Abstract
Background
Reduced responsiveness to positive incentives is a central feature of Major Depressive Disorder (MDD). In the present study, we compared neural correlates of monetary incentive processing in unmedicated depressed participants and never-depressed controls.
Methods
Fourteen currently depressed and twelve never-depressed participants underwent fMRI while participating in a monetary incentive delay task. During the task, participants were cued to anticipate and respond to a rapidly presented target in order to gain or avoid losing varying amounts of money.
Results
Depressed and never-depressed participants did not differ in nucleus accumbens (NAcc) activation or in affective or behavioral responses during gain anticipation. Depressed participants did, however, exhibit increasing anterior cingulate activation during anticipation of increasing gains, whereas never-depressed participants showed increasing anterior cingulate activation during anticipation of increasing loss. Depressed subjects also showed reduced discrimination of gain versus nongain outcomes.
Conclusions
The present findings indicate that while unmedicated depressed individuals have the capacity to experience positive arousal and recruit NAcc activation during gain anticipation, they also exhibit increased ACC activation, suggestive of increased conflict during anticipation of gains, in addition to showing reduced discrimination of gain versus nongain outcomes.
Keywords: reward, depression, incentive, accumbens, prefrontal, cingulate, human, FMRI
Depressive disorders are prevalent and burdensome, imposing enormous costs on individuals and society (1), since 16% of the general population develops clinically significant depression (2), and 80% of these individuals experiences recurrent depressive episodes (3). Major Depressive Disorder (MDD) is characterized by two primary affective symptoms: sustained negative affect and reduced positive affect. Cognitive and motivational research has traditionally focused on the first of these symptoms. Findings from this research suggest that depressed individuals attend more to negative than to neutral or positive material, and remember it better (4; 5). Such negative biases have been proposed to account for the development and maintenance of depression (6; 7).
Fewer studies have focused on the role of diminished positive affect in depression (8; 9). These few studies suggest that relative to nondepressed counterparts, depressed individuals report experiencing reduced positive affect (10) and show less autonomic and nonverbal (e.g., facial) responsiveness to positive material (11–13). Depressed individuals also show poorer memory for positive material (14) and fail to behaviorally respond faster for monetary reward (15–17). Finally, among depressed individuals, those who respond to positive material exhibit better symptomatic improvement over the following year, independent of initial symptom severity (12; 18; 19).
A substantial body of animal research implicates subcortical circuitry along the ascending trajectory of mesolimbic dopamine projections in appetitive motivation (20; 21). This mesolimbic circuit includes midbrain nuclei that produce dopamine (e.g., the ventral tegmental area), as well as their subcortical (e.g., the nucleus accumbens (NAcc)), and cortical target regions (e.g., the mesial and orbital frontal cortices) (22). Unresponsiveness in this mesolimbic circuit has been hypothesized to contribute to depression (23). In fact, early studies utilizing electroencephalography revealed decreased resting activity in the left prefrontal cortex of depressed individuals (24–28), which was interpreted to reflect reduced appetitive motivation (29). More recent studies using functional magnetic resonance imaging (FMRI), which enables visualization of changes in activation in small subcortical regions, have revealed reduced mesolimbic responsiveness to positive material in depressed individuals (30–33).
Incentive processing unfolds over time and includes multiple stages (e.g., cue identification, anticipation, behavioral execution, outcome processing, adjustment). Minimally, anticipation of incentives can be distinguished from consumption (34; 35). The second-to-second resolution of event-related FMRI allows investigators detect changes in subcortical activity during these distinct phases of incentive processing in behaving humans (36). Using this method, investigators have found evidence for specialization within the mesolimbic circuit: while anticipation of both primary (e.g., juice) and secondary (e.g., money) rewards increases activation in ventral striatum (including the NAcc), rewarding outcomes instead increase activation in the mesial prefrontal cortex (MPFC), dorsomedial caudate, and posterior cingulate (37; 38). To date, neural responses during reward anticipation and outcomes have not been examined in depressed individuals (although anticipatory activation has been examined in depressed children (39)). Thus, the primary goal of this preliminary study was to examine neural responses to anticipated and actual gain outcomes in a sample of unmedicated adults diagnosed with Major Depressive Disorder (MDD).
In addition to the NAcc, recent findings have also implicated the dorsal anterior cingulate cortex (ACC) in incentive processing and, particularly in conflict monitoring, engagement of control, and incentive-guided behavioral selection (40; 41). Investigators have proposed that the ACC activates under conditions of risk (i.e., involving potential gains but also potential loss) when behavioral errors are more likely (42). In this context, a second goal of the present study was to examine ACC activation in a situation with the potential for affectively conflicting outcomes (i.e., anticipation of gain) in depressed individuals. To the extent that anticipation of gain introduces mood-incongruent conflict in depressed individuals (43), we predicted that they would show increased ACC activation.
Methods
Participants
Fourteen individuals (five male) diagnosed with Major Depressive Disorder (MDD) but no other current Axis I Disorders using the Structured Clinical Interview for DSM (44) and twelve individuals (four male) with no history of any Axis I disorder participated in the present study. All participants spoke fluent English and ranged in age from 18 to 48 years. Approximately half of the MDD participants were recruited from two outpatient university hospital psychiatry clinics, while the other half were self-referred from the community. Participants reported no reported lifetime history of brain injury or primary psychotic ideation, no current diagnoses of panic disorder or social phobia, and no behavioral indications of impaired mental status or mental retardation. Participants were also excluded if they met criteria for alcohol or substance dependence or showed signs of alcohol or substance abuse within the past six months. Participants who were currently taking psychotropic medication (including antidepressants) or who had taken psychotropic medication less than three months prior to the scan were excluded, so that potential group differences could not be attributed to medication effects. No participants had received electroconvulsive therapy. Potential control (CTL) participants were excluded from the study based on the same general and medical criteria adopted for MDD participants, or if they had a lifetime diagnosis of any Axis I disorder. CTL and MDD participants did not differ in terms of age, handedness, or verbal ability (a proxy for general intelligence; see Table 1).
Table 1.
Demographic and clinical information, and behavioral results
| CTL (n=12) | MDD (n=14) | |
|---|---|---|
| Age (years) | 28.67 (4.25) | 30.71 (8.80) |
| Handedness (EHI) | 37.11 (20.49) | 49.91 (10.13) |
| Shipley Vocabulary | 34.75 (1.07) | 34.62 (3.46) |
| GAF | 86.92 (3.80) | 51.79 (7.74)** |
| BDI | 0.50 (0.80) | 25.38 (7.88)** |
| Total earnings ($) | 53.94 (19.80) | 65.07 (29.97) |
| Hit rate (% overall) | 68 (15) | 74 (14) |
| RT (sec overall) | 202.81 (19.31) | 201.15 (18.46) |
significant difference at p<.001 (two-tailed); CTL = never-depressed control participants; MDD = Major Depressive Disorder; EHI = Edinburgh Handedness Inventory; GAF = Global Assessment of Functioning, BDI = Beck Depression Inventory; RT = reaction time
Three trained psychology graduate students and two post-baccalaureate research assistants administered the SCID to all participants. Based on a random sample of 15 diagnostic interviews, inter-rater reliability for the SCID was r=.96. The Global Assessment of Functioning Scale (GAF, Axis V, DSM-IV; American Psychiatric Association, 1994) was also administered to all participants. The GAF provides a reliable rating of psychological, social, and occupational functioning that correlates robustly with other measures of illness severity (12; 45). Inter-rater reliability for the GAF in the present study was high (r=.92). Participants also completed the Beck Depression Inventory II (46), which provided a continuous measure of depressive symptoms.
Monetary Incentive Delay (MID) Task
The MID task was designed to elicit neural responses to monetary incentive anticipation and outcomes (47). Each of two MID task runs consisted of 90 6-second trials, yielding a total of 180 trials. During each trial, subjects saw one of nine cue shapes (cue; 250 msec), fixated on a crosshair as they waited a variable interval (anticipation; 2000–2500 msec), and then attempted to respond with a button press during the presentation of a white target of variable duration (target; 160–360 msec). Feedback (outcome; 1650 msec) followed the disappearance of the target, which notified subjects how much money they had gained or lost that trial as well as their cumulative total up to that point. On incentive trials, subjects could either gain or avoid losing money by pressing the button during target presentation. Task difficulty was based on reaction times collected during the practice session prior to scanning and set such that participants would succeed on approximately 66% of their target responses. FMRI volume acquisitions were time-locked to cue offset and thus were acquired during anticipatory delay and outcome periods (48).
Cues signaled potential gains (n=72, denoted by circles), potential losses (n=72; denoted by squares), or no response requirement (n=36; denoted by triangles). Gain cues signaled the possibility of winning $0.00 (n=18; no lines), $0.20 (n=18; one horizontal line), $1.00 (n=18; two horizontal lines), or $5.00 (n=18; three horizontal lines). Similarly, loss cues signaled the possibility of losing $0.00 (n=18; no lines), $0.20 (n=18; one horizontal line), $1.00 (n=18; two horizontal lines), or $5.00 (n=18; three horizontal lines). “No response” trials (n=36; a triangle) indicated that the subject should not respond during that trial, and instead should wait until the cue signaling the next trial appeared. Trial types were pseudo-randomly ordered within each run, and runs were counterbalanced across subjects. Subjects were trained for at least ten minutes, tested for explicit cue comprehension, and shown the cash they could make during the task prior to entering the scanner.
FMRI Acquisition
Imaging was performed using a 1.5-T General Electric MRI scanner with a standard quadrature head coil. Twenty-four 4-mm-thick slices (in-plane resolution 3.75 × 3.75 mm, no gap) extended axially from the mid-pons to the top of the skull, providing adequate spatial resolution of subcortical regions of interest (e.g., midbrain, ventral striatum), and omitting only the base of the cerebellum or crown of the skull in some subjects. Functional whole brain scans were acquired every 2 sec with a T2*-sensitive in-/out- spiral pulse sequence (TE=40 ms, flip=90°) designed to minimize signal dropout at the base of the brain (49). Thus, even in artifact-prone regions (e.g., orbitofrontal cortex, ventral striatum, and amygdala), signal-to-noise ratio was > 40 x and percent maximum signal was > 65%. High-resolution structural scans were subsequently acquired using a T1-weighted spoiled grass sequence (TR=100 ms; TE=7 ms, flip=90°), which facilitated subsequent localization and coregistration of functional data.
FMRI analysis
Analyses focused on changes in blood oxygen level dependent contrast (or “activation”) that occurred during anticipatory and outcome periods, and were conducted using Analysis of Functional Neural Images (AFNI) software (50). For preprocessing, voxel time series were concatenated across task sessions, interpolated to correct for non-simultaneous slice acquisition within each volume (using sinc interpolation and the most ventral slice as a reference), corrected for three-dimensional motion (using the third volume of the first session as a reference), and slightly spatially smoothed (kernel FWHM=4 mm). Visual inspection of motion correction estimates ensured that no subject’s head moved more than 2.0 mm in any dimension from one volume acquisition to the next Data were preprocessed with bandpass filtering (admitting frequencies from 6–90 sec), and computation of percent signal change (calculated with respect to the mean activation over the entire experiment in each voxel).
Preprocessed time series data for each individual were analyzed with multiple regression (51). The regression model included a set of four orthogonal regressors of interest: anticipation of gain (i.e., +$0.20, +$1.00, or +$5.00) versus nongain (i.e., +$0.00, still requiring a response), anticipation of loss (i.e., +$0.20, +$1.00, or +$5.00) versus nonloss (−$0.00), gain versus nongain outcomes, and nonloss versus loss outcomes. Additional covariates included one regressor that contrasted anticipation of making a response (i.e., on incentive trials) versus no response; two orthogonal regressors highlighting each trial period of interest (i.e., anticipation and outcome); six regressors describing residual motion; and six regressors modeling baseline, linear, and quadratic trends for each experimental session. Regressors of interest were convolved with a gamma-variate function that modeled a prototypical hemodynamic response (52) prior to inclusion in the model. Maps of t-statistics representing each of the regressors of interest were transformed into Z-scores, slightly spatially smoothed (kernel FWHM=4 mm), and spatially normalized by warping to Talairach space. Statistical maps were then generated for the CTL and MDD groups using a meta-analytic formula, and thresholded using a criterion adopted in prior studies of the MID task to correct for multiple comparisons in subcortical, anterior insular, and mesial prefrontal gray matter regions (Z>3.88, p<.05 corrected for 500 comparisons, minimum cluster=four 4 mm3 voxels) (47).
Group data were compared in two ways. First, direct t-tests compared contrast coefficient maps across groups. Four 8-mm diameter spherical volumes of interest (VOIs) were compared in these t-tests for gain versus nongain anticipation: bilateral NAcc, MPFC, and dorsal ACC. T-test comparisons tested for significant group differences in averaged activation extracted from each of these bilaterally averaged VOIs at p<.0167 (correcting for three comparisons). Second, for verification, peak signal change (4 sec lag) was extracted from these VOIs and averaged by trial type (53). Peak signal change was then compared using mixed-model analyses of variance (ANOVAs) with incentive valence (positive, negative) and magnitude ($0.00, $0.20, $1.00, $5.00) as within-subject factors, and diagnostic group (CTL, MDD) as the between-subjects factor.
Behavior and Affect
Reaction times and hit rates were recorded on each trial of the MID task. Mixed-model ANOVAs of hit rates and reaction times were conducted for different trial types, with incentive valence (gain, loss) and magnitude ($0.00, $0.20, $1.00, $5.00) as within-subject factors and diagnostic group (CTL, MDD) as the between-subjects factor. After completing the MID task, participants rated their affective reactions to each of the incentive cues (i.e., happiness, excitement, unhappiness, fear) on 4-point Likert scales. Ratings for positive (i.e., happiness and excitement) and negative (i.e., unhappiness and fear) arousal were averaged to maximize reliability. Mixed-model ANOVAs of hit rate and affect were conducted, with incentive valence (gain, loss) and magnitude ($0.00, $0.20, $1.00, $5.00) as within-subjects factors and group (CTL, MDD) as the between-subjects factor. We also examined possible group differences in head motion by conducting t-tests on the standard deviations of motion estimates (i.e., R-L, A-P, and S-I displacements).
Results
Participant Characteristics
As expected, MDD participants scored lower in general functioning and higher in depressive symptomatology than CTL participants (see Table 1). Whereas the GAF scores of the MDD participants indicated the presence of serious symptoms and impairment, the GAF scores of the CTL participants reflected absent or minor symptoms. The MDD participants had a mean of four previous depressive episodes. The groups did not differ in terms of age, handedness, or vocabulary scores.
Behavior and Affect
The three-way ANOVA conducted on hit rate yielded no significant main effects or interactions, indicating comparable performance on the MID task in the two groups. Similarly, the three-way ANOVA conducted on reaction time yielded only a significant main effect of magnitude (F(3,69)=6.65, p<.001); with no other significant effects. The three-way ANOVA conducted on cue-elicited positive arousal yielded significant main effects of valence (F(1,23)=25.56, p<.001) and magnitude (F(3,69)=19.70, p<.001), and the predicted interaction of valence X magnitude (F(3,69)=7.57, p<.001), but no effects of diagnosis. Similarly, the ANOVA conducted on cue-elicited negative arousal also yielded only significant main effects of valence (F(1,23)=23.11, p<.001) and magnitude (F(3,69)=13.05, p<.001), with a trend towards the predicted interaction of valence X magnitude (F(3,69)=2.72, p<.06), but no effects of diagnosis. Finally, t-tests indicated that there were no significant group differences in overall head motion in any of the three dimensions. Together, these findings indicated that MDD and CTL participants showed similar behavioral performance, similar affective reactions to cues, and similarly low levels of movement across different incentive conditions.
Brain Activation
Gain versus nongain anticipation
Anticipation of gain (all amounts) versus nongain activated foci in the NAcc in both CTL and MDD participants (see Figure 1), extending to other parts of the striatum (i.e., caudate and putamen) and thalamus. In addition, MDD participants showed prominent activation in dorsal mesial cortical regions extending from the ACC through the supplementary motor area to the more posterior motor cortex. MDD participants also showed increased activation at foci in the parahippocampal gyri and parietal cortex (see Supplementary Tables S1 & S2).
Figure 1.
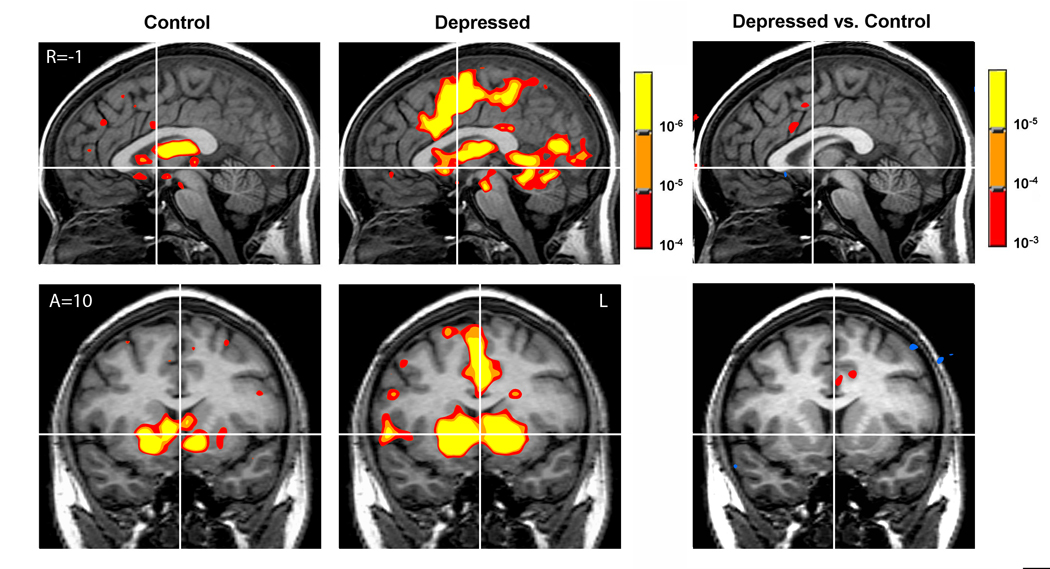
Gain versus nongain anticipation contrasts for control (n=12; left), depressed (n=14; middle), and depressed versus control participants (right).
Loss versus nonloss anticipation
Anticipation of loss (all amounts) versus nonloss activated foci in lateral cortical regions including the middle and inferior frontal gyri and parietal regions, as well as subcortical foci in the insula, caudate, and thalamus for both CTL and MDD participants.
Gain versus nongain outcomes
Gain versus nongain outcomes activated foci in the MPFC and posterior cingulate cortex in both CTL and MDD participants, as well as subcortical foci in the caudate and hippocampus. In addition, the putamen and sublenticular extended amygdala were activated in CTL participants.
Nonloss versus loss outcomes
Nonloss versus loss outcomes activated the middle frontal gyri, parietal cortex, and sublenticular extended amygdala and putamen in CTL participants. Only the caudate head was activated in MDD participants.
Group comparisons
Group analyses suggested greater activation in the anterior cingulate for MDD participants during gain anticipation and possibly in the striatum for CTL participants in response to gain outcomes. To verify these potential group differences, we conducted t-tests to directly compare CTL and MDD participants’ activation in bilateral volumes of interest in the NAcc, MPFC, and ACC. Consistent with the single group maps, these direct comparisons revealed greater activations for MDD than for CTL participants during gain versus nongain anticipation contrasts not in the NAcc, but rather in regions occupying the mesial wall of the prefrontal cortex, including the dorsal ACC (see Table 2). CTL participants showed greater activation than did MDD participants in the MPFC, putamen, and insula in response to gain outcomes. There were no significant group differences in activation of these volumes of interest for other contrasts (see Figure 1).
Table 2.
Comparison of depressed versus control participants (independent t; p<.016, uncorrected, cluster=4; positive Z indicates depressed > control, negative Z indicates control > depressed).
| Region | R | A | S | Peak Z | |
|---|---|---|---|---|---|
| Gain vs Non Anticipation | L Superior Frontal Gyrus (BA 8) | −31 | 17 | 50 | −4.10 |
| L Anterior Cingulate (BA 32) | −11 | 11 | 34 | 3.21 | |
| L Precentral Gyrus (BA 6) | −51 | −3 | 24 | 3.71 | |
| R Postcentral Gyrus (BA 6) | 43 | −15 | 32 | 5.05 | |
| Loss vs Non Anticipation | N/A | ||||
| Gain vs Non Outcome | R MFPC (BA 32) | 8 | 40 | 4 | −3.20 |
| L Insula (BA 47) | −31 | 25 | −6 | −4.32 | |
| R Putamen | 13 | 9 | 8 | −3.57 | |
| L Putamen | −19 | 5 | 6 | −4.48 | |
| L Superior Frontal Gyrus (BA 6) | −17 | 3 | 62 | −3.95 | |
| L Insula (BA 13) | −41 | −25 | 16 | −3.30 | |
| L Postcentral Gyrus (BA 3) | −33 | −33 | 58 | −3.74 | |
| L Inferior Parietal Lobe (BA 40) | −47 | −39 | 30 | −3.91 | |
| Non vs Loss Outcome | L Parahipp. Gyrus | −38 | −45 | −3 | −3.49 |
Volumes of interest
Nucleus Accumbens (NAcc; ±10, 10, −2)
To verify an absence of group differences in NAcc activation during gain anticipation, we directly analyzed peak activation extracted from NAcc VOIs during anticipation. A mixed-model ANOVA (valence by magnitude by diagnostic group) yielded significant main effects of valence (F(1,24)=6.14, p<.05) and magnitude (F(3,72)=12.78, p<.001), and a significant interaction of valence by magnitude (F(3,72)=3.53, p<.05), but no main effect or interactions involving diagnostic group (see Figure 2).
Figure 2.
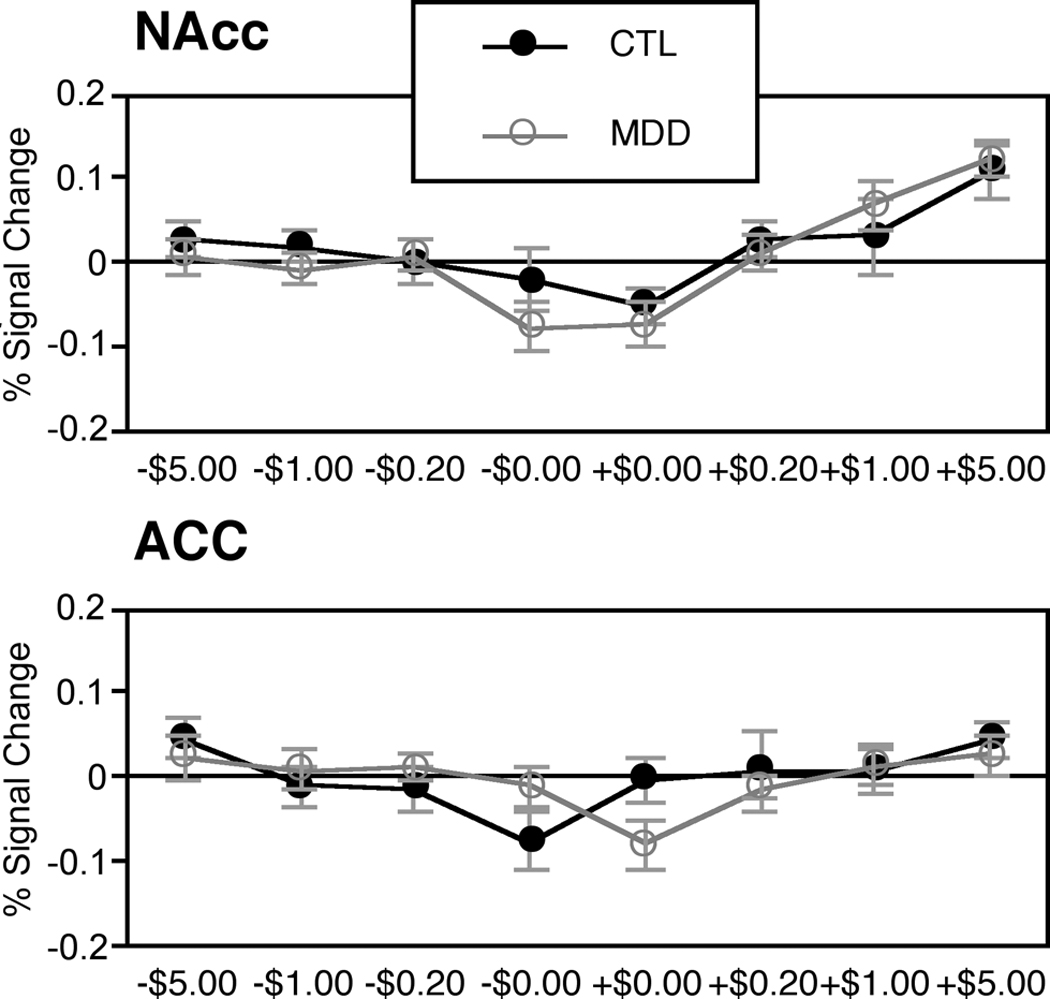
Peak activations by group in nucleus accumbens (top) and anterior cingulate (bottom) volumes of interest (lag = 4 sec; mean ± SEM)
Anterior Cingulate (ACC; ±8, 11, 34)
To examine group differences in ACC activation, we directly analyzed peak activation extracted from ACC VOIs during anticipation. A mixed-model ANOVA (valence by magnitude by diagnostic group) yielded a significant main effect of magnitude (F(3,72)=4.43, p<.01), qualified by a significant interaction of valence and diagnostic group (F(1,24)=4.70, p<.05). A linear trend analysis indicated that whereas CTL participants showed a linear increase in ACC activation during anticipation of losses, MDD participants instead showed a linear increase in ACC activation during anticipation of gains (F(1,24)=4.25, p=.05; see Figure 2).
Mesial Prefrontal Cortex (MPFC; ±4, 50, −4)
To examine potential group differences in MPFC activation, we directly analyzed peak activation extracted from MPFC VOIs in response to large gain (i.e., +$5.00) versus nongain (i.e., +$0.00) outcomes following anticipation of a +$5.00 gain. A mixed model ANOVA (outcome by diagnostic group) yielded a main effect of outcome (F(1,24)=5.03, p<.05), but no significant main effect of diagnosis or interaction of diagnosis by outcome. Thus, unlike statistical maps in the other VOIs, analysis of MPFC peak activation did not support a robust interaction of depression status with responses to gain outcomes.
Brain / Affect Correlations
For each of the large incentive conditions which generated maximum signal (i.e., +$5.00 and −$5.00), cue-induced positive arousal and negative arousal were correlated with cue-induced anticipatory brain activation in the NAcc and ACC VOIs. Replicating previous findings, +$5.00 cue-induced positive arousal correlated with peak NAcc activation after presentation of the +$5.00 cue across groups (r(25)=.53, p<.01). This positive association did not significantly differ for CTL versus MDD subjects (see Figure 3). On the other hand, +$5.00 cue-induced negative arousal did not significantly correlate with peak NAcc activation. Neither did −$5.00 cue-induced positive or negative arousal correlate with peak NAcc activation. There were no significant correlations between +$5.00 or −$5.00 cue-induced positive or negative arousal and corresponding anterior cingulate activation in either group.
Figure 3.
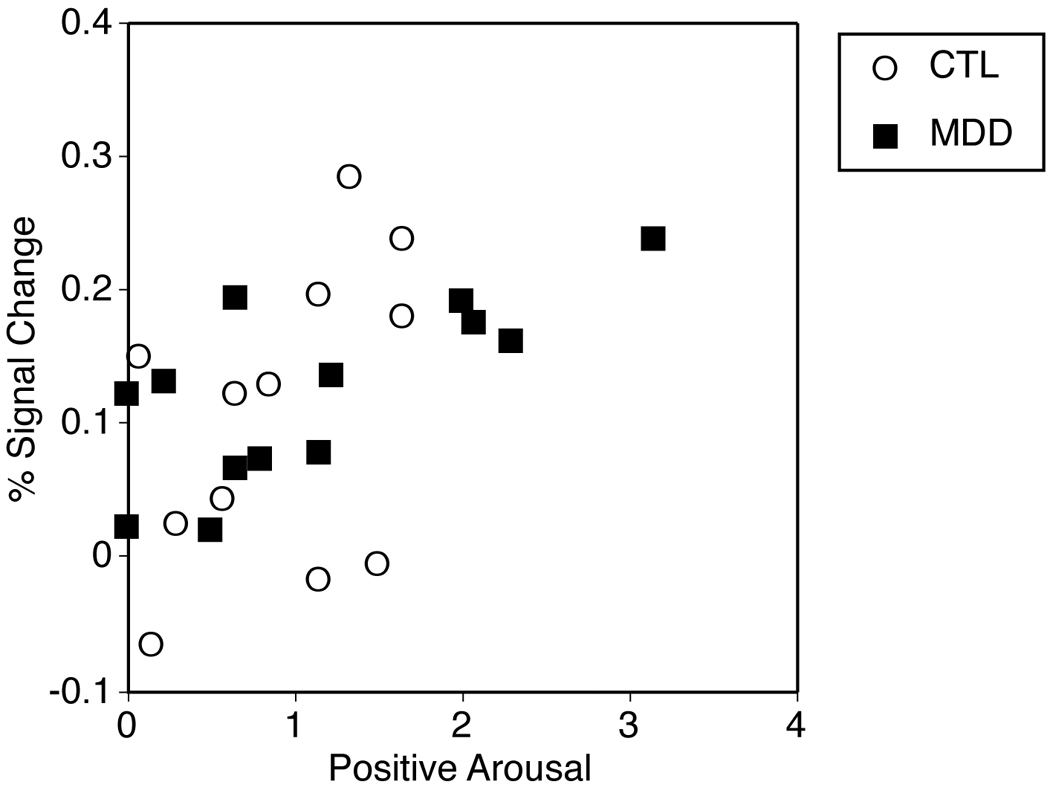
Correlation of +$5.00 cue-elicited peak activation with +$5.00 cue-elicited positive arousal for depressed and control participants (r(25)=.53, p<.01)
Discussion
The present study was designed to contrast neural and subjective responses to monetary incentives in unmedicated depressed participants and never-depressed participants. Because affective disturbances are central symptoms of major depressive disorder, incentive processing might be altered. Moreover, anticipation represents a critical phase of incentive processing because it has the potential to influence subsequent thought and behavior (54).
This research yielded three relevant results. First, because depressed individuals have been found to report reduced positive affect (10; 12), we predicted that they would show less NAcc activation and positive arousal during anticipation of monetary gains. In this sample of depressed participants, however, findings did not support our hypothesis. Neither NAcc activation nor self-reported levels of positive arousal differentiated depressed from never-depressed individuals during gain anticipation. Instead, both groups of participants showed increased NAcc activation and positive arousal while anticipating large monetary gains, and individual differences in NAcc activation correlated with positive arousal in both groups.
This lack of a difference between depressed and healthy individuals stands in contrast to recent findings comparing clinical samples of unmedicated schizophrenics with healthy adults. In event-related FMRI experiments featuring similar-sized samples and the same MID task, unmedicated schizophrenics showed marked blunting of NAcc activation during gain anticipation. Further, in schizophrenic individuals, the degree of blunting correlated with severity of anhedonic symptoms (55; 56). In contrast, the present findings suggest that unmedicated depressed individuals can recruit both NAcc activation and positive arousal during gain anticipation, at least in a highly structured and rapidly-paced task with clearly-defined monetary incentives. In the present sample of depressed individuals, anhedonic symptoms may not have been as prominent as in the sample of schizophrenics described above. Thus, it will be important for futures studies to examine the effects of anhedonic symptoms in depressed individuals on incentive processing.
A second finding involved the anterior cingulate cortex (ACC). Relative to controls, depressed participants exhibited increasing dorsal ACC activation as they anticipated increasing gains. Controls, on the other hand, exhibited increasing dorsal ACC activation as they anticipated increasing losses. ACC activation has been observed in healthy individuals under conditions involving uncertainty and conflict, when errors are likely (40; 57). Activation in a more dorsal and posterior region relative to ACC has been implicated in motor conflict. Because the same button press response was required in all incentive trials, and depressed and never-depressed groups did not differ in reaction times or performance across different conditions, it is unlikely that differences in ACC activation were due to increased motor conflict (as reflected by reaction time). The present findings suggest that whereas healthy individuals experience more affective conflict during anticipation of avoidable losses, depressed individuals experience more affective conflict during anticipation of attainable gains. If affective conflict in the face of uncertain gains characterizes depression, such a neural marker warrants further investigation.
Indeed, abnormal cingulate function has been implicated in previous research in depression. Cingulotomies (lesions of the ACC near regions observed in this study) are one of the few psychosurgical procedures used to treat intractable and therapeutically unresponsive depression (58). Further, positron emission tomography studies of depressed patients have documented increased resting ACC activity in a more subgenual region (59; 60), and inhibition of subgenual ACC activity can ameliorate refractory depression (61). Subgenual ACC activity has also been found to distinguish depressed from nondepressed individuals during exposure to emotional faces (62), and to predict therapeutic response depressed individuals (63; 64). The present study utilized event-related fMRI, which resolves faster changes in activation than do other imaging modalities (e.g., PET, resting EEG, or block design fMRI). Further research must determine whether rapid changes in dorsal ACC activation observed in this study can predict therapeutic response or remission. Some brain imaging evidence points to decreased ACC activation in depressed individuals, but these findings may reflect activation in response to positive outcomes, rather than anticipatory activation (30).
A third finding involved the mesial prefrontal cortex (MPFC). Although both depressed and nondepressed individuals showed MPFC and dorsal striatal responses to gain outcomes, direct comparisons suggested that this response was weaker for depressed individuals. Volume of interest analyses in predicted regions, however, did not yield a significant group difference, suggesting that this finding requires replication and further exploration. Nonetheless, such a finding would provide a replication in depressed adults of research suggesting reduced activation to gain outcomes in depressed children (39).
In the present study, we examined incentive processing in an unselected sample of individuals diagnosed with MDD. Because previous research suggests that the degree of NAcc activation during gain anticipation might specifically vary with anhedonic symptom profiles (17; 33; 55; 65), future studies might profitably focus on depressed individuals with anhedonic symptom profiles. A strength of the present study is that none of the depressed participants was currently taking psychotropic medication. Future studies might also investigate depressed participants on versus off medication. Because NAcc activation has been linked to dopamine release, it is possible that pharmacotherapeutic interventions that target dopaminergic function might have a more pronounced effect on NAcc activation than do drugs that target serotonergic function (66).
In conclusion, the present findings indicate that while carefully diagnosed unmedicated depressed individuals show similarities to never-depressed control participants in their neural and affective responses to monetary incentives, they also show some differences involving increased recruitment of cortical midline structures during gain anticipation, and decreased neural responsiveness to gain outcomes. Further research is needed to further clarify the role of these differences in the maintenance of, and recovery from, depression.
Supplementary Material
Acknowledgements
We thank G. Elliott Wimmer and Jenny Pegg for assistance in data collection and analysis, as well as Jutta Joormann, John Krystal, and three anonymous reviewers for helpful feedback on prior drafts of this manuscript. This research was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (BK) and National Institute of Mental Health Grant MH59259 (IHG).
Footnotes
Financial Disclosure The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;290:2218. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Keller MB, Boland RJ. Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biol Psychiatry. 1998;44:348–360. doi: 10.1016/s0006-3223(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 4.Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. J Abn Psychol. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- 5.Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behav Res Ther. 1996;34:695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT. Cognitive therapy and the emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- 7.Gotlib IH, Krasnoperova E. Biased information processing as a vulnerability factor in depression. Behav Therapy. 1998;29:603–617. [Google Scholar]
- 8.Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abn Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 9.Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Ann Rev Psychol. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- 10.Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnorm Psychol. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- 11.Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J Abnorm Psychol. 2001;110:488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- 12.Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- 13.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in Major Depressive Disorder. J Abn Psychol. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 14.Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: Difficulties in repairing sad mood with happy memories? J Abn Psychol. 2004;113:179–188. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abn Psychol. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 16.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emot. 2000;14:711–724. [Google Scholar]
- 17.Pizzagalli D, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol Psychiatry. 2005:57. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. NeuroReport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 19.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- 20.Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- 21.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuys R. Chemoarchitecture of the brain. New York: Springer Verlag; 1985. [Google Scholar]
- 23.Meehl P. Hedonic capacity: Some conjectures. Bull Menn Clinic. 1975:295–307. [PubMed] [Google Scholar]
- 24.George MS, Ketter TA, Post RM. Activation studies in mood disorders. Psychiatr Ann. 1994;24:648–652. [Google Scholar]
- 25.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abn Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 26.Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal glucose metabolism common to three types of depression. Arch Gen Psychiatr. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 27.Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia -- focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 28.Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emot. 1998;12:449–478. [Google Scholar]
- 29.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- 30.Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- 31.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatr. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatr. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 33.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 36.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 37.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related FMRI. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 38.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 39.Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: An FMRI study. J Child Psychol Psychiatr. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Science. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 41.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 43.Malhi Gs, Laopoulos J, Sachdev P, Mitchell PB, Ivanovski B, Parker GB. Cognitive generation of affect in hypomania: An fMRI study. Bipolar Dis. 2004;6:271–285. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II): I. Description. 1995;9:83–91. [Google Scholar]
- 45.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory - II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 47.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 48.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related FMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 49.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 50.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance images. Comp Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 51.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. Fourth ed. Chicago: Irwin; 1996. [Google Scholar]
- 52.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 53.Kuhnen CM, Knutson B. The neural basis of financial risk-taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 56.Juckel G, Schlagenhauf F, Filonov D, Wustenberg T, Villringer A, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical but not atypical neuroleptics. Psychopharmacology (Berl) 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 57.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll DC, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of perofrmance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 58.Spangler WJ, Cosgrove GR, Ballantine HT, Cassem EH, Rauch SL, Nierenberg A, et al. Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery. 1996;38:1071–1076. [PubMed] [Google Scholar]
- 59.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatr. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 60.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 61.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. NeuroReport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 63.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam KM. Depression: Perspectives from affective neuroscience. Ann Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 64.Mayberg HS. Modulating dyfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–210. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 65.Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, et al. Neural response to pleasant stimuli in anhedonia: An fMRI study. NeuroReport. 2003;4:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- 66.Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl.) 2007;91:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


