Abstract
Rats allowed extended daily access (6hrs) to cocaine, consume high doses of the drug and escalate their cocaine intake over days, resembling the pattern of cocaine use seen in human addicts. The current study was designed to test whether such animals would also demonstrate the heightened motivation to seek cocaine seen in human addicts. Rats were trained to lever-press for i.v. cocaine (0.25 mg/infusion) over a 5-day period of 1h sessions. Subjects were then assigned to either a brief-access (1h/day) or an extended-access condition for an additional 10 days. Control rats lever-pressed for i.v. saline. Following the final self-administration session animals were tested for their motivation to receive cocaine in an operant runway apparatus. Extended-access animals exhibited significantly higher motivation for cocaine in the runway (where they received 1.0 mg/kg cocaine i.v. upon goal box entry) as was evident by faster run times and less ambivalence about entering the goal box (i.e. retreat behavior) than either brief-access or control subjects. Brief and extended- access animals, tested in the Elevated Plus Maze, exhibited comparable and significant increases in anxiety following a single 1.0 mg/kg IV injection of cocaine, as compared to saline control animals that were challenged with i.v. saline infusion. Together, these data suggest that extended access to cocaine results in an especially high motivation for the drug that is not accounted for by reductions in the anxiogenic properties of cocaine.
Keywords: Addiction, anxiety, elevated plus maze, rats, runway
1. Introduction
Animals given one hour of daily access to cocaine (brief-access) consume low amounts of the drug each day and maintain stable intake over days. In contrast, rats given six hours of daily access to cocaine (extended access) consume large amounts of the drug each day and escalate their intake over days (Ahmed & Koob, 1998). Thus, the extended-access condition mimics important aspects of cocaine use in human addicts (i.e., i.v. self-administration of cocaine, consumption of large amounts of the drug in a single session, and escalation of use over time) and has therefore been suggested as an animal model for cocaine addiction (Ahmed & Koob, 1998). This hypothesis is bolstered by the demonstration that extended-access animals are neurochemically distinguishable from control and brief-access animals (Ben-Shahar et. al., 2004, 2005, 2006, 2007; Ary et al., 2006). We have therefore hypothesized that the extended-access condition leads to distinct neuroadaptations which render animals less able to control their drug consumption – a characteristic of human cocaine addiction. If this hypothesis is correct, then one would expect that subjects provided extended daily access to cocaine would come to exhibit greater motivation to seek the drug than either naïve control subjects or subjects with a history of only brief daily access to cocaine.
To test this hypothesis we employed a runway model of self-administration developed in our laboratory. In this procedure, animals are trained to traverse a straight alley once each day to obtain an i.v. injection of drug reinforcer delivered upon entry into the goal box. The time required for the un-drugged subject to return to a place (the goal box), where on prior trials drug reinforcement was delivered, provides an index of the subject's motivation to seek the drug. The operant runway has been successfully employed to study motivation of subjects to seek a wide variety of natural and drug reinforcers including food (Ettenberg & Camp 1986b), water (Ettenberg & Camp, 1986a), amphetamine (Ettenberg, 1990), nicotine (Cohen & Ettenberg, 2007), and heroin (Ettenberg et al., 1996). In contrast to that observed with other reinforcing stimuli, subjects running for cocaine develop over trials a unique approach-avoidance behavior in which they run quickly toward the goal box, stop at the threshold of the goal box, and then turn and “retreat” all the way back to the start box (Ettenberg & Geist, 1991, 1993). Such “retreats” are reversed by pretreatment with anxiolytic drugs such as diazepam or buspirone (Ettenberg & Geist, 1991; Ettenberg & Bernardi, 2006), and are thought to reflect ambivalence in animals about entering a goal box associated with cocaine that result from the drug's concurrent positive and negative (anxiogenic) properties (Ettenberg, 2004). The current project used the runway paradigm to assess cocaine-seeking motivation of animals previously exposed to self-administered saline or cocaine (brief- or extended-access). Additionally, since runway performance could be altered by changes in either the positive and/or negative properties of cocaine, the three treatment groups were also assessed for their anxiogenic response to cocaine as measured in the Elevated Plus Maze.
2. Methods
2.1. Subjects
The subjects (n=85, only 74 of which finished the experiment, due to catheter failure and/or lack of significant baseline cocaine self-administration) were male albino Sprague-Dawley rats weighing 275−325g at the beginning of the experiment and obtained from Charles River Laboratories (Hollister, CA). The animals were housed in pairs in plastic hanging cages located within a temperature-controlled (22°C), 12/12 h light/dark cycle (lights on at 2000) environment in the Psychology Department vivarium at UCSB. Subjects had ad libitum access to food and water, except during operant training for food reinforcement (i.e. see Food Training below). During operant training for food reinforcement subjects were food restricted to maintain 85% of their baseline weight. All procedures were conducted in strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the UCSB Institutional Animal Care and Use Committee.
2.2. Surgeries
Rats were implanted with a chronic silastic catheter in the right jugular vein under Isoflurane gas anesthesia (Abbott Laboratories, North Chicago, IL; 4% for induction; 2.0 − 2.5% for maintenance). Atropine (0.04 mg/kg IM) was administered to minimize respiratory congestion during anesthesia. Banamine (2 mg/kg SC), a non-opiate analgesic, was provided to treat post-surgical pain. Each catheter consisted of 13.5 cm long silastic tubing (0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning Corporation, Midland, MI), which was secured to a threaded 22 gauge metal guide cannula (Plastics One, Roanoke, VA) that was secured in place along the midline of the animal's back perpendicular to the dorsal surface. An obdurator was screwed down into the cannula to protect the open end from contamination. The cannula itself was held in place via a small swatch of Bard Mesh (C.R. Bard Inc., Cranston, RI) to which it was glued. The mesh was in turn laid flat subcutaneously on the animal's back. The other end of the catheter ran subcutaneously around the shoulder to the neck where it was inserted into the jugular vein and secured in place by sutures. Animals were allowed 7 days for recovery. Catheter patency was maintained by flushing the i.v. system with 0.1 ml of sterile heparin+timentin/saline (60 IU/ml and 100mg/ml, respectively) solution each day.
2.3. Apparatus
2.3.1. Self-Administration Boxes
Eleven (29 cm wide × 25 cm long × 30 cm high) operant chambers (Med Associates Inc., St. Albans, VT) were used for self-administration training and testing. Each chamber was equipped with two retractable levers, each positioned 7.0 cm above the grid floor on either side of a food pellet trough that was situated 2 cm above the grid floor. Food dispensers were located outside the chambers. A center house light (2.8 W) was situated 28 cm above the grid floor in the center of the back panel. A cue light (2.8 W) was located 6−7 cm above each lever. During self-administration, only the right cue light was used. All behavioral testing equipment and data acquisition were controlled by a desktop personal computer running Med Associates software (MED-PC for Windows, Version 1.17). A liquid swivel (375−22PS, Instech Laboratories Inc.) was located above the center of each operant chamber permitting the animals to freely move about the chamber without strain on the PE tubing. The inlet of the liquid swivel was connected with polyethylene tubing (Plastics One; outer diameter 0.127 cm, inner diameter 0.058 cm) to a 10-ml syringe containing the self-administration solutions and seated in a syringe pump (Med Associates Inc., St. Albans, VT). An additional length of PE tubing passed through a cannula connector (C313CT Plastic One) from the swivel overhead to the animal where it was connected to the external cannula on the animal's back. Intravenous infusions were administered by activation of the syringe pump.
2.3.2. Runways
Two identical wooden straight-arm runways measuring 160 cm long × 12 cm wide × 44 cm high were used to study cocaine-seeking behavior. Attached at opposite ends of the alley were a start box and goal box of equal dimensions (23 × 20 × 44 cm). Both start and goal boxes were separated from the alley by means of sliding doors. The floor of the apparatus consists of 3 mm diameter steel rods laid in parallel 1.2 cm apart and oriented perpendicular to the sidewalls of the runway. Thirteen infrared photocell emitter–detector pairs were distributed along the runway. The first pair was located within the start box, the final pair was located within the goal box, and the 11 remaining pairs were equally spaced along the walls of the alley approximately 15 cm apart from one another and 2.5 cm above the floor. These infrared sensors were wired to a custom built interface (Hamilton-Kinder Co.) that was in turn connected to a desktop computer. The computer ran the custom software that recorded the precise location of the animal in the runway in real time throughout each trial. This software also controlled the opening of the start box door, the closing of the goal box door, and the delivery of the drug reinforcer (i.e., activation of the syringe pump). Suspended above the alley were two long bar magnets aligned in parallel along the entire length of the apparatus. The magnets served as rails, between which a liquid swivel (375−22PS, Instech Laboratories Inc.) was located. The top of the swivel was connected by PE 20 tubing to a 10-ml drug-filled syringe and its bottom was connected by PE 20 tubing to the guide cannula on the animal's back. A flat circular disk served as a collar around the swivel and prevented it from falling through the space between the two magnetic rails. The swivel permitted the animal to turn and move throughout the apparatus without disconnecting itself from the drug delivery system and without tangling the PE tubing that ran from the swivel to the animal's-back. As the subject ran down the alley, it essentially was pulling behind and above it the swivel that was moving along between the two magnetic rails. To minimize the weight and hence the pull of the swivel on the animal's back, a pot magnet was affixed to the underside of the swivel's collar. The polarity of the pot magnet and the polarity of the rails were aligned so as to repel one another. As a result, the swivel literally floated slightly above the top of the rails and thereby produced minimal resistance to the animal as it was moving about within the runway below.
2.3.3. Elevated Plus-Maze
The Plus-Maze (custom made from 2 cm thick plywood) was elevated 60 cm above the floor. The four arms were each 50 cm long and 10 cm wide and emanated from a 10 by 10 cm center square. Two of the arms were “closed” (had 40 cm high walls), and two were open (no walls). The maze was located inside a closed room and animal's movements in the apparatus were recorded by a Panasonic CCTV camera (Stoelting, Wood Dale, Illinois) secured 180 cm above the running surface of the maze. The input from the camera was fed into a desktop computer and analyzed by ANY-maze™ Video Tracking System (copyright 1999−2006 Stoelting Co. CMU) that identified the time spent in each of the four arms of the maze. In this apparatus, the time spent in the open “insecure” arms has been shown to serve as an index of the subjects’ general level of anxiety (e.g. File et al., 1993; Rodgers & Dalvi, 1997).
2.4. Drugs
Cocaine hydrochloride was dissolved in 0.9% physiological saline in a concentration of 0.25 mg/0.1 ml (for the lever-press self-administration phase; comparable on average to 0.73 mg/kg) or 1 mg/0.1 ml/kg (for the runway and plus maze portions of the study). Drug or vehicle solutions were infused in a volume of 0.1 ml over a 4s period. The dose of 1.0 mg/kg i.v. cocaine was selected for the runway phase because this was found to be the most reinforcing in previous dose-response analyses (i.e. Raven et al., 2000).
2.5. Procedures
All training and testing were conducted during the dark phase of the light/dark cycle at the same time each day.
2.5.1. Self-Administration
To facilitate acquisition of operant responding for cocaine, rats were initially trained to lever press for 45mg food pellets during 1-hour test sessions in the operant chambers. Rats were food deprived for 24h before the initiation of food training and maintained on a restricted diet (15 g of lab chow per day) for the duration of food training (one week on average). Each right-lever press resulted in delivery of one 45mg food pellet. Once the lever-press operant was acquired food was again made available ad libitum in the animal's home cage. Surgical implantations of i.v. catheters were performed one to two days after a rat completed the food-training regimen. Seven days after surgery, cocaine self-administration (SA) training began. Training consisted of 1-h daily sessions each of which was initiated by the extension of the levers into the operant chamber and terminated by their subsequent withdrawal. Each right-lever press resulted in an infusion of either 0.1ml physiological saline or 0.25mg cocaine dissolved in 0.1ml physiological saline, and in the illumination of the right cue light for 20s. During the presentation of the cue light, additional right-lever presses were recorded but produced no scheduled consequences. Responses at the left lever were recorded throughout the session but had no effects. After seven days of training, rats were assigned to either a “brief-access” or to an “extended-access” group. Cocaine rats were matched for drug intake per session, such that the overall mean amount of drug intake per session was the same in the brief- and extended-access conditions. The brief-access groups experienced ten additional one-hour daily sessions during which they earned the same i.v. compound as during training: cocaine (Coc1h group) or saline (Saline control group). The extended-access group was provided ten additional sessions of 6 hrs/day access to cocaine (Coc6h group). At the end of this ten-day period, the rats were left alone in their home cages until the next stage of behavioral testing begun.
2.5.2. Runway
Three days following the last self-administration session in the operant boxes a subset of the subjects began a fourteen-day period of cocaine administration in the runway. Subjects received one trial a day. A trial consisted of placing the subject in the start box, opening the start box door, and allowing the rat to traverse the runway and enter the goal box after which, the goal-box door was closed and a single infusion of cocaine (1mg/kg i.v.) was administered. Rats were allowed 15 minutes to enter the goal box once the start box door was opened. Both Run-Times (i.e. the time required to transverse the alley and fully enter the goal-box) and Retreat frequencies were recorded on every trial.
2.5.3. Elevated Plus Maze
Seventeen days (which was parallel to the last day of training in the runway) following the last self-administration session the remaining subjects (i.e. a second subset of subjects) were tested once for cocaine-induced anxiety in the Elevated Plus Maze. Saline control animals (i.e. the animals that received 1h of daily access to saline in the self-administration phase) were each administered either saline 1ml/kg i.v. (SalS group) or cocaine 1.0 mg/kg i.v. (SalC). Brief- and extended-access animals were each administered 1.0 mg/kg cocaine i.v.. Immediately following the saline or cocaine i.v. injection animals were placed on the center-square of the elevated plus maze and allowed to move freely in the maze for 10 minutes. The location of the animal within the elevated plus maze was recorded by the video camera. Each arm of the maze was defined as a separate zone or compartment as was the middle square. An animal was defined as being “within” a particular zone of the maze when the center of its body was inside that zone. Total time spent in the open arms alone (i.e. excluding center time) was calculated. The animals were considered more anxious to the extent that they spent less time in the open arms.
3. Results
3.1. Self-Administration
Saline control animals (n=32), lever-pressing for saline infusions, showed low levels of self-administration (10 infusions per session on average with no significant change over days – Figure 1). Brief-access animals (n=21) maintained relatively constant levels of cocaine self-administration over a ten day period, self-administering on average 18.6 infusions on the first day and 21.5 infusions on the last day (Figure 1). In contrast, extended-access animals (n=21) escalated their cocaine intake over days self-administering on average 15 infusions during the first hour and 77.1 infusions over the entire session on the first day, and 26.3 infusions during the first hour and 118.8 infusion over the entire session on the last day (Figure 1). The data from the first hour of the daily session for the cocaine groups was subjected to a Two-Way ANOVA with repeated measures that yielded a significant main effect for Day (F(1,40)=55.343, p<.0001) and a significant Day × Group interaction (F(1,40)=19.394, p<.0001). Subsequent analysis (i.e. One-Way ANOVAs) revealed no difference between the first and last day for the brief-access group, and no difference between the brief- and extended-access on the first day, but a significant difference between first and last day for the extended-access group (F(1,20)=83.942, p<.0001). Response rates during the 6-hours of the daily session were subjected to a One-Way ANOVA with repeated measures that yielded a significant main effect for Day (F(9,162)=17.025, p<.0001). Subsequent analysis revealed a significant difference between response rates on first vs. the last day for the extended-access animals (F(1,20)=87.111, p<.0001).
Figure 1.
Mean (+SEM) number of cocaine self-administered infusions over days are depicted in the main graph. Significant increases in number of self-administered cocaine infusions in the first hour and over the whole session were observed in the Coc6h group by the third day. * Represents significant difference from the first day of testing (p < 0.01). Mean (+SEM) number of cocaine self-administered infusions on the first (light bars) and tenth (dark bars) day of testing for the saline, coc1h, and coc6h (first hour and whole session) is depicted in the inset graph. * Represents significant difference between first and tenth day of testing (p < 0.001).
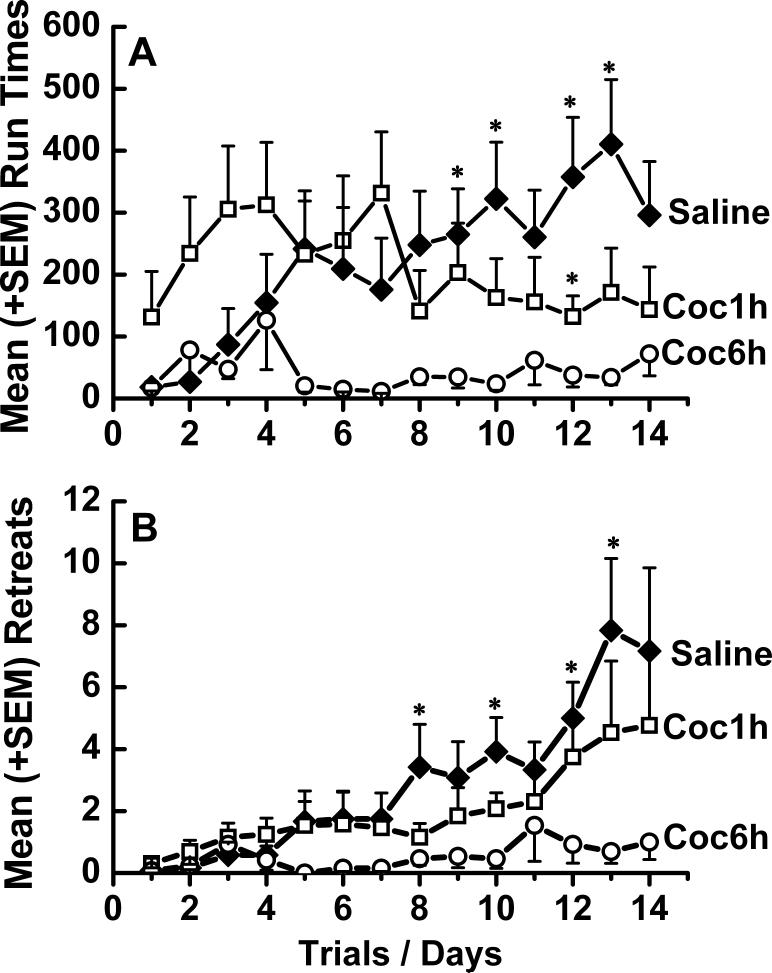
3.2. Runway
Saline control animals (n=12) traversing the runway once a day for i.v. cocaine showed progressively slower Run Times over days that were paralleled by increased numbers of retreats over days (Figure 2). Brief-access animals (n=13) running for a single injection of i.v. cocaine produced run times and retreat behaviors that were qualitatively and quantitatively comparable to that of saline controls. In contrast, extended-access (n=13) animals traversed the alley significantly faster than either the brief access or saline control animals and exhibited significantly fewer retreats compared to saline control animals (Figure 2). Thus, a Two-Way ANOVA with repeated measures computed on the data presented in Figure 2 Panel A yielded a significant main effect for Day (F(13,403)=2.235, p<.008), a significant main effect for Group (F(2,31)=4.778, p<.016), and a significant Group × Day interaction (F(26,403)=2.453, p<.001). While there was no difference between Run Times for the three groups on the first day, LSD Post Hoc tests revealed that the extended-access animals had faster Run Times than both saline control (p < .009) and brief-access (p < .018) animals, but no significant difference between these latter two conditions. Similarly, a Two-Way ANOVA with repeated measures analyzing Retreats, yielded a significant main effect for Day (F(13,403)=7.549, p<.0001), a significant main effect for Group (F(2,31)=4.432, p<.02), and a significant Group × Day interaction (F(26,403)=2.033, p<.002). Post Hoc tests again confirmed no difference between the three groups on the first day and that the extended-access animals had fewer retreats than both control (p < .007) and brief-access (p < .046) animals, while these latter two conditions again were not different from one another.
Figure 2.
Mean (±SEM) times to (Panel A), or number of retreats performed while rats (Panel B), traverse the runway for a single IV cocaine infusion over 14 consecutive days. * Represents significant difference from Saline (p < 0.05).
3.3. Elevated Plus Maze
During either the first 5 minutes of testing or the entire ten minutes, saline control animals challenged with cocaine (SalC; n=11) spent marginally less time in the open arms than did control animals challenged with saline (SalS; n=9). Coc1h (n=8) and Coc6h (n=8) animals spent significantly less time in the open arms than did SalS subjects (see Figure 3). A One-Way ANOVA analyzing these data (for the entire testing period) revealed a significant main effect for Group (F(3,42)=4.098, p< .013), and LSD Post Hoc analysis revealed significant differences between SalS animals and Coc1h animals (p < .004), and between SalS animals and Coc6h animals (p < .004). Percent time spent in the open arms, total number of entries, and percent entries into the open arms, were not significantly different between groups.
Figure 3.
Mean (+SEM) number of seconds spent in the open arms. * Represents significant difference from the SalS group (p < 0.02).
4. Discussion
Brief daily access to cocaine i.v. resulted in stable and moderate levels of cocaine self-administration, while extended daily access to the drug resulted in escalated and high levels of cocaine consumption. These results serve to replicate those of Ahmed & Koob (1998), and our own prior work (Ben-Shahar et al. 2004, 2005, 2006, 2007). Extended access animals traversed the runway significantly faster and with fewer retreats than both the brief access and saline control animals. These data are consistent with the notion that extended access to cocaine results in heightened motivation to seek the drug. Finally, cocaine-induced anxiety, as measured in the Elevated Plus Maze, was similar in the three experimental groups (i.e. saline control, brief access, and extended access animals). These data suggest that the difference in runway performance exhibited by the three experimental groups did not result from a differential anxiogenic response to cocaine.
In the runway, control animals that in the operant boxes self-administered saline, when given the opportunity to run for single daily i.v. injections of 1.0 mg/kg cocaine, behaved in a way that is comparable to that observed in previous studies (Ettenberg & Geist, 1991, 1993). Over trials, these animals took longer and longer to enter the goal box, a result stemming from the increasing ambivalence about entering the goal box (i.e. increased retreats over trials) which in turn reflects the drug's known positive (reinforcing) and negative (anxiogenic) properties (See Ettenberg, 2004). Brief-access subjects tended to traverse the runway at a slower speed initially, but at a faster speed toward the last trials compared to Saline control animals. However, retreat behavior was not different in these two groups. Thus, is spite of the similar increase in retreat behavior seen in both the Coc1h and Saline animals over the last sessions in the runway, Coc1h animals tended to enter the goal box faster than controls. Since, in the runway model of cocaine administration, Run Times reflect the motivation of the subject to seek cocaine, these data imply that brief-access animals experienced increased motivation to re-administer cocaine during the last sessions in the runway. Thus, previous experience with brief daily access to cocaine affected these animals’ motivation to seek cocaine in the runway.
Extended-access animals traversed the alley significantly faster, and exhibited significantly fewer retreats, than either the brief-access or control animals. The significantly shorter Run Times with which the Coc6h animals traversed the runway for one infusion of cocaine show that the Coc6h animals were more motivated to seek cocaine compared to either of the other two groups. Whether this enhanced compulsion to seek cocaine stems from the escalation of cocaine intake over days or simply from their significantly increased accumulated drug intake, is not clear. In either case, the current data are consistent with the notion that the extended-access group reflects an enhanced compulsion to seek cocaine – as is seen in human addicts (Gawin & Kleber, 1985, 1988; Gawin, 1991).
Data from other laboratories have already demonstrated that extended-access animals manifest higher motivation for cocaine. However, such increased motivation was demonstrated either when under the influence of the drug (i.e. higher break points for cocaine in the progressive ratio paradigm; Paterson & Markou, 2003) or in response to the context in which cocaine was self-administered in the past (i.e. higher rates of reinstatement of cocaine seeking behavior; Ahmed & Cador, 2006; Kippin et al. 2006; Mantsch et al. 2004). Our data greatly strengthen and extend these results by showing increased motivation to seek cocaine under conditions in which relapse to cocaine is usually observed in human cocaine-addicts. More specifically, our data shows that extended-access animals will show higher motivation for seek cocaine when not under the influence of either the drug or conditioned stimuli to it, but rather when they are drug free and in the presence of a context that signals the availability of the drug. The current results, therefore, strongly reinforce the argument that the extended access condition result in an enhanced compulsion to seek cocaine when not under the influence of the drug (i.e. to relapse), thus mimicking another very important aspect of addiction in humans that was not fully addressed previously.
In addition to cocaine's well documented reinforcing actions, there is now considerable evidence from both human/clinical and animal/preclinical studies that repeated cocaine administration is also associated with the onset of negative anxiogenic side effects (e.g. Spealman, 1979; Washton & Gold, 1984; Anthony et al., 1989; Rogerio & Takahashi, 1992; Yang et al., 1992; DeVries & Pert, 1998). More recently, the anxiogenic response of rats previously given extended access to cocaine has been assessed with somewhat variable results. For example, Sorge and Stewart (2005) found a small but reliable decrease in shock-induced relapse in extended access animals at 1 day, but not at 10 or 60 days of withdrawal. However, Aujla et al. (2007) reported that extended access rats exhibited a significant increase in defensive burying at 1, 14, and 42 days of withdrawal compared to both brief access, or control, animals. In another study, Mantsch et al. (2007) found reduced basal plasma corticosterone levels but an augmented response to restraint compared to saline controls at 24 days of withdrawal. Finally, Mantsch et al. (2008) found that baseline levels of anxiety, as measured in the Elevated Plus Maze, were statistically similar in saline control animals as compared to both short or extended access animals, but lower in extended access compared to short access animals, after 10 days/sessions of extinction training. Thus, it seems that a history of extended access to self-administered cocaine alters the anxiogenic response of animals differentially depending on the testing procedure.
In the runway, the concurrent reinforcing and anxiogenic effects of cocaine are reflected in the development of approach-avoidance conflict (retreats) about entering the goal box where the drug is delivered (Ettenberg, 2004; Ettenberg & Bernardi, 2006; Ettenberg & Geist, 1991, 1993). In other words, on any given session the speed at which a subject enters the goal box and the number of retreats it executes, directly reflect the strength of cocaine-induced reinforcement and anxiety experienced on previous days/sessions by this subject in the goal box. Thus, in the present experiment, the significant reduction in retreats exhibited by the extended access animals, as compared to the two other experimental groups, could reflect either that: 1. cocaine-induced reinforcement experienced by these animals in the goal box is stronger; or that 2. cocaine-induced anxiety experienced by these animals in the goal box is weaker. In order to test for the second option, namely did the extended access animals experience less anxiety in response to cocaine infusion in the goal box we directly measured cocaine-induced anxiety in our different experimental groups, using the Elevated Plus Maze. In this test, the amount of time spent in the “unprotected” open arms of the maze is used as an index of anxiety (Rodgers & Dalvi, 1997). Cocaine has been shown to reduce the time spent in the open arms, an effect reflective of its anxiogenic actions (Rogerio & Takahasi, 1992).
Subjects were challenged in the Elevated Plus Maze with the same dose used in the runway (i.e., 1 mg/kg cocaine i.v.) so that we are able to determine whether or not the differences observed in runway performance between the three experimental groups might be attributable to a differential sensitivity to the anxiogenic effects of cocaine. Cocaine treated control subjects exhibited a small elevation in anxiety as evidenced by a marginal decrease in the time spent in the open arms compared to control animals challenged with saline. However, both the brief and extended-access groups exhibited significant and similar increases in cocaine-induced anxiety. It would seem then that both groups were experiencing comparable levels of drug-induced anxiety following an i.v. injection of 1.0 mg/kg cocaine, that were marginally higher than that experienced by control animals. This would in turn suggest that the significant reduction in retreats exhibited by the extended-access animals (compared to both the brief-access and control animals) was not the result of lower sensitivity to the anxiogenic effects of cocaine. Rather, extended-access animals were more willing or more motivated to obtain cocaine in spite of the drug's negative anxiogenic actions. It is important to note a significant procedural difference between the runway and elevated plus maze experiments. In the runway animals were exposed to a single dose of cocaine each day for 14 days, whereas in the Elevated Plus Maze animals were not exposed to such cocaine treatments during the intervening period between the end of self-administration and the last/only test. However, it remains the case that 17 days following the cessation of operant lever-press access to cocaine, the drug produced a similar response in all animals. The willingness to experience negative consequences in order to get to the drug is, of course, one of the hallmarks of human drug addiction (Gawin & Kleber, 1985, 1988; Gawin, 1991; American Psychiatric Association, 2000).
5. Conclusions
Extended access animals that self-administered (lever-pressed for) large quantities of cocaine in traditional operant boxes, later exhibited increased motivation to seek cocaine when placed un-drugged in a runway where entry into the goal box had resulted in cocaine delivery on previous trials. Such behavior is reminiscent of the increased cravings observed in detoxified cocaine addicts when placed in an environment in which they had previously got cocaine. In addition, such increased motivation was present in spite of a significant anxiogenic response to cocaine suggesting that these animals were willing to overcome more anxiety in order to self-administer cocaine. We therefore conclude that the extended access condition mimics the compulsive drug seeking behavior observed in cocaine addicts. More importantly, these data are consistent with the notion that excessive cocaine self-administration is sufficient to cause compulsive cocaine use similar to that seen in cocaine addicts.
Acknowledgment
This work was supported by National Institute of Drug Abuse grant DA017104 awarded to OBS and by grant DA05041 awarded to AE.
Abbreviations
- Coc1h
A condition in which rats received one hour of daily access to self-administered cocaine
- Coc6h
A condition in which rats received six hours of daily access to self-administered cocaine
- SA
Self-administration
- SalC
control animals that were challenged with cocaine before testing in the Elevated Plus Maze
- SalS
control animals that were challenged with saline before testing in the Elevated Plus Maze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set-point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. p. 950. [Google Scholar]
- Anthony JC, Tein AY, Petronis KR. Epidemiological evidence on cocaine use and panic attacks. Am. J. Epidemiol. 1989;129:543–549. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK, Ben-Shahar O. Brief- versus extended-access to cocaine differentially alters the expression of Group 1 mGluRs and Homer proteins in the prefrontal cortex of rats. Neuroscience Meeting Planner 2006. 2006 Program No. 294.2. [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with Extended Access to Cocaine Exhibit Increased Stress Reactivity and Sensitivity to the Anxiolytic-Like Effects of the mGluR 2/3 Agonist LY379268 during Abstinence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301588. In Press. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to IV cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–153. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Ettenberg A. Motivational effects of nicotine as measured in a runway model of drug self-administration. Behav Pharmacol. 2007;18:265–271. doi: 10.1097/FBP.0b013e3281f19b3c. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology. 1998;137:333–340. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Haloperidol prevents the reinstatement of amphetamine-rewarded runway responding in rats. Pharmacol Biochem Behav. 1990;36:635–638. doi: 10.1016/0091-3057(90)90268-m. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci. Biobehav. Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85:393–399. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. A partial reinforcement extinction effect in water-reinforced rats intermittently treated with haloperidol. Pharmacol Biochem Behav. 1986a;25:1231–1235. doi: 10.1016/0091-3057(86)90117-6. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav. 1986b;25:813–821. doi: 10.1016/0091-3057(86)90392-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, MacConell LA, Geist TD. Effects of haloperidol in a response-reinstatement model of heroin relapse. Psychopharmacology. 1996;124:205–210. doi: 10.1007/BF02246658. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Viana M, Graeff FG. Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology. 1993;111:491–494. doi: 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Cocaine use in a treatment population: patterns and diagnostic distinctions. NIDA Res Monogr. 1985;61:182–192. [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Evolving conceptualizations of cocaine dependence. Yale J Biol Med. 1988;61:123–136. [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl) 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Behavior maintained by termination of a schedule of self-administered cocaine. Science. 1979;204:1231–1233. doi: 10.1126/science.109920. [DOI] [PubMed] [Google Scholar]
- Washton AM, Gold MS. Chronic cocaine abuse: evidence for adverse effects on health and functioning. Psychiatr Ann. 1984;14:733–743. [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]