Abstract
Disruption of the oxidant/antioxidant balance in the lung is thought to be a key step in the development of many airway pathologies. Hence, antioxidant enzymes play key roles in controlling or preventing pulmonary diseases related to oxidative stress. The superoxide dismutases (SOD) are a family of enzymes that play a pivotal role protecting tissues from damage by oxidant stress by scavenging superoxide anion, which prevents the formation of other more potent oxidants such as peroxynitrite and hydroxyl radical. Extracellular SOD (EC-SOD) is found predominantly in the extracellular matrix of tissues and is ideally situated to prevent cell and tissue damage initiated by extracellularly produced ROS. EC-SOD has been shown to be protective in several models of interstitial lung disease, including pulmonary fibrosis. In addition, alterations in EC-SOD expression are also present in human idiopathic pulmonary fibrosis (IPF). This review discusses EC-SOD regulation in response to pulmonary fibrosis in animals and humans and reviews possible mechanisms by which EC-SOD may protect against fibrosis.
Introduction
Pulmonary fibrosis can result from a variety of insults to the lung including toxins, fibers/particles, autoimmune reactions, drugs, infections, and traumatic injuries. The resulting histopathological changes in the lung can be diverse with overlapping features, characterized by varying degrees of inflammation and fibrosis. If the etiological agent is known, simple avoidance of the agent may result in spontaneous resolution. However, in the majority of the cases the etiology is unknown (idiopathic pulmonary fibrosis; IPF) and there is an unrelenting progression of pulmonary fibrosis that results in increasing symptoms and eventually death in the majority of patients.
IPF is a chronic interstitial lung disease, characterized by parenchymal cell injury and fibrosis of the alveolar parenchyma and a low grade mixed inflammatory infiltrate. The pathogenesis of IPF is known to involve destruction of epithelium and the underlying basement membrane, type II cell hyperplasia, fibroblastic/myofibroblasic foci, and excessive extracellular matrix deposition. Several inflammatory mediators have been implicated in the pathogenesis of IPF. These include cytokines, chemokines, growth factors, and reactive oxygen species (ROS)(43, 73, 125). It has been proposed that the cellular redox state and the balance of oxidants/antioxidants play a significant role in the progression of pulmonary fibrosis in animal models and many studies suggest this is also true for human IPF as well (Fig. 1). Superoxide radical and hydrogen peroxide are generated continuously under normal physiologic conditions and are easily metabolized by numerous antioxidants and antioxidant enzymes. Under pathologic conditions, including pulmonary fibrosis, the production of ROS can be augmented by a variety of mechanisms, and additional oxidants such as hydroxyl radicals and reactive nitrogen species (RNS) can also be formed. Notably, the lung, like other tissues, has highly specialized and compartmentalized antioxidant defenses to protect against ROS and RNS. Thus, the location of the oxidant production and the protective antioxidants will play an important role in mediating the pathologic response to injuries. This article reviews the importance of extracellular oxidative stress and the role of extracellular superoxide dismutase (EC-SOD) in both experimental animal models of pulmonary fibrosis and human IPF.
FIG. 1. Potential roles of reactive oxygen species (ROS) in the pathogenesis of pulmonary fibrosis.
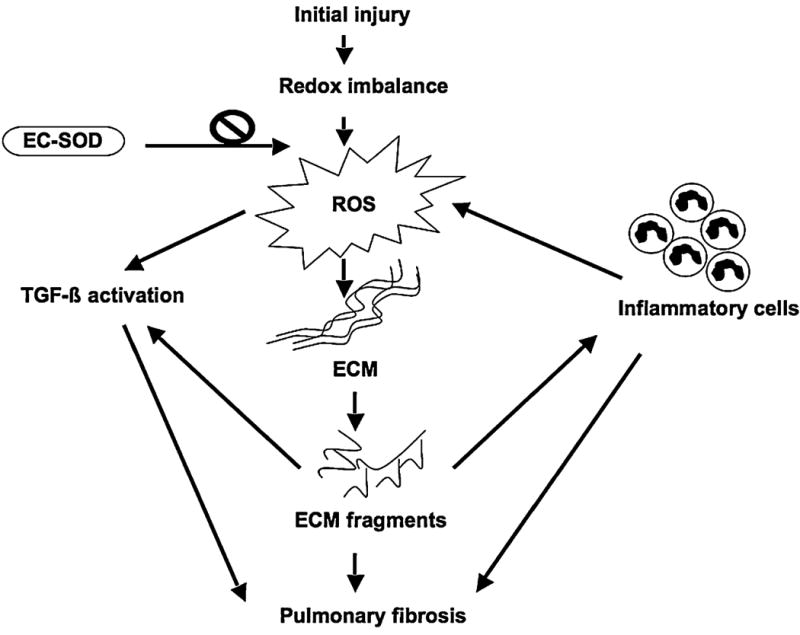
Multiple factors create a redox imbalance, resulting in the production of ROS. ROS can degrade several components of the extracellular matrix, causing ECM remodeling. The ECM fragments produced can lead to inflammatory cell recruitment to the site and further increases ROS production. Meanwhile, persistent inflammation can also trigger the fibrogenic process. ROS and ROS-induced ECM fragmentation products can also activate fibrotic cytokines/growth factors such as TGF-β, thus enhancing this process. EC-SOD can significantly reduce the oxidative stress in the lung parenchyma both in experimental models of lung fibrosis and in human lung.
Biochemical and Molecular Characteristics of EC-SOD
Marklund and coworkers first discovered EC-SOD, a slightly hydrophobic glycoprotein with a molecular weight of 135,000 kDa, in 1982. Although it presents in various organisms as a tetramer (33, 103), a dimer (45), or larger multimers (33, 103), EC-SOD primarily exists as a homotetramer. An important characteristic of EC-SOD is that it contains a heparin/matrix-binding domain, which consists of a cluster of positively charged arginine and lysine residues in the C-terminal of each subunit (Fig. 2). These matrix binding domains enable EC-SOD to bind to the matrix and to cell surfaces in tissues (32). Notably, the heparin/matrix-binding domain is sensitive to proteolysis. Thus, proteases can act to release EC-SOD from tissue matrices and directly alter oxidant/antioxidant balances. In addition to binding heparan-sulfate proteoglycans on the matrix and cell surfaces, the polycationic sequence of EC-SOD may also give it high affinity to other negatively charged components in the matrix (see below).
FIG. 2. This schematic diagram illustrates the heparin-binding affinity patterns of EC-SOD as well as a naturally occurring EC-SOD mutation.

Tissues contain predominantly type C EC-SOD, which consists of a tetramer in which all four subunits contain the carboxy terminal heparin/matrix-binding domain (underlined sequence). The intersubunit disulfide bond links the heparin/matrix-binding domains of the subunits together. Proteolytic removal of these domains does not alter the enzymatic activity of EC-SOD, but results in progressive reduction of it's affinity to the matrix and leads to clearance of the protein from the tissue into the plasma. There is a naturally occurring polymorphism/mutation within the coding sequence of the heparin/matrix binding domain (C760G), which results in an amino acid change from an arginine to a glycine at amino acid residue 213. This amino acid is in the center of the cluster of positive amino acids in the heparin/matrix-binding domain and results in a lower affinity of the enzyme to heparin. This decreased heparin affinity results in elevated serum levels of EC-SOD (adapted from Refs. 33, 102).
EC-SOD contains one copper and one zinc atom per subunit that are required for enzymatic activity (80, 133). Although EC-SOD activity can be inhibited by a variety of agents, including azide, cyanide, diethyldithiocarbamate (DDC), and hydrogen peroxide (81), the EC-SOD protein is very stable and displays marked resistance to high temperatures, pH extremes, and high urea concentrations (132). The EC-SOD gene is ∼60% homologous to Cu-Zn SOD, especially in the region of the active site (145), but shows minimal homology with MnSOD (49). The genomic structure and chromosomal localization of the EC-SOD gene has been mapped in humans to chromosome 4q21 (39), and in mouse to chromosome 5 (40). The expression of EC-SOD mRNA and protein, unlike CuZn-SOD and Mn-SOD, is cell- and tissue- specific and is prominent in the lung, heart, blood vessels, placenta, and kidney. The primary location of EC-SOD in tissues is in the extracellular matrix (79, 102) and on cell surfaces, where it is found at much higher concentrations than are present in the plasma. Indeed, tissue EC-SOD is thought to account for 90–99% of the EC-SOD in the body (78, 79). EC-SOD is also synthesized and secreted by a variety of fibroblast cell lines, glial cell lines, and endothelial cell lines (77, 79). High levels of EC-SOD expression in the lung are primarily attributed to type II epithelial cells (40) and to the smooth-muscle region surrounding blood vessels and airways (102). As the only antioxidant that enzymatically scavenges superoxide in the extracellular compartment, EC-SOD has an important role in a number of lung diseases, where it modulates oxidant injury, inflammation, and fibrosis.
Regulation of EC-SOD in Experimental Animal Models of Pulmonary Fibrosis
Highly localized productions of low levels of ROS/RNS are essential to normal physiological functions in the lung, such as smooth muscle relaxation in airways, blood vessels, and immune responses. However, disruption of the oxidant/antioxidant balance in the lung is thought to be a key step in the development of pulmonary pathologies. Therefore, the regulation of antioxidants such as EC-SOD may be important in modulating or preventing the pathogenesis of many pulmonary diseases. Studies show that exposure to 100% oxygen for 72 h resulted in a significant decrease in EC-SOD levels in the lungs and bronchoalveolar lavage fluid of mice (105). This correlated with a significant depletion of EC-SOD from the alveolar parenchyma and an increase in the ratio of proteolyzed (lacking heparin/matrix binding domain) to unproteolyzed (containing heparin/matrix binding domain) EC-SOD, which suggests that hyperoxia depletes EC-SOD from the alveolar parenchyma by cutting the heparin-binding domain. Proteolytic depletion of EC-SOD may enhance hyperoxic pulmonary injury by altering the oxidant–antioxidant balance in alveolar spaces (105). The role of EC-SOD in the pathogenesis of acute lung injury has also been investigated in mouse models expressing altered levels of EC-SOD. Mice null for EC-SOD were phenotypically normal until stressed (18). However, exposure of EC-SOD-null mice to >99% oxygen caused significant reduction in viability and an earlier onset of severe lung edema as compared to wild-type mice (18). This suggests that, under normal physiological conditions, the animals are capable of compensating for the lack of EC-SOD; but, when exposed to oxidative stress, these mice are unable to compensate and show increased sensitivity (18). These studies indicate that extracellular oxidative stress contributes to the pathogenesis of acute lung injury and that native EC-SOD provides some protection. Furthermore, these studies suggest that loss of EC-SOD from pulmonary matrices may contribute to enhanced pathology.
Bleomycin-Induced Pulmonary Fibrosis Model
The bleomycin-treated mouse is a well-described model system of pulmonary fibrosis in which oxidative stress is known to contribute to pathology. Treatment of mice with bleomycin results in a fibrotic response that occurs in two distinct phases. First, there is an acute phase characterized by an influx of inflammatory cells, in particular, macrophages and polymorphonuclear leukocytes (PMN). This is followed by a chronic stage characterized by extracellular matrix remodeling and collagen deposition (3, 30). An important feature of this model is that activated phagocytes release large amounts of reactive oxygen species (ROS) after bleomycin administration. The ROS generated include the superoxide anion (23, 128), hydroxyl radicals (19, 48), nitric oxide (44, 143) and hydrogen peroxide (72). By using ROS production deficient p47phox −/− (KO) mice, Manoury et al. found that ROS play a central role in the pathogenesis of bleomycin-induced lung injury as the absence of ROS production resulted in protection of the mice against bleomycin-induced pulmonary fibrosis (75).
The role of EC-SOD in bleomycin-induced pulmonary fibrosis has been examined using both the EC-SOD-transgenic(12) and EC-SOD-null mouse models (31). Targeted over-expression of human EC-SOD in the lungs of mice significantly protects these mice against bleomycin-induced lung injury (12) while enhanced bleomycin-induced pulmonary damage occurs in mice lacking EC-SOD (31). Bleomycin-injury in wild-type mice leads to a significant loss of interstitial EC-SOD and an accumulation of EC-SOD in the alveolar lining fluid (31). The finding that knockout mice lacking EC-SOD have increased fibrosis in response to bleomycin suggests that the depletion of interstitial EC-SOD after bleomycin injury may contribute to further oxidative stress in the extracellular matrix that further promotes the fibrotic response.
Asbestos-Induced Pulmonary Fibrosis Model
Asbestos is a group of naturally occurring mineral fibers that are associated with the development of both malignant (lung cancer, mesothelioma) and nonmalignant (asbestosis) diseases in the lung and pleura (93, 94). Asbestos fibers can be divided into two groups, serpentine (curly fibers) and amphiboles (straight fibers). Chrysotile asbestos is the only member of the serpentine group, and these fibers are curly especially when >10 microns in length and frequently have splayed ends. In contrast, crocidolite (blue asbestos) is one of several types of the amphiboles that are straight fibers that do not have splayed ends and do not have a tendency for longitudinal splitting commonly observed with chrysotile. Amphiboles such as crocidolite asbestos are more potent than chrysotile asbestos in inducing asbestos-associated diseases. Clinical manifestations of crocidolite asbestos-induced lung injury are very similar to those described for idiopathic pulmonary fibrosis (IPF), but are generally less severe and slower in progression. A main factor in determining the surface and biological reactivity of asbestos fibers is their ability to participate in redox reactions that generate free radicals. Free radicals generated from asbestos fibers and/or tissue/cellular damage induced by the fibers are linked to cell signaling, inflammation, and a plethora of other responses (mutagenesis, proliferation, etc.) associated with the pathogenesis of asbestos-associated diseases (120). As a result, the asbestos-induced pulmonary fibrosis model is also commonly used to study the oxidant-antioxidant imbalance in the lower respiratory tract in the pathogenesis of pulmonary injury (31, 61, 105, 109).
Intratracheal instillation of crocidolite asbestos causes free radical generation in rodent lungs (46) and induces pulmonary inflammation and fibrosis in rats (53, 54) and mice (126). In addition, studies directly show an increase in superoxide production in patients with asbestos induced lung disease (129). Studies also demonstrated that EC-SOD knockout mice show enhanced lung damage, inflammation, and fibrosis compared to wild-type mice in asbestos-induced lung injuries (31, 35, 126). Similar to bleomycin-injury, asbestos injury also leads to a rapid loss of interstitial EC-SOD with accumulation of the proteolyzed protein, lacking the matrix binding domain, in the alveolar lining fluid (126). This loss of interstitial EC-SOD may augment oxidative stress in the alveolar matrix and further contribute to increased fibrosis. Taken together, these studies once again suggest that oxidants play an important role in the development of interstitial pulmonary injuries and antioxidant enzymes like EC-SOD play an important role in protecting against pulmonary injury.
Mechanisms of EC-SOD Protection in Pulmonary Fibrosis
EC-SOD is expressed in high levels in the lung compared to other tissues (39, 40, 101). The enzyme is found in the matrix of conducting airways and blood vessels, and is also found in the matrix and surface of alveolar septa (102). The alveolar parenchyma is the primary location of fibrosis in human IPF and in mouse models of pulmonary fibrosis. Thus, the matrix and cell surface localization of EC-SOD in the alveolar matrix suggest that its effects on pulmonary fibrosis are likely modulated in these locations. As many matrix components are sensitive to oxidative modification/degradation (66) and one of the hallmarks of pulmonary fibrosis is increased turnover of the ECM, it is likely that one of the primary mechanisms in which EC-SOD protects against pulmonary fibrosis is by preventing oxidant mediated matrix degradation.
Extracellular Matrix
Collagen
Collagen deposition is involved in many lung injuries including asthma (10, 22), emphysema (41, 82), and fibrosis. In the normal lung, type I collagen fibers are localized to the interstitium of alveolar septa in delicate and irregular patterns, while in fibrotic lungs there is a marked increase in type I collagen in thickened septa (74). In addition, epithelial injury and degradation of the basement membrane, primarily comprised of type IV collagen, are known to contribute to the pathogenesis of pulmonary fibrosis (138, 141). Previously, it was shown that type 1 collagen is sensitive to degradation by the superoxide anion both directly and indirectly through the activation of latent collagenases in neutrophils (14, 87, 89, 123). Recently, in vitro studies have shown that type IV collagen is also sensitive to degradation by reactive oxygen species (unpublished observations). Therefore, it can be hypothesized that one way to attenuate the collagen turnover and oxidative damage is to quench these oxidants.
The original immunochemical studies of EC-SOD in the lung found that EC-SOD is associated with high levels with type I collagen in the alveolar septa (102). This observation suggested that EC-SOD might have an important role in protecting collagen from oxidative stress. Subsequent studies demonstrated that EC-SOD specifically binds to type I collagen through the heparin/matrix-binding domain and the bound EC-SOD significantly protects type I collagen from oxidative fragmentation (108). Similarly, recent investigations in our laboratory have shown that EC-SOD also directly binds with type IV collagen and protects it from oxidative damage (unpublished observations). Recent studies have also observed that oxidative fragmentation of collagen occurs in vivo after bleomycin-induced lung injury (31). Using 2-pyrrolidone as an in vivo marker for oxidative fragmentation of collagen at proline residues, this study showed that EC-SOD knockout mice had higher levels of this marker compared to wild-type mice and thus suggests that EC-SOD reduces oxidative fragmentation of collagen(31).
Notably, type I collagen fragments are potent chemoattractants and activators of inflammatory cells (20, 42, 67, 88, 90, 110, 115). We have recently shown that oxidant-derived fragments of type I and type IV collagen are also potent chemoattractants and that EC-SOD can inhibit this process (unpublished observations). Overall, inhibitions of type I and type IV collagen fragmentation is likely to be one mechanism in which EC-SOD inhibits inflammation and fibrosis in models of interstitial lung injury.
Heparan sulfate (HS)
Heparan sulfate is an ubiquitously expressed polysaccharide that appears on cell surfaces and in the extracellular matrices as a proteoglycan. It is a highly sulfated polysaccharide consisting of the repeating disaccharide unit of 1 to 4-linked glucosamine and glucuronic/iduronic acid. It has great structural diversity and has been implicated in numerous biological processes (29, 134). One of the primary functions of heparan sulfate is to bind and localize growth factors and other proteins, including EC-SOD to the tissue matrix (9a). Although the effects of heparan sulfate are not very clear in pulmonary fibrosis, its function and interaction with EC-SOD are greatly discussed and studied in other organ dysfunctions including vascular diseases (2, 50, 55a). EC-SOD's interaction with heparan sulfate was originally recognized when EC-SOD was first purified (80), and it is known to interact with heparan sulfate on cell surfaces. EC-SOD binds to negatively charged heparin/HS through its positively charged C-terminal heparin/matrix-binding domain (34, 55). Matrilysin (MMP7) has recently been shown to be an important mediator of pulmonary fibrosis (24, 69). Syndecan-1, a heparan sulfate proteoglycan, is the primary substrate of MMP7 and once released from cell surfaces contributes to inflammation in the lung (27). Previous studies have indicated that superoxide contributes to ROS-mediated degradation of heparan sufate in vitro. In addition, we have found that there is increased accumulation of several syndecans (heparan sulfate proteoglycans) in the alveolar lining fluid of EC-SOD knockout mice after asbestos injury compared to wild-type mice (62a). Thus, inhibition of oxidative fragmentation of syndecans may yet be another mechanism in which EC-SOD inhibits fibrosis in the lung.
Hyaluronan (HA)
HA is a large unsulfated polyanionic glycosaminoglycan consisting of repeating disaccharide units of N-acetyl glucosamine and glucuronic acid, that is expressed abundantly in the extracellular matrix, as well as on cell surfaces (139). Under physiologic conditions, HA exists as a high molecular weight polymer in excess of 106 Da. It does not induce inflammatory or proliferative genes as a native high molecular mass polymer, but it can activate protein tyrosine cascades in endothelial cells at low levels (71). A significant property of hyaluronan is its capacity to bind huge amounts of water (1000-fold of its own weight). The biosynthesis of hyaluronan differs markedly from that of the other glycosaminoglycans. It occurs in the plasma membrane and does not require the presence of a core protein as a primer. Its biosynthesis is not inhibited by most of the chemicals that inhibit the synthesis of other glycosminoglucans, which supports the concept that hyaluronan synthesis is separate from general glycosaminoglycan synthesis. Many growth factors (epidermal growth factor, platelet-derived growth factor, transforming growth factor-β) are known to activate the biosynthesis of hyaluronan (142). HA can be depolymerized by enzymatic and nonenzymatic processes (68). The enzymes that degrade HA are hyaluronidases, chondroitinases, and hexosaminidases (68). Most hyaluronidases are lysosomal enzymes and require an acid pH for maximal activity (63). Hyaluronan can also be degraded into smaller fragments by exposure to free radicals (26). This is an important mechanism for generating HA fragments at sites of inflammation (116). Studies show that hyaluronan turnover and degradation increase during inflammation and lower molecular weight species of hyaluronan accumulate (86, 92). As HA is a polyanionic matrix component, it is likely that EC-SOD will also have high affinity for this matrix component. Indeed, we have found that EC-SOD does bind to HA and can directly inhibit oxidative degradation of this matrix and cell surface component both in vitro and in vivo (unpublished observation). Hyaluronan accumulation has also been reported in bleomycin (95–97) and asbestos-induced lung injury and fibrosis (16). In fact, BAL fluid from bleomycin-treated animals shows increased concentrations of both the native high molecular weight HA, as well as lower molecular weight HA fragments (95). Furthermore, immunohistochemistry of bleomycin-injured rat lungs shows increased concentrations of HA in the alveolar septa as well as increased HA in alveolar macrophages (97, 119). Low molecular weight hyaluronan fragments have been shown to be capable of activating macrophages and inducing the expression of genes whose functions are relevant to chronic inflammation (83). Meanwhile, inflammatory alveolar macrophages from bleomycin-injured rat lungs express metalloproteinase murine metalloelastase (MME) mRNA, and this expression of MME is further enhanced by HA fragments, which suggest that HA-induced MME may play a role in pulmonary fibrosis (51). Thus, inhibiting HA degradation may yet be another important mechanism in which EC-SOD protects against fibrosis.
Inflammation
Uncontrolled ROS production by activated inflammatory cells can lead to parenchymal, epithelial, and endothelial cell injury, and also to ECM component degradation (59, 122). Degradation and turnover of ECM components are also important factors in inducing inflammatory responses. EC-SOD is a potent inhibitor of inflammation in a number of injury models. EC-SOD attenuates inflammation and neutrophil influx in LPS models (11, 70) and in response to hyperoxia (4, 38). In addition, EC-SOD inhibits inflammation in several models of pulmonary fibrosis (32, 35, 127). We have hypothesized that one of the mechanisms in which EC-SOD inhibits inflammation is by interacting with the components in the extracellular matrix and inhibiting the production of fragments that act as chemoattractants for inflammatory cells.
Noble et al. found that HA has several functions in lung injury and repair. After lung injury, HA fragments accumulate and stimulate alveolar macrophages to produce chemokines that recruit subsets of inflammatory cells. These HA fragments are then cleared from the inflamed lung by alveolar macrophages in a CD44-dependent manner (98). Failure to clear hyaluronan fragments leads to unremitting inflammation, and hematopoietic CD44 is necessary to clear hyaluronan fragments that are produced after lung injury (85, 99, 100). However, in the absence of CD44, alveolar macrophages continue to produce chemokines in response to hyaluronan fragments, implicating another receptor system in controlling macrophage function. The authors also found that Toll-like receptors 2 and 4 (TLR2 and TLR4) are responsible for macrophage inflammatory gene expression in response to hyaluronan fragments (98). In addition, the interaction of receptor for HA-mediated motility (RHAMM, CD168) with HA is a critical component of the recruitment of inflammatory cells to the lung after injury (144). Separate studies also suggest that high molecular form HA is broken down by ROS to form low molecular weight fragments that signal via RHAMM to stimulate ciliary beat frequency (CBF) in the airways; the degradation of HA in this process can also be prevented by SOD (76).
Heparan sulfate also plays an important role in inflammation: it is a potential ligand for P- and L-selectin, two key molecules involved in the adhesion of leukocytes to the inflamed endothelium (47). Heparan sulfate can also bind pro-inflammatory chemokines, transport them across the endothelium, and present them to leukocytes (84). Furthermore, collagen fragments are known to be both chemoattractants and activators of neutrophils (88), and, thus, may enhance inflammatory reactions. Thus, inhibiting oxidative fragmentation of matrix components may be another mechanism in which EC-SOD inhibits inflammation in response to fibrotic injuries.
Interestingly, inflammatory cells infiltrating the areas of injury have been found to contain intracellular EC-SOD (13, 127), especially in neutrophils and macrophages. These cells are the predominant source of EC-SOD that accumulates in the alveolar lining fluid after asbestos injury. As the EC-SOD released from these cells lack the heparin/matrix binding domain, it is incapable of binding to the matrix or cell surfaces, but may represent a mechanism to help restore antioxidant levels to areas after an inflammatory response.
Cytokine Regulation
Fibrotic cytokine overproduction is another hallmark of lung fibrosis. Transforming growth factor-β (TGF-β) is believed to be a critical mediator involved in the fibrotic response through several possible mechanisms. First, it regulates ECM production (121). The synthesis, deposition, and turnover of matrix components by fibroblasts are controlled by soluble mediators and growth factors such as TGF-β and basic fibroblast growth factor (FGF-2) (6). Elevations in TGF-β mRNA and protein content precede the increased expression of collagen in bleomycin-induced pulmonary fibrosis (52). TGF-β also enhances the production of type I and type III collagen in various cell types (36, 124, 135) and hyaluronan in human lung fibroblasts (140).
TGF-β also has potent chemotactic effects on inflammatory cells. Previous studies have shown that TGF-β, if released soon after injury, acts primarily as a pro-inflammatory molecule and later, TGF-β function switches to resolution of inflammation and initiation of repair (137). It has been hypothesized that the persistence of chronic fibrosis may be due to unabated continuation of repair processes after resolution of the inflammatory response. TGF-β therefore may be an important mediator of chronic but abnormal repair.
Another characteristic of TGF-β is its potential interactions with oxidants/antioxidants in the lung. For example, TGF-β-differentiated myofibroblasts can themselves serve as a source of oxidant production (131). Furthermore, in vitro studies have shown that ROS increases the release of TGF-β from pulmonary epithelial cells and can directly activate TGF-β by disrupting its interaction with latency-associated peptide (8, 9), suggesting an oxidant-mediated positive feedback mechanism within the myofibroblast microenvironment. TGF-β has been shown to activate NADPH oxidase in human fibroblasts, leading to increased production of ROS (130).
EC-SOD can prevent lung injury by decreasing TGF-β activation. In an acute radiation-induced lung injury model, overexpression of EC-SOD confers protection, in part via an attenuation of the macrophage response, and also decreases TGF-β1 activation with a subsequent downregulation of the profibrotic TGF-β pathway (112). EC-SOD can also stabilize the ECM components by preventing ROS-induced degradation and further prevent TGF-β activation through ECM-stimulated mechanisms. Thus, modulation of TGF-β activity may be yet another mechanism in which EC-SOD protects against pulmonary fibrosis.
EC-SOD and Human IPF
Characteristic features of human idiopathic pulmonary fibrosis
Human idiopathic pulmonary fibrosis (IPF) (histopathology of usual interstitial pneumonia, UIP) has special features and differs from many other human interstitial lung disorders that may cause fibrosis (1, 57). The characteristic features of human UIP/IPF are the patchy temporally heterogeneous fibrotic involvement of the lung (Fig. 3), a poor response to all medical therapies, and a poor prognosis. In contrast, other fibrotic interstitial lung diseases such as those caused by drugs, radiation, or allergen exposure (extrinsic allergic alveolitis/“hypersensitivity pneumonitis”) have a more diffuse lung involvement and a slower progression, especially when the exposure has been eliminated. Experimental models of lung fibrosis show greater degrees of inflammation than human IPF and are frequently reversible (i.e., bleomycin).
FIG. 3.

In a high resolution computerized tomography (HRCT), subpleural honeycombing as a marker of advanced lung damage/fibrosis is seen bilaterally in the basal areas of IPF lungs (left). In idiopathic pulmonary fibrosis, subpleural fibroblast proliferation and epithelial atypia are considered as hallmarks of the disease (right). Alpha-actin positive cells show red staining in photomicrographs (200× magnification). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Human UIP can be differentiated from other interstitial/fibrotic lung diseases by lung histopathology (Fig. 3). Typically effected regions contain both well-preserved areas with normal lung architecture and damaged areas with ongoing immature fibrosis, as well as end-stage fibrosis (temporal heterogeneity). The fibrosis usually begins in subpleural areas of both lungs, but spreads more centrally with time. The areas of immature fibrosis are the fibroblastic/myofibroblastic foci, and these areas are believed to be the driving force promoting the fibrotic process. The poor prognosis of UIP/IPF is associated with the number of fibroblast foci in the lung (37, 58). Another characteristic feature in UIP/IPF is a low-grade neutrophilic inflammation, especially if compared to other human interstitial lung diseases, which also lack fibroblastic foci. The major features of human UIP/ IPF are listed in Fig. 4.
FIG. 4.
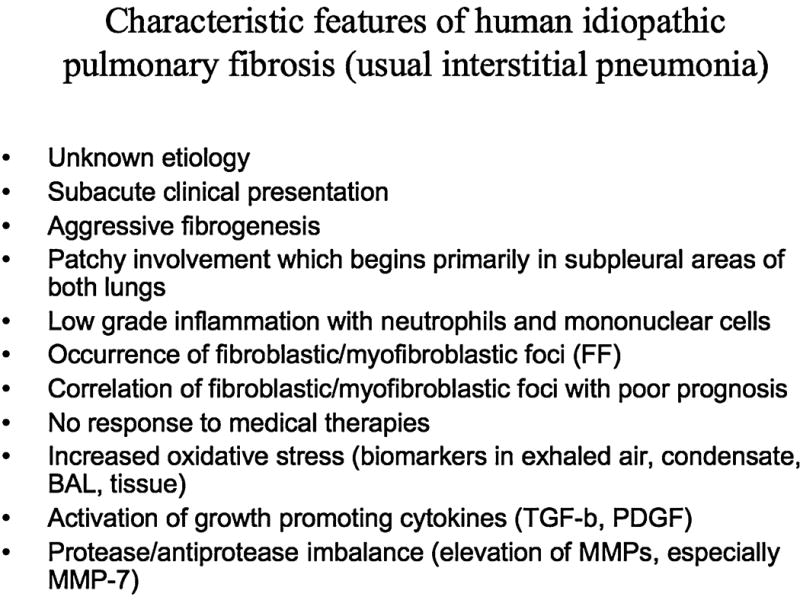
Characteristic features of human idiopathic pulmonary fibrosis (usual interstitial pneumonia).
There is clear evidence of increased oxidative stress in IPF, but it is still unclear why irritants/oxidants lead to fatal lung fibrosis in only a small number of individuals. The current concept of IPF pathogenesis suggests that potential contributors to the development of human IPF include local injury to alveolar epithelium with a subsequent abnormal repair process that includes epithelial cell plasticity, and aberrant myofibroblast differentiation and proliferation. Since no current therapy has any significant effects on the progression of IPF or survival of the patients, there has been escalating interest for the development of new drug therapies for this disease, and these new insights are highly focused on growth factor signaling or activation. Two prominent growth-regulatory cytokines linked to IPF are TGF-β and platelet-derived growth factor (PDGF). TGF-β ligand, receptors, and intracellular signals have been shown to be directly or indirectly activated by reactive oxygen metabolites, and PDGF receptor activation is stringently regulated via H2O2 production (5, 21, 56, 146). Increased oxidant burden is a prominent feature in the IPF lung (15, 17, 61, 64, 65, 91), and one hypothesis in the pathogenesis of this disease is the activation of growth-regulatory cytokines by oxidant–antioxidant imbalances leading to progressive fibrotic events. Differences in susceptibility to oxidant stress-induced fibrotic events could be explained [i.e., by individual differences in the production and activation of TGF-β (7, 28)] and other growth-promoting proteins.
EC-SOD Polymorphism and Human IPF
Another contributing factor to the individual susceptibility to IPF might be variability and polymorphisms of potential antioxidant/detoxification enzymes against exogenous irritants that can lead to fibrosis. EC-SOD has been considered to be one of most important antioxidant enzymes of human lung (34). As described above, experimental studies also emphasize the importance of EC-SOD as a fibroprotective enzyme (12, 32, 35, 38, 126). The human EC-SOD gene contains a single nucleotide polymorphism (G to C) that is known to result in a functionally significant amino acid change from arginine to glycine (Arg213Gly) (118, 145). This polymorphism is not infrequent (3–6% in various populations). This EC-SOD polymorphism is in the center of the polycationic sequence of amino acid residues in the heparin/matrix binding domain and causes decreased anchoring of EC-SOD to heparin/matrix proteins in the lung interstitium. This leads to diffusion of matrix EC-SOD into the plasma and thereby decreases the lung antioxidant defense capacity. In a recent study on UIP/IPF patients (n = 63) and controls (n = 61), this polymorphism appeared to have no influence on patient's clinical course, but that specific study was not powered to exclude the possibility that EC-SOD polymorphism may have a role in the development of lung fibrosis (62). Larger series of patients are needed to confirm the importance of this or other EC-SOD polymorphisms or individual genetic variability in other antioxidant enzymes in UIP/IPF.
Expression of EC-SOD in Human IPF
EC-SOD is expressed in the bronchial and alveolar epithelium, macrophages, vessels, and interstitium in the human lung (104). In IPF lung tissue, the expression of EC-SOD is very similar in alveolar macrophages and airway epithelial cells as in the normal lung (62). However, EC-SOD expression has been found to be low/absent in the fibrotic areas and fibroblastic foci in UIP lungs, compared to less damaged nonfibrotic areas in the same lung, further suggesting that there is increased oxidative stress in the fibrotic areas of the lung (Fig. 5). Low EC-SOD levels may be explained by various mechanisms such as downregulation of this enzyme by growth factors or proteolysis of EC-SOD's heparin/matrix binding domain in the damaged areas of the lung, which leads to diffusion of EC-SOD from the matrix. Another interesting finding has been the high level of EC-SOD expression in the interstitial mast cells in the UIP lung (62). The high number of mast cells has been documented in fibrotic lung (107). The significance of EC-SOD in these cells remains to be investigated, but may represent a source to replenish EC-SOD similar to that seen with inflammatory cells in animal models of fibrosis (see above). Overall, the human lung has highly specific localization of EC-SOD that is disturbed in UIP/IPF lungs.
FIG. 5.
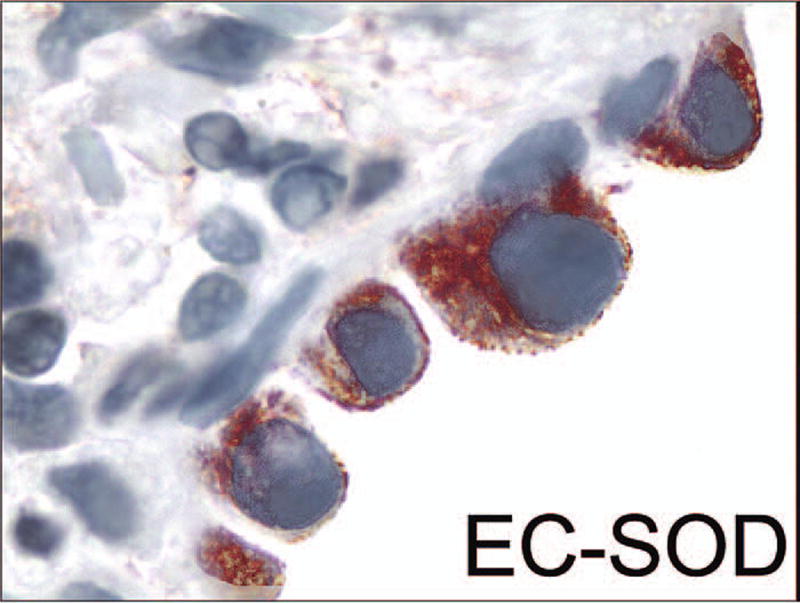
EC-SOD-positive activated epithelial cells (red) are seen on the top of an EC-SOD negative fibroblast focus (1000× magnification). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Exogenous Antioxidants and SOD Mimetics in Pulmonary Fibrosis
Exogenous antioxidants and antioxidant mimetics have been widely investigated in several diseases related to free radical-induced tissue injury. The most widely investigated antioxidants include glutathione, NAC, and SODs. Initially, SODs and their derivatives have been shown to offer significant protection in animal models that lead to fibrosis (60). Several synthetic small-molecular-weight SOD mimetics have been developed (25, 117), and these agents decrease oxidative stress, lung inflammation, and significantly protect the lung in a wide range of animal models, including bleomycin and radiation-induced pulmonary fibrosis (106, 113, 136). More recently, α-phenyl-N-tert-butyl nitrone, a catalytic antioxidant (ECSOD mimetic), manganese (III) mesotetrakis (N,N′-diethyl-1,3-imidazolium-2-yl) porphyrin (AEOL 10150 and AEOL 10113), and a SOD mimetic M40419 have also been reported to inhibit cigarette smoke-induced inflammatory responses in vivo (114). Although synthetic antioxidants might therefore be useful in suppressing the inflammatory responses in the human lung, these compounds have, however, not yet been tested in human lung fibrosis. More studies will be needed not only to examine these compounds in the context of the various stages of IPF but also to evaluate several classes of the new synthetic antioxidants (e.g., SOD mimetics), alone or in combination with other treatment interventions in IPF.
Summary
Accumulating evidence indicates that pulmonary fibrosis represents a spectrum of tissue responses to identified/unidentified injurious agents that result in varying degrees of inflammation and fibrosis. The human IPF, however, differs from most models of experimental pulmonary fibrosis by having a less prominent inflammatory infiltrate and patchy fibrosis, containing areas of new (fibroblastic foci) and late fibrosis. EC-SOD is the only antioxidant enzyme in the ECM and extracellular space that is known to enzymatically scavenge superoxide radicals and thereby prevent the formation of many other reactive oxygen metabolites. This enzyme is expressed at a high level in the lung, compared to most other tissues. Its ability to directly bind several components in the ECM allows it to exist in high concentrations with specific matrix components. This specific localization may be central to its protective effect by preventing oxidant-induced ECM degradation and abolishing fibrotic cytokine TGF-β activation. Importantly, EC-SOD has been shown to protect against experimental pulmonary fibrosis, and its levels are low in the fibrotic areas of human IPF. This low level of interstitial EC-SOD in IPF lungs may therefore contribute to antioxidant imbalances that result in further progression of this relentless disease.
Acknowledgments
The project described was supported by National Institute of Health Grants R01 HL63700 (TDO), R21 ES013986 (TDO), American Heart Association Established Investigator Award and NIEHS grant F32 ES015383-01 (FG) and by The Finnish Antituberculosis Association Foundation and EVO Funding of Helsinki University Hospital (VLK, MM).
Abbreviations
- CBF
ciliary beat frequency
- ECM
extracellular matrix
- EC-SOD
extracellular superoxide dismutase
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- HA
hyaluronan
- HS
heparan sulfate
- IPF
idiopathic pulmonary fibrosis
- MME
murine metalloelastase
- PDGF
platelet-derived growth factor
- PMN
human polymorphic neutrophil
- RHAMM
receptor for HA-mediated motility
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TGF-β
transforming growth factor-β
References
- 1.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Yamazaki N, Tasaki H, Toyokawa T, Yamashita K, Hirano K. Changes in the heparin affinity of extracellular-superoxide dismutase in patients with coronary artery atherosclerosis. Biol Pharm Bull. 1998;21:1090–1093. doi: 10.1248/bpb.21.1090. [DOI] [PubMed] [Google Scholar]
- 3.Adamson IY, Bowden DH. The pathogenesis of bloemycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed MN, Suliman HB, Folz RJ, Nozik–Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med. 2003;167:400–405. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 5.Alejandre–Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla–Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L537–549. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 6.Asplin IR, Wu SM, Mathew S, Bhattacharjee G, Pizzo SV. Differential regulation of the fibroblast growth factor (FGF) family by alpha(2)-macroglobulin: evidence for selective modulation of FGF-2-induced angiogenesis. Blood. 2001;97:3450–3457. doi: 10.1182/blood.v97.11.3450. [DOI] [PubMed] [Google Scholar]
- 7.Awad MR, El–Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation. 1998;66:1014–1020. doi: 10.1097/00007890-199810270-00009. [DOI] [PubMed] [Google Scholar]
- 8.Barcellos—Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 9.Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C, Cadranel J, Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol. 1999;21:128–136. doi: 10.1165/ajrcmb.21.1.3379. [DOI] [PubMed] [Google Scholar]
- 9a.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 10.Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:1308–1313. doi: 10.1164/ajrccm.162.4.9910051. [DOI] [PubMed] [Google Scholar]
- 11.Bowler RP, Nicks M, Tran K, Tanner G, Chang LY, Young SK, Worthen GS. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol. 2004;31:432–439. doi: 10.1165/rcmb.2004-0057OC. [DOI] [PubMed] [Google Scholar]
- 12.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 13.Brady TC, Chang LY, Day BJ, Crapo JD. Extracellular superoxide dismutase is upregulated with inducible nitric oxide synthase after NF-kappa B activation. Am J Physiol. 1997;273:L1002–1006. doi: 10.1152/ajplung.1997.273.5.L1002. [DOI] [PubMed] [Google Scholar]
- 14.Burkhardt H, Hartmann F, Schwingel ML. Activation of latent collagenase from polymorphonuclear leukocytes by oxygen radicals. Enzyme. 1986;36:221–231. doi: 10.1159/000469298. [DOI] [PubMed] [Google Scholar]
- 15.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 16.Cantin AM, Larivee P, Martel M, Begin R. Hyaluronan (hyaluronic acid) in lung lavage of asbestos-exposed humans and sheep. Lung. 1992;170:211–220. doi: 10.1007/BF00174118. [DOI] [PubMed] [Google Scholar]
- 17.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79:1665–1673. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakrabarti S, Makrigiorgos GM, O'Brien K, Bump E, Kassis AI. Measurement of hydroxyl radicals catalyzed in the immediate vicinity of DNA by metal-bleomycin complexes. Free Radic Biol Med. 1996;20:777–783. doi: 10.1016/0891-5849(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 20.Chang C, Houck JC. Demonstration of the chemotactic properties of collagen. Proc Soc Exp Biol Med. 1970;134:22–26. doi: 10.3181/00379727-134-34719. [DOI] [PubMed] [Google Scholar]
- 21.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 22.Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ, Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med. 1998;158:1936–1944. doi: 10.1164/ajrccm.158.6.9712073. [DOI] [PubMed] [Google Scholar]
- 23.Conley NS, Yarbro JW, Ferrari HA, Zeidler RB. Bleomycin increases superoxide anion generation by pig peripheral alveolar macrophages. Mol Pharmacol. 1986;30:48–52. [PubMed] [Google Scholar]
- 24.Dave NB, Kaminski N. Analysis of microarray experiments for pulmonary fibrosis. Methods Mol Med. 2005;117:333–358. doi: 10.1385/1-59259-940-0:333. [DOI] [PubMed] [Google Scholar]
- 25.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 26.Deguine V, Menasche M, Ferrari P, Fraisse L, Pouliquen Y, Robert L. Free radical depolymerization of hyaluronan by Maillard reaction products: role in liquefaction of aging vitreous. Int J Biol Macromol. 1998;22:17–22. doi: 10.1016/s0141-8130(97)00084-6. [DOI] [PubMed] [Google Scholar]
- 27.Ding K, Lopez–Burks M, Sanchez–Duran JA, Korc M, Lander AD. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J Cell Biol. 2005;171:729–738. doi: 10.1083/jcb.200508010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El—Gamel A, Awad MR, Hasleton PS, Yonan NA, Hutchinson JA, Campbell CS, Rahman AH, Deiraniya AK, Sinnott PJ, Hutchinson IV. Transforming growth factor-beta (TGF-beta1) genotype and lung allograft fibrosis. J Heart Lung Transplant. 1999;18:517–523. doi: 10.1016/s1053-2498(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 29.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 30.Fasske E, Morgenroth K. Experimental bleomycin lung in mice. A contribution to the pathogenesis of pulmonary fibrosis. Lung. 1983;161:133–146. doi: 10.1007/BF02713855. [DOI] [PubMed] [Google Scholar]
- 31.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 32.Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198–1207. doi: 10.1016/s0891-5849(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 33.Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun. 2000;275:542–548. doi: 10.1006/bbrc.2000.3327. [DOI] [PubMed] [Google Scholar]
- 34.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 35.Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2006;40:601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine A, Goldstein RH. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987;262:3897–3902. [PubMed] [Google Scholar]
- 37.Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, Murray S, Thannickal VJ, Kazerooni EA, Gross BH, Lynch JP, 3rd, Martinez FJ. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–1415. doi: 10.1164/rccm.200204-373OC. [DOI] [PubMed] [Google Scholar]
- 38.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folz RJ, Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- 40.Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol. 1997;17:393–403. doi: 10.1165/ajrcmb.17.4.2826. [DOI] [PubMed] [Google Scholar]
- 41.Gardi C, Martorana PA, Calzoni P, van Even P, de Santi MM, Cavarra E, Lungarella G. Lung collagen synthesis and deposition in tight-skin mice with genetic emphysema. Exp Mol Pathol. 1992;56:163–172. doi: 10.1016/0014-4800(92)90033-8. [DOI] [PubMed] [Google Scholar]
- 42.Garnotel R, Rittie L, Poitevin S, Monboisse JC, Nguyen P, Potron G, Maquart FX, Randoux A, Gillery P. Human blood monocytes interact with type I collagen through alpha × beta 2 integrin (CD11c-CD18, gp150-95) J Immunol. 2000;164:5928–5934. doi: 10.4049/jimmunol.164.11.5928. [DOI] [PubMed] [Google Scholar]
- 43.Gauldie J, Jordana M, Cox G. Cytokines and pulmonary fibrosis. Thorax. 1993;48:931–935. doi: 10.1136/thx.48.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genovese T, Cuzzocrea S, Di Paola R, Failla M, Mazzon E, Sortino MA, Frasca G, Gili E, Crimi N, Caputi AP, Vancheri C. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerlach D, Reichardt W, Vettermann S. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol Lett. 1998;160:217–224. doi: 10.1111/j.1574-6968.1998.tb12914.x. [DOI] [PubMed] [Google Scholar]
- 46.Ghio AJ, Kadiiska MB, Xiang QH, Mason RP. In vivo evidence of free radical formation after asbestos instillation: an ESR spin trapping investigation. Free Radic Biol Med. 1998;24:11–17. doi: 10.1016/s0891-5849(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 47.Gotte M. Syndecans in inflammation. FASEB J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 48.Gutteridge JM, Rowley DA, Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of ‘free’ iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981;199:263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho YS, Howard AJ, Crapo JD. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol. 1991;4:278–286. doi: 10.1165/ajrcmb/4.3.278. [DOI] [PubMed] [Google Scholar]
- 50.Horiuchi M, Tsutsui M, Tasaki H, Morishita T, Suda O, Nakata S, Nihei S, Miyamoto M, Kouzuma R, Okazaki M, Yanagihara N, Adachi T, Nakashima Y. Upregulation of vascular extracellular superoxide dismutase in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 2004;24:106–111. doi: 10.1161/01.ATV.0000104240.56460.AB. [DOI] [PubMed] [Google Scholar]
- 51.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162:4171–4176. [PubMed] [Google Scholar]
- 52.Hoyt DG, Lazo JS. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther. 1988;246:765–771. [PubMed] [Google Scholar]
- 53.Kamp DW, Israbian VA, Preusen SE, Zhang CX, Weitzman SA. Asbestos causes DNA strand breaks in cultured pulmonary epithelial cells: role of iron-catalyzed free radicals. Am J Physiol. 1995;268:L471–480. doi: 10.1152/ajplung.1995.268.3.L471. [DOI] [PubMed] [Google Scholar]
- 54.Kamp DW, Israbian VA, Yeldandi AV, Panos RJ, Graceffa P, Weitzman SA. Phytic acid, an iron chelator, attenuates pulmonary inflammation and fibrosis in rats after intratracheal instillation of asbestos. Toxicol Pathol. 1995;23:689–695. doi: 10.1177/019262339502300606. [DOI] [PubMed] [Google Scholar]
- 55.Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988;255:223–228. [PMC free article] [PubMed] [Google Scholar]
- 55a.Karlsson K, Sandstrom J, Edlund A, Edlund T, Marklund SL. Pharmacokinetics of extracellular-superoxide dismutase in the vascular system. Free Radic Biol Med. 1993;14:185–190. doi: 10.1016/0891-5849(93)90009-j. [DOI] [PubMed] [Google Scholar]
- 56.Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 59.Kinnula VL. Production and degradation of oxygen metabolites during inflammatory states in the human lung. Curr Drug Targets Inflamm Allergy. 2005;4:465–470. doi: 10.2174/1568010054526368. [DOI] [PubMed] [Google Scholar]
- 60.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 61.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinnula VL, Hodgson UA, Lakari EK, Tan RJ, Sormunen RT, Soini YM, Kakko SJ, Laitinen TH, Oury TD, Paakko PK. Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology. 2006;49:66–74. doi: 10.1111/j.1365-2559.2006.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62a.Kliment, et al. Extracellular superoxide dismutase protests against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2007;10:000–000. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreil G. Hyaluronidases—a group of neglected enzymes. Protein Sci. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 65.Lakari E, Soini Y, Saily M, Koistinen P, Paakko P, Kinnula VL. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol. 2002;117:132–142. doi: 10.1309/w7t9-hw9v-v94b-r9km. [DOI] [PubMed] [Google Scholar]
- 66.Larios JM, Budhiraja R, Fanburg BL, Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276:17437–17441. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 67.Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol. 1986;39:255–266. doi: 10.1002/jlb.39.3.255. [DOI] [PubMed] [Google Scholar]
- 68.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 69.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 70.Loenders B, Van Mechelen E, Nicolai S, Buyssens N, Van Osselaer N, Jorens PG, Willems J, Herman AG, Slegers H. Localization of extracellular superoxide dismutase in rat lung: neutrophils and macrophages as carriers of the enzyme. Free Radic Biol Med. 1998;24:1097–1106. doi: 10.1016/s0891-5849(97)00434-6. [DOI] [PubMed] [Google Scholar]
- 71.Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J Biol Chem. 2000;275:27641–27649. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]
- 72.Lower EE, Strohofer S, Baughman RP. Bleomycin causes alveolar macrophages from cigarette smokers to release hydrogen peroxide. Am J Med Sci. 1988;295:193–197. doi: 10.1097/00000441-198803000-00006. [DOI] [PubMed] [Google Scholar]
- 73.MacNee W, Rahman I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50 1:S53–58. doi: 10.1136/thx.50.suppl_1.s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madri JA, Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980;11:353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- 75.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marklund SL. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990;266:213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984;74:1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci USA. 1982;79:7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marklund SL. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984;220:269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin–Mosquero C, Peces–Barba G, Rubio ML, Ortega M, Rodriguez–Nieto MJ, Martinez Galan L, Gonzalez–Mangado N. Increased collagen deposition correlated with lung destruction in human emphysema. Histol Histopathol. 2006;21:823–828. doi: 10.14670/HH-21.823. [DOI] [PubMed] [Google Scholar]
- 83.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Middleton J, Neil S, Wintle J, Clark–Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 85.Miyake K, Underhill CB, Lesley J, Kincade PW. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990;172:69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Modig J, Hallgren R. Increased hyaluronic acid production in lung—a possible important factor in interstitial and alveolar edema during general anesthesia and in adult respiratory distress syndrome. Resuscitation. 1989;17:223–231. doi: 10.1016/0300-9572(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 87.Monboisse JC, Bellon G, Dufer J, Randoux A, Borel JP. Collagen activates superoxide anion production by human polymorphonuclear neutrophils. Biochem J. 1987;246:599–603. doi: 10.1042/bj2460599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monboisse JC, Bellon G, Randoux A, Dufer J, Borel JP. Activation of human neutrophils by type I collagen. Requirement of two different sequences. Biochem J. 1990;270:459–462. doi: 10.1042/bj2700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monboisse JC, Braquet P, Randoux A, Borel JP. Non-enzymatic degradation of acid-soluble calf skin collagen by superoxide ion: protective effect of flavonoids. Biochem Pharmacol. 1983;32:53–58. doi: 10.1016/0006-2952(83)90651-2. [DOI] [PubMed] [Google Scholar]
- 90.Monboisse JC, Garnotel R, Randoux A, Dufer J, Borel JP. Adhesion of human neutrophils to and activation by type-I collagen involving a beta 2 integrin. J Leukoc Biol. 1991;50:373–380. doi: 10.1002/jlb.50.4.373. [DOI] [PubMed] [Google Scholar]
- 91.Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med. 1998;158:1524–1527. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- 92.Monzon ME, Casalino–Matsuda SM, Forteza RM. Identification of glycosaminoglycans in human airway secretions. Am J Respir Cell Mol Biol. 2006;34:135–141. doi: 10.1165/rcmb.2005-0256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mossman BT, Gee JB. Asbestos-related cancer and the amphibole hypothesis. The hypothesis is still supported by scientists and scientific data. Am J Public Health. 1997;87:689–690. 690–681. doi: 10.2105/ajph.87.4.689. author reply. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mossman BT, Gee JB. Asbestos-related diseases. N Engl J Med. 1989;320:1721–1730. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- 95.Nettelbladt O, Bergh J, Schenholm M, Tengblad A, Hallgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989;139:759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- 96.Nettelbladt O, Hallgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am Rev Respir Dis. 1989;140:1028–1032. doi: 10.1164/ajrccm/140.4.1028. [DOI] [PubMed] [Google Scholar]
- 97.Nettelbladt O, Scheynius A, Bergh J, Tengblad A, Hallgren R. Alveolar accumulation of hyaluronan and alveolar cellular response in bleomycin-induced alveolitis. Eur Respir J. 1991;4:407–414. [PubMed] [Google Scholar]
- 98.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993;91:2368–2377. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ookawara T, Imazeki N, Matsubara O, Kizaki T, Oh–Ishi S, Nakao C, Sato Y, Ohno H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275:C840–847. doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- 102.Oury TD, Chang LY, Marklund SL, Day BJ, Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 103.Oury TD, Crapo JD, Valnickova Z, Enghild JJ. Human extracellular superoxide dismutase is a tetramer composed of two disulphide-linked dimers: a simplified, high-yield purification of extracellular superoxide dismutase. Biochem J. 1996;317:51–57. doi: 10.1042/bj3170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- 105.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- 106.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 107.Pesci A, Bertorelli G, Gabrielli M, Olivieri D. Mast cells in fibrotic lung disorders. Chest. 1993;103:989–996. doi: 10.1378/chest.103.4.989. [DOI] [PubMed] [Google Scholar]
- 108.Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, Enghild JJ. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 109.Porter DW, Millecchia LL, Willard P, Robinson VA, Ramsey D, McLaurin J, Khan A, Brumbaugh K, Beighley CM, Teass A, Castranova V. Nitric oxide and reactive oxygen species production causes progressive damage in rats after cessation of silica inhalation. Toxicol Sci. 2006;90:188–197. doi: 10.1093/toxsci/kfj075. [DOI] [PubMed] [Google Scholar]
- 110.Postlethwaite AE, Kang AH. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976;143:1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int. 2000;57:385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 112.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase red uces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rabbani ZN, Batinic–Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rahman I, Kilty I. Antioxidant therapeutic targets in COPD. Curr Drug Targets. 2006;7:707–720. doi: 10.2174/138945006777435254. [DOI] [PubMed] [Google Scholar]
- 115.Riley DJ, Kerr JS, Guss HN, Curran SF, Laskin DL, Berg RA. Intratracheal instillation of collagen peptides induces a neutrophil influx in rat lungs. Trans Assoc Am Physicians. 1984;97:290–295. [PubMed] [Google Scholar]
- 116.Saari H. Oxygen derived free radicals and synovial fluid hyaluronate. Ann Rheum Dis. 1991;50:389–392. doi: 10.1136/ard.50.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulm Pharmacol Ther. 2002;15:439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- 118.Sandstrom J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 119.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23:475–484. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 120.Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 121.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 122.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 123.Sorsa T, Saari H, Konttinen YT, Suomalainen K, Lindy S, Uitto VJ. Non-proteolytic activation of latent human neutrophil collagenase and its role in matrix destruction in periodontal diseases. Int J Tissue React. 1989;11:153–159. [PubMed] [Google Scholar]
- 124.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Strausz J, Muller–Quernheim J, Steppling H, Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 126.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- 127.Tan RJ, Lee JS, Manni ML, Fattman CL, Tobolewski JM, Zheng M, Kolls JK, Martin TR, Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol. 2006;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tarnell EB, Oliver BL, Johnson GM, Watts FL, Thrall RS. Superoxide anion production by rat neutrophils at various stages of bleomycin-induced lung injury. Lung. 1992;170:41–50. doi: 10.1007/BF00164754. [DOI] [PubMed] [Google Scholar]
- 129.Teramoto S, Fukuchi Y, Uejima Y, Shu CY, Orimo H. Superoxide anion formation and glutathione metabolism of blood in patients with idiopathic pulmonary fibrosis. Biochem Mol Med. 1995;55:66–70. doi: 10.1006/bmme.1995.1033. [DOI] [PubMed] [Google Scholar]
- 130.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 131.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 132.Tibell L, Aasa R, Marklund SL. Spectral and physical properties of human extracellular superoxide dismutase: a comparison with CuZn superoxide dismutase. Arch Biochem Biophys. 1993;304:429–433. doi: 10.1006/abbi.1993.1371. [DOI] [PubMed] [Google Scholar]
- 133.Tibell L, Hjalmarsson K, Edlund T, Skogman G, Engstrom A, Marklund SL. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci USA. 1987;84:6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- 135.Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vujaskovic Z, Batinic–Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 137.Wahl SM, Hunt DA, Wakefield LM, McCartney–Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei JG, Cui GB, Wang W, Wei LX, Liang GM, Song LJ, Xu JK. Relationship between bleomycin-induced pulmonary fibrosis and vascular endothelial cell injury. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2004;22:354–357. [PubMed] [Google Scholar]
- 139.Weissmann B, Meyer K, Sampson P, Linker A. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem. 1954;208:417–429. [PubMed] [Google Scholar]
- 140.Westergren–Thorsson G, Sarnstrand B, Fransson LA, Malmstrom A. TGF-beta enhances the production of hyaluronan in human lung but not in skin fibroblasts. Exp Cell Res. 1990;186:192–195. doi: 10.1016/0014-4827(90)90227-2. [DOI] [PubMed] [Google Scholar]
- 141.Woods LW, Wilson DW, Segall HJ. Manipulation of injury and repair of the alveolar epithelium using two pneumotoxicants: 3-methylindole and monocrotaline. Exp Lung Res. 1999;25:165–181. doi: 10.1080/019021499270376. [DOI] [PubMed] [Google Scholar]
- 142.Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol. 2004;122:631–639. doi: 10.1111/j.0022-202X.2004.22332.x. [DOI] [PubMed] [Google Scholar]
- 143.Yoshimura S, Nishimura Y, Nishiuma T, Yamashita T, Kobayashi K, Yokoyama M. Overexpression of nitric oxide synthase by the endothelium attenuates bleomycin-induced lung fibrosis and impairs MMP-9/TIMP-1 balance. Respirology. 2006;11:546–556. doi: 10.1111/j.1440-1843.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 144.Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC. Expression and Role of the Hyaluronan Receptor RHAMM in Inflammation after Bleomycin Injury. Am J Respir Cell Mol Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 146.Zhou Q, Meng D, Yan B, Jiang BH, Fang J. Transactivation of epidermal growth factor receptor by insulin-like growth factor 1 requires basal hydrogen peroxide. FEBS Lett. 2006;580:5161–5166. doi: 10.1016/j.febslet.2006.08.068. [DOI] [PubMed] [Google Scholar]


