Abstract
We investigated the role of the cerebellar flocculus in mediating the adaptive changes that occur in the intrinsic properties of brainstem medial vestibular nucleus (MVN) neurons during vestibular compensation. Ipsi-lesional, but not contra-lesional, flocculectomy prevented the compensatory increase in intrinsic excitability (CIE) that normally occurs in the de-afferented MVN neurons within 4 h after unilateral labyrinthectomy (UL). Flocculectomy did not, however, prevent the down-regulation of efficacy of GABA receptors that also occurs in these neurons after UL, indicating that these responses of the MVN neurons to deafferentation are discrete, parallel processes. CIE was also abolished by intra-floccular microinjection of the metabotropic glutamate receptor (mGluR) antagonist AIDA, and the protein kinase C inhibitor bisindolymaleimide I (BIS-I). The serene-threonine kinase inhibitor H-7 had no effect when microinjected at the time of de-afferentation, but abolished CIE if microinjected 2 h later. These cellular effects are in line with the recently reported retardatory effects of BIS-I and H-7 on behavioural recovery after UL. They demonstrate that the increase in intrinsic excitability in MVN neurons during vestibular compensation is cerebellum dependent, and requires mGluR activation and protein phosphorylation in cerebellar cortex. Furthermore, microinjection of the glucocorticoid receptor (GR) antagonist RU38486 into the ipsi-lesional flocculus also abolished CIE in MVN neurons. Thus an important site for glucocorticoids in facilitating vestibular compensation is within the cerebellar cortex. These observations ascribe functional significance to the high levels of GR and 11-β-HSD Type 1 expression in cerebellum.
Recent studies have identified two cellular mechanisms of plasticity in medial vestibular nucleus (MVN) neurons during ‘vestibular compensation’, the behavioural recovery that takes place after de-afferentation of the vestibular receptors of one inner ear. Following unilateral labyrinthectomy (UL), neurons in the rostral region of the MVN on the lesioned side (the ‘ipsi-lesional side’) develop a sustained increase in their intrinsic excitability. This is observed as a significant elevation of their mean spontaneous discharge rate in isolated slices of the MVN in vitro (Cameron & Dutia, 1997, 1999; Johnston et al. 2001; Ris et al. 2001; Him & Dutia, 2001), and which is revealed in intracellular experiments to be due to changes in the intrinsic membrane properties of ‘Type B’, but not ‘Type A’, MVN neurons (Him & Dutia, 2001). Intriguingly, the development of this ‘compensatory increase in intrinsic excitability’ (CIE; Cameron & Dutia, 1999) is conditional upon the activation of nuclear glucocorticoid receptors (GRs). Thus, the development of CIE in MVN neurons is prevented by systemic administration of the GR antagonist RU38486, and does not develop in animals that remain anaesthetized after UL; however in anaesthetized animals that are treated with the synthetic GR agonist dexamethasone simultaneously with UL, CIE develops in ipsi-lesional MVN neurons (Cameron & Dutia, 1999). Thus the acute stress that normally accompanies the severe behavioural symptoms immediately after UL facilitates the neuronal plasticity underlying vestibular compensation (Cameron & Dutia, 1999; reviews, Darlington & Smith, 2000; Seemungal et al. 2001). The locus of this striking glucocorticoid-mediated plasticity is uncertain.
Secondly, in parallel with the development of CIE, there is a rapid down-regulation of the functional efficacy of GABA receptors (Yamanaka et al. 2000; Johnston et al. 2001), and glycine receptors (Vibert et al. 2000), in ipsi-lesional MVN neurons after UL. These post-lesional changes in the MVN neurons are appropriate to counteract the disfacilitation and increased commissural inhibition that silences these cells immediately after UL (for discussion see Yamanaka et al. 2000; Graham & Dutia, 2001). Although other adaptive processes may also be involved in the initial stages of vestibular compensation, these changes in intrinsic properties of MVN neurons are likely to be important in the early restoration of their resting discharge after de-afferentation, which is necessary for behavioural recovery.
Several lines of evidence indicate that the cerebellar flocculus-paraflocculus complex (floccular lobe, FL) plays an important role in vestibular compensation. Ablation of the vestibulocerebellum (paraflocculus-flocculus, uvula and nodulus), or disruption of climbing fibre inputs to flocculus, severely delays compensation (Llinas et al. 1975; Azzena et al. 1979; Jeannerod et al. 1981; Courjon et al. 1982; review, Darlington & Smith, 2000). Flocculectomy after compensation causes the reappearance of UL-induced behavioural deficits (decompensation; Kitahara et al. 1997). Recent studies have implicated cerebellar protein kinase C (PKC) activation in the early stages of vestibular compensation. Goto et al. (1997) reported an asymmetry in PKC expression in ipsi- and contra-lesional FL 6 h post-UL, which returned to normal 24 h post-UL. Subsequently Barmack et al. (2001) showed changes in intracellular distribution of PKC-δ, but not PKC-γ, in vestibulocerebellar Purkinje cells within 12 h post-UL, and suggested that this may result in a reduction in synaptic GABA release within the ipsi-lesional MVN. Behaviourally, Balaban et al. (1999) showed that intracerebroventricular injection of the PKC inhibitor BIS-I delayed compensation of spontaneous nystagmus at 8 h post-UL but not at later times, and suggested this could be due to inhibition of cerebellar long-term depression (LTD). Here, we investigated the hypothesis that cerebellar cortical plasticity, possibly involving LTD in flocculus after UL, may have a role in mediating post-lesional plasticity in ipsi-lesional MVN neurons in the early stages of compensation (4 and 48 h post-UL). The effects of flocculectomy and intra-floccular microinjection of a mGluR antagonist or a PKC inhibitor to inhibit LTD were investigated. Furthermore, the cerebellum has a very high density of GR whose function is presently unknown, but which may also regulate LTD; we also explored their possible role in mediating MVN plasticity.
Methods
Slices of the ipsi-lesional MVN were prepared from Sprague-Dawley rats (80-120 g), that had undergone a left unilateral labyrinthectomy (UL) either 4 or 48 h previously. The surgical techniques for UL and the methods for slice preparation were identical to those described in our earlier studies (Dutia et al. 1992; Cameron & Dutia, 1997, 1999; Yamanaka et al. 2000; Johnston et al. 2001). Procedures were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986. In summary, the labyrinthectomy was carried out under surgical anaesthesia and aseptic conditions by opening the horizontal semi-circular canal duct in the temporal bone with a 0.7 mm diameter dental drill, following the open canal duct anteriorly to the vestibule of the inner ear, and aspirating the contents of the vestibule, which was then rinsed with 100 % ethanol. Either 4 or 48 h later, the animal was decapitated by guillotine under halothane anaesthesia and the whole brain quickly removed into ice-cold artificial cerebrospinal fluid (aCSF; composition (mm): 124 NaCl, 5 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3 and 10 d-glucose, equilibrated with 95 % O2-5 % CO2). The brainstem was isolated and cemented with the fourth ventricle uppermost to the stage of a Vibroslice (Campden Instruments, Loughborough, UK). Horizontal slices of 350 μm thickness containing the MVN were cut. Each slice was cut down the midline and trimmed to give two isolated medial vestibular nuclei. Care was taken to unambiguously identify the left (ipsi-lesional) and right (contra-lesional) nuclei. The ipsi-lesional MVN was transferred to an interface-type incubation chamber that was continually perfused with aCSF (flow rate 1.8 ml min−1) bubbled with 95 % O2-5 % CO2 and maintained at 33 ± 0.2 °C. After an incubation period of 1 h, conventional glass extracellular microelectrodes filled with 2 mNaCl (impedance 10-15 MΩ) coupled to an Axoclamp 2B amplifier (Axon Instruments, Union City, CA, USA) were used to explore the MVN for tonically firing neurons. The spontaneous discharge of tonically active MVN neurons was displayed and analysed online using a micro 1401 A/D interface (CED, Cambridge, UK) linked to a PC running Spike 2 software (CED, Cambridge, UK). As in previous studies, MVN neurons showed stable spontaneous resting discharge rates in vitro, with no evidence to indicate a decline in spontaneous activity over time.
Two series of experiments were carried out. In the first series (‘recovery experiments’) we investigated the effects of ablation of the flocculus-paraflocculus complex by aspiration, in animals that underwent UL under avertin anaesthesia (300 mg kg−1 tribromoethanol i.p.; Cameron & Dutia, 1997, 1999; Yamanaka et al. 2000). These animals began to recover from anaesthesia 30-40 min after induction, and were returned to their home cages for either 4 or 48 h post-UL at which time they were killed for the preparation of MVN slices. These animals all showed the characteristic signs of unilateral vestibular loss upon recovery from anaesthesia (spontaneous nystagmus, barrel rolling, ipsilateral limb flexion and contralateral extension). Within 1-2 h these animals were able to locomote, eat and drink, and showed no ill effects other than those due to the vestibular loss. The effects of ipsi-lesional, contra-lesional and bilateral flocculectomy were investigated.
The second series of experiments (‘non-recovery experiments’) was carried out on animals that underwent UL under urethane anaesthesia (ethyl carbamate, 1.25 g kg−1i.p.), and which remained anaesthetised in a warm environment for 4 h post-UL (Cameron & Dutia, 1999). In these experiments we investigated the effects of intra-floccular microinjection of drugs through a cannula stereotaxically implanted in the ipsi-lesional FL (10 mm caudal and 4.5 mm lateral to bregma, implanted to a depth of 7.0 mm). The correct positioning of the cannula tip within the FL, and the restricted diffusion of the injected solution within the complex, was confirmed microscopically using the dye 5 % Alcian blue. In previous studies we have shown that under these experimental conditions, the development of CIE in the rostral ipsi-lesional MVN cells is dependent on the activation of glucocorticoid receptors (GR) (Cameron & Dutia, 1999). Accordingly the synthetic GR agonist dexamethasone 21-phosphate (Sigma, Poole, UK) was administered to the urethane-anaesthetized animals (5 mg kg−1i.p. 15 min before UL; Cameron & Dutia, 1999).
Drugs
Dose-response relationships for the inhibitory effects of GABA agonists on the tonic in vitro discharge of MVN neurons in slices were obtained as in previous studies (Dutia et al. 1992; Yamanaka et al. 2000). The GABAA agonist, muscimol (5-aminomethyl-3-hydroxyisoxazole) and GABAB agonist baclofen (4-amino-3-(4-chlorophenyl)-butanoic acid) were obtained from Sigma. Aliquots (500 μl) of stock solutions of the drug were made in distilled water and frozen until used. Test solutions of the agonists were made by diluting the stock solution in oxygenated aCSF immediately before use, and applied to the slices by switching the perfusion inlet tube to the appropriate reservoir containing the agonist. The inhibitory response of each cell to a 60 s application of agonist was measured as the maximal decrease in discharge rate expressed as a percentage of the resting discharge rate (Dutia et al. 1992).
Drugs for microinjection into the FL (non-recovery experiments) were made up in sterile physiological saline and administered using a 5 μl volume Hamilton syringe, at a rate of 0.1 μl min−1 over 10 min (total volume, 1 μl). Stock solutions of the specific and potent PKC inhibitor bisindolymaleimide I hydrochloride (BIS-I; purchased from Calbiochem, Nottingham, UK; Toullec et al. 1991), the broad-spectrum serene-threonine protein kinase inhibitor, H-7 dihydrochloride ((±)-1-(5-isoquinolinesulphonyl)-2-methylpiperazine; purchased from Tocris, Bristol, UK; Hidaka et al. 1984) and the group 1 mGluR antagonist (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA; purchased from Tocris, Bristol, UK; Moroni et al. 1997) were made up in distilled water and frozen until required. A stock solution of the glucocorticoid receptor antagonist RU38486 was made up in sterile peanut oil. Test solutions of these drugs were made up immediately before use by diluting the stock solution in sterile physiological saline. The final concentrations used for microinjection were AIDA, 1 mm; BIS-I, 25 μm; H-7, 25 mm; and RU38486, 50 μm.
Data were analysed using SigmaStat for Windows (SPSS, Chicago, IL, USA), and Graphpad Prism version 3 (GraphPad Software, San Diego, CA, USA). Values are presented as means ± s.e.m. as appropriate.
Results
The effects of flocculectomy on MVN neuronal plasticity after UL
In animals that were subjected to a left UL under avertin anaesthesia (‘recovery experiments’, Methods), the effects of ablating either the ipsi-lesional (left) or contra-lesional (right) FL at the time of UL were investigated. Figure 1 shows the mean in vitro discharge rates of rostral ipsi-lesional MVN neurons in slices prepared from animals that had undergone vestibular compensation for either 4 or 48 h post-UL. In slices prepared from UL animals in which the bilateral flocculi were left intact (UL4h and UL48h groups, Fig. 1; n = 5 animals in each case), the in vitro discharge rate of the rostral MVN neurons was significantly higher than in control slices, confirming our previous reports of the development of CIE in these neurons during vestibular compensation (Cameron & Dutia, 1997, 1999; Johnston et al. 2001). However in animals that were bilaterally flocculectomised at the time of UL (bFX+UL4h group, Fig. 1; n = 5), CIE failed to develop, and the mean in vitro firing rate of the MVN neurons was not different from normal. Bilateral flocculectomy itself, without UL (bFX group, Fig. 1; n = 4) had no effect on the in vitro firing rates of the MVN neurons.
Figure 1. Ipsi-lesional flocculectomy abolishes the increase in intrinsic excitability of MVN neurons during compensation.
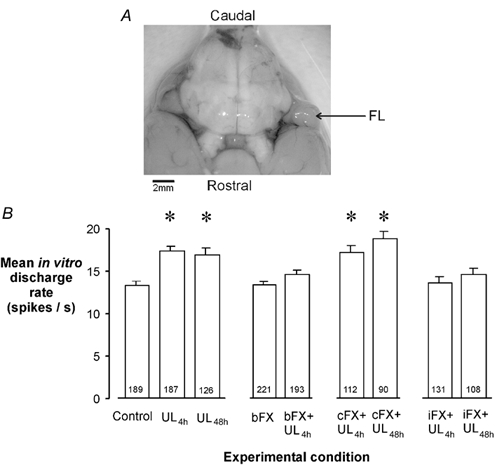
A, photomicrograph of the brain from a representative left-flocculectomised rat (ventral view). Note that the left flocculus-paraflocculus lobe (FL) is absent. B, mean resting in vitro discharge rates (± s.e.m.) of rostral ipsi-lesional MVN neurons in slices that were prepared from animals that had undergone either 4 or 48 h of vestibular compensation after unilateral labyrinthectomy (UL4h, UL48h), in combination with either bilateral flocculectomy (bFX), contra-lesional flocculectomy (cFX) or ipsi-lesional flocculectomy (iFX). The number of cells recorded in each experimental condition is shown at the base of the columns. In this and following figures, asterisks indicate where the mean in vitro discharge rate of the MVN cells is significantly elevated compared to control (P < 0.05, Mann-Whitney rank sum test).
In animals that underwent contra-lesional flocculectomy at the time of UL, CIE developed in the ipsi-lesional MVN neurons in the same way as in cerebellar intact animals (cFX+UL4h and cFX+UL48h groups, Fig. 1; n = 3 and 4, animals respectively). By contrast, in animals that underwent an ipsi-lesional flocculectomy at the time of UL, CIE failed to develop either at 4 or at 48 h post-UL (iFX+UL4h and iFX+UL48h groups, Fig. 1; n = 3 and 4 animals, respectively).
We investigated whether ipsi-lesional flocculectomy also prevented the down-regulation of GABA receptor functional efficacy that normally occurs in the MVN neurons after UL. The inhibitory effects of the GABAA agonist muscimol and the GABAB agonist baclofen were investigated in slices of the ipsi-lesional MVN prepared either from normal animals or animals that underwent iFX+UL4h. In control slices both muscimol (1-10 μm, n = 12 cells) and baclofen (0.6-10 μm, n = 18 cells) caused a reversible, dose-dependent inhibition of the tonic discharge of all MVN neurons tested (Fig. 2A), in a similar way to that demonstrated in our previous studies (Dutia et al. 1992; Yamanaka et al. 2000; Johnston et al. 2001). As shown in Fig. 2B, in slices from iFX+UL4h animals the inhibitory effects of both muscimol and baclofen were significantly lower than normal at each dose tested, and there was a rightward shift of the fitted dose response relationships for both agonists (muscimol: n = 17 cells from 10 animals; EC50 in control slices = 1.7 μm, in iFX+UL4h slices = 6.4 μm; baclofen: n = 19 cells from 11 animals, EC50 in control slices = 2.5 μm, in iFX+UL4h slices = 19.0 μm). Thus, ipsi-lesional flocculectomy, which abolished the development of CIE in rostral MVN neurons (Fig. 1), did not prevent the down-regulation of GABAA and GABAB receptor efficacy in these cells after UL.
Figure 2. Ipsi-lesional flocculectomy does not prevent adaptive changes in the functional efficacy of GABA receptors in MVN neurons during compensation.
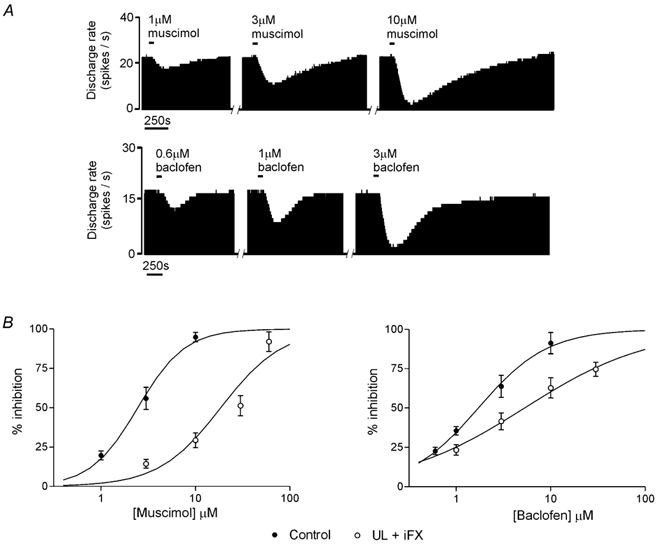
A, firing rate histograms showing the dose-dependent inhibition of the spontaneous discharge of two MVN neurons by bath-application of muscimol (upper panel) and baclofen (lower panel). The bars above the data indicate the 60 s period of application of the agonists. The inhibitory response was measured as the maximal inhibition of firing rate expressed as a percentage of the resting discharge rate for each cell. B, dose-response relationships for the inhibitory response to muscimol (left panel) and baclofen (right panel), of MVN neurons in control slices (○) and in slices prepared from animals that underwent iFX+UL 4 h earlier (•). Data are means and s.e.m.
Flocculus mechanisms mediating the development of CIE in ipsi-lesional MVN neurons
To investigate possible mechanisms in the flocculus that mediate the development of CIE in the ipsi-lesional MVN neurons after UL, we performed microinjections of the specific group I mGluR antagonist AIDA, or the potent PKC inhibitor BIS-I, or the serene-threonine protein kinase inhibitor, H-7 into the ipsi-lesional FL at the time of UL (see Methods). Microinjections were carried out in animals that remained at a stable level of anaesthesia under urethane for 4 h post-UL, at which time they were killed for the preparation of MVN slices (‘non-recovery experiments’, Methods). We have shown previously that under urethane anaesthesia, the activation of GRs by systemic administration of dexamethasone is required for the development of CIE in rostral MVN neurons (Cameron & Dutia, 1999). Accordingly these animals were administered dexamethasone (5 mg kg−1) i.p. at the time of UL (Methods). As shown in Fig. 3A, the mean in vitro discharge rate of MVN cells in slices from urethane-anaesthetised animals was significantly higher in dexamethasone-treated animals than in controls, confirming our previous results (UL4h-Dex group vs. UL4h+DexIP group, Fig. 3A; n = 4 animals in each case).
Figure 3. Inhibition of cerebellar plasticity in ipsi-lesional flocculus prevents the increase in intrinsic excitability of MVN neurons during compensation.
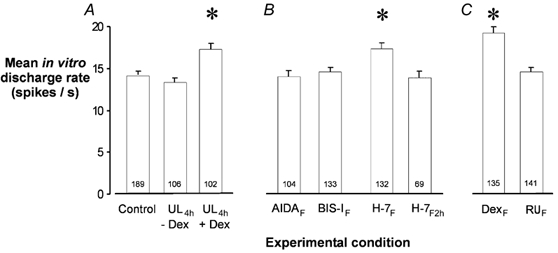
Mean in vitro discharge rates (±s.e.m.) of rostral ipsi-lesional MVN neurons in slices prepared from animals that were unilaterally labyrinthectomised under urethane anaesthesia, and which remained anaesthetised for 4 h post-UL. The number of cells recorded in each experimental condition is shown at the base of the columns. A, in animals treated with dexamethasone i.p. at the time of UL (UL4h+ Dex), the mean in vitro discharge rate of the MVN neurons was significantly elevated compared to that in control MVN slices, while in slices from animals that did not receive dexamethasone i.p. (UL4h - Dex) the mean in vitro discharge rate is not different from control. B, effects of intrafloccular micro-injection of the metabotropic glutamate receptor antagonist AIDA, the PKC inhibitor BIS-I and the serene-threonine kinase inhibitor H-7 at the time of UL, on the mean in vitro discharge rates of MVN neurons in slices prepared 4 h post-UL. In each of these groups dexamethasone was given i.p. at the time of UL. Intrafloccular micro-injection of AIDA and BIS-I abolished the expected increase in intrinsic excitability of the MVN neurons (AIDAF, BIS-IF groups). H-7 had no effect when micro-injected at the time of UL (H-7F group), but did abolish the increase in intrinsic excitability when micro-injected 2 h post-UL (H-7F2h group). C, ffects of intra-floccular microinjection of dexamethasone (DexF group) and the GR antagonist RU38486 (RUF group). The DexF group did not receive dexamethasone i.p.; instead dexamethasone was micro-injected into the ipsi-lesional floccular lobe at the time of UL. The RUF group received dexamethasone i.p. at the time of UL, but RU38486 was micro-injected into the ipsi-lesional flocculus to block GR.
Intra-floccular microinjection of either AIDA or BIS-I at the time of UL prevented the development of CIE in the ipsi-lesional MVN neurons at 4 h post-UL (AIDAF and BIS-IF groups, respectively, Fig. 3B; n = 4 animals in each case). By contrast, microinjection of H-7 at the time of UL had no effect (H-7F group, Fig. 3B; n = 4). Interestingly, microinjection of H-7 with a delay of 2 h post-UL, was effective in abolishing CIE in the MVN neurons (H-7F2h group, Fig. 3B; n = 4). Intra-floccular microinjection of vehicle (sterile physiological saline) had no effect on development of CIE in the MVN neurons (n = 4 animals; data not shown).
Finally, we investigated whether activation of glucocorticoid receptors (GRs) in the flocculus was necessary for the development of CIE in the ipsi-lesional MVN neurons. Slices were prepared at 4 h post-UL from urethane-anaesthetised animals, in which dexamethasone was not administered i.p.; instead, 1 μl of 1 mm dexamethasone was microinjected directly into the flocculus at the time of UL (DexF group, Fig. 3C; n = 5 animals). The mean firing rate of the ipsi-lesional MVN cells in these slices was significantly higher than in controls, demonstrating that intra-floccular administration of dexamethasone is sufficient to induce CIE in the MVN neurons after UL. In the converse experiment, we prepared slices at 4 h post-UL from urethane-anaesthetised animals in which dexamethasone was again administered i.p. at the time of UL, but in which an intra-floccular microinjection of 1 μl of the GR antagonist RU38486 (50 μm) was made at the time of UL. In these animals, the expected development of CIE the ipsi-lesional MVN cells failed to occur (RUF group, Fig. 3C; n = 5 animals).
Discussion
These results show that the compensatory increase in intrinsic excitability (CIE) that develops in ipsi-lesional MVN neurons during vestibular compensation (Cameron & Dutia, 1997, 1999; Johnston et al. 2001; Ris et al. 2001; Him & Dutia, 2001), is cerebellar-dependent. Ipsi-lesional flocculectomy, as well as intra-floccular microinjections of drugs that inhibit cerebellar cortical plasticity, prevented the development of CIE in MVN neurons after UL. These results suggest a novel role for the flocculus in mediating vestibular neuronal plasticity at the cellular level, and provide the first evidence that protein phosphorylation, and GR and mGluR1 activation in the flocculus, mediate sustained changes in the intrinsic excitability of brainstem neurons in the MVN, to which the FL projects.
Cellular plasticity in vestibular compensation
Ipsi-lesional flocculectomy prevented CIE in ipsi-lesional MVN neurons, but did not prevent the simultaneous down-regulation of functional efficacy of GABA receptors that also occurs after UL (Fig. 1 and Fig. 2). This confirms our earlier suggestion that these two post-lesional responses in the de-afferented MVN neurons are mediated by independent cellular mechanisms (Yamanaka et al. 2000). Down-regulation of GABA receptor efficacy after UL is likely to occur in response to the sustained increase in commissural inhibition of the ipsi-lesional neurons by the hyperactive MVN cells on the contra-lesional side (Smith & Curthoys, 1989; Ris & Godaux, 1998; Yamanaka et al. 2000; review, Curthoys & Halmagyi, 1995). By contrast, the development of CIE in these neurons is dependent on the integrity of the ipsilateral FL, and is abolished by intra-floccular microinjections of the PKC inhibitor BIS-I, the mGluR antagonist AIDA and the GR antagonist RU38486 (Fig. 3).
It could be argued that ipsi-lesional flocculectomy may dis-inhibit the MVN neurons on that side, and so reduce the post-UL imbalance in firing rates of the MVNs of the two sides and remove the necessity for CIE to be expressed in the MVN neurons. However the fact that the adaptive changes in GABA receptor efficacy still occur in ipsi-lesional cells strongly indicates that flocculectomy does not alleviate the marked imbalance in activity of the MVN neurons after UL in rat. Evidence from behavioural studies, in which flocculectomy impairs rather than accelerates the compensation of static vestibular symptoms, also suggests that the imbalance in commissural inhibition persists after flocculectomy. This is supported by the good agreement between the effects of BIS-I and H-7 on behavioural compensation reported by Balaban et al. (1999), and our present results. Thus BIS-I given i.c.v. retarded the compensation of spontaneous nystagmus after UL (Balaban et al. 1999), and also abolished CIE in ipsi-lesional neurons when microinjected into the ipsi-lesional flocculus. In addition H-7 had no effect on behavioural compensation if administered at the time of UL, but retarded it when given 6 h post-UL (Balaban et al. 1999), while in this study intrafloccular H-7 had no effect on CIE in ipsi-lesional MVN neurons if given at the time of UL, but abolished CIE if given 2 h post-UL. Taken together, these findings implicate cerebellar PKC-mediated mechanisms in vestibular compensation immediately post-UL, while at later times other kinase (possibly PKA/PKG)-mediated mechanisms in flocculus appear to be more important.
Cerebellar cortical and sub-cortical plasticity in vestibular compensation
Micro-injection of BIS-I, AIDA and the GR antagonist RU38486 into the ipsi-lesional flocculus at the time of UL, prevented the development of CIE in ipsi-lesional MVN neurons. To our knowledge, these results are the first to show a sustained up-regulation of intrinsic excitability in extra-cerebellar, sub-cortical neurons dependent upon cortical protein phosphorylation, and glutamate- and glucocorticoid-receptor activation in vivo. The results support the hypothesis that cerebellar cortical plasticity after UL, involving the expression of long-term depression (LTD) in the ipsi-lesional flocculus, leads to the induction of CIE in MVN neurons. Thus, cerebellar cortical LTD is dependent upon mGluR and PKC activation (Xia et al. 2000), while GR activation regulates PKC pathways and promotes LTD in the hippocampus (ffrench-Mullen, 1995; Pavlides et al. 1995; Xu et al. 1998; Dwivedi & Pandey, 1999). PKC activation in cerebellar Purkinje neurons is also required for VOR adaptation induced by visual-vestibular mismatch (de Zeeuw et al. 1998), while stress and GR activation are implicated in eye-blink associative conditioning, another model of cerebellar-dependent motor plasticity (Xu et al. 1998; Shors, 2001).
Interestingly, the present results show that after unilateral vestibular de-afferentation, CIE in MVN neurons develops even in urethane-anaesthetised animals, provided that cerebellar cortical GRs are activated by systemic administration or intra-floccular microinjection of dexamethasone. While the flocculus is known to be involved in mediating vestibulo-ocular reflex plasticity in response to changes in visual feedback, in these anaesthetised animals there is no spontaneous nystagmus or oculomotor reflex function. Instead, a likely signal to drive flocculus-mediated plasticity may simply be the large mismatch in the resting activity of brainstem MVN neurons on the lesioned and intact sides.
Recent evidence indicates that ≈15-20 % of neurons in the rostral MVN are flocculus target neurons (FTNs) receiving monosynaptic inhibition from the ipsilateral flocculus, and intracellularly all recorded FTNs so far have been ‘Type B’ MVN neurons (du Lac et al. 1995; Babalian & Vidal, 2000). In the only intracellular study of CIE in MVN neurons to date, we showed that CIE also develops selectively in a subset of Type B neurons (Him & Dutia, 2001). Thus, CIE does not occur in Type A neurons, or in Type B neurons with prominent plateau potentials (Type BDP neurons) and Type B neurons with low-threshold Ca2+ spikes (Type BLTS neurons); instead the in vitro discharge rate of the ‘remaining’ Type B cells (≈50 % of all Type B neurons) is significantly higher than normal post-UL (Him & Dutia, 2001). Since Type A cells form about 30 % of all MVN neurons (Johnston et al. 1994), the ‘remaining’ Type B cells amount to ≈35 % of all MVN cells. While this is a higher proportion than that observed for FTNs in vivo (15-20 %), this is not inconsistent with the hypothesis that CIE may be induced after UL in MVN neurons that receive floccular synaptic input.
How sustained changes in the intrinsic properties of MVN neurons may result from floccular plasticity involving mGluR1 and GR activation and protein phosphorylation is an intriguing question. Recent studies have shown that deep cerebellar nucleus (DCN) neurons possess spontaneous activity and intrinsic membrane properties similar to MVN neurons (Aizenman & Linden, 1999; Czubayko et al. 2001), and that persistent changes in their intrinsic excitability occur after high-frequency stimulation of their synaptic inputs in vitro (Aizenmann & Linden, 2000). DCN neurons are anatomically analogous to MVN neurons, in that both receive direct Purkinje cell projections from their respective areas of cerebellar cortex. As discussed by Aizenmann & Linden (2000), during cerebellar-dependent motor learning such alterations in the intrinsic properties of sub-cortical neurons, in parallel with cortical synaptic plasticity, may provide a powerful and versatile means of modifying signal processing within the cerebellar cortico-nuclear complex. At present it is not known if alterations in intrinsic excitability of sub-cortical neurons, as observed here after de-afferentation in the flocculus- MVN system, are a common feature of cerebellar plasticity. Possible mechanisms that might induce persistent changes in the intrinsic properties of MVN neurons include altered patterning of synaptic inhibitory inputs from the ipsi-lesional flocculus after UL, or the post-synaptic actions of, for example, peptides co-released with GABA at the Purkinje cell synaptic terminals in the MVN.
Stress, glucocorticoid receptors and cerebellar plasticity
The present results show that intrafloccular microinjection of dexamethasone is sufficient to enable CIE in ipsi-lesional MVN neurons in urethane-anaesthetised animals, while intrafloccular microinjection of the GR antagonist RU38486 prevents the expected development of CIE in anaesthetised animals given systemic dexamethasone. These findings support recent behavioural and cellular studies implicating stress, corticosteroids and GRs in vestibular compensation (Yamanaka et al. 1995; Cameron & Dutia, 1999; Yamamoto et al. 2000; review, Seemungal et al. 2001), and suggest that a major site of glucocorticoid action is within the cerebellar cortex. There is particularly high GR expression in cerebellar cortex (Sousa et al. 1989), with very high levels of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1; Moisan et al. 1990a,b), an intracellular enzyme that potently regenerates active glucocorticoids within cerebellar and hippocampal neurons (Yau et al. 2001). Glucocorticoids exert profound effects on neuronal and synaptic function in the hippocampus and limbic system (Joels, 2001), but the function of the high levels of cerebellar GR and 11β-HSD1 have been enigmatic. The present results indicate for the first time, a role of cerebellar GR in mediating MVN neuronal plasticity in vivo, and raise the possibility that broadly analogous mechanisms may be involved in glucocorticoid modulation of other forms of cerebellum-dependent motor plasticity (e.g. Xu et al. 1998; Shors, 2001).
Acknowledgments
The support of the Wellcome Trust is gratefully acknowledged. A.R.J. is supported by a Wellcome Career Development Fellowship.
References
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. Journal of Neurophysiology. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature Neuroscience. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Azzena GB, Mameli O, Tolu E. Cerebellar contribution in compensating the vestibular function. Progress in Brain Research. 1979;50:599–606. doi: 10.1016/S0079-6123(08)60858-4. [DOI] [PubMed] [Google Scholar]
- Babalian AL, Vidal PP. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. Journal of Neurophysiology. 2000;84:2514–2528. doi: 10.1152/jn.2000.84.5.2514. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Freilino M, Romero GG. Protein kinase C inhibition blocks the early appearance of vestibular compensation. Brain Research. 1999;845:97–101. doi: 10.1016/s0006-8993(99)01958-7. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Qian ZY, Kim HJ, Yoshimura J. Activity-dependent distribution of protein kinase C-delta within rat cerebellar Purkinje cells following unilateral labyrinthectomy. Experimental Brain Research. 2001;141:6–20. doi: 10.1007/s002210100855. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Cellular basis of vestibular compensation: changes in intrinsic excitability of MVN neurons. NeuroReport. 1997;8:2595–2599. doi: 10.1097/00001756-199707280-00035. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurons dependent on glucocorticoid receptor activation. Journal of Physiology. 1999;518:151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courjon JH, Flandrin JM, Jeannerod M, Schmid R. The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Research. 1982;239:251–257. doi: 10.1016/0006-8993(82)90847-2. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. Journal of Vestibular Research. 1995;5:67–107. [PubMed] [Google Scholar]
- Czubayko U, Sultan F, Thier P, Schwarz C. Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. Journal of Neurophysiology. 2001;85:2017–2029. doi: 10.1152/jn.2001.85.5.2017. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Progress in Neurobiology. 2000;62:313–325. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annual Review of Neuroscience. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Johnston AR, McQueen DS. Tonic activity of rat medial vestibular nucleus neurons in vitro and its inhibition by GABA. Experimental Brain Research. 1992;88:466–472. doi: 10.1007/BF00228176. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Administration of dexamethasone up-regulates protein kinase C activity and the expression of gamma and epsilon protein kinase C isozymes in the rat brain. Journal of Neurochemistry. 1999;72:380–387. doi: 10.1046/j.1471-4159.1999.0720380.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Mullen JM. Cortisol inhibition of calcium currents in guinea pig hippocampal CA1 neurons via G-protein-coupled activation of protein kinase C. Journal of Neuroscience. 1995;15:903–911. doi: 10.1523/JNEUROSCI.15-01-00903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto MM, Romero GG, Balaban CD. Transient changes in flocculonodular lobe protein kinase C expression during vestibular compensation. Journal of Neuroscience. 1997;17:4367–4381. doi: 10.1523/JNEUROSCI.17-11-04367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BP, Dutia MB. Cellular basis of vestibular compensation: analysis and modelling of the role of the commissural inhibitory system. Experimental Brain Research. 2001;137:387–396. doi: 10.1007/s002210100677. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Him A, Dutia MB. Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation. Brain Research. 2001;908:58–66. doi: 10.1016/s0006-8993(01)02600-2. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Courjon JH, Flandrin JM, Schmid R. Supravestibular control of vestibular compensation after hemilabyrinthectomy in the cat. In: Flohr H, Precht W, editors. Lesion-Induced Neuronal Plasticity in Sensorimotor Systems. Berlin: Springer; 1981. pp. 208–220. [Google Scholar]
- Joels M. Corticosteroid actions in the hippocampus. Journal of Neuroendocrinology. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABAA and GABAB receptors during vestibular compensation. NeuroReport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Johnston AR, MacLeod NK, Dutia MB. Ionic conductances contributing to spike repolarization and after- potentials in rat medial vestibular nucleus neurons. Journal of Physiology. 1994;481:61–77. doi: 10.1113/jphysiol.1994.sp020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H. Role of the flocculus in the development of vestibular compensation: immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience. 1997;76:571–580. doi: 10.1016/s0306-4522(96)00374-0. [DOI] [PubMed] [Google Scholar]
- Llinas R, Walton K, Hillman DE, Sotelo C. Inferior olive: its role in motor learing. Science. 1975;190:1230–1231. doi: 10.1126/science.128123. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Seckl JR, Brett LP, Monder C, Agarwal AK, White PC, Edwards CRW. 11β-hydroxysteroid dehydrogenase mRNA expression, bioactivity and immunoreactivity in rat cerebellum. Journal of Neuroendocrinology. 1990a;2:853–858. doi: 10.1111/j.1365-2826.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Seckl JR, Edwards CRW. 11 beta-hydroxysteroid dehydrogenase bioactivity and messenger RNA expression in rat forebrain: localization in hypothalamus, hippocampus, and cortex. Endocrinology. 1990b;127:1450–1455. doi: 10.1210/endo-127-3-1450. [DOI] [PubMed] [Google Scholar]
- Moroni F, Lombardi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Torregrossa SA, Pellegrini-Giampietro DE, Luneia R, Pellicciari R. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. Journal of Pharmacology and Experimental Therapeutics. 1997;281:721–729. [PubMed] [Google Scholar]
- Pavlides C, Kimura A, Magarinos AM, McEwen BS. Hippocampal homosynaptic long-term depression/depotentiation induced by adrenal steroids. Neuroscience. 1995;68:379–385. doi: 10.1016/0306-4522(95)94332-s. [DOI] [PubMed] [Google Scholar]
- Ris L, Capron B, Vibert N, Vidal PP, Godaux E. Modification of the pacemaker activity of vestibular neurons in brainstem slices during vestibular compensation in the guinea pig. European Journal of Neuroscience. 2001;13:2234–2240. doi: 10.1046/j.0953-816x.2001.01603.x. [DOI] [PubMed] [Google Scholar]
- Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. Journal of Neurophysiology. 1998;80:2352–2367. doi: 10.1152/jn.1998.80.5.2352. [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Gresty MA, Bronstein AM. The endocrine system, vertigo and balance. Current Opinion in Neurology. 2001;14:27–34. doi: 10.1097/00019052-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Research Reviews. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Sousa RJ, Tannery NH, Lafer EM. In situ hybridization mapping of glucocorticoid receptor messenger ribonucleic acid in rat brain. Molecular Endocrinology. 1989;3:481–494. doi: 10.1210/mend-3-3-481. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. Journal of Biological Chemistry. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Vibert N, Beraneck M, Bantikyan A, Vidal PP. Vestibular compensation modifies the sensitivity of vestibular neurons to inhibitory amino acids. NeuroReport. 2000;11:1921–1927. doi: 10.1097/00001756-200006260-00023. [DOI] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proceedings of the National Academy of Sciences of the USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yamanaka T, Matsunaga T. The effect of stress application on vestibular compensation. Acta Otolaryngologica. 2000;120:504–507. doi: 10.1080/000164800750046009. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurons after unilateral labyrinthectomy. Journal of Physiology. 2000;523:413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Sasa M, Amano T, Miyahara H, Matsunaga T. Role of glucocorticoid in vestibular compensation in relation to activation of vestibular nucleus neurons. Acta Otolaryngologica Suppl. 1995;519:168–172. doi: 10.3109/00016489509121895. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ, Seckl JR. Lack of tissue glucocorticoid reactivation in 11beta -hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proceedings of the National Academy of Sciences of the USA. 2001;98:4716–4721. doi: 10.1073/pnas.071562698. [DOI] [PMC free article] [PubMed] [Google Scholar]


