCO3−/CO2 (see Bevensee et al. 1996; Smith et al. 1998). They are also consistent with previous reports (Raley-Susman et al. 1993; Schwiening & Boron, 1994; Baxter & Church, 1996) that Na+-independent and Na+-dependent Cl−-HCO3− exchange contribute to base and acid extrusion, respectively, from rat hippocampal neurons. The former transport mechanism is most active at high pHi values whereas the latter is most active at low pHi values and, in many cell types, the two exchangers act in concert to determine steady-state pHi (e.g. Olsnes et al. 1987; Boyarsky et al. 1988; Cassel et al. 1988; Ganz et al. 1989; Green et al. 1990; Kikeri et al. 1990; Mugharbil et al. 1990; Tønnessen et al. 1990; Kramhøft et al. 1994; Leem et al. 1999). The fact that, depending on the initial pHi in Hepes-buffered medium, pHi could increase or decrease upon exposure to HCO3−, suggested the possibilities that activation of Na+-independent Cl−-HCO3− exchange might contribute to the HCO3−-induced fall in pHi in cells which, in Hepes, had high resting pHi values, and that activation of Na+-dependent Cl−-HCO3− exchange might contribute to the HCO3−-induced rise in pHi in cells which, in Hepes, had low resting pHi values. In this regard, we (Baxter & Church, 1996) and others (Schwiening & Boron, 1994) have failed to uncover a contribution from Na+-HCO3− cotransport to the regulation of pHi in rat CA1 neuron somata (also see Schmitt et al. 2000).
Both Na+-dependent and Na+-independent Cl−-HCO3− exchangers are typically sensitive to DIDS. We have shown previously that DIDS fails to affect steady-state pHi in rat CA1 neurons under nominally HCO3−/CO2-free, Hepes-buffered conditions (Smith et al. 1998). However, applied in the presence of HCO3−/CO2, DIDS elicited decreases and increases in pHi in neurons with low (n = 26) and high (n = 11) initial pHi values, respectively (Fig. 1C); in seven additional cells with pHi values in the range 7.05-7.31, DIDS failed to affect steady-state pHi. The DIDS-evoked changes in pHi were plotted against the pHi values measured prior to its addition and a least squares fit to the data points had a positive slope and an x-intercept at pHi 7.17 (Fig. 1D), which is similar to that obtained from the regression line relating the changes in pHi evoked by the addition of HCO3− to initial pHi values under Hepes-buffered conditions (pHi 7.21; Fig. 1B). Thus, the addition of DIDS under HCO3−/CO2-buffered conditions elicited qualitatively opposite changes in pHi to those seen upon the transition from a Hepes- to a HCO3−/CO2-buffered medium at a constant pHo.
Taken together, the findings are consistent with previous reports (Schwiening & Boron, 1994; Baxter & Church, 1996; Bevensee et al. 1996; Smith et al. 1998) that HCO3−-dependent, DIDS-sensitive mechanism(s) contribute to the regulation of pHi in rat hippocampal neurons.
Effect of modulating PKA activity on pHi under HCO3−/CO2-buffered conditions
In rat CA1 neurons under nominally HCO3−/CO2-free conditions at 37 °C, activation of the cAMP/PKA second messenger pathway produces an alkaline shift in the pHi dependence of Na+-H+ exchange and thereby increases steady-state pHi; in contrast, inhibiting the pathway fails to affect either Na+-H+ exchange activity or steady-state pHi (Smith et al. 1998). In light of the above findings, we therefore examined whether modulating the activity of the cAMP/PKA pathway evokes DIDS-sensitive changes in pHi in the presence of HCO3−/CO2.
In agreement with the results of Smith et al. (1998), inhibiting PKA with 50 μm Rp-cAMPS under Hepes-buffered conditions failed to affect pHi in 10 and seven neurons with initial pHi values ≤ 7.20 and > 7.20, respectively (data not shown). In contrast, under HCO3−/CO2-buffered conditions, Rp-cAMPS elicited changes in pHi that were dependent on the resting pHi prior to the modulation of PKA activity. Thus, as illustrated in Fig. 2A, Rp-cAMPS increased pHi in neurons with low initial pHi values and decreased pHi in neurons with high initial pHi values. When the Rp-cAMPS-evoked changes in pHi were plotted against initial pHi values (Fig. 2B), the regression fit to the data points had a negative slope and a similar x-intercept (pHi 7.26) as the fit representing the change in pHi that occurred on the transition from a Hepes- to a HCO3−/CO2-buffered medium (Fig. 1B). The changes in pHi evoked by Rp-cAMPS in the presence of HCO3− were attenuated by pre-treatment with DIDS (Fig. 2B) and were mimicked by the adenylate cyclase inhibitor DDA (100 μm; n = 11; data not shown). Next, the effects of activating PKA were examined. As illustrated in Fig. 2C, under Hepes-buffered conditions 25 μm Sp-cAMPS elicited a 0.21 ± 0.03 (n = 8) pH unit increase in pHi in neurons with initial pHi values ≤ 7.20, which is in agreement with the findings of Smith et al. (1998). However in the presence of HCO3−, 25 μm Sp-cAMPS caused a 0.11 ± 0.01 pH unit increase in pHi in nine neurons also with low initial pHi values (P < 0.02 for the difference to the change in pHi observed in the absence of HCO3−). The effect of HCO3− to decrease the magnitude of the rise in pHi evoked by Sp-cAMPS in low pHi neurons was inhibited by DIDS; in six neurons pre-treated with DIDS for 10-15 min, 25 μm Sp-cAMPS evoked a larger increase in pHi (0.23 ± 0.03 pH units) than observed in the absence of the stilbene (P < 0.01). In contrast to results obtained in low pHi neurons, the rise in pHi evoked by 25 μm Sp-cAMPS in cells with initial pHi values > 7.20 was smaller in the absence (a 0.10 ± 0.02 pH unit increase; n = 7) than in the presence (a 0.26 ± 0.02 pH unit increase; n = 6) of HCO3−/CO2 (P < 0.02; Fig. 2C). These data are summarized in Fig. 2D, which illustrates that Sp-cAMPS tended to evoke larger rises in pHi under Hepes- than under HCO3−/CO2-buffered conditions in neurons with initial pHi values ≤7.20 whereas, in neurons with initial pHi values > 7.20, the opposite was true. The effects of Sp-cAMPS on steady-state pHi were mimicked by the adenylate cyclase activator forskolin (25 μm; n = 11 and 23 under HCO3−-containing and nominally HCO3−-free conditions, respectively) whereas an inactive analogue of forskolin, 1′,9′-dideoxyforskolin (25 μm; n = 6 in each case), failed to influence pHi (data not shown; also see Smith et al. 1998).
Figure 2. Effects of Rp- and Sp-cAMPS on pHi.
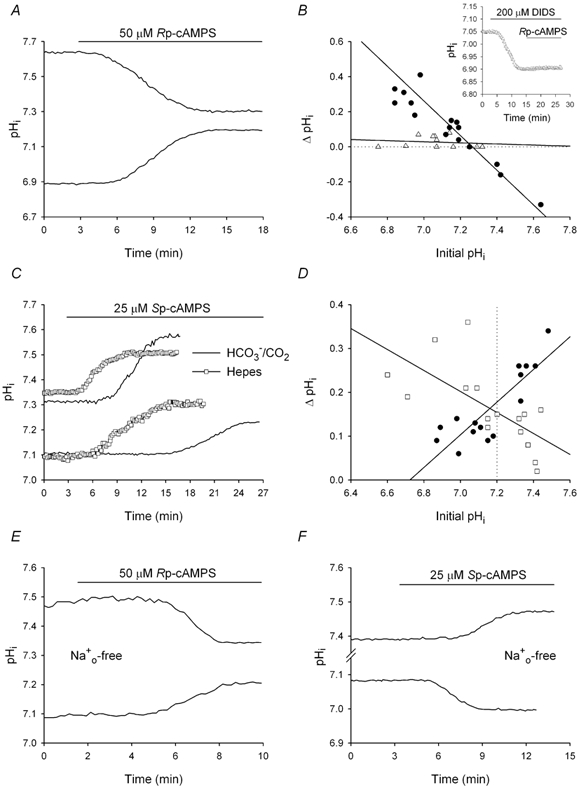
A, Rp-cAMPS, applied under HCO3−/CO2-buffered conditions (pHo 7.35) for the period indicated by the bar above the traces, evoked increases and decreases in pHi in two different neurons with low and high initial pHi values, respectively, prior to the addition of the PKA inhibitor. B, the changes in pHi (ΔpHi) elicited by 50 μm Rp-cAMPS under HCO3−/CO2-buffered control conditions (•) or following 10-15 min pre-treatment with 200 μm DIDS (▵) were plotted against pHi values measured prior to the addition of the PKA inhibitor and each set of data points was fitted by least squares regression (r2≥ 0.80). The changes in pHi evoked by Rp-cAMPS were dependent upon the initial pHi and, as illustrated in the inset, were attenuated by DIDS. C, four different neurons, two with similarly low initial pHi values and two with similarly high initial pHi values, were exposed to 25 μm Sp-cAMPS under HCO3−/CO2- (continuous line) or Hepes- (□) buffered conditions. In neurons with initial pHi values ≤ 7.20, Sp-cAMPS evoked a greater increase in pHi under Hepes- than under HCO3−/CO2-buffered conditions; in contrast, in neurons with initial pHi values > 7.20, Sp-cAMPS evoked a smaller increase in pHi under Hepes- than under HCO3−/CO2-buffered conditions. D, ΔpHi elicited by 25 μm Sp-cAMPS in the nominal absence (□) or presence (•) of HCO3−/CO2 were plotted against initial values of pHi and a regression line was fitted to each set of data points. The vertical dotted line represents the division between ‘low’ (initial pHi≤ 7.20) and ‘high’ (initial pHi > 7.20) pHi neurons. E, the same experiment as that shown in A, but conducted in the absence of external Na+. Rp-cAMPS evoked an increase and a decrease in pHi in two different neurons with low and high initial pHi values, respectively. F, under Na+o-free, HCO3−-buffered conditions, the addition of 25 μm Sp-cAMPS caused pHi to increase in a neuron with a high initial pHi, and pHi to decrease in a different neuron with a low initial pHi.
To further explore the effects of PKA modulators on steady-state pHi under HCO3−/CO2-buffered conditions, experiments were repeated in the absence of external Na+, under which condition forward Na+-H+ and Na+-dependent Cl−-HCO3− exchange are blocked. Similar to observations made in the presence of Na+o, 50 μm Rp-cAMPS applied under Na+o-free conditions evoked a rise in pHi of 0.15 ± 0.03 pH units in nine neurons with low initial pHi values and a fall in pHi of 0.18 ± 0.06 pH units in four neurons with high initial pHi values (Fig. 2E); these effects were abolished by pre-treatment with DIDS (n = 4, low pHi neurons and n = 3, high pHi neurons). In contrast, 25 μm Sp-cAMPS elicited a 0.07 ± 0.01 pH unit decrease in pHi in five neurons with initial pHi values ≤ 7.20 and a 0.12 ± 0.03 pH unit increase in pHi in four neurons with initial pHi values > 7.20 (Fig. 2F). Thus, the directions of the pHi changes evoked by Sp-cAMPS under Na+o-free conditions in low and high pHi neurons were opposite to and the same as those observed in the presence of Na+o, respectively (see Fig. 2C). The pHi changes evoked by Sp-cAMPS in low (n = 3) and high (n = 5) pHi cells in the absence of external Na+ were abolished by pre-treatment with DIDS. Finally, applied under Na+o- and Cl−o-free conditions (where all known acid/base transporters in rat CA1 neurons are inactive), neither 50 μm Rp-cAMPS (n = 4 low pHi neurons and n = 2 high pHi neurons) nor 25 μm Sp-cAMPS (n = 5 low pHi neurons and n = 2 high pHi neurons) significantly affected steady-state pHi.
PKA-dependent modulation of Na+-independent and Na+-dependent Cl−-HCO3− exchange
The above findings indicate that modulating the activity of the cAMP/PKA second messenger pathway leads to HCO3−-dependent, DIDS-sensitive changes in steady-state pHi, the magnitudes and/or directions of which depend on the initial pHi. Insofar as Na+-dependent and Na+-independent Cl−-HCO3− exchange are the only HCO3−-dependent, DIDS-sensitive mechanisms found to date to participate in pHi regulation in rat CA1 neurons (see Introduction), together with the fact that these exchangers are active over a wide, overlapping range of physiologically relevant pHi values (e.g. Boron et al. 1979; Olsnes et al. 1987; Boyarsky et al. 1988; Cassel et al. 1988; Kikeri et al. 1990; Tønnessen et al. 1990; Kramhøft et al. 1994; Strazzabosco et al. 1997; Leem et al. 1999), four possibilities could account for the observed effects of modulating PKA activity on steady-state pHi under HCO3−/CO2-buffered conditions (Table 1). In brief, HCO3−-dependent, DIDS-sensitive increases in pHi may in part reflect an increased rate of acid extrusion via Na+-dependent anion exchange and/or a decreased rate of acid loading via Na+-independent anion exchange. Conversely, stimulation of Na+-independent Cl−-HCO3− exchange and/or inhibition of Na+-dependent Cl−-HCO3− exchange may contribute to HCO3−-dependent, DIDS-sensitive decreases in pHi. Therefore, in the third series of experiments, we examined the effects of modulating PKA activity on Na+-independent and Na+-dependent Cl−-HCO3− exchange.
Table 1.
Changes in pHi evoked by modulating PKA activity under HCO3−/CO2-buffered conditions and potential underlying alterations in Cl−-HCO3− exchanger activities
| Initial pHi | Treatment | Observed change in pHi | Potential alteration in Cl−–HCO3− exchanger activities | ||
|---|---|---|---|---|---|
| Na+–independent | Na+–dependent | ||||
| ≤ 7.20 | Rp-cAMPS | ↑ | ↓ | and/or | ↑ |
| Sp-cAMPS | ↓* | ↑ | and/or | ↓ | |
| > 7.20 | Rp-cAMPS | ↓ | ↑ | and/or | ↓ |
| Sp-cAMPS | ↑* | ↓ | and/or | ↑ | |
Rp–cAMPS and Sp–cAMPS were applied at 50 μm and 25 μm, respectively.
Relative to the change in pHi evoked by Sp-cAMPS under nominally HCO3−/CO2– free, Hepes-buffered conditions.
Na+-independent Cl−-HCO3− exchange
Forward Na+-independent Cl−-HCO3− exchange
Consistent with the participation of Na+-independent Cl−-HCO3− exchange in the recovery of pHi from base loading in CA1 neurons, pHi recovery from internal alkali loads was inhibited by DIDS (n = 6) or under Cl−o-free conditions (n = 8) (Fig. 3A), and could proceed in the absence of Na+o (n = 7; see Fig. 3D and Fig. 4C). Alkali loads were then imposed in the absence and presence of 50 μm Rp-cAMPS or 25 μm Sp-cAMPS; all neurons in these experiments had pHi values ≤ 7.20 prior to modulating PKA activity. The overall rate of pHi recovery from alkali loads decreased ≈4.5-fold in the presence compared to the absence of Rp-cAMPS in 14 cells under Na+o-containing conditions (Fig. 3B). As shown in Fig. 3C, Rp-cAMPS reduced rates of pHi recovery (P < 0.05 at all absolute values of pHi) and shifted the pHi dependence of the rate of pHi recovery in the alkaline direction; similar effects were observed in the absence of Na+o (n = 7; Fig. 3D). In contrast to the effects of Rp-cAMPS, activation of PKA with Sp-cAMPS increased rates of pHi recovery from alkali loads under Na+o-containing (n = 9; Fig. 4A and B) and Na+o-free (n = 5; Fig. 4C) conditions.
Figure 3. Effects of Rp-cAMPS on pHi recovery from base loading in the presence and absence of Na+o.
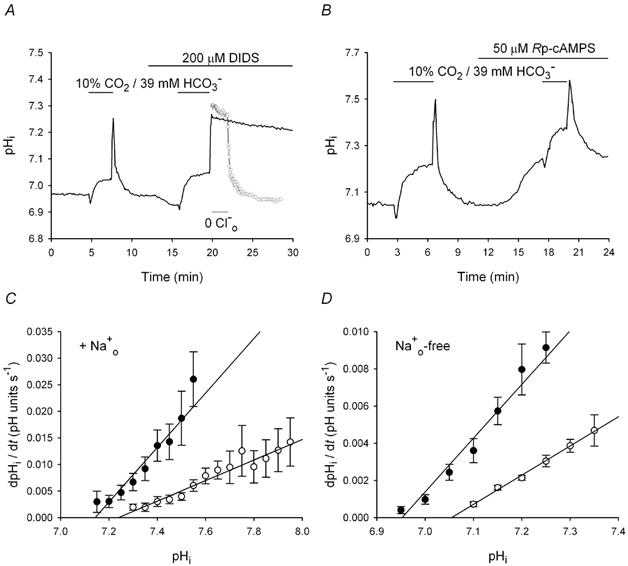
All data were obtained from neurons with initial pHi values ≤ 7.20 under HCO3−/CO2-buffered conditions. Alkali loads were imposed by applying and withdrawing a 10 % CO2/39 mm HCO3− solution, as indicated by the short bars above the traces in A and B. A, following an initial alkali load, pHi was allowed to recover and a second alkali load was imposed in the presence of DIDS (applied for the period indicated by the long bar above the trace). Superimposed is a record (○) obtained from a different cell that was exposed to Cl−-free medium (applied for the period indicated by the short bar beneath the trace) at the peak of the second alkali load (the first part of the record from this experiment has been omitted for clarity). B, after recovery from an initial alkali load imposed under control conditions, the neuron was exposed to 50 μm Rp-cAMPS. Rp-cAMPS evoked a rise in pHi (see Fig. 2A) and a second alkali load was then imposed; the rate of pHi recovery was decreased in the presence of Rp-cAMPS. C, mean rates of pHi recovery following alkali loads imposed in the absence (•) and presence (○) of 50 μm Rp-cAMPS plotted against absolute values of pHi. Data points were obtained from 14 experiments of the type illustrated in B; error bars represent s.e.m.D, mean rates of pHi recovery from alkali loads obtained in the absence (•) and presence (○) of 50 μm Rp-cAMPS under Na+o-free conditions plotted against absolute values of pHi. Data points were obtained from seven experiments of the type illustrated in B, except in the absence of external Na+; error bars represent s.e.m.
Figure 4. Effects of Sp-cAMPS on pHi recovery from base loading in the presence and absence of Na+o.
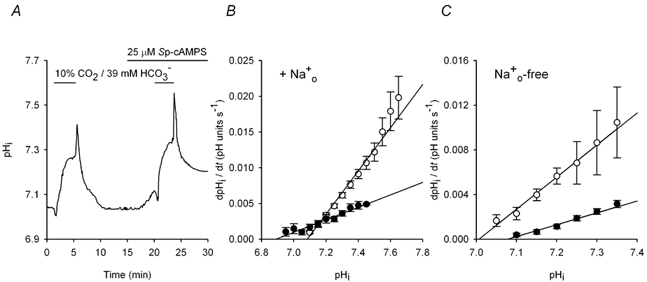
All data were obtained from neurons with initial pHi values ≤ 7.20 under HCO3−/CO2-buffered conditions. A, after the recovery of pHi from a control alkali load imposed under Na+o-containing conditions, 25 μm Sp-cAMPS evoked a slow increase in steady-state pHi (see Fig. 2C). A second alkali load was then imposed and pHi recovered at a faster rate, and to a new steady-state level, in the continued presence of Sp-cAMPS. B, the pHi dependence of pHi recovery under Na+o-containing conditions in the absence (•) and presence (○) of 25 μm Sp-cAMPS. Data points were obtained from nine experiments of the type illustrated in A; error bars represent s.e.m. The alkaline shift in the pHi-dependence of pHi recovery from alkali loads evoked by Sp-cAMPS reflects the increase in pHi elicited by Sp-cAMPS in the presence of Na+o in neurons with resting pHi values ≤ 7.20. C, mean rates of pHi recovery from base loading in the absence (•) and presence (○) of 25 μm Sp-cAMPS under Na+o-free conditions plotted against absolute values of pHi. Data points were obtained from five experiments of the type illustrated in A, except in the absence of external Na+. The acidic shift in the pHi-dependence of pHi recovery from alkali loads in the presence of Sp-cAMPS reflects the decrease in pHi elicited by Sp-cAMPS in the absence of Na+o in neurons with resting pHi values ≤ 7.20 (see Fig. 2F).
Reverse Na+-independent Cl−-HCO3− exchange
In cultured rat hippocampal neurons, acute exposure to Cl−-free, HCO3−-buffered medium elicits an internal alkalinization that is blocked by DIDS and persists in the absence of external Na+, which is consistent with reverse Na+-independent Cl−-HCO3− exchange activity (Baxter & Church, 1996; also see Boyarsky et al. 1988; Ganz et al. 1989; Raley-Susman et al. 1993). In the present study in acutely isolated adult neurons, the acute removal of Cl−o elicited a reversible 0.13 ± 0.01 pH unit rise in pHi (n = 31 low pHi neurons; Fig. 5) that was dependent on HCO3− and blocked by DIDS (n = 8 in each case; data not shown). Also in neurons with pHi values ≤ 7.20 prior to the removal of Cl−o, exposure to Cl−-free medium in the presence of 50 μm Rp-cAMPS or 25 μm Sp-cAMPS evoked increases in pHi of 0.07 ± 0.01 (n = 4; Fig. 5A) and 0.42 ± 0.07 pH units (n = 6; Fig. 5B), respectively (P < 0.05 in each case, compared to the alkalinizations observed in the absence of a test treatment). The increases in pHi observed during the acute removal of Cl−o in the presence of Rp- or Sp-cAMPS were abolished by DIDS (n = 4 in each case; see Fig. 5B) and, as shown in Fig. 5C, differences in pHi values prior to the introduction of Cl−-free medium could not account for the observed effects of the PKA modulators on the DIDS-sensitive alkalinizations induced in low pHi cells by Cl−o removal.
Figure 5. Effects of Rp- and Sp-cAMPS on changes in pHi evoked by removal of Cl−o.
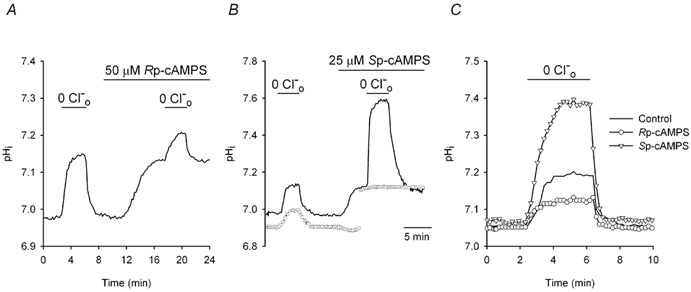
All data were obtained from neurons with initial pHi values ≤ 7.20 under HCO3−/CO2-buffered conditions. A, the acute removal of Cl−o (for the period indicated by the short bar above the trace) evoked an increase in pHi which recovered upon the reintroduction of the anion. Rp-cAMPS (50 μm) was then applied and, after pHi increased to a new steady-state level (see Fig. 2A), the removal of Cl−o evoked an increase in pHi that was smaller than in the absence of the PKA inhibitor. Increases in pHi evoked by the acute removal of Cl−o in the presence of a PKA modulator were measured as the difference between the maximum pHi observed in the absence of Cl−o and the plateau pHi value observed following the reintroduction of Cl−o in the continued presence of the PKA modulator. B, a neuron with a low initial pHi (continuous line) was exposed to Cl−-free medium, which caused an increase in pHi. Sp-cAMPS (25 μm) was then applied; the PKA activator evoked an increase in pHi (see Fig. 2C) and subsequent exposure to Cl−-free medium caused a large internal alkalinization. Superimposed is a record (□) obtained from a different low pHi neuron in which 25 μm Sp-cAMPS was co-applied with 200 μm DIDS; the gap in the trace represents a 5.5 min break in the record. The rise in pHi observed during exposure to Cl−-free medium in the presence of Sp-cAMPS was blocked by DIDS. C, the increases in pHi evoked by transient exposure to Cl−-free medium are shown in three different neurons with similar initial pHi values under control conditions (continuous line) and following pre-treatment with 25 μm Sp-cAMPS (▿) or 50 μm Rp-cAMPS (○).
Na+-dependent Cl−-HCO3− exchange
pHi recovery from acid loads
Under HCO3−/CO2-buffered conditions at 37 °C, the effect of activating PKA on rates of pHi recovery from acid loads will reflect not only potential changes in Na+-dependent Cl−-HCO3− exchange activity but also changes in the activity of the acid-extruding Na+-H+ exchanger (Smith et al. 1998). However, Na+-H+ exchange in rat hippocampal neurons is insensitive to amiloride, amiloride derivatives and guanidinium compounds (Raley-Susman et al. 1991; Schwiening & Boron, 1994; Baxter & Church, 1996; Bevensee et al. 1996), precluding the use of selective pharmacological inhibitors of Na+-H+ exchange to isolate the effect of a test treatment on Na+-dependent Cl−-HCO3− exchange. Nevertheless, Na+-H+ exchange activity in rat hippocampal neurons is markedly inhibited at RT, compared to 37 °C, and under reduced temperature conditions Na+-dependent Cl−-HCO3− exchange becomes the dominant mechanism whereby pHi recovers from internal acid loads (Baxter & Church, 1996; Sheldon & Church, 2002). In agreement with the latter reports, in control experiments conducted as part of the present study, rates of pHi recovery from acid loads imposed in acutely isolated adult rat CA1 neurons at 37 °C were similar in the presence and nominal absence of HCO3−/CO2 ((4.88 ± 0.65) × 10−3 pH units s−1, n = 4 and (4.66 ± 0.94) × 10−3 pH units s−1, n = 5, respectively, measured at a common test pHi of 6.65). In contrast, at RT, not only were rates of pHi recovery significantly faster in the presence ((2.48 ± 0.40) × 10−3 pH units s−1, n = 4) than in the absence ((0.82 ± 0.40) × 10−3 pH units s−1, n = 5) of HCO3−/CO2 but also the higher rates of pHi recovery observed in the presence of HCO3− were reduced to 0.98 ± 0.60 × 10−3 pH units s−1 (n = 3) in the presence of DIDS. Therefore, to assess the effects of modulating PKA activity on Na+-dependent Cl−-HCO3− exchange in relative isolation, the majority of experiments in this series were performed at RT.
As previously described (Smith et al. 1998), under Hepes-buffered conditions at 37 °C, 25 μm Sp-cAMPS increased the overall rate of pHi recovery from acid loads by 148 ± 16 % (n = 5; not shown); however at 20-22 °C, an increase of only 2 ± 10 % (n = 9) was observed (Fig. 6A and B). These findings are consistent with suggestions (see above) that Na+-H+ exchange plays only a limited role in the recovery of pHi from acid loads at RT. In contrast to the lack of effect of Sp-cAMPS under Hepes-buffered conditions at RT, under HCO3−/CO2-buffered conditions at RT Sp-cAMPS evoked a 58 ± 3 % (n = 6) decrease in the overall rate of pHi recovery in neurons with initial pHi values ≤ 7.20 (Fig. 6C). The effect of Sp-cAMPS to decrease rates of pHi recovery from acid loads imposed in the presence of HCO3− at RT in low pHi neurons was blocked by DIDS (the overall change in the rate of pHi recovery evoked by Sp-cAMPS in the presence of DIDS was a 16 ± 7 % increase; n = 9). In contrast to results obtained in low pHi neurons, in neurons with initial pHi values > 7.20, Sp-cAMPS evoked an overall increase of 385 ± 42 % (n = 6) in rates of pHi recovery from acid loads imposed under HCO3−/CO2-buffered conditions at RT (Fig. 6D); pre-treatment with DIDS attenuated the increase to 26 ± 7 % (n = 6). The results are consistent with the possibility (Table 1) that Sp-cAMPS inhibits Na+-dependent Cl−-HCO3− exchange in neurons with initial pHi values ≤ 7.20 and also suggest that, in neurons with initial pHi values > 7.20, activation of PKA may stimulate Na+-dependent Cl−-HCO3− exchange.
Figure 6. Effects of Sp-cAMPS on pHi recovery from acid loads at room temperature.
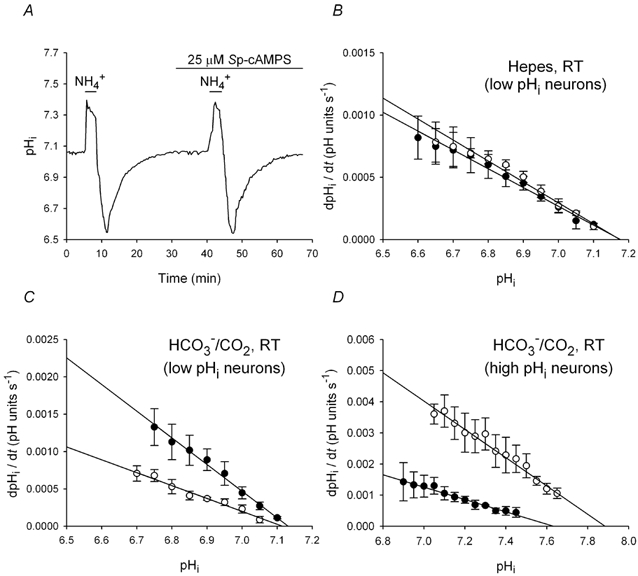
A, two consecutive acid loads, the second in the presence of 25 μm Sp-cAMPS, were imposed on a neuron with a low initial pHi under Hepes-buffered conditions. Rates of pHi recovery from both acid loads were similar and, in contrast to the effects of Sp-cAMPS under Hepes-buffered conditions at 37 °C (see Smith et al. 1998), pHi failed to recover to a higher steady-state level in the presence of Sp-cAMPS. B, rates of pHi recovery from acid loads performed in the absence (•) and presence (○) of 25 μm Sp-cAMPS plotted against absolute values of pHi; data points were obtained from nine experiments of the type shown in A (error bars represent s.e.m.). Sp-cAMPS failed to significantly affect the rate of pHi recovery at any absolute value of pHi. C, the pHi dependence of pHi recovery under HCO3−/CO2-buffered conditions at RT in the absence (•) and presence (○) of 25 μm Sp-cAMPS; data points were obtained from six experiments of the type shown in A, except in the presence of HCO3−/CO2. Rates of pHi recovery were significantly reduced in the presence of Sp-cAMPS at all absolute values of pHi. D, rates of pHi recovery from acid loads imposed on high pHi neurons (n = 6) under HCO3−/CO2-buffered conditions at RT in the absence (•) and presence (○) of 25 μm Sp-cAMPS plotted against absolute values of pHi. In contrast to its effect in low pHi neurons, Sp-cAMPS increased rates of pHi recovery in neurons with high initial pHi values.
Next, acid loads were imposed in the absence and presence of 50 μm Rp-cAMPS. As observed previously at 37 °C (Smith et al. 1998), at RT application of Rp-cAMPS failed to significantly change rates of pHi recovery from acid loads imposed in low pHi neurons in the absence of HCO3− (n = 10; data not shown). In contrast, under HCO3−/CO2-buffered conditions, Rp-cAMPS increased overall rates of pHi recovery in neurons with initial pHi values ≤ 7.20 by 239 ± 39 % (n = 12; Fig. 7A and B) and 193 ± 33 % (n = 8; Fig. 7C and D) at RT and at 37 °C, respectively. The effect of Rp-cAMPS to increase rates of pHi recovery from acid loads imposed in low pHi neurons under HCO3−/CO2-buffered conditions was attenuated by pre-treatment with DIDS; the overall increases in rates of pHi recovery evoked by Rp-cAMPS in the presence of DIDS at RT and at 37 °C were 19 ± 21 % (n = 3) and 11 ± 14 % (n = 4), respectively. Thus, in low pHi neurons, Rp-cAMPS increased rates of pHi recovery from acid loads in a HCO3−-dependent, DIDS-sensitive manner. The results are consistent with the possibility (Table 1) that inhibition of PKA stimulates Na+-dependent Cl−-HCO3− exchange in neurons with initial pHi values ≤ 7.20. No neurons with initial pHi values > 7.20 were encountered in this series of experiments.
Figure 7. Effects of Rp-cAMPS on pHi recovery from acid loads in low pHi neurons.
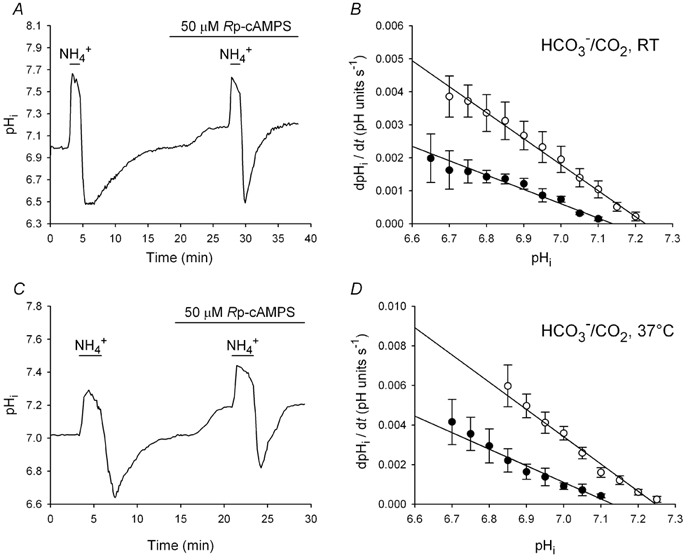
A, an acid load was imposed on a low pHi neuron under HCO3−/CO2-buffered conditions at RT and, after recovery of pHi, 50 μm Rp-cAMPS was applied. Rp-cAMPS increased both steady-state pHi and the rate of pHi recovery from the second acid load. B, the pHi dependence of the rate of pHi recovery from acid loads imposed under HCO3−/CO2-buffered conditions at RT in the absence (•) and presence (○) of 50 μm Rp-cAMPS; data points were obtained from 12 experiments of the type shown in A. C, the same experiment as shown in A, but conducted at 37 °C. D, the pHi dependence of the rate of pHi recovery from acid loads performed at 37 °C in the absence (•) and presence (○) of 50 μm Rp-cAMPS; data points were obtained from eight experiments of the type shown in C. In contrast to observations made in the nominal absence of HCO3−/CO2, Rp-cAMPS applied to low pHi neurons in the presence of HCO3−/CO2 increased rates of pHi recovery at all absolute values of pHi and shifted the pHi dependence of the rate of pHi recovery in an alkaline direction.
Effects of depleting internal Cl−
In a second set of experiments designed to examine the effects of PKA modulators on Na+-dependent Cl−-HCO3− exchange, we employed a technique in which Na+-dependent Cl−-HCO3− exchange is repeatedly activated in the absence of external Cl−, resulting in the gradual depletion of Cl−i and inhibition of the exchange mechanism (see Schwiening & Boron, 1994). We reasoned that Na+-dependent Cl−-HCO3− exchange would ‘run-down’ more quickly in the presence of PKA modulators that stimulate the exchange mechanism, whereas the opposite would be true for PKA modulators that inhibit the transporter. All neurons examined in these experiments had initial pHi values ≤ 7.20.
As illustrated in Fig. 8A, an initial application of HCO3−/CO2 in the absence of external Cl− elicited a transient acidification (due to CO2 entry) followed by a 0.10 ± 0.02 pH unit (n = 7) increase in pHi, which returned to the initial steady-state value upon the removal of HCO3−/CO2. Second, third and fourth exposures to HCO3−/CO2, in the continued absence of Cl−o, caused 0.08 ± 0.02, 0.06 ± 0.02 and 0.05 ± 0.02 pH unit increases in pHi, respectively. Instantaneous rates of change in pHi during each exposure to HCO3−/CO2 were calculated at an absolute pHi value of 7.00 and were then normalized to the mean rate of change in pHi observed during the first application of HCO3−/CO2 (Fig. 8C). The progressive decline in the normalized rates of alkalinization evoked by repeated HCO3−/CO2 applications in the absence of Cl−o suggests that Na+-dependent Cl−-HCO3− exchange activity was declining progressively due to depletion of Cl−i (see Schwiening & Boron, 1994).
Figure 8. Effects Rp- and Sp-cAMPS on alkalinizations evoked by HCO3−/CO2 under Cl−o-free conditions.
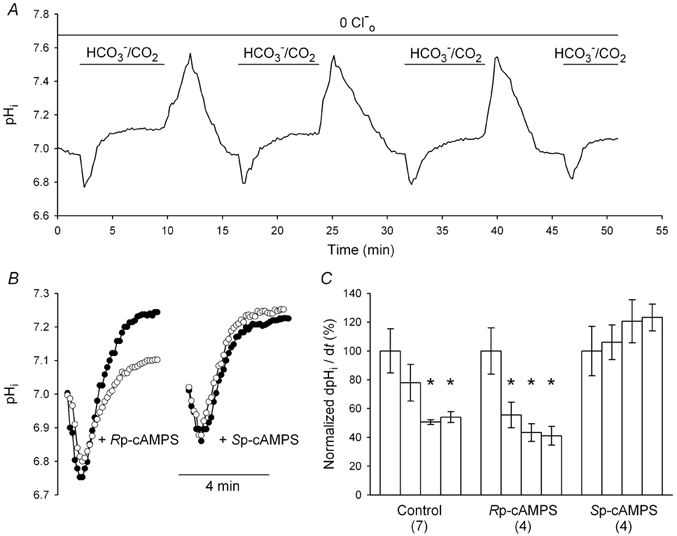
A, under Cl−o-free conditions, repeated exposures to HCO3−/CO2 caused pHi to transiently decrease and then increase to a new steady-state level; the magnitude of the HCO3−/CO2-evoked increase in pHi declined with each successive application, resulting in a progressive reduction in the rate at which pHi reached each new steady-state level (see C, Control). B, the effects on pHi of exposing two different low pHi neurons to HCO3−/CO2 under Cl−o-free conditions in the presence of 50 μm Rp-cAMPS (left-hand side) or 25 μm Sp-cAMPS (right-hand side). Under each condition, filled circles identify the record obtained during the first exposure to HCO3−/CO2; traces identified by open circles represent the changes in pHi observed during the second (Rp-cAMPS) or fourth (Sp-cAMPS) exposures to HCO3−/CO2 in the respective series. C, rates of alkalinization observed during consecutive applications of HCO3−/CO2 in the continuous absence of Cl−o were calculated at an absolute pHi value of 7.00 and normalized to the mean rate of alkalinization observed during the first application of HCO3−/CO2 under each experimental condition. The resulting normalized rates of alkalinization are presented as percentage values (± s.e.m.) under control conditions and in the presence of 50 μm Rp-cAMPS and 25 μm Sp-cAMPS. (Number of neurons examined under each experimental condition). *P < 0.05 for the difference between the first normalized rate of alkalinization under a given experimental condition.
Similar experiments were then performed in the presence of 50 μm Rp-cAMPS or 25 μm Sp-cAMPS (n = 4 in each case; see Fig. 8B), and normalized rates of change of pHi were calculated at an absolute pHi value of 7.00 during four consecutive exposures to HCO3−/CO2 under each experimental condition. In the absence of a PKA modulator, the normalized rate of alkalinization became significantly decreased during the third exposure to HCO3−/CO2 (Fig. 8C). However, in the presence of Rp-cAMPS, the normalized rate of alkalinization became significantly decreased during the second exposure to HCO3−/CO2, suggesting that internal Cl− was being depleted faster in the presence than in the absence of Rp-cAMPS. In contrast, normalized rates of alkalinization obtained in the presence of Sp-cAMPS failed to decline in response to four successive exposures to HCO3−/CO2, suggesting that internal Cl− was not being depleted. The results are consistent with those obtained in the acid load recovery experiments and further support the possibilities (Table 1) that inhibition and stimulation of PKA, increases and decreases, respectively, Na+-dependent Cl−-HCO3− exchange activity in rat CA1 neurons with initial pHi values ≤ 7.20.
Discussion
The results of the study indicate that: (a) HCO3−-dependent, DIDS-sensitive mechanisms are important determinants of pHi in adult rat CA1 neurons at physiological pHo; (b) inhibiting or activating PKA under HCO3−/CO2-buffered conditions leads to DIDS-sensitive changes in pHi, the directions and magnitudes of which are related to the initial pHi of a neuron prior to the modulation of PKA activity; and (c) the effects of modulating PKA on steady-state pHi in the presence of HCO3−/CO2 may in part be mediated by alterations in Na+-dependent and Na+-independent Cl−-HCO3− exchange activity (and, in the case of PKA activation, Na+-H+ exchange).
Steady-state pHi in the presence of HCO3−/CO2
It has previously been shown that Na+-dependent Cl−-HCO3− exchange contributes to acid extrusion from rat CA1 neurons and that activation of this mechanism underlies the sustained increases in pHi that occur in low pHi neurons upon the addition of HCO3− at a constant pHo (Schwiening & Boron, 1994; Smith et al. 1998; also see Baxter & Church, 1996; Bevensee et al. 1996). The present study extends these findings to show that, under HCO3−-containing conditions, the application of DIDS to neurons with initial pHi values ≤ 7.20 leads to reductions in pHi. In contrast, in neurons with initial pHi values > 7.20, the transition from a Hepes- to a HCO3−/CO2-buffered medium caused pHi to decrease, while pHi increased following the application of DIDS under HCO3−-containing conditions. The latter observations, together with the fact that the magnitudes of the HCO3−- and DIDS-induced changes in pHi in neurons with initial pHi values > 7.20 were greater the more alkaline the initial pHi, are most easily explained as being due to alterations in Na+-independent Cl−-HCO3− exchange activity. In many non-neuronal cell types, this transport mechanism is most active at high pHi values and contributes to pHi recovery from alkali loads (e.g. Vaughan-Jones, 1986; Olsnes et al. 1987; Boyarsky et al. 1988; Cassel et al. 1988; Ganz et al. 1989; Green et al. 1990; Kikeri et al. 1990; Mugharbil et al. 1990; Tønnessen et al. 1990; Kramhøft et al. 1994; Leem et al. 1999). In rat hippocampal neurons, Na+-independent Cl−-HCO3− exchange has been inferred on the basis of the increase in pHi observed upon acute reversal of the transmembrane Cl− gradient (Raley-Susman et al. 1993; Baxter & Church, 1996). The present study confirms that pHi recovery from base loading in CA1 neurons is inhibited by DIDS or the removal of Cl−o and can proceed in the absence of Na+o.
The fact that the regression fits relating initial pHi values to the changes in pHi observed on the addition of HCO3− to Hepes-buffered medium (Fig. 1B) or the addition of DIDS to HCO3−-buffered medium (Fig. 1D) intersected the respective abscissae at a similar absolute value of pHi suggests that this value may reflect one towards which CA1 neurons regulate their pHi when HCO3− is available and HCO3−-dependent pHi regulating mechanisms are operative. In a manner analogous to Na+-H+ exchangers, which possess internal H+ modifier site(s) that modulate transport activity, the full length isoform of the AE3 anion exchanger, which probably mediates Na+-independent Cl−-HCO3− exchange in hippocampal neurons (Kopito et al. 1989; Raley-Susman et al. 1993), may also possess a pH sensor that confers pHi sensitivity (Lee et al. 1991; also see Olsnes et al. 1987; Kopito et al. 1989; Green et al. 1990; Mugharbil et al. 1990; Ludt et al. 1991; Zhang et al. 1996; Stewart et al. 2001). Sodium-dependent Cl−-HCO3− exchange activity is also regulated by pHi (e.g. Boron et al. 1979; Boyarsky et al. 1988; Kikeri et al. 1990; Tønnessen et al. 1990); although the identity of the presumed pH sensor remains unknown, histidine-rich regions are present in the cytoplasmic N-terminus of NDCBE1 (which is expressed at the somatic level in rat hippocampal neurons, where it probably mediates Na+-dependent Cl−-HCO3− exchange; Wang et al. 2000; Grichtchenko et al. 2001a,b).
PKA modulation of Na+-dependent and Na+-independent Cl−-HCO3− exchange
In rat CA1 neurons under Hepes-buffered conditions, Sp-cAMPS stimulates Na+-H+ exchange and thereby increases pHi, whereas Rp-cAMPS fails to affect either parameter (Smith et al. 1998). In contrast, in the present study, both PKA modulators were found to elicit HCO3−-dependent, DIDS-sensitive changes in pHi, the directions and magnitudes of which were related to the initial pHi prior to their application. Although alterations in Na+-dependent and Na+-independent Cl−-HCO3− exchange activity (which may themselves be pHi-dependent; see below) probably contribute to the latter effects, changes in the activities of other, as yet uncharacterized, pHi regulating mechanisms (see Bevensee et al. 1996; Sheldon & Church, 2002) cannot be excluded. Nevertheless, in light of many of the results presented here, these mechanisms are likely to be HCO3−-dependent and DIDS-sensitive.
The involvement of PKA in the control of Na+-independent Cl−-HCO3− exchange is suggested by the following observations. First, Rp- and Sp-cAMPS exerted effects on the Cl−o-dependent, DIDS-sensitive recovery of pHi from alkali loads that persisted in the absence of Na+o. Second, Rp- and Sp-cAMPS modulated the magnitude of the HCO3−-dependent, DIDS-sensitive alkalosis seen on acute exposure to Cl−-free medium. With regard to Na+-dependent Cl−-HCO3− exchange, examination of the effects of PKA modulators on the activity of this transport mechanism in rat CA1 neurons is greatly complicated by the lack of a selective Na+-H+ exchange inhibitor. Nevertheless, complementary results were obtained in two distinct experimental protocols, suggesting that PKA does indeed participate in the control of Na+-dependent Cl−-HCO3− exchange in CA1 neurons. Thus, examined at RT, Sp- and Rp-cAMPS exerted HCO3−-dependent effects on rates of pHi recovery from internal acid loads that were attenuated by DIDS. In addition, Rp- and Sp-cAMPS affected the rapidity with which rates of alkalinization in response to repeated applications of HCO3−/CO2 in the absence of Cl−o declined.
The control of the activities of neuronal HCO3−-dependent pHi regulating mechanisms by intracellular second messengers has not previously been investigated. However, cAMP and/or PKA are known to modify anion exchange activity in a variety of non-neuronal cell types. For example, Na+-dependent Cl−-HCO3− exchange is stimulated by increases in [cAMP]i in barNaCle muscle fibres (Boron et al. 1978) and human bile duct cells (Strazzabosco et al. 1997). In contrast, Na+-independent Cl−-HCO3− exchange may be stimulated, inhibited or unaffected by activation of the cAMP/PKA pathway (e.g. Reuss, 1987; Vigne et al. 1988; Green & Kleeman, 1992; Strazzabosco et al. 1997; Spirl” et al. 1998; Alvarez et al. 2001), which emphasizes that the second messenger control of the activity of a given pHi regulating mechanism is highly dependent on the cell type in which a given exchanger isoform is expressed. In this regard, both NDCBE1 and AE3 contain consensus sites for phosphorylation by protein kinases, including PKA. (Rat AE3 (GenBank accession number P23348), mouse NCBE (GenBank accession number BAB17922) and human NDCBE1 (GenBank accession number AAC82380) protein sequences were analysed using the ScanProsite sequence analysis tool http://ca.expasy.org/tools/scnpsit1.html). In the present study, Rp- and Sp-cAMPS shifted the pHi dependence of pHi recovery from alkali and acid loads and/or changed the slopes of the pHivs. dpHi/dt relationships, suggesting that phosphorylation events might contribute to the effects of modulating PKA activity on Na+-independent and Na+-dependent Cl−-HCO3− exchange (see Wakabayashi et al. 1997). However, we have no evidence to suggest whether the modulation of anion exchanger activities by PKA involves direct phosphorylation of the exchange proteins themselves or of associated regulatory proteins (see Alvarez et al. 2001).
Dependence of the PKA modulation of anion exchange activity on initial pHi
Three intriguing features of the modulation of Cl−-HCO3− exchange by PKA in adult rat CA1 neurons are suggested by the present study (see Table 1). First, in neurons with low (pHi ≤ 7.20), and possibly high (pHi > 7.20), initial pHi values, inhibition and activation of PKA lead to opposite effects on the activities of the Na+-dependent and Na+-independent anion exchangers. Second, the effects of Rp- and Sp-cAMPS on the activity of a given type of Cl−-HCO3− exchanger (i.e. Na+-dependent or Na+-independent) appear to depend on the initial pHi of a neuron prior to the modulation of PKA activity. Third, the directions of the reciprocal changes in anion exchange activities (inhibition or stimulation) evoked by Sp- and Rp-cAMPS may be opposite in cells with low vs. high initial pHi values. Given the relative lack of experimental data obtained from high pHi neurons in experiments designed to assess the effects of PKA modulators on the activities of the Na+-dependent and Na+-independent Cl−-HCO3− exchangers in relative isolation, these possibilities must remain tentative. Nevertheless, there is some precedence in the literature for the first two of these features. Thus, in guinea-pig ventricular myocytes, not only are the Na+-HCO3− symport and Na+-H+ antiport oppositely coupled to α1-adrenoceptors but also coupling of the two mechanisms to β-receptors is the reverse of that to α1-receptors (Lagadic-Gossmann & Vaughan-Jones, 1993). And, in Vero cells, non-steroidal anti-inflammatory drugs stimulate Na+-independent Cl−-HCO3− exchange at pHi < 7.0 whereas, at pHi > 7.0, the antiport is inhibited (cf. the effects of Sp-cAMPS on Na+-independent anion exchange in the present study); these effects appeared to reflect an action of the drugs to alter the transition from the low to the high activity state of the antiport via drug-induced changes in the activity of an intracellular regulatory system (protein kinase C; Tønnessen et al. 1989).
While the mechanism(s) which might underlie the differential pHi-dependent modulation of Na+-dependent and Na+-independent Cl−-HCO3− exchange by PKA are unknown, this feature provides a potential explanation for the observed effects of PKA modulators on steady-state pHi under HCO3−-buffered conditions (see Table 1). As noted above, activation of PKA stimulates Na+-H+ exchange in both low and high pHi neurons and thereby evokes a rise in pHi even in the absence of HCO3− (Smith et al. 1998). However in the presence of HCO3−, the pHi-dependent effects of Sp-cAMPS on the activities of the Na+-dependent and Na+-independent Cl−-HCO3− exchangers could modulate the magnitude of the pHi increase in a DIDS-sensitive manner, accounting for the experimental results shown in Fig. 2C and D. Thus, in neurons with initial pHi values ≤ 7.20, the increase in pHi elicited by Sp-cAMPS under HCO3−-buffered conditions is less than that observed in the absence of HCO3−, being limited by the concurrent activation of Na+-independent Cl−-HCO3− exchange and inhibition of Na+-dependent Cl−-HCO3− exchange. Conversely, in neurons with initial pHi values > 7.20, a Sp-cAMPS-evoked decrease in Na+-independent Cl−-HCO3− exchange and increase in Na+-dependent Cl−-HCO3− exchange could act to augment the rise in pHi caused by the concomitant activation of Na+-H+ exchange. In contrast to Sp-cAMPS, Rp-cAMPS does not affect Na+-H+ exchange activity in rat CA1 neurons (Smith et al. 1998). However in low pHi neurons in the presence of HCO3−, Rp-cAMPS concurrently stimulates Na+-dependent Cl−-HCO3− exchange and inhibits Na+-independent Cl−-HCO3− exchange, resulting in the DIDS-sensitive increase in pHi observed experimentally (Fig. 2A and B). Conversely, the DIDS-sensitive decrease in pHi evoked by Rp-cAMPS in neurons with initial pHi values > 7.20 (Fig. 2A and B) could potentially reflect concomitant stimulation of Na+-independent Cl−-HCO3− exchange and inhibition of Na+-dependent Cl−-HCO3− exchange.
We can only speculate on the potential functional significance of the present findings, although a number of possibilities exist. For example, rapid increases in [cAMP]i occur in rat CA1 neurons in slice preparations following anoxia/ischaemia or the application of excitotoxins (e.g. Small et al. 1996). Given the low pHi values typically observed under such conditions, the concurrent effects of PKA activation on Na+-H+ exchange and Na+-dependent and -independent Cl−-HCO3− exchange in low pHi neurons could contribute to findings that activation of not only Na+-H+ exchange but also HCO3−-dependent, DIDS-sensitive acid loading occurs in hippocampal neurons after anoxia (Diarra et al. 1999; Yao et al. 2001; Sheldon & Church, 2002), and that both ischaemia and the application of excitotoxins induce rises in [Cl−]i (Inglefield & Schwartz-Bloom, 1998). It is also noteworthy that Cl−-HCO3− exchangers have the potential to regulate the internal concentrations of both anions (Cl− and HCO3−) that are physiologically permeant through GABAA receptor-operated channels (see Boron et al. 1978; Vaughan-Jones, 1986). According to the model presented above, activation of PKA in neurons with pHi values ≤ 7.20 could cause a positive shift in EGABAA by stimulating not only Na+-independent Cl−-HCO3− exchange but also Na+-H+ exchange (see Kaila, 1994). This would reduce hyperpolarizing GABAA responses or even convert them to excitatory ones, with attendant, potentially detrimental, increases in [Ca2+]i (e.g. Autere et al. 1999). Indeed, it has been suggested that Na+-independent Cl−-HCO3− exchange contributes to the accumulation of Cl−i that underlies the excitatory effects of GABAA receptor activation in hippocampal neurons (Sipilä et al. 2000).
Summary
In summary, PKA regulates the activities of the Na+-dependent and Na+-independent Cl−-HCO3− exchangers in adult rat CA1 neurons. Inhibition or activation of PKA exerts opposite effects on the activities of the Na+-dependent and Na+-independent anion exchangers in neurons with low (pHi ≤ 7.20) and possibly high (pHi > 7.20) resting pHi values. Furthermore, the directions of the reciprocal changes in anion exchange activities (inhibition or stimulation) evoked by Sp- and Rp-cAMPS may be opposite in cells with low vs. high resting pHi values, suggesting that pHi itself may act as a modulator in second messenger-mediated events. Although the precise relationships between the effects of modulating PKA activity on the activities of the transport mechanisms remain to be determined, alterations in Na+-dependent and Na+-independent Cl−-HCO3− exchange probably contribute to the effects of modulating PKA activity on steady-state pHi in CA1 neurons under physiological (i.e. HCO3−/CO2-buffered) conditions, that are themselves dependent on the initial pHi prior to the modulation of PKA activity. It will be of interest to determine whether additional second messenger systems contribute to the regulation of the activities of Cl−-HCO3− exchangers in hippocampal neurons, as reported in other cell types (e.g. Ludt et al. 1991; Green & Kleeman, 1992; Pucéat et al. 1998; Alvarez et al. 2001).
Acknowledgments
Financial support was provided by an operating grant to J.C. from the Canadian Institutes of Health Research.
References
- Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circulation Research. 2001;89:1246–1253. doi: 10.1161/hh2401.101907. [DOI] [PubMed] [Google Scholar]
- Autere A-M, Lamsa K, Kaila K, Taira T. Synaptic activation of GABAA receptors induces neuronal uptake of Ca2+ in adult rat hippocampal slices. Journal of Neurophysiology. 1999;81:811–816. doi: 10.1152/jn.1999.81.2.811. [DOI] [PubMed] [Google Scholar]
- Baxter KA, Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. Journal of Physiology. 1996;493:457–470. doi: 10.1113/jphysiol.1996.sp021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. Journal of Physiology. 1996;494:315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, McCormick WC, Roos A. pH regulation in barNaCle muscle fibers: dependence on intracellular and extracellular pH. American Journal of Physiology. 1979;237:C185–193. doi: 10.1152/ajpcell.1979.237.3.C185. [DOI] [PubMed] [Google Scholar]
- Boron WF, Russell JM, Brodwick MS, Keifer DW, Roos A. Influence of cyclic AMP on intracellular pH regulation and chloride fluxes in barNaCle muscle fibers. Nature. 1978;276:511–513. doi: 10.1038/276511a0. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl−-HCO3− exchangers. American Journal of Physiology. 1988;255:C857–869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Hanssen C, Clyne LA. Inadequacy of high K+/nigericin for calibrating BCECF. II. Intracellular pH dependence of the correction. American Journal of Physiology. 1996;271:C1146–1156. doi: 10.1152/ajpcell.1996.271.4.C1146. [DOI] [PubMed] [Google Scholar]
- Brett CL, Church J. Modulation of the activity of intracellular pH regulating mechanisms in rat hippocampal CA1 neurones: the cAMP/PKA pathway. Canadian Journal of Physiology and Pharmacology. 1998;76:Aii. [Google Scholar]
- Cassel D, Scharf O, Rotman M, Cragoe EJ, Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl−/HCO3− exchange systems in Chinese hamster lung fibroblasts. Journal of Biological Chemistry. 1988;263:6122–6127. [PubMed] [Google Scholar]
- Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93:1003–1016. doi: 10.1016/s0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- Ganz MB, Boyarsky G, Sterzel RB, Boron WF. Arginine vasopressin enhances pHi regulation in the presence of HCO3− by stimulating three acid-base transport systems. Nature. 1989;337:648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Green J, Kleeman CR. Role of calcium and cAMP messenger systems in intracellular pH regulation of osteoblastic cells. American Journal of Physiology. 1992;262:C111–121. doi: 10.1152/ajpcell.1992.262.1.C111. [DOI] [PubMed] [Google Scholar]
- Green J, Yamaguchi DT, Kleeman CR, Muallem S. Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions. Journal of General Physiology. 1990;95:121–145. doi: 10.1085/jgp.95.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. Journal of Biological Chemistry. 2001a;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Risso Bradley S, Rojas JD, Richerson GB, Boron WF. Localization of the Na+-driven Cl/HCO3 exchanger protein (NDCBE1) in rat brain. Society for Neuroscience Abstracts. 2001b;27:527.12. [Google Scholar]
- Inglefield JR, Schwartz-Bloom RD. Activation of excitatory amino acid receptors in the rat hippocampal slice increases intracellular Cl− and cell volume. Journal of Neurochemistry. 1998;71:1396–1404. doi: 10.1046/j.1471-4159.1998.71041396.x. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAa receptor channel function in the nervous system. Progress in Neurobiology. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kelly T, Brett CL, Church J. Regulation of HCO3−/Cl− exchange by cAMP-dependent protein kinase (PKA) in rat hippocampal CA1 neurons. Society for Neuroscience Abstracts. 2000;26:625.3. [Google Scholar]
- Kikeri D, Zeidel ML, Ballermann BJ, Brenner BM, Hebert SC. pH regulation and response to AVP in A10 cells differ markedly in the presence vs. absence of CO2/HCO3−. American Journal of Physiology. 1990;259:C471–483. doi: 10.1152/ajpcell.1990.259.3.C471. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989;59:927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kramhøft B, Hoffmann EK, Simonsen LO. pHi regulation in Ehrlich mouse ascites tumor cells: role of sodium-dependent and sodium-independent chloride-bicarbonate exchange. Journal of Membrane Biology. 1994;138:121–132. doi: 10.1007/BF00232640. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Vaughan-Jones RD. Coupling of dual acid extrusion in the guinea-pig isolated ventricular myocyte to α1- and β-adrenoceptors. Journal of Physiology. 1993;464:49–73. doi: 10.1113/jphysiol.1993.sp019624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BS, Gunn RB, Kopito RR. Functional differences among nonerythroid anion exchangers expressed in a transfected human cell line. Journal of Biological Chemistry. 1991;266:11448–11454. [PubMed] [Google Scholar]
- Leem C-H, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. Journal of Physiology. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludt J, Tønnessen TI, Sandvig K, Olsnes S. Evidence for involvement of protein kinase C in regulation of intracellular pH by Cl−/HCO3− antiport. Journal of Membrane Biology. 1991;119:179–186. doi: 10.1007/BF01871417. [DOI] [PubMed] [Google Scholar]
- Mugharbil A, Knickelbein RG, Aronson PS, Dobbins JW. Rabbit ileal brush-border membrane Cl-HCO3 exchanger is activated by an internal pH-sensitive modifier site. American Journal of Physiology. 1990;259:G666–670. doi: 10.1152/ajpgi.1990.259.4.G666. [DOI] [PubMed] [Google Scholar]
- Olsnes S, Ludt J, Tønnessen TI, Sandvig K. Bicarbonate/chloride antiport in Vero cells: II. Mechanisms for bicarbonate-dependent regulation of intracellular pH. Journal of Cellular Physiology. 1987;132:192–202. doi: 10.1002/jcp.1041320203. [DOI] [PubMed] [Google Scholar]
- Phillips KP, Baltz JM. Intracellular pH regulation by HCO3−/Cl− exchange is activated during early mouse zygote development. Developmental Biology. 1999;208:392–405. doi: 10.1006/dbio.1999.9199. [DOI] [PubMed] [Google Scholar]
- Pucéat M, Roche S, Vassort G. Src family tyrosine kinase regulates intracellular pH in cardiomyocytes. Journal of Cell Biology. 1998;141:1637–1646. doi: 10.1083/jcb.141.7.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raley-Susman KM, Cragoe EJ, Jr, Sapolsky RM, Kopito RR. Regulation of intracellular pH in cultured hippocampal neurons by an amiloride-insensitive Na+/H+ exchanger. Journal of Biological Chemistry. 1991;266:2739–2745. [PubMed] [Google Scholar]
- Raley-Susman KM, Sapolsky RM, Kopito RR. Cl−/HCO3− exchange function differs in adult and fetal rat hippocampal neurons. Brain Research. 1993;614:308–314. doi: 10.1016/0006-8993(93)91049-x. [DOI] [PubMed] [Google Scholar]
- Reuss L. Cyclic AMP inhibits Cl−/HCO3− exchange at the apical membrane of Necturus gallbladder epithelium. Journal of General Physiology. 1987;90:173–196. doi: 10.1085/jgp.90.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiological Reviews. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. Journal of Neuroscience. 2000;20:6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. American Journal of Physiology – Renal Physiology. 2001;280:F739–747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. Journal of Physiology. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C, Church J. Intracellular pH response to anoxia in acutely dissociated adult rat hippocampal CA1 neurons. Journal of Neurophysiology. 2002;87:2209–2224. doi: 10.1152/jn.2002.87.5.2209. [DOI] [PubMed] [Google Scholar]
- Sipilä S, Uusisaari M, Voipio J, Kaila K. GABAa-mediated excitation and inhibition of spontaneously active neonatal hippocampal neurons in the absence of synaptic transmission. Society for Neuroscience Abstracts. 2000;26:37.11. [Google Scholar]
- Small DL, Monette R, Chakravarthy B, Durkin J, Barbe G, Mealing G, Morley P, Buchan AM. Mechanisms of 1S, 3R-ACPD-induced neuroprotection in rat hippocampal slices subjected to oxygen and glucose deprivation. Neuropharmacology. 1996;35:1037–1048. doi: 10.1016/s0028-3908(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Smith GAM, Brett CL, Church J. Effects of noradrenaline on intracellular pH in acutely dissociated adult rat hippocampal CA1 neurones. Journal of Physiology. 1998;512:487–505. doi: 10.1111/j.1469-7793.1998.487be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirlì C, Granato A, Zsembery A, Anglani F, Okolicsànyi L, Larusso NF, Crepaldi G, Strazzabosco M. Functional polarity of Na+/H+ and Cl−/HCO3− exchangers in a rat cholangiocyte cell line. American Journal of Physiology. 1998;275:G1236–1245. doi: 10.1152/ajpgi.1998.275.6.G1236. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. American Journal of Physiology - Cell Physiology. 2001;281:C1344–1354. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M, Joplin R, Zsembery A, Wallace L, Spirlì C, Fabris L, Granato A, Rossanese A, Poci C, Neuberger JM, Okolicsànyi L, Crepaldi G. Na+-dependent and -independent Cl−/HCO3− exchange mediate cellular HCO3− transport in cultured human intrahepatic bile duct cells. Hepatology. 1997;25:976–985. doi: 10.1002/hep.510250431. [DOI] [PubMed] [Google Scholar]
- Tønnessen TI, Aas AT, Sandvig K, Olsnes S. Effect of anti-inflammatory analgesic drugs on the regulation of cytosolic pH by anion antiport. Journal of Pharmacology and Experimental Therapeutics. 1989;248:1197–1206. [PubMed] [Google Scholar]
- Tønnessen TI, Sandvig K, Olsnes S. Role of Na+-H+ and Cl− -HCO3− antiports in the regulation of cytosolic pH near neutrality. American Journal of Physiology. 1990;258:C1117–1126. doi: 10.1152/ajpcell.1990.258.6.C1117. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones RD. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. Journal of Physiology. 1986;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P, Breittmayer J-P, Frelin C, Lazdunski M. Dual control of the intracellular pH in aortic smooth muscle cells by a cAMP-sensitive HCO3−/Cl− antiporter and a protein kinase C-sensitive Na+/H+ antiporter. Journal of Biological Chemistry. 1988;263:18023–18029. [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouysségur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Wang C-Z, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger. Journal of Biological Chemistry. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu X-Q, Douglas RM, Haddad GG. Role of Na+/H+ exchanger during O2 deprivation in mouse CA1 neurons. American Journal of Physiology. 2001;281:C1205–1210. doi: 10.1152/ajpcell.2001.281.4.C1205. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. Journal of Biological Chemistry. 1996;271:5741–5749. doi: 10.1074/jbc.271.10.5741. [DOI] [PubMed] [Google Scholar]


