Abstract
In June 2005 a WHO-IPCS expert meeting was held in Geneva during which the toxic equivalency factors (TEFs) for dioxin like compounds, including some polychlorinated biphenyls (PCBs), were re-evaluated. For this re-evaluation process the refined TEF database recently published by Haws and coworkers (Toxicol. Sci. 2006, 89:4-30) was used as a starting point. Decisions about a TEF value were made based on a combination of unweighted relative effect potency (REP) distributions from this database, expert judgement and point estimates. Previous TEFs were assigned in increments of 0.01, 0.05, 0.1, etc., but for this re-evaluation it was decided to use half order of magnitude increments on a logarithmic scale of 0.03, 0.1, 0.3 etc. Changes were decided by the expert panel for 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) (TEF=0.3), 1,2,3,7,8-pentachlorodibenzofuran (PeCDF) (TEF=0.03), octachlorodibenzo-p-dioxin (OCDD) and octachlorodibenzofuran (OCDF) (TEFs=0.0003), 3,4,4’,5-tetrachlorbiphenyl (PCB 81) (TEF=0.0003), 3,3’,4,4’,5,5’-hexachlorobiphenyl (PCB 169) (TEF=0.03) and a single TEF value (0.00003) for all relevant mono-ortho substituted PCBs. Additivity, an important prerequisite of the TEF concept was again confirmed by results from recent in vivo mixture studies. Some experimental evidence shows that nondioxin-like aryl hydrocarbon receptor (AhR) agonists/antagonists are able to impact the overall toxic potency of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds and this needs to be investigated further. Certain individual and groups of compounds were identified for possible future inclusion in the TEF concept, including 3,4,4’-TCB (PCB 37), polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs), mixed polyhalogenated dibenzo-p-dioxins and dibenzofurans, polyhalogenated naphthalenes and polybrominated biphenyls (PBBs). Concern was expressed about direct application of the TEF/TEQ approach to abiotic matrices such as soil, sediment etc., for direct application in human risk assessment. This is problematic, as the present TEF scheme and TEQ methodology is primarily intended for estimating exposure and risks via oral ingestion (e.g., by dietary intake). A number of future approaches to determine alternative or additional TEFs were also identified. These included the use of a probabilistic methodology to determine TEFs that better describe the associated levels of uncertainty and ‘systemic’ TEFs for blood and adipose tissue and total toxic equivalency (TEQ) for body burden.
Keywords: Dioxins, Dibenzofurans, PCBs, TEFs, Re-evaluation, WHO
Introduction
Polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and biphenyls (PCBs) are persistent organic pollutants that are omnipresent in the global environment. Many of these hydrophobic and lipophilic compounds are highly resistant to metabolism in vertebrate species, including humans. As a result of these properties, biomagnification occurs through the food chain and high tissue concentrations can often occur in top predator species. Most if not all toxic and biological effects of these compounds are mediated through the aryl hydrocarbon receptor (AhR), a cytosolic receptor protein present in most vertebrate tissues with high affinity for 2,3,7,8-substituted PCDD/Fs and some non ortho substituted PCBs (Poland et al., 1985; Safe et al., 1985; Safe, 1986). Hundreds of congeners are formed during synthetic processes such as combustion and certain industrial activities (Hutzinger et al., 1985). Thus, human exposure either through food or the environment results in the uptake of a large number of these compounds. As a result humans retain dozens of PCB congeners in their tissues, blood and milk (Schecter et al., 1994; Liem et al., 2000). Most PCDD and PCDF congeners with a 2,3,7,8 chlorine substitution pattern are also strongly retained (Van den Berg et al., 1994). Thus, risk assessment of these compounds involves a complex mixture of PCDD, PCDF and PCB compounds that are AhR agonists sharing a common mechanism of action and should not be done for only one specific congener.
During the last few decades data from many experimental studies with mixtures of these compounds are consistent with an additive model, although deviations up to a factor of two, and sometimes more, from additivity are not uncommon (Safe et al., 1985; Safe, 1986; Barnes et al., 1991; Barnes, 1991; Birnbaum and DeVito, 1995; Zabel et al., 1995; Safe, 1997; Safe, 1998; Van den Berg et al., 1998). As a result of this generally accepted additivity, the toxic equivalency concept was developed during the mid 1980s (Safe et al., 1985; Safe, 1986; Barnes et al., 1991; Barnes, 1991). It uses the relative effect potency (REP) determined for individual PCDD, PCDF and PCB compounds for producing toxic or biological effects relative to a reference compound, usually 2,3,7,8-TCDD. The total Toxic Equivalent (TEQ) is operationally defined by the sum of the products of the concentration of each compound multiplied by it’s TEF value, and is an estimate of the total 2,3,7,8-TCDD-like activity of the mixture.
Since the early 1990s, the World Health Organization (WHO) has organized expert meetings with the objective to harmonize the toxic equivalency factors (TEFs) for dioxin and dioxin-like compounds on the international level, thereby giving recommendations to national regulatory authorities. Prior to 2005 two WHO (re-) evaluations of the TEFs were conducted. In 1993, the first evaluation was done that resulted in human and mammalian WHO TEFs for all 2,3,7,8-PCDDs and PCDFs, but also a recommended TEF value for several PCBs (Ahlborg et al., 1994). A WHO TEF (re-)evaluation was again done in 1997, which led to the revision of several mammalian TEF values of important congeners and withdrawal of the di-ortho PCBs from the TEF concept for dioxin-like compounds. In addition, the first WHO TEF values for birds and fish were proposed during this meeting (Van den Berg et al., 1998). To support this meeting the Karolinska Institutet in Stockholm (Sweden) prepared a database with results from all studies for which relative effect potency (REP) values were known at that time, and they were used to determine the WHO 1998 TEF values. This REP database was recently used as a starting point to compile a much more extensive database for relative effect potency (REP) values (Haws et al., 2006). In June 2005 a third WHO expert meeting to re-evaluate current mammalian TEF values was held in Geneva, Switzerland. Preceding this meeting a one day public hearing took place with stakeholders, interested parties and members of the expert panel, during which the panel members were able to discuss various aspects of the TEF and TEQ concept with the participants and use this information during the actual evaluation process. Besides the re-evaluation of the WHO 1998 TEF values, the validity, criteria and correct use of the TEF/TEQ concept, methods for proper identification of TEF values and possible compounds for future inclusion were discussed. This report presents the results of this meeting including the TEF values that now are proposed as WHO 2005 TEFs for human risk assessment of these compounds.
Validity and criteria of the TEF concept
During the 2005 WHO re-evaluation of the 1998 WHO TEF values, both the general TEF concept and REP criteria were extensively discussed. The criteria for inclusion of a compound in the TEF concept at this meeting were similar to those used at the two earlier WHO expert meetings (Ahlborg et al., 1994; Van den Berg et al., 1998). These criteria are that for inclusion in the TEF concept a compound must:
-
1)
show a structural relationship to the PCDDs and PCDFs;
-
2)
bind to the Ah receptor (AhR);
-
3)
elicit AhR mediated biochemical and toxic responses;
-
4)
be persistent and accumulate in the foodchain.
It was recognized that the vast amount of literature available in this field provides many examples of uncertainties associated with the determination of REPs. In addition, high variation can sometimes be found in REP values for the same congener and for similar endpoints in different species (e.g., rats versus mice).
The 2005 WHO re-evaluation of the TEF values made extensive use of the review and REP database of Haws and coworkers (Haws et al., 2006) in which a set of criteria was developed to identify, include, or exclude REPs for dioxin-like compounds. Extensive consultations between the compilers of this database with the WHO represented by M. van den Berg and R.E. Peterson took place. However, it must be emphasized that for this 2005 TEF re-evaluation the expert panel used all available REPs, either included or excluded in this database, and made their own assessment (Haws et al., 2006). Studies published since the 1997 re-evaluation were also fully evaluated.
When reviewing the database of mammalian REPs for dioxin-like compounds it was observed, that even for the most thoroughly studied congeners like 2,3,4,7,8-PeCDF and PCB126, significant gaps in knowledge exist (Haws et al., 2006). Reasons for significant differences in REPs for the same congener can be caused by the use of different dosing regimens (acute versus subchronic), different endpoints, species, and mechanisms (e.g., tumor promotion caused by at least two different mechanisms as for mono-ortho substituted PCBs), as well as different methods used for calculating REPs. Thus, different methodological approaches used in different studies clearly provide uncertainties when deriving and comparing REPs. If future study designs to derive REPs were more standardized and similar, the variation in REPs when using the same congener, endpoint, and species might be expected to be smaller.
At this 2005 meeting the ‘ideal’ REP study design was discussed, as previous WHO TEF evaluations did not provide sufficient information regarding the criteria that needed to be met to establish a REP value and give an expert panel the greatest degree of confidence in a particular REP. The following general guidelines for a future ‘ideal’ dose response study used to determine an in vivo REP resulted from the workshop:
-
○
A full dose response curve for both the congener and for 2,3,7,8-TCDD should be determined.
-
○
The congener and 2,3,7,8-TCDD should be administered by the same route to animals of the same species, strain, sex, and age, and the animals should be housed, fed the same diet, and maintained under the same conditions in the same laboratory.
-
○
Ideally, the absolute maximal response (efficacy) should be similar for both the congener and for 2,3,7,8-TCDD and their dose response curves should be parallel, but in practice this is often not observed for various reasons.
-
○
If the above dose response criteria are met, the REP should be calculated by dividing the ED50 of 2,3,7,8-TCDD by the ED50 of the congener.
-
○
If full dose response relationships are not attained and determination of ED50s is not possible, lowest observed effect doses (LOEDs) or concentrations (LOECs) or benchmark doses could be used to determine the REP. However, such a REP has more uncertainty than if ED50s were used.
For studies that are designed to determine REPs it is clear that in vivo studies have the highest priority, because they combine both toxicokinetic as well as toxicodynamic aspects. Therefore in vivo studies should preferably be used for setting TEFs. Nevertheless, in vitro studies can contribute significantly to establish the AhR mediated mechanism of action of a compound and explain possible differences in species sensitivity, especially with respect to that of humans versus experimental animal species. For in vitro studies stricter criteria should be applied as these are from an experimental design point of view usually easier to accommodate than in vivo studies. For in vitro studies the following experimental design is suggested to determine a REP:
-
○
A vehicle group and at least four graded concentrations of a congener and four graded concentrations of 2,3,7,8-TCDD should be selected.
-
○
For congener and 2,3,7,8-TCDD treatment groups, three of these concentrations should elicit a response that falls between the EC20 (effective dose 20%) and EC80 for the congener and for 2,3,7,8-TCDD.
-
○
At least one concentration should elicit a maximal response (EC100) and the concentration response curves should be parallel.
-
○
The REP should be based on the EC50 of 2,3,7,8-TCDD and the EC50 of the congener.
In general, 2,3,7,8-TCDD has been used as the reference compound of choice, but in several studies PCB 126 has been used instead of 2,3,7,8-TCDD. Based on available data from the literature it was concluded that PCB 126 could indeed be used as a reference compound in rat studies with a REP value of 0.1. Recent studies have confirmed this value for multiple endpoints (Toyoshiba et al., 2004; Walker et al., 2005). However, it should be examined in more detail if the same REP for PCB 126 is applicable as a reference compound for mouse studies. The REP values for some endpoints such as enzyme induction tend to be significantly lower in mice than in rats (Harper et al., 1993; Birnbaum and DeVito, 1995; van Birgelen et al., 1996a; DeVito et al., 2000). In this respect it should be noted that mice studies in which PCB 126 was used as a reference compound were excluded from the database and from further consideration because of other methodological reasons (Haws et al., 2006)
Literature data also indicate that the PCB 126 REP for enzyme induction in human cell systems, including primary hepatocytes, breast cancer cell lines and primary lymphocytes, may be one or two orders of magnitude lower (Zeiger et al., 2001; van Duursen et al., 2003). In addition, the apparent binding affinity of 2,3,7,8-TCDD to the human AhR is generally 1/10th that of the AhR of the more sensitive rodent species, but significant variation among individual humans occurs (Roberts et al., 1990; Ema et al., 1994; Poland et al., 1994; Harper et al., 2002; Ramadoss and Perdew, 2004). It has been suggested that on average humans are among the more dioxin-resistant species, but the human data set is too limited to be conclusive (Harper et al., 2002; Okey et al., 2005). A study with AhR-humanized mice may indicate lower responsiveness towards toxic effects of 2,3,7,8-TCDD (Moriguchi et al., 2003) Taken together this information warrants more research into REP values in human systems to establish if the present TEFs based on rodent studies are indeed also valid for humans.
Additivity is an important prerequisite of the TEF concept and this aspect was re-visited in detail by the 2005 expert panel. It was concluded that results from recent in vivo mixture studies with dioxin-like compounds are consistent with additivity and support the TEF approach (Gao et al., 1999; Fattore et al., 2000; Hamm et al., 2003; Walker et al., 2005). Gao and co-workers (1999) studied the relative potency and additivity of 2,3,7,8-TCDD, 1,2,3,7,8-PeCDD and 1,2,3,4,7,8-HxCDD in a rat ovulation model; their results confirmed both parallel dose response curves and mixture additivity. Fattore and coworkers (2000) measured hepatic vitamin A reduction in rats after subchronic dietary exposure to a low dose mixture containing 1,2,3,7,8-PeCDD, 2,3,4,7,8-PeCDF and 1,2,3,6,7,8-HxCDF to test additivity. The effects of this mixture showed that the predicted results based on WHO 1998 TEFs were approximately 2 fold higher. Hamm and co-workers (2003) studied a mixture of nine dioxins, furans, and coplanar PCBs and looked at developmental reproductive endpoints in rats, comparing results of the mixture to that of 2,3,7,8-TCDD alone. The results showed that the experimental estimated TEQ was within a factor of two of that predicted from the WHO 1998 TEFs. A mixture study from the National Toxicology Program was also examined by the expert panel and again the results generally supported additivity and parallel dose response curves for complex and long term neoplastic and non neo-plastic endpoints (Walker et al., 2005).
Thus, results in these recent mixture studies could be predicted rather well with the WHO 1998 TEFs, within a factor of two or less. This degree of accuracy was somewhat surprising in view of the complicated experimental situation present in subchronic toxicity studies, where congener-specific toxicodynamics and kinetics are intermingled and can influence the final outcome. In addition, the WHO 1998 TEFs were derived from a range of REPs using different biological models or endpoints and were therefore estimates with an order of magnitude uncertainty (Van den Berg et al., 1998).
Process used to determine TEF values: point estimates, expert judgement and probabilistic distribution
Both the WHO 1993 and 1997 TEF re-evaluations used point estimates derived by expert judgement from a wide range of REPs (Ahlborg et al., 1994; Van den Berg et al., 1998). In the 2005 TEF re-evaluation it was decided by the expert panel to use the REP database from Haws et al. for initial assessment of a TEF value (Haws et al., 2006). This recently published database and applied criteria were a refinement of the criteria and database that were developed to support the two previous WHO TEF re-evaluations (Ahlborg et al., 1994; Van den Berg et al., 1998). The criteria for inclusion or exclusion of a REP in this database (Haws et al., 2006) were accepted by the expert panel. These criteria can be summarized as follows:
-
○
At least one test congener and a valid reference compound must have been included in the study or the reference compound must have been included in an identical experiment from the same laboratory, but in another study.
-
○
The endpoint must have been an established Ah receptor mediated response known to be affected by both the test congener and the reference compound.
-
○
In the REP database, in vivo and in vitro studies were separated.
-
○
Repetitive endpoints (i.e., measures of the same biological response) were identified in all studies in the database and the most representative REP value was retained for re-evaluation of a TEF.
-
○
Those studies that used only a single dose level of either the test and/or reference compound were filtered out of the REP database and not used in the TEF re-evaluation process
-
○
Results from non-peer reviewed studies were not used in re-evaluating a TEF value and consequently did not contribute to the distribution of REPs for individual congeners.
-
○
REPs based on biological responses that were statistically significant were included in the 2005 REP database and contributed to the distribution of REPs for individual congeners used to re-evaluate TEFs. However, when there was a very limited data set for an individual congener, the panel also considered biological responses that were not statistically significant as part of the overall expert judgment in re-evaluating a TEF value.
-
○
REPs based on quantitative structure activity relationship (QSAR) studies were included in the REP database
When using this database the primary focus of the TEF re-evaluation was on in vivo studies (Haws et al., 2006). In vitro studies were only used for support in those situations where no or very few in vivo REP data were available. For in vitro REPs only established AhR mediated responses were used to assign REP values..
During the TEF re-evaluation the expert panel considered using REP distributions available from the REP database (Haws et al., 2006) when re-evaluating a TEF value. However, the REP distributions in this study are unweighted (Haws et al., 2006) and it was decided that establishing a weighting criteria for REPs generated in different types of studies (in vivo, in vitro, chronic, acute, etc.) was not feasible at this meeting. In addition, it was concluded that REP distributions for a specific congener in this database could not be used to derive a TEF value, because a fixed percentile would have to be used as a cut off point. Such an approach would be like using a single point estimate, but with lower biological or toxicological relevance. This is because all types of in vivo studies (acute, subchronic, etc.) and different endpoints have been combined and associated REP distributions are shown as a single box plot. Thus, with only unweighted distributions of REPs available, a final expert judgment in the TEF re-evaluation process involving the type and quality of the study had preference over the unweighted REP distributions (Haws et al., 2006). Nevertheless, it was recognized by the expert panel that in the future weighted REP distributions could be used for derivation of TEF values, but establishing consensus values for these REP weighting factors would require additional expertise.
The WHO expert panel decided that a combination of these unweighted REP distributions, expert judgement and point estimates would be used to re-evaluate a TEF. Figure 1 shows the unweighted distribution of in vivo and in vitro REPs and WHO 1998 TEF values for PCDDs, PCDFs, non-ortho PCBs and mono-ortho PCBs (Haws et al., 2006). These unweighted REP distributions were used to start the selection and decision process for a TEF re-evaluation. The 75th percentile of the in vivo REP distribution for an individual congener was used as an initial decision point to review the WHO 1998 TEF for that congener. If the WHO 1998 TEF was below the 75th percentile of the in vivo REP distribution the data driving this TEF value was extensively re-evaluated. If the WHO 1998 TEF value was above the 75th percentile, a quick review was done regarding the decision made at the 1997 WHO meeting with respect to those studies that had been driving the 1998 WHO TEF value. In addition, results of new studies conducted after 1997 or old information missed at the 1997 WHO meeting were evaluated to determine if these would influence the WHO 1998 TEF value for that congener. Based on the combined information a possible new TEF value was considered. Special attention was also given to validity of WHO 1998 TEF values that were near or higher than the 90th percentile, e.g. 1,2,3,7,8-PeCDF. Thus, the above TEF re-evaluation process provided a way both to increase as well as to decrease a TEF value. Figure 2 illustrates the decision scheme used at the expert meeting for the initial re-evaluation process of the TEFs. For transparency, the expert judgment process and rationale used by the expert panel for a possible newly assigned WHO 2005 TEF value is explained in the next paragraph. This is followed by subsequent paragraphs devoted exclusively to each congener re-evaluated.
Figure 1.
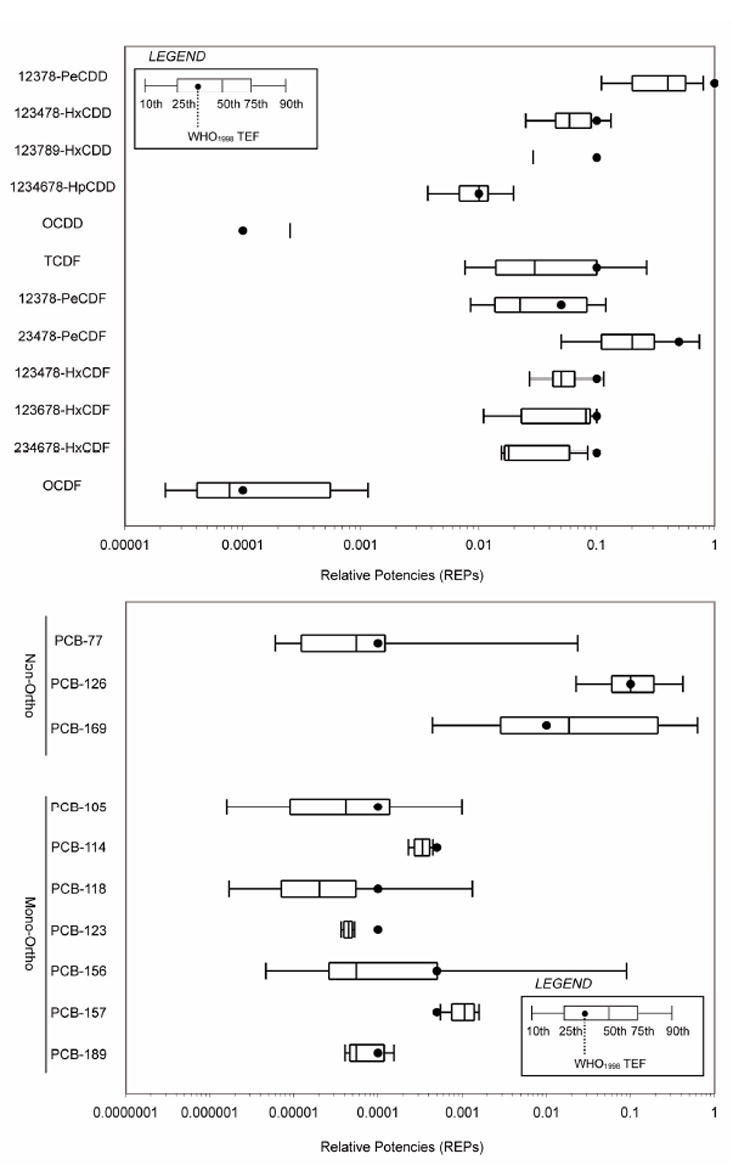
Distribution of in vivo unweighted REP values in the REP2004 database. Reprinted with permission from (Haws et al., 2006).
Figure 2.
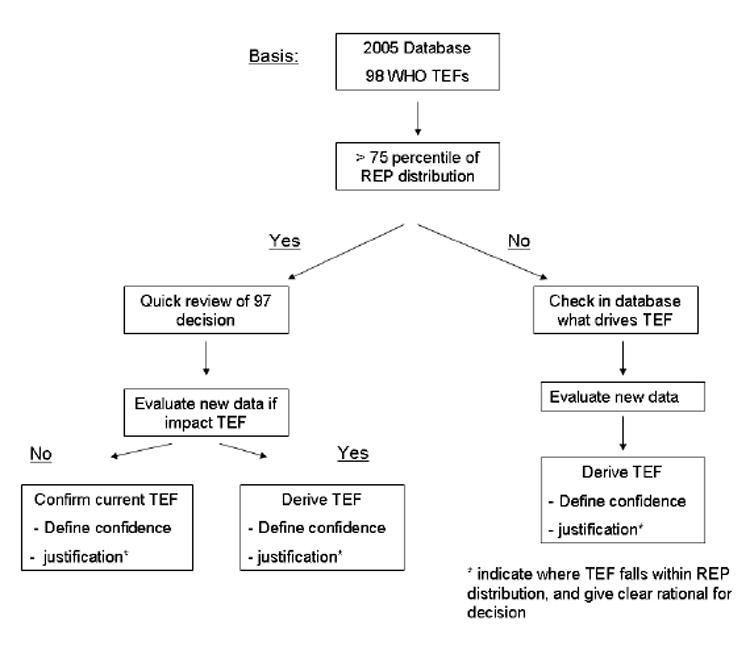
Decision scheme used in the 2005 re-evaluation of the 1998 WHO TEF values (Van den Berg et al., 1998) assigned to individual PCDD, PCDF, and PCB congeners.
As in previous WHO TEF consultations, it was decided by the expert panel to use a stepwise scale for assigning TEFs values. However, instead of assigning TEFs in the increments used previously (0.01, 0.05, 0.1, etc.), it was decided to use half order of magnitude increments on a logarithmic scale at (0.03, 0.1, 0.3 etc). As a result all (non)revised 2005 WHO TEFs were fitted on a logarithmic scale. This decision to assign TEFs as half order of magnitude estimates may be useful in describing, with statistical methods, the uncertainty of TEFs in the future. Thus, as a default, all TEF values are assumed to vary in uncertainty by at least one order of magnitude, depending on the congener and its REP distribution. Consequently, a TEF of 0.1 infers a degree of uncertainty bounded by 0.03 and 0.3. For a TEF value of 0.3, a degree of uncertainty bounded by 0.1 and 1 was used. Thus, the TEF is a central value with a degree of uncertainty assumed to be at least +/- half a log, which is one order of magnitude. However, it should be realized that TEF assignments are usually within the 50th to 75th percentile of the REP distribution, with a general inclination towards the 75th percentile in order to be health protective. However, the latter approach was also influenced by the type and quality of study, e.g. single versus multiple dose, that could not been discerned from the REP distributions shown in Figure 1. This more conservative and health protective approach practically means that for a TEF value the likelihood of a half log error too low is less than the likelihood of half a log error too high. Due to the new ‘spacing’ to express TEFs on a half-log scale it was also necessary in the final review process to evaluate each individual TEF value for those congeners for which there were no new data available.
WHO 2005 TEF values
1,2,3,7,8-PeCDD
The WHO 1998 TEF was set at 1.0, which is above the 90th-percentile of the REP distribution of twelve in vivo studies. New studies indicate a REP between 0.1 and 1.0 for this compound (Fattore et al., 2000; Johnson et al., 2000; Simanainen et al., 2002). The vitamin A and tumor promotion studies provide REPs of 0.7 and 1 (Waern et al., 1991; Fattore et al., 2000). Results from acute toxicity studies result in REPs closer to 0.5, but, in general, REPs increase in subchronic toxicity studies (Haws et al., 2006). Therefore, the consensus WHO 2005 TEF value remained at 1.
1,2,3,4,7,8-HxCDD
The WHO 1998 TEF was set at 0.1, which is around the 80th percentile of the REP distribution of five in vivo studies. One new study determined REPs ranging from 0.04 to 0.12 (Gao et al., 1999), while two other recent studies observed REPs between 0.06 and 0.4 (Simanainen et al., 2002; Takagi et al., 2003). Very little data indicate that the TEF value should be changed to either 0.3 or 0.03. Therefore it was decided to keep the WHO 2005 TEF value at 0.1
1,2,3,6,7,8-HxCDD
The WHO 1998 TEF was set at 0.1. No in vivo studies are available for this HxCDD isomer. This TEF value is above the 75th percentile of the REP distribution of four in vitro studies (Haws et al., 2006). A more recent in vitro study (Bols, 1997) supports this TEF value and therefore no change for the WHO 2005 TEF was decided.
1,2,3,7,8,9-HxCDD
Similar to the above previous hexa isomers the WHO 1998 TEF was set at 0.1. It was noted that very little in vivo data is available with a recent study giving a REP of 0.029 (Takagi et al., 2003). In addition, four in vitro studies produced REPs up to 0.07 (Schrenk et al., 1991; Lipp et al., 1992), which is above the 75th percentile of the distribution. The expert panel considered decreasing the TEF value to 0.03 but decided that there was not enough data to support such a change. In vitro data were observed to be consistent between HxCDD isomers. In view of the homology between the HxCDD isomers it was therefore decided to retain the old value of 0.1 as the WHO 2005 TEF value.
1,2,3,4,6,7,8-HpCDD
The WHO 1998 TEF was set at 0.01, which is at the 50th percentile of the REP distribution range of four in vivo studies. New studies again point toward a REP of 0.01 for this congener (Viluksela et al., 1994; Viluksela et al., 1997a; Viluksela et al., 1997b; Simanainen et al., 2002). An earlier tumor promotion study also indicated a similar REP (Schrenk et al., 1994). It was also discussed whether or not the available information from the important studies mentioned above would be sufficient to increase the TEF to 0.03, which is well above the 90th percentile of the REP distribution. This suggestion was rejected by the expert panel. It was decided to retain the WHO 2005 TEF value as 0.01.
OCDD
The WHO 1998 TEF was set at 0.0001, which is well outside the 10th percentile of the range of in vivo and in vitro REP values (Haws et al., 2006). At present the only in vivo REPs meeting the stringent conditions of the database (Haws et al., 2006) are based on one study that was reported in two different papers using different endpoints (Wermelinger et al., 1990; Fattore et al., 2000). It was discussed whether or not this TEF should be increased to bring it in line with the results of the subchronic toxicity study (Wermelinger et al., 1990; Fattore et al., 2000). The new in vivo REP data from Fattore and coworkers were evaluated and these would support a TEF greater than 0.0001. One concern that was expressed within the expert panel was that the animals used in a more recent publication (Fattore et al., 2000) were the same animals used in an earlier study (Wermelinger et al., 1990), and this OCDD was reported to be contaminated with other more potent 2,3,7,8 substituted congeners such as 2,3,4,7,8-PeCDF. Using the NTP data now available for 2,3,4,7,8-PeCDF (Walker et al., 2005), it was calculated that the reported contamination of 2.5 ppm (pg/μg) 2,3,4,7,8-PeCDF was not of toxicological relevance for the results (see footnote for calculation)1. Overall, it was concluded that there is very limited in vivo information available with only one subchronic toxicity study (Wermelinger et al., 1990; Fattore et al., 2000). The expert panel decided that the information provided by both in vivo studies derived from only one experiment did not provide a solid basis to increase the TEF value for this compound to 0.001, but the combined information from in vivo and in vitro data (Haws et al., 2006) did justify a raise in TEF value. Therefore it was decided to increase the WHO 2005 TEF value to 0.0003. The expert panel is aware of the implications that the increase in this WHO TEF value for OCDD might have from a regulatory and risk management point of view. However, with respect to the high concentrations of OCDD in some environmental matrices a number of critical remarks regarding the inappropriate use of the present WHO TEFs are made in the section on the use of TEQ for abiotic environmental matrices.
2,3,7,8-TCDF
The WHO 1998 TEF was set at 0.1. This value is at the 75th percentile of the REP distribution of nine in vivo studies for this compound (Haws et al., 2006). Only one new study has been reported (Takagi et al., 2003) and a REP of 0.07 was found for increased cleft palate formation, which is close to the TEF of 0.1. Consequently it was decided that the WHO 2005 TEF should remain at 0.1
1,2,3,7,8-PnCDF
The WHO 1998 TEF was set at 0.05 which is within the 50th and 75th percentile of the REP distribution of eight in vivo studies. A new study by Fattore et al. (2000) found a REP of 0.01 for effects on hepatic vitamin A reduction, but a study by Takagi et al. reported a REP of 0.045 for cleft palate (Takagi et al., 2003). The majority of the vivo studies report a REP value below 0.1 but many relevant studies have REPs above 0.01. Therefore it was decided that the 2005 WHO TEF should become 0.03.
2,3,4,7,8-PnCDF
The WHO 1998 TEF was set at 0.5 which is well above the 75th percentile of the REP distribution of eight in vivo studies. Results from the long term NTP study in female Sprague Dawley rats using many different endpoints are now available to evaluate this earlier TEF value more closely. The REPs for neoplastic endpoints from the NTP study (Walker et al., 2005) are around 0.2 to 0.3, while nonneoplastic endpoints have REPs that range from 0.7 to 1.1 An older subchronic study by Pluess and coworkers pointed towards a REP of 0.4 (Pluess et al., 1998). More recent studies using hepatic vitamin A reduction and immunological effects as endpoints also point toward a TEF below 0.5 (Fattore et al., 2000; Johnson et al., 2000). In view of this new information it was decided by consensus of the expert panel to change the WHO 2005 TEF to 0.3.
1,2,3,4,7,8-HxCDF
The WHO 1998 TEF was set at 0.1 which is above the 75th percentile of the REP distribution of six in vivo studies. No new in vivo studies have been published since 1997 and in view of the limited data there was no reason to change this value. Thus, the WHO 2005 TEF value remains 0.1.
1,2,3,6,7,8-HxCDF
The WHO 1998 TEF was also set at 0.1 and this value is above the 75th percentile of the distribution of three in vivo REPs and when the results of in vitro and in vivo studies with the PCDF are combined, REP values lie within the 50th and 75th percentile. A new study reported a REP of 0.03 for hepatic vitamin A reduction (Fattore et al., 2000). However, the animals analyzed were from an older study from which a REP of 0.1 for subchronic toxicity was reported (Pluess et al., 1998). In view of the limited number of studies available and the fact that WHO 1998 TEFs of 0.1 for most HxCDFs were all around the 50th to 75th percentile (Haws et al., 2006) the expert panel decided not to discriminate between TEF values for these congeners. As a result, the 2005 WHO TEF remains at 0.1.
1,2,3,7,8,9-HxCDF
The WHO 1998 TEF for this HxCDF was set at 0.1. There are no in vivo results and only two older in vitro studies for this congener with REPs of 0.2 and 0.1 (Tysklind et al., 1994; Brown, 2001) supporting the 0.1 TEF value similar to the other HxCDFs. Consequently the 2005 WHO TEF remains as 0.1
2,3,4,6,7,8-HxCDF
The WHO 1998 TEF value is 0.1 and it is around the 50th percentile of the REP distribution range of the combined in vivo and five in vitro studies (Haws et al., 2006). Most in vitro studies suggest a TEF value slightly above 0.1 (Bandiera et al., 1984; Mason et al., 1987; Tysklind et al., 1994; Brown, 2001). There is only one in vivo study for this hexa-isomer indicating REPs for different endpoints ranging from 0.02 to 0.1. Given this weak and limited REP database and approximate similarities in responses for this and certain other HxCDFs, there was consensus in the expert panel to retain the 2005 WHO TEF at 0.1
1,2,3,4,6,7,8- and 1,2,3,4,7,8,9-HpCDFs
The WHO 1998 TEFs for both HpCDFs were set at 0.01. Since 1997 there are no new in vivo studies published. Only two in vitro studies have been published (Tysklind et al., 1994; Brown, 2001) reporting REPs, respectively, of 0.02 and 0.3 for 1,2,3,4,6,7,8-HpCDF and 0.04 and 0.02 for 1,2,3,4,7,8,9-HpCDF. Although these in vitro results do suggest a slightly higher TEF than 0.01 the expert panel thought there was too much uncertainty in this limited database to raise the TEF. In addition, it was expected that in vivo there would be low absorption of these HpCDFs from the GI tract, thereby reducing their relative potency below that of the in vitro REPs. Based on these arguments It was decided that the WHO 2005 TEFs would remain the same for both isomers, 0.01.
OCDF
The WHO 1998 TEF value of 0.0001 is within the 50th and 75th percentile of the REP distribution range of three in vivo studies, but when these data are combined with in vitro results it falls below the 50th percentile (Haws et al., 2006). The recent study by Fattore and coworkers (Fattore et al., 2000) using the same animals as Wermelinger and coworkers (Wermelinger et al., 1990) indicate a REP for OCDF greater than 0.0001 based on hepatic vitamin A reductions. Some earlier in vivo studies also indicated a REP higher than the WHO 1998 TEF (Waern, 1995; van Birgelen et al., 1996a; DeVito et al., 1998). As with OCDD, there was originally concern among the expert panel about impurities with 2,3,7,8 chlorine-substituted congeners (Wermelinger et al., 1990; Fattore et al., 2000), but calculations indicated that the reported contamination with 1,2,3,4,6,7,8-HpCDF was of no toxicological concern. When the limited number of in vivo and in vitro REPs (<10) are reviewed, REPs range from 4×10-6 to 0.0028 with a 50th percentile of 0.0007 (Haws et al., 2006). As with OCDD the expert panel decided that the limited in vivo information available would not warrant a factor of 10 increase of the WHO 1998 TEF value, but increasing the WHO 2005 TEF value to 0.0003 is appropriate in view of some of the higher in vivo REPs reported. This would also be in line with comparable REP values obtained in a recent study including both OCDD and OCDF (Fattore et al., 2000).
PCB 77
The WHO 1998 TEF value of 0.0001 is just below the 75th percentile in a very nonhomogenous distribution of six in vivo REPs. The available subchronic toxicity studies are all around the 75th percentile (Hakansson H. et al., 1994; Chu et al., 1995). Immunotoxicological studies with mice were given less weight (Mayura et al., 1993; Harper et al., 1995) because these were acute studies involving the ip route of exposure and no information on purity was provided. It was decided by the expert panel that the subchronic study was still the most representative (Chu et al., 1995). As a consequence the WHO 2005 TEF value remained at 0.0001.
PCB 81
The WHO 1998 TEF value was 0.0001. PCB 81 has been observed in wildlife and human milk (Kumar et al., 2001) confirming the validity of inclusion of this PCB in the TEF scheme. There are no new in vivo data for this PCB congener. Older in vivo data were excluded because these involved single dose studies from which the expert panel believed no reliable REP value could be determined. Various in vitro studies with human hepatoma HepG2 cells and monkey hepatocytes indicate that PCB 81 is more potent than PCB 77 (Pang et al., 1999; van der Burght et al., 1999; Brown, 2001; Zeiger et al., 2001). Based on the in vitro REP distribution, it is noticeable that the WHO 1998 TEF is located at the very low end of the REP distribution range (Haws et al., 2006). Thus, based on the information that PCB 81 is more potent in vitro and more persistent than PCB 77, the expert panel decided to raise the WHO 2005 TEF value to 0.0003. However, the expert panel expressed its low confidence in the PCB 81 REP database because it lacks in vivo REP data.
PCB 126
The WHO 1998 TEF was set at 0.1, which is at the median of the REP distribution range of twenty in vivo studies. This 1998 TEF value was mainly driven by the tumor promotion study with this compound (Hemming et al., 1995). New in vivo studies from the NTP covering many endpoints (Johnson et al., 2000; Walker et al., 2005) support the TEF of 0.1. With respect to rat studies the expert panel recognized the tight range of REPs for this congener (Haws et al., 2006), which supports the use of PCB 126 as reference compound with a TEF of 0.1 when comparing rat studies. Information from mice studies and some human in vitro systems (especially for enzyme induction) suggest that the REP for PCB 126 might be lower than 0.1 (Harper et al., 1993; Birnbaum and DeVito, 1995; van Birgelen et al., 1996a; DeVito et al., 2000; Zeiger et al., 2001; van Duursen et al., 2003). Clearly more information is necessary regarding this issue. Although concern was expressed about interspecies variability in REPs, the expert panel considered the present information too limited to make a decision other than to retain 0.1 as the WHO 2005 TEF.
PCB 169
The WHO 1998 TEF was set at 0.01, which is below the median in the REP distribution range of seven in vivo studies. The 1998 TEF was mainly driven by a four week repeated dose mouse study measuring enzyme induction and generating a REP of less than 0.001 (DeVito et al., 1998). On the other hand, REPs from several other in vivo studies ranged from less than 0.01 to 0.7 (Yoshimura et al., 1979; Parkinson et al., 1981; Harper et al., 1993). Thus, large differences in REPs have been observed for PCB 169 between both species and endpoints.. In view of the fact that the WHO 1998 TEF was also below the median of the in vivo REP distribution (Haws et al., 2006), the expert panel decided it was appropriate to raise the TEF between the 50th and 75th percentile (see Figure 1). Nevertheless many single dose studies were observed to have significantly higher REPs (around 0.1) than those observed in a thirteen week study. In view of these significant differences between single and multiple dose studies the expert panel judged that the WHO 2005 TEF for PCB169 of 0.03 would be more appropriate than a potentially overly conservative REP of 0.1.
Mono-ortho substituted PCBs 105, 114, 118, 123, 156, 157, 167 and 189
The WHO 1998 TEF values for the mono-ortho PCBs ranged from 0.00001 to 0.0005. A major issue with the REP values for the different mono-ortho PCBs is that they span four to five orders of magnitude, depending on the congener. In Figure 3 this wide variation in REPs is illustrated. Even if only in vivo studies are considered, the 90% distribution range is extremely large (see Figure 1). This great variation in REP values was of serious concern to the expert panel. The panel considers possible, inconsistent, low level contamination of the mono-ortho PCBs with more potent dioxin-like compounds to play, at least in part, a role in causing this large variation. De Vito and coworkers (2003) found that less than 1% contamination of PCB 77 by PCB126 significantly impacted the apparent REP of PCB 77 (De Vito, 2003). Shortly before the WHO 2005 TEF re-evaluation meeting, laboratories of panel members performed a number of in vitro experiments in an attempt to elucidate the possible impact of impurities on REPs for the mono-ortho PCBs. Peters and coworkers (2006) recently showed that after being purified on charcoal the mono-ortho PCBs 105, 118, 156 and 167 did not cause AhR-mediated activation and CYP1A1 induction in two genetically modified rodent hepatoma CAFLUX cell lines at concentrations that would generally justify a REP larger than 0.0001 (Peters et al., 2006). Based on the combined information the expert panel expressed low confidence in the higher REP values for certain mono-ortho PCBs. It was concluded that the unusually wide variability of REP values for mono-ortho PCBs can probably be explained by the occurrence of impurities with 2,3,7,8 chlorine-substituted PCDDs and PCDFs or PCB126. As the occurrence of these impurities clearly depends on the route of synthesis and the degree of clean-up, it was not possible to make a general statement about how it occurs in all cases. It was concluded that for future studies with mono-ortho PCBs or any other weak AhR agonists a purity of >99% is clearly not sufficient to establish a reliable REP. The expert panel compiled Figure 3 to make a decision on the TEF values of the mono-ortho PCBs, acknowledging the impurity issue and that the earlier decision scheme with ≥ 75th percentile (Figure 2) was not appropriate. In this case the most environmentally relevant mono-ortho PCBs are 105, 118, and 156 and it was decided to use the medians of the REP distribution range of these PCB congeners as a guide. This resulted in a recommended TEF of 0.00003 for these three mono-ortho PCBs. A differentiation for all other remaining mono-ortho PCBs was considered not feasible by the expert panel due to the lack of sufficient experimental data. Consequently the recommended WHO 2005 TEF for all mono-ortho PCBs is 0.00003.
Figure 3.
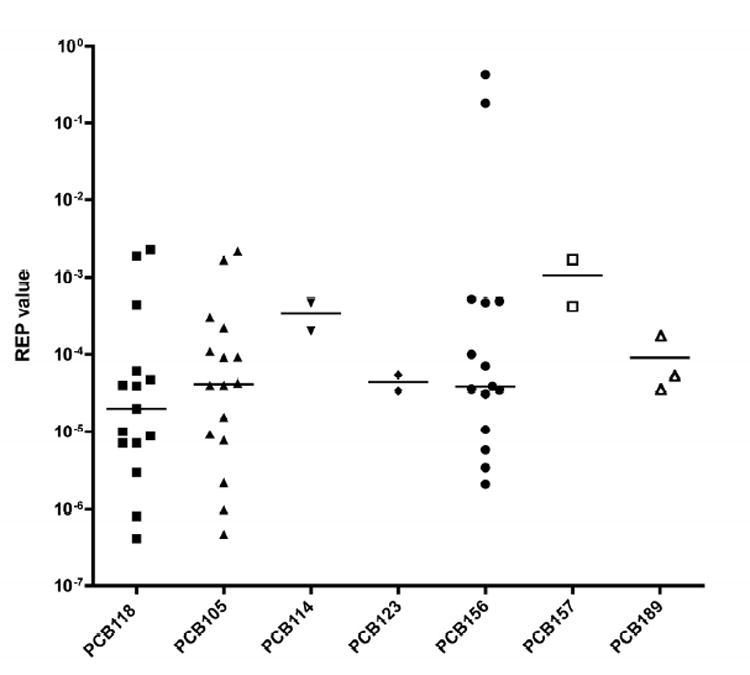
Distribution of REP values for the different mono-ortho PCBs based on AhR mediated effects.
Other compounds discussed for possible inclusion in the TEF scheme
3,4,4’-TCB (PCB 37)
PCB 37 is commonly found in the environment (Hansen, 1998). It has also been detected in edible fish species at levels comparable with PCB 77 and 81 (Sapozhnikova et al., 2004). In seals it has been measured in relatively high levels, indicating possible bioaccumulative properties in the foodchain (Addison et al., 1999). It has also been found in human milk (Hansen, 1998). In an in vitro study with the human MCF-7 breast carcinoma and HepG2 hepatoma cell lines no induction of CYP1A1 or 1B1 could be found. However, PCB 37 was found to be a significant catalytic inhibitor of both CYP activities (Pang et al., 1999). In view of the above information there is a clear need for more in vivo and in vitro information to decide if this PCB needs to be included in the TEF scheme.
Polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs)
Both in vitro and in vivo studies have shown that PBDDs and PBDFs have AhR agonist properties and cause dioxin-like effects (Mason et al., 1987; Birnbaum et al., 2003). Emerging data from Japan and the Baltic Sea indicate that PBDDs and PBDFs can be found in sediment, mussels and higher trophic species like the cormorant (Choi, 2003; Watanabe et al., 2004; Malmvarn et al., 2005; Takigami et al., 2005). In addition, there is limited recent information showing that these compounds are found in human milk and adipose tissue at levels that can contribute significantly to the total amount of TEQ (Choi, 2003; Kotz et al., 2005; Ohta et al., 2005). It appears that environmental levels might be significantly lower than those of the PCDDs, PCDFs and PCBs already in the TEF scheme. However a better exposure assessment especially with regard to humans is needed. If the presence of PBDDs and PBDFs in human food as well as in people is more extensively demonstrated there will be a clear need for assigning TEFs to these compounds. At present it is unclear to what extent the ongoing use of brominated flame retardants, especially polybrominated diphenylethers (PBDEs), could lead to an increase in human and environmental exposure to PBDDs and PBDFs. Therefore it is recommended by the expert panel to perform a more thorough exposure analysis for humans. In addition, it was concluded that among all compounds proposed in this paragraph for development of WHO TEFs, the PBDDs and PBDFs should be given high priority. More REP studies on PBDDs and PBDFs are urgently needed.
Mixed halogenated dibenzo-p-dioxins (PXCDDs) and dibenzofurans (PXCDFs)
Due to the extremely high number of congeners, analysis of PXCDDs and PXCDFs is still a major problem. Very little is known about the possible relevance of these compounds for human exposure (Birnbaum et al., 2003). If the mixed halogenated (bromine- and chlorine-substituted) dioxins and dibenzofurans are indeed detected in humans and their food, these should definitely be considered for inclusion in the TEF scheme. Early in vitro studies suggest that these compounds follow the same structure activity rules as the PCDDs and PCDFs (Mason et al., 1987; Weber and Greim, 1997; Behnisch et al., 2001).
Hexachlorobenzene (HCB)
It has been suggested that HCB fulfills the criteria for inclusion in the TEF concept (van Birgelen, 1998), although arguments for doing so have been criticized (Schwab, 1999; Vos, 2000; Pohl et al., 2001). HCB has mixed inducer properties in analogy with the mono-ortho PCBs. Before inclusion in the TEF concept is considered, it should be confirmed that highly purified HCB has indeed AhR agonistic properties, as contamination of HCB with PCDDs and PCDFs has been reported (Goldstein, 1979) (see footnote below). Thus, results from older HCB studies could have an impurity problem similar to that observed for the mono-ortho PCBs. Priority should thus be given to confirm the compound‘s dioxin-like properties using highly purified HCB with measured absence of 2,3,7,8 chlorine-substituted dioxins and dibenzofurans or dioxin-like PCBs2.
Polychlorinated and brominated naphthalenes (PCNs and PBNs)
Based on recent published data there was agreement by the expert panel that these compounds definitely should be considered for inclusion in the TEF concept, as PCNs are actually reported in food and humans (Williams et al., 1993; Hayward, 1998; Lunden and Noren, 1998; Weistrand and Noren, 1998; Domingo et al., 2003; Falandysz, 2003). Earlier in vivo studies demonstrated that PCNs and PBNs were able to induce dioxin-like effects, such as cleft palate and hydronephrosis (McKinney and McConnell, 1982; Birnbaum et al., 1983; Miller and Birnbaum, 1986). Further arguments for inclusion would be that multiple PCN and PBN congeners have distinct in vitro AhR activities that show analogy with PCDDs and PCDFs, but are less potent (Robertson et al., 1982; Robertson et al., 1984; Blankenship et al., 2000; Villeneuve et al., 2000; Behnisch et al., 2003; Darnerud, 2003). However, as with mono-ortho PCBs and HCB, the possible impurity issue should be addressed thoroughly before inclusion in the TEF concept is decided.
Polybrominated biphenyls (PBBs)
Certain polybrominated biphenyls (PBBs) have been reported to be AhR active in both in vitro and in vivo experiments (Robertson et al., 1982; Robertson et al., 1984; Darnerud, 2003). It was noted by the expert panel that some human background exposure to PBBs is still occurring. However, human exposure data outside the “Michigan episode” is surprisingly scarce. Recently, PBB exposure has been reported in bird species at the top of the food chain from Japan and Norway (Lindberg et al., 2004; Watanabe et al., 2004; Herzke et al., 2005). This occurrence in top predator wildlife species also stresses the need to further identify present human background exposure to PBBs. Thus, based on the AHR mechanism of action, inclusion of certain PBB congeners in the TEF scheme is appropriate, but further human exposure analysis should identify the possible relevance of PBBS to the total TEQ.
Polybrominated diphenylethers (PBDEs)
The expert panel accepted that PBDEs by themselves do not have AhR agonist properties and should not be included in the TEF concept (Chen and Bunce, 2003; Peters et al., 2004; Sanders et al., 2005). However, commercial mixtures of PBDEs can contain polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) that express significant AhR-mediated activities, such as CYP1A1 induction (Birnbaum et al., 2003; Hakk and Letcher, 2003). The expert panel had concerns about earlier results in the literature indicating that PBDEs cause AhR-mediated effects because of the possible impurity issue similar to that described for the mono-ortho PCBs. In addition, it was also recognized that photochemical and combustion processes of PBDEs can also produce PBDDs and PBDFs. In conclusion, it was recommended that TEFs should not be assigned for PBDEs.
‘Non dioxin-like’ AhR Ligands and the TEF Concept
The AhR can bind and be activated by a structurally diverse range of synthetic and naturally occurring chemicals (Denison and Heath-Pagliuso, 1998; Heath-Pagliuso et al., 1998; Nagy et al., 2002; Jeuken et al., 2003). These chemicals are widely distributed in dietary vegetables, fruits, teas and dietary herbal supplements sometimes at relatively high concentrations (Herzog et al., 1993a; Herzog et al., 1993b; Formica and Regelson, 1995; Berhow et al., 1998; Amakura et al., 2002; Jeuken et al., 2003). The ability of metabolically-labile phytochemicals to induce or inhibit induction of CYP1A1-dependent activities by 2,3,7,8-TCDD in cell culture model systems have been reported by numerous laboratories (Williams et al., 1993; Amakura et al., 2002; Jeuken et al., 2003; Zhang et al., 2003). However, the majority of toxicity studies demonstrated that these naturally occurring AhR agonists fail to produce AhR-dependent toxicity (Pohjanvirta et al., 2002; Leibelt et al., 2003) , although some developmental dioxin-like effects have been reported for indole-3-carbinol (I3C) (Wilker et al., 1996). In addition, naturally occurring AhR ligands, such as I3C and diindolymethane (DIM), have been reported to inhibit 2,3,7,8-TCDD-dependent in vivo induction of CYP1A1 and immunotoxicity (Chen et al., 1995; Chen et al., 1996).
The ability of some nondioxin-like PCBs and PCDFs to inhibit 2,3,7,8-TCDD-induced CYP1A1 activity and immunotoxicity in C57BL/6J mice has also been reported (Bannister and Safe, 1987; Davis and Safe, 1988; Biegel et al., 1989; Morrissey et al., 1992; Smialowicz et al., 1997; Loeffler and Peterson, 1999; Chen and Bunce, 2004; Crofton et al., 2005) whereas other studies have shown synergistic effects on dioxin toxicity of nondioxin-like compounds, e.g. thyroid hormones, porphyrins, reproductive toxicity and immunotoxicity (Birnbaum et al., 1986; Bannister and Safe, 1987; van Birgelen et al., 1996b; Loeffler and Peterson, 1999; Crofton et al., 2005).
The above studies provide evidence that nondioxin-like compounds that are weak AhR agonists can modulate the overall toxic potency of 2,3,7,8-TCDD and related compounds. If occurring under natural background situations these interactions might impact the magnitude and overall toxic effect(s) produced by a defined amount of TEQ (i.e., from intake or present in the body), but not impact the determination of individual relative potency (REP) or TEF values for dioxin-like chemicals. The potential impact of these nondioxin-like natural compounds on the risk of toxicity posed by exposure to a particular level of TEQs should be further investigated.
The use of TEQ for abiotic environmental matrices
Concurrent with the development of the TEF and TEQ approach has been its application to environmental matrices such as soil, sediment, industrial wastes, soot, fly-ash from municipal incinerators, waste water effluents, etc. As such, the TEQ approach has been and continues to be used to give a single value to complex environmental matrices (Barnes et al., 1991; Barnes, 1991), usually without taking into consideration whether this is actually a risk-based number. The expert panel emphasized that correct application of the present TEF scheme (see table 1) and TEQ methodology in human risk assessment is only intended for estimating exposure to dioxin-like chemicals from consumption of food products and breast milk, etc. This limitation is derived from the fact that those REP studies that have been considered most relevant for the determination of the present TEFs are largely based on oral intake studies, often through the diet. In fact experimental toxicological studies using abiotic matrices with dioxin-like compounds that would allow for the determination of environmental matrice-based REPs s (e.g. soil or sediment) are almost nonexistent. Furthermore, the issue of matrix specific bioavailability of these chemicals from abiotic environmental samples leads to a high degree of uncertainty for risk assessment as this is largely dependent upon the organic carbon content and age of the particles. For example, direct application of these WHO TEFs for assessment of OCDD or OCDF present in soil, sediment or fly ash would lead to inaccurate assessment of the potential toxic potency of the matrix. This derives primarily from the fact that the highly hydrophobic PCDDs and PCDFs bind strongly to particles thereby significantly reducing their bioavailability for living organisms (Van den Berg et al., 1994). As a result, application of these WHO TEFs for calculating the TEQ for e.g. OCDD and OCDF in abiotic environmental matrices has limited toxicological relevance and use for risk assessment unless the aspect of reduced bioavailability is taken into consideration. Nevertheless, the expert panel recognized that it is now common practice, to use the TEQ and associated TEFs directly to characterize and compare contamination by dioxin-like chemicals of abiotic environmental samples and is even codified in national and international legislation, e.g. the Stockholm Convention on Persistent Organic Pollutants (POPs).
Table 1.
Summary of WHO 1998 and WHO 2005 TEF values
| Compound | WHO 1998 TEF | WHO 2005 TEF |
|---|---|---|
| chlorinated dibenzo-p-dioxins | ||
| 2,3,7,8-TCDD | 1 | 1 |
| 1,2,3,7,8-PeCDD | 1 | 1 |
| 1,2,3,4,7,8-HxCDD | 0,1 | 0,1 |
| 1,2,3,6,7,8-HxCDD | 0,1 | 0,1 |
| 1,2,3,7,8,9-HxCDD | 0,1 | 0,1 |
| 1,2,3,4,6,7,8-HpCDD | 0,01 | 0,01 |
| OCDD | 0,0001 | 0,0003 |
| chlorinated dibenzofurans | ||
| 2,3,7,8-TCDF | 0,1 | 0,1 |
| 1,2,3,7,8-PeCDF | 0,05 | 0,03 |
| 2,3,4,7,8-PeCDF | 0,5 | 0,3 |
| 1,2,3,4,7,8-HxCDF | 0,1 | 0,1 |
| 1,2,3,6,7,8-HxCDF | 0,1 | 0,1 |
| 1,2,3,7,8,9-HxCDF | 0,1 | 0,1 |
| 2,3,4,6,7,8-HxCDF | 0,1 | 0,1 |
| 1,2,3,4,6,7,8-HpCDF | 0,01 | 0,01 |
| 1,2,3,6,7,8,9-HpCDF | 0,01 | 0,01 |
| OCDF | 0,0001 | 0,0003 |
| non-ortho substituted PCBs | ||
| 3,3’,4,4’-tetraCB (PCB 77) | 0,0001 | 0,0001 |
| 3,4,4’,5-tetraCB (PCB 81) | 0,0001 | 0,0003 |
| 3,3’,4,4’,5-pentaCB (PCB 126) | 0,1 | 0,1 |
| 3,3’,4,4’,5,5’-hexaCB (PCB 169) | 0,01 | 0,03 |
| mono-ortho substituted PCBs | ||
| 2,3,3’,4,4’-pentaCB (PCB 105) | 0,0001 | 0,00003 |
| 2,3,4,4’,5-pentaCB (PCB 114) | 0,0005 | 0,00003 |
| 2,3’,4,4’,5-pentaCB (PCB 118) | 0,0001 | 0,00003 |
| 2’,3,4,4’,5-pentaCB (PCB 123) | 0,0001 | 0,00003 |
| 2,3,3’,4,4’,5-hexaCB (PCB 156) | 0,0005 | 0,00003 |
| 2,3,3’,4,4’,5’-hexaCB (PCB 157) | 0,0005 | 0,00003 |
| 2,3’,4,4’,5,5’-hexaCB (PCB 167) | 0,00001 | 0,00003 |
| 2,3,3’,4,4’,5,5’-heptaCB (PCB 189) | 0,0001 | 0,00003 |
*Bold values indicate a change in TEF value.
In relation to this use of the TEQ it should be emphasized that while these values by themselves do not have any toxicological implications or direct use in risk assessment, they can be a useful tool to compare concentrations within similar abiotic matrices and serve a prioritization function. Accordingly, it is recommended that when a human risk assessment is to be done from abiotic matrices, factors such as fate, transport, and bioavailibility from each matrix be specifically considered before a final estimate of the toxicological relevant TEQ is made. If a human risk assessment is done for abiotic matrices, the expert panel recognized that it would be preferable to use congener-specific equations throughout the whole model rather than base it on total TEQ in an abiotic matrix.
Future recommendations for determination of TEF values
Previous WHO TEF re-evaluations have used expert judgement and point estimates to establish congener-specific TEF values. In addition, the 1997 expert meeting indicated that TEF values were order of magnitude estimates (Van den Berg et al., 1998). This statement was given irrespective of the type of congener, even though large differences are present in the REP studies of individual compounds (Haws et al., 2006). When using point estimates and expert judgment, an advantage is that selection of a TEF can be made from those studies which are most relevant for human exposure (e.g., in vivo long term or subchronic). A disadvantage is that such an approach does not describe the range of REPs and may reflect a bias in judgment within the expert panel.
Recently, several authors have published papers in which they advocated the use of a probabilistic approach to determine TEFs (Finley et al., 2003; Haws et al., 2006). In using such an approach, there is a clear advantage because it will better describe the level of uncertainty present in a TEF value. The distribution of REPs can be expressed in terms of minimum and maximum values combined with percentiles at different levels (e.g., 25th and 75th percentiles). A disadvantage could be that such an approach lumps all data together and gives similar weight to all types of studies. In part, this problem could be avoided by separating in vitro from in vivo REPs (Haws et al., 2006).
However, if probabilistic approaches for setting a future TEF are used, it is essential that weighting factors be applied to REPs that are determined from different types of studies. These weighted REP values could then be used to determine weighted REP distributions in the risk assessment process. Clearly, unweighted REP distribution ranges that bracket the TEFs incorporate biological and toxicological uncertainty. For this reason, in the WHO 2005 TEF reevaluation, unweighted REP distribution ranges, expert judgment, and point estimates were used in combination to assign TEFs. The sole use of a probabilistic approach to determine TEF values also includes other decision points, such as establishing a range instead of a point estimate for the TEF value. However, the use of a TEF range might cause problems for regulatory authorities and international harmonization of TEF values because one or more TEF values could then be selected for risk assessment calculations. This might easily lead to different TEFs being used by different countries depending on the level of conservatism used in the risk management process by national authorities. In this respect the choice, for example, of a 50th, 75th or 95th percentile of the REP distribution range to assign a TEF is a risk management decision.
Similar to the use of WHO 2005 TEFs and TEQ with abiotic matrices, the application of these values to human tissue samples must be carried out with caution. This is because the present WHO TEF concept is, by default, primarily designed for intake situations. There is emerging evidence suggesting that the relative potency of certain dioxin-like compounds may differ when the REP is determined based on administered dose versus tissue concentration (DeVito et al., 2000; Chen et al., 2001; Hamm et al., 2003). As a result the use of systemic TEFs and TEQ has been suggested as an additional approach to the present WHO TEFs. From a biological and toxicological point of view the development and use of systemic TEFs is recommended, but the expert panel was of the opinion that at present there is insufficient data to allow the development of systemic TEFs. If systemic TEFs would be developed in the future, TEF values based on blood lipid concentration might be the preferred choice. However, the use of intake TEFs from food is a valid approach for estimation of human body burdens, since many of the concerns with issues of fate and transport when dealing with abiotic matrices do not exist and many of the pharmacokinetic issues are already (partially) dealt with during bioaccumulation and biomagnification up the food chain.
With respect to the use of systemic TEFs it would also be useful to determine if in vitro derived TEFs can potentially be used as surrogates for systemic TEFs derived from in vivo studies. If such a relationship does exist, this would allow a better use of the vast amount of in vitro data that has been obtained for dioxin-like compounds over the last few decades. In view of their direct biological relevance to humans, the expert panel proposed that systemic or body burden TEFs for humans should be developed in the near future. These body burden/systemic TEFs would allow a more accurate quantitative human dose response assessment. However, it also was concluded by the expert panel that such systemic TEFs should be used in the future along with the 2005 WHO TEFs derived for ingestion situations, as both types of TEFs have different valid applications. The TEQ based on intake TEFs can be used to monitor intervention programs, while systemic or body burden TEFs would be more applicable for biomonitoring systemic levels of dioxin-like chemicals in humans. In addition, body burden TEFs can also be used as the dose metric for interspecies extrapolation. At present the WHO 2005 TEFs that are based on intake can be applied for characterization of exposure to dioxin-like chemicals in human blood or tissues and comparisons across populations, but these derived TEQ values have certain caveats from a risk assessment point of view.
Conclusions
Additivity, an important prerequisite of the TEF concept was found to be consistent with results from recent in vivo mixture studies (Gao et al., 1999; Fattore et al., 2000; Hamm et al., 2003; Walker et al., 2005). These studies showed that WHO 1998 TEF values predicted mixture toxicity within a factor two or less. Such accuracy is almost surprising in view of the fact that TEFs are derived from a range of REPs using different biological models or endpoints and are considered estimates with an order of magnitude uncertainty (Van den Berg et al., 1998).
The expert panel recognized that there are studies providing evidence that nondioxin-like AhR agonists and antagonists are able to increase or decrease the toxicity of 2,3,7,8-TCDD and related compounds. Accordingly their possible effect on the overall accuracy of the estimated magnitude of the TEQ needs to be investigated further, but it does not impact the experimental determination of individual REPs or TEFs.
For this TEF re-evaluation process the expert panel made extensive use of the refined TEF database that was recently published by Haws and coworkers (Haws et al., 2006). Decisions about a TEF value were based on a combination of unweighted REP distributions, expert judgement and point estimates. The use of solely unweighted REP distributions to set a TEF value was rejected because a specific percentile would have to be used as a cut off, which could equally well be considered as a point estimate. However, such a percentile would have a lower biological or toxicological relevance than that obtained by expert judgment.
Previous TEFs were assigned in increments of 0.01, 0.05, 0.1, etc., but for this reevaluation it was decided to use half order of magnitude increments on a logarithmic scale at 0.03, 0.1, 0.3 etc. This should be more useful in describing, with statistical methods, the uncertainty of TEFs in the future. In Table 1 the WHO 1998 and 2005 TEF values are summarized.
Figure 4 gives some indication of the quantitative impact of the 2005 changes on WHO TEF values in some selected biotic samples. The changes are shown as the ratio between the 2005 and 1998 WHO TEF values. In general it can be concluded that the changes in 2005 values have a limited impact on the total TEQ of these samples with an overall decrease in TEQ ranging between 10 and 25%. The exception being the chicken where the decrease of the TEF for 2,3,4,7,8-PeCDF (from 0.5 to 0.3) and of lower TEFs for the mono-ortho PCBs resulted in an almost 50% decrease of total TEQ. In view of this average impact of 10 to 25% it should be realized that many duplicate GC-MS analyses for these compounds also have an uncertainty that can fall in the range of 10 to 25%.
Figure 4.
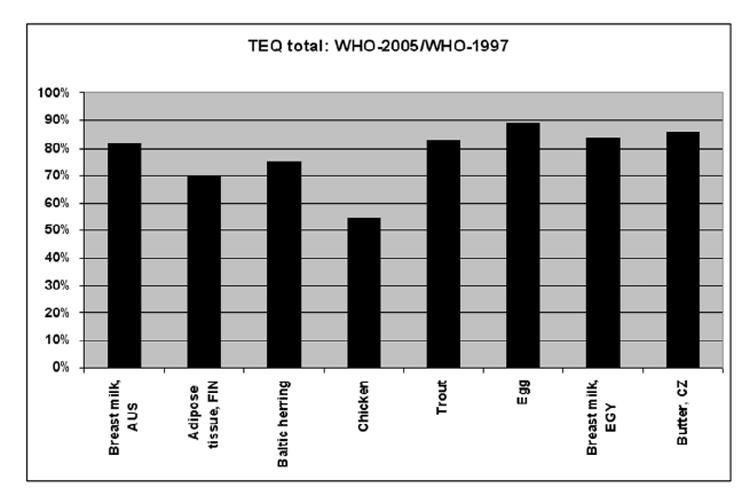
Percent reduction in total TEQ levels calculated for the same biotic samples when 2005 TEFs rather than 1997 TEFs are used. For each biotic sample shown the height of the bar is the percent that the total TEQ level determined using WHO 2005 TEFs is of the total TEQ level determined using WHO 1997 TEFs.
Several groups of compounds were identified for possible future inclusion in the TEF/TEQ concept. Based on mechanistic considerations 3,4,4’-PCB (PCB 37), PBDDs, PBDFs, PXCDDs, PXCDFs, PCNs, PBNs and PBBs undoubtedly belong in the TEF concept. However, for most if not all of these compounds there is a distinct lack of human exposure data. Therefore, preliminary exposure assessments should be done in the near future to indicate if these compounds are relevant for humans with respect to TEQ dietary intake. In addition, hexachlorobenzene could be a possible candidate for inclusion in the TEF/TEQ concept, but only if it is unequivocally shown that impurities have not been the cause of earlier dioxin-like effects observed in experimental models. With respect to polybrominated diphenylethers (PBDEs), it was concluded that there is no reason for their inclusion in the TEF/TEQ concept.
Concern is expressed about the application of the TEF/TEQ approach to abiotic environmental matrices such as soil, sediment, etc. The present TEF scheme (see Table 1) and TEQ methodology is primarily meant for estimating exposure via dietary intake situations because present TEFs are based largely on oral uptake studies often through diet. Application of these ‘intake or ingestion’ TEFs for calculating the TEQ in abiotic environmental matrices has limited toxicological relevance and use for risk assessment, unless the aspect of reduced bioavailability and environmental fate and transport of the various dioxin-like compounds are taken into account. If human risk assessment is done for abiotic matrices it is recommended that congener-specific equations be used throughout the whole model, instead of using a total TEQ-basis, because fate and transport properties differ widely between congeners.
A number of future approaches to determine alternative or additional TEFs were identified. The use of a probabilistic methodology to determine TEFs has the advantage that it better describes the level of uncertainty in a TEF. The disadvantage could be that this approach lumps data together and gives similar weight to all studies, a problem that can only partly be avoided by separating in vitro from in vivo REPs. In addition, the sole use of a probabilistic approach includes other decision points, e.g. establishing a range of values from which one or more TEF values could be selected for risk assessment. Clearly, such an approach might cause problems for regulatory authorities and international harmonization of TEFs. Furthermore, choosing a specific percentile (e.g. 50th, 75th or 95th) would, in fact, not be far different from using a point estimate.
The use of the present TEF values with body burden matrices as blood and adipose tissue have certain caveats from a risk assessment point of view, as they were determined from intake situations. There is emerging experimental evidence which suggests that some REPs may differ when based on administered dose versus tissue concentration. The development and use of systemic TEFs and TEQ is recommended as an additional approach to the present TEF concept, but at present there are insufficient data to develop these systemic TEFs.
Footnotes
Calculation of 2,3,4,7,8-PnCDF contamination in OCDD Fattore et al study (2000). 2.5 ppm = 2.5pg/μg OCDD. Highest OCDD dose 800 ppb = 800ng/g = 0.8ug/g feed. At this dose level the PnCDF dose must have been 2.5 pg PeCDF/0.8ugOCDD/g feed, which is equivalent with 2 pg PnCDF /g feed. Assuming a rat of 200 g with 20 g feed per day the PnCDF dose must have been 40pg PnCDF/200g rat, which is equivalent with 200 pg PnCDF/kg/day or 0.2 ng PnCDF/kg/day. This dose is two orders of magnitude lower than the lowest dose (20 ng PnCDF/kg/d) used in the National Toxicology Program and well below the NOEL of all endpoints that were looked at.
Analysis of hexachlorobenzene done for the UK medical research council indicated levels of 16000 ng OCDD/g, 6000 ng OCDF/g, 1000 ng HpCDF/g and 88ngTCDD/g in HCB of high chemical quality (M. Rose pers.comm..)
The contents of this paper reflect the opinions and views of the authors and do not necessarily represent the official views or policies of NIEHS, NIH, USEPA, UNEP or WHO. The mention of trade names and commercial products does not constitute endorsement or use recommendation.
References
- Addison RF, Ikonomou MG, Stobo WT. Polychlorinated dibenzo-p-dioxins and furans and non-ortho- and mono-ortho-chlorine substituted polychlorinated biphenyls in grey seals (Halichoerus grypus) from Sable Island, Nova Scotia, in 1995. Marine Environmental Research. 1999;47:225–240. [Google Scholar]
- Ahlborg UG, Becking GC, Birnbaum LS, Brouwer A, Derks HJGM, Feeley M, Golog G, Hanberg A, Larsen JC, Liem AK et al. Toxic equivalency factors for dioxin-like PCBs: Report on WHO-ECEH and IPCS consultation. Chemosphere. 1994;28:1049–1067. [Google Scholar]
- Amakura Y, Tsutsumi T, Nakamura M, Kitagawa H, Fujino J, Sasaki K, Yoshida T, Toyoda M. Preliminary screening of the inhibitory effect of food extracts on activation of the aryl hydrocarbon receptor induced by 2, 3,7,8-tetrachlorodibenzo-p-dioxin. Biol Pharm Bull. 2002;25:272–274. doi: 10.1248/bpb.25.272. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Sawyer T, Romkes M, Zmudzka B, Safe L, Mason G, Keys B, Safe S. Polychlorinated dibenzofurans (PCDFs): effects of structure on binding to the 2,3,7,8-TCDD cytosolic receptor protein, AHH induction and toxicity. Toxicology. 1984;32:131–144. doi: 10.1016/0300-483x(84)90132-x. [DOI] [PubMed] [Google Scholar]
- Bannister R, Safe S. Synergistic interactions of 2,3,7,8-TCDD and 2,2’,4,4’,5,5’-hexachlorobiphenyl in C57BL/6J and DBA/2J mice: role of the Ah receptor. Toxicology. 1987;44:159–169. doi: 10.1016/0300-483x(87)90146-6. [DOI] [PubMed] [Google Scholar]
- Barnes D, Alford-Stevens A, Birnbaum L, Kutz FW, Wood W, Patton D. Toxicity equivalency factors for PCBs? Qual Assur. 1991;1:70–81. [PubMed] [Google Scholar]
- Barnes DG. Toxicity equivalents and EPA’s risk assessment of 2,3,7,8-TCDD. Sci Total Environ. 1991;104:73–86. doi: 10.1016/0048-9697(91)90008-3. [DOI] [PubMed] [Google Scholar]
- Behnisch PA, Hosoe K, Sakai S. Bioanalytical screening methods for dioxins and dioxin-like compounds a review of bioassay/biomarker technology. Environ Int. 2001;27:413–439. doi: 10.1016/s0160-4120(01)00028-9. [DOI] [PubMed] [Google Scholar]
- Behnisch PA, Hosoe K, Sakai S. Brominated dioxin-like compounds: in vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ Int. 2003;29:861–877. doi: 10.1016/s0160-4120(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Berhow M, Tisserat B, Kanes K, Vandercook C. Survey of phenolic compounds produced in citrus. Department of Agriculture ; United States: 1998. [Google Scholar]
- Biegel L, Harris M, Davis D, Rosengren R, Safe L, Safe S. 2,2’,4,4’,5,5’-hexachlorobiphenyl as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist in C57BL/6J mice. Toxicol Appl Pharmacol. 1989 ;97:561–571. doi: 10.1016/0041-008x(89)90261-5. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Darcey DJ, McKinney JD. Hexabromonaphthalene contaminants of polybrominated biphenyls: chemical composition and disposition in the rat. J Toxicol Environ Health. 1983;12:555–573. doi: 10.1080/15287398309530449. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105:391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Harris MW, Miller CP, Pratt RM, Lamb JC. Synergistic interaction of 2,3,7,8,-tetrachlorodibenzo-p-dioxin and hydrocortisone in the induction of cleft palate in mice. Teratology. 1986;33:29–35. doi: 10.1002/tera.1420330106. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF, Diliberto JJ. Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) Environ Int. 2003;29:855–860. doi: 10.1016/S0160-4120(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Blankenship AL, Kannan K, Villalobos SA, Villeneuve DL, Falandyz J, Imagawa T, Jakobsson E, Giesy JP. Relative potencies of individual polychlorinated naphthalenes and halowax mixtures to induce Ah receptor-mediated responses. Environ Sci Technol. 2000;34:3153–3158. [Google Scholar]
- Bols . Ecotoxicology: Responses, Biomarkers and Risk Assessment. SOS Publications; Fairhaven: 1997. UNKNOWN; p. 329. [Google Scholar]
- Brown et al. UNKNOWN. Organohalogen Compounds. 2001;53:211–214. [Google Scholar]
- Chen CY, Hamm JT, Hass JR, Birnbaum LS. Disposition of polychlorinated dibenzo-p-dioxins, dibenzofurans, and non-ortho polychlorinated biphenyls in pregnant long evans rats and the transfer to offspring. Toxicol Appl Pharmacol. 2001;173:65–88. doi: 10.1006/taap.2001.9143. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Polybrominated diphenyl ethers as Ah receptor agonists and antagonists. Toxicol Sci. 2003;76:310–320. doi: 10.1093/toxsci/kfg236. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Interaction between halogenated aromatic compounds in the Ah receptor signal transduction pathway. Environ Toxicol. 2004;19:480–489. doi: 10.1002/tox.20053. [DOI] [PubMed] [Google Scholar]
- Chen I, Harper N, Safe S. Inhibition of TCDD-induced responses in B6C3F1mice and hepa1c1c7cells by indole-3-carbinol. Organohalogen Compounds. 1995;25:57–60. [Google Scholar]
- Chen I, Safe S, Bjeldanes L. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem Pharmacol. 1996;51:1069–1076. doi: 10.1016/0006-2952(96)00060-3. [DOI] [PubMed] [Google Scholar]
- Choi J-W, Fujimaki S, Kitamura K, Hashimoto S, Ito H, Suzuki N, Sakai S-I, Morita M. Polybrominated Dibenzo-p-dioxins, Dibenzofurans, and Diphenyl Ethers in Japanese Human Adipose Tissue. Environ Sci Technol. 2003;37:817–821. doi: 10.1021/es0258780. [DOI] [PubMed] [Google Scholar]
- Chu I, Villeneuve DC, Yagminas A, Lecavalier P, Hakansson H, Ahlborg UG, Valli VE, Kennedy SW, Bergman A, Seegal RF, et al. Toxicity of PCB 77 (3,3’,4,4’-tetrachlorobiphenyl) and PCB 118 (2,3’,4,4’5-pentachlorobiphenyl) in the rat following subchronic dietary exposure. Fundam Appl Toxicol. 1995;26:282–292. doi: 10.1006/faat.1995.1099. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, Carter WH, Jr, DeVito MJ. Thyroid-hormone-disrupting chemicals: evidence for dose-dependent additivity or synergism. Environ Health Perspect. 2005;113:1549–1554. doi: 10.1289/ehp.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- Davis D, Safe S. Immunosuppressive activities of polychlorinated dibenzofuran congeners: quantitative structure-activity relationships and interactive effects. Toxicol Appl Pharmacol. 1988;94:141–149. doi: 10.1016/0041-008x(88)90344-4. [DOI] [PubMed] [Google Scholar]
- De Vito M. The influence of chemical impurity on estimating relative potency factors for PCBs. Organohalogen Compounds. 2003;65:288–291. [Google Scholar]
- Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Menache MG, Diliberto JJ, Ross DG, Birnbaum LS. Dose-response relationships for induction of CYP1A1 and CYP1A2 enzyme activity in liver, lung, and skin in female mice following subchronic exposure to polychlorinated biphenyls. Toxicol Appl Pharmacol. 2000;167:157–172. doi: 10.1006/taap.2000.9010. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Ross DG, Dupuy AE, Jr, Ferrario J, McDaniel D, Birnbaum LS. Dose-response relationships for disposition and hepatic sequestration of polyhalogenated dibenzo-p-dioxins, dibenzofurans, and biphenyls following subchronic treatment in mice. Toxicol Sci. 1998;46:223–234. doi: 10.1006/toxs.1998.2530. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Falco G, Llobet JM, Casas C, Teixido A, Muller L. Polychlorinated Naphthalenes in Foods: Estimated Dietary Intake by the Population of Catalonia, Spain. Environ Sci Technol. 2003;37:2332–2335. doi: 10.1021/es030009b. [DOI] [PubMed] [Google Scholar]
- Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- Falandysz J. Chloronaphthalenes as food-chain contaminants: a review. Food Addit Contam. 2003;20:995–1014. doi: 10.1080/02652030310001615195. [DOI] [PubMed] [Google Scholar]
- Fattore E, Trossvik C, Hakansson H. Relative potency values derived from hepatic vitamin A reduction in male and female Sprague-Dawley rats following subchronic dietary exposure to individual polychlorinated dibenzo-p-dioxin and dibenzofuran congeners and a mixture thereof. Toxicol Appl Pharmacol. 2000;165:184–194. doi: 10.1006/taap.2000.8943. [DOI] [PubMed] [Google Scholar]
- Finley BL, Connor KT, Scott PK. The use of toxic equivalency factor distributions in probabilistic risk assessments for dioxins, furans, and PCBs. J Toxicol Environ Health A. 2003;66:533–550. doi: 10.1080/15287390306353. [DOI] [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gao X, Son DS, Terranova PF, Rozman KK. Toxic equivalency factors of polychlorinated dibenzo-p-dioxins in an ovulation model: validation of the toxic equivalency concept for one aspect of endocrine disruption. Toxicol Appl Pharmacol. 1999 ;157:107–116. doi: 10.1006/taap.1999.8649. [DOI] [PubMed] [Google Scholar]
- Goldstein JA. The structure-activity relationships of halogenated biphenyls as enzyme inducers. Ann N Y Acad Sci. 1979;320:164–178. [PubMed] [Google Scholar]
- Hakansson H, Manzoor E, Trossvik C, Ahlborg UG, Chu I, Villenueve D. Effect on tissue vitamin A levels in the rat following subchronic exposure to four individual PCB congeners (IUPAC 77, 118, 126, and 153) Chemosphere. 1994;29:2309–2313. doi: 10.1016/0045-6535(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Hakk H, Letcher RJ. Metabolism in the toxicokinetics and fate of brominated flame retardants--a review. Environ Int. 2003;29:801–828. doi: 10.1016/S0160-4120(03)00109-0. [DOI] [PubMed] [Google Scholar]
- Hamm JT, Chen CY, Birnbaum LS. A mixture of dioxins, furans, and non-ortho PCBs based upon consensus toxic equivalency factors produces dioxin-like reproductive effects. Toxicol Sci. 2003;74:182–191. doi: 10.1093/toxsci/kfg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106 (Suppl 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper N, Connor K, Safe S. Immunotoxic potencies of polychlorinated biphenyl (PCB), dibenzofuran (PCDF) and dibenzo-p-dioxin (PCDD) congeners in C57BL/6 and DBA/2 mice. Toxicology. 1993;80:217–227. doi: 10.1016/0300-483x(93)90183-s. [DOI] [PubMed] [Google Scholar]
- Harper N, Connor K, Steinberg M, Safe S. Immunosuppressive activity of polychlorinated biphenyl mixtures and congeners: nonadditive (antagonistic) interactions. Fundam Appl Toxicol. 1995;27:131–139. doi: 10.1006/faat.1995.1116. [DOI] [PubMed] [Google Scholar]
- Harper PA, Wong JY, Lam MS, Okey AB. Polymorphisms in the human AH receptor. Chem Biol Interact. 2002;141:161–187. doi: 10.1016/s0009-2797(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Haws LC, Su SH, Harris M, Devito MJ, Walker NJ, Farland WH, Finley B, Birnbaum LS. Development of a Refined Database of Mammalian Relative Potency Estimates for Dioxin-like Compounds. Toxicol Sci. 2006;89:4–30. doi: 10.1093/toxsci/kfi294. [DOI] [PubMed] [Google Scholar]
- Hayward D. Identification of bioaccumulating polychlorinated naphthalenes and their toxicological significance. Environ Res. 1998;76:1–18. doi: 10.1006/enrs.1997.3777. [DOI] [PubMed] [Google Scholar]
- Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- Hemming H, Bager Y, Flodstrom S, Nordgren I, Kronevi T, Ahlborg UG, Warngard L. Liver tumour promoting activity of 3, 4,5,3’,4’pentachlorobiphenyl and its interaction with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Pharmacol. 1995;292:241–249. doi: 10.1016/0926-6917(95)90028-4. [DOI] [PubMed] [Google Scholar]
- Herzke D, Berger U, Kallenborn R, Nygard T, Vetter W. Brominated flame retardants and other organobromines in Norwegian predatory bird eggs. Chemosphere. 2005;61:441–449. doi: 10.1016/j.chemosphere.2005.01.066. [DOI] [PubMed] [Google Scholar]
- Herzog MG, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetable and 9 fruits commonly consumed in the Netherlands. J Agr Food Chem. 1993a;41:2379–2383. [Google Scholar]
- Herzog MG, Hollman PCH, Van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J Agr Food Chem. 1993b;41:1242–1246. [Google Scholar]
- Hutzinger O, Choudhry GG, Chittim BG, Johnston LE. Formation of polychlorinated dibenzofurans and dioxins during combustion, electrical equipment fires and PCB incineration. Environ Health Perspect. 1985;60:3–9. doi: 10.1289/ehp.85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem. 2003;51:5478–5487. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- Johnson CW, Williams WC, Copeland CB, DeVito MJ, Smialowicz RJ. Sensitivity of the SRBC PFC assay versus ELISA for detection of immunosuppression by TCDD and TCDD-like congeners. Toxicology. 2000;156:1–11. doi: 10.1016/s0300-483x(00)00330-9. [DOI] [PubMed] [Google Scholar]
- Kotz A, Malisch R, Kypke K, Oehme M. PBDE, PBDD/F and mixed chlorinated-brominated PXDD/F in pooled human milk samples from different countries. Organohalogen Compounds. 2005;67:1540–1544. [Google Scholar]
- Kumar KS, Kannan K, Paramasivan ON, Shanmuga Sundaram VP, Nakanishi J, Masunaga S. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and polychlorinated biphenyls in human tissues, meat, fish, and wildlife samples from India. Environ Sci Technol. 2001;35:3448–3455. doi: 10.1021/es010555+. [DOI] [PubMed] [Google Scholar]
- Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE. Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3, 3’-diindolylmethane in sprague-dawley rats. Toxicol Sci. 2003;74:10–21. doi: 10.1093/toxsci/kfg103. [DOI] [PubMed] [Google Scholar]
- Liem AK, Furst P, Rappe C. Exposure of populations to dioxins and related compounds. Food Addit Contam. 2000;17:241–259. doi: 10.1080/026520300283324. [DOI] [PubMed] [Google Scholar]
- Lindberg P, Sellstrom U, Haggberg L, deWit CA. Higher Brominated Diphenyl Ethers and Hexabromocyclododecane Found in Eggs of Peregrine Falcons (Falco peregrinus) Breeding in Sweden. Environ Sci Technol. 2004;38:93–96. doi: 10.1021/es034614q. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Schrenk D, Wiesmuller T, Hagenmaier H, Bock KW. Assessment of biological activities of mixtures of polychlorinated dibenzo-p-dioxins (PCDDs) and their constituents in human HepG2 cells. Arch Toxicol. 1992;66:220–223. doi: 10.1007/BF01974019. [DOI] [PubMed] [Google Scholar]
- Loeffler IK, Peterson RE. Interactive effects of TCDD and p,p’-DDE on male reproductive tract development in in utero and lactationally exposed rats. Toxicol Appl Pharmacol. 1999;154:28–39. doi: 10.1006/taap.1998.8572. [DOI] [PubMed] [Google Scholar]
- Lunden A, Noren K. Polychlorinated naphthalenes and other organochlorine contaminants in Swedish human milk, 1972-1992. Arch Environ Contam Toxicol. 1998;34:414–423. doi: 10.1007/s002449900338. [DOI] [PubMed] [Google Scholar]
- Malmvarn A, Zebuhr Y, Jensen S, Kautsky L, Greyerz E, Nakano T, Asplund L. Identification of Polybrominated Dibenzo-p-dioxins in Blue Mussels (Mytilus edulis) from the Baltic Sea. Environ Sci Technol. 2005;39:8235–8242. doi: 10.1021/es0513281. [DOI] [PubMed] [Google Scholar]
- Mason G, Zacharewski T, Denomme MA, Safe L, Safe S. Polybrominated dibenzo-p-dioxins and related compounds: quantitative in vivo and in vitro structure-activity relationships. Toxicology. 1987;44:245–255. doi: 10.1016/0300-483x(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Mayura K, Spainhour CB, Howie L, Safe S, Phillips TD. Teratogenicity and immunotoxicity of 3, 3’,4,4’,5-pentachlorobiphenyl in C57BL/6 mice. Toxicology. 1993;77:123–131. doi: 10.1016/0300-483x(93)90143-g. [DOI] [PubMed] [Google Scholar]
- McKinney JD, McConnell EE. Structural specificity and the dioxin receptor. Pergamon; New York: 1982. [Google Scholar]
- Miller CP, Birnbaum LS. Teratologic Evalution of Hexabrominated Naphthalenes in C57BL/6N mice. Fundam Appl Toxicol. 1986:393–405. doi: 10.1016/0272-0590(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Motohashi H, Hosoya T, Nakajima O, Takahashi S, Ohsako S, Aoki Y, Nishimura N, Tohyama C, Fujii-Kuriyama Y, Yamamoto M. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc Natl Acad Sci U S A. 2003;100:5652–5657. doi: 10.1073/pnas.1037886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey RE, Harris MW, Diliberto JJ, Birnbaum LS. Limited PCB antagonism of TCDD-induced malformations in mice. Toxicol Lett. 1992;60:19–25. doi: 10.1016/0378-4274(92)90043-j. [DOI] [PubMed] [Google Scholar]
- Nagy SR, Liu G, Lam KS, Denison MS. Identification of novel Ah receptor agonists using a high-throughput green fluorescent protein-based recombinant cell bioassay. Biochemistry. 2002;41:861–868. doi: 10.1021/bi011373v. [DOI] [PubMed] [Google Scholar]
- Ohta S, Okumura T, Nakao T, Aozasa O, Miyata H. Contamination levels of organic bromine compoounds (BFRs, dioxins) in mother’s milk and daily milk products. Organohalogen Compounds. 2005;67:562–564. [Google Scholar]
- Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, Korkalainen M, Tuomisto J, Pohjanvirta R. Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: The case of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 2005;207:43–51. doi: 10.1016/j.taap.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Pang S, Cao JQ, Katz BH, Hayes CL, Sutter TR, Spink DC. Inductive and inhibitory effects of non-ortho-substituted polychlorinated biphenyls on estrogen metabolism and human cytochromes P450 1A1 and 1B1. Biochem Pharmacol. 1999;58:29–38. doi: 10.1016/s0006-2952(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Robertson LW, Safe L, Safe S. Polychlorinated biphenyls as inducers of hepatic microsomal enzymes: effects of di-ortho substitution. Chem Biol Interact. 1981;35:1–12. doi: 10.1016/0009-2797(81)90059-4. [DOI] [PubMed] [Google Scholar]
- Peters AK, Leonards PE, Zhao B, Bergman A, Denison MS, Van den Berg M. Determination of In Vitro Relative Potency (REP) Values For mono-ortho Polychlorinated Biphenyls after Purification with Active Charcoal. Toxicol Lett. 2006 doi: 10.1016/j.toxlet.2006.04.005. submitted. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AK, van Londen K, Bergman A, Bohonowych J, Denison MS, van den Berg M, Sanderson JT. Effects of polybrominated diphenyl ethers on basal and TCDD-induced ethoxyresorufin activity and cytochrome P450-1A1 expression in MCF-7, HepG2, and H4IIE cells. Toxicol Sci. 2004;82:488–496. doi: 10.1093/toxsci/kfh284. [DOI] [PubMed] [Google Scholar]
- Pluess N, Poiger H, Hohbach C, Schlatter C. Subchronic toxicity of some chlorinated dibenzofurans (PCDFs) and a mixture of PCDFs and chlorinated dibenzodioxins (PCDDs) in rats. Chemosphere. 1998;17:973–984. [Google Scholar]
- Pohjanvirta R, Korkalainen M, McGuire J, Simanainen U, Juvonen R, Tuomisto JT, Unkila M, Viluksela M, Bergman J, Poellinger L, Tuomisto J. Comparison of acute toxicities of indolo[3,2-b]carbazole (ICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in TCDD-sensitive rats. Food Chem Toxicol. 2002;40:1023–1032. doi: 10.1016/s0278-6915(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Pohl HR, McClure PR, Fay M, Holler J, De Rosa CT. Public health assessment of hexachlorobenzene. Chemosphere. 2001;43:903–908. doi: 10.1016/s0045-6535(00)00451-3. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson J, Glover E. Studies on the mechanism of action of halogenated aromatic hydrocarbons. Clin Physiol Biochem. 1985;3:147–154. [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- Roberts EA, Johnson KC, Harper PA, Okey AB. Characterization of the Ah receptor mediating aryl hydrocarbon hydroxylase induction in the human liver cell line Hep G2. Arch Biochem Biophys. 1990;276:442–450. doi: 10.1016/0003-9861(90)90743-i. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Parkinson A, Bandiera S, Lambert I, Merrill J, Safe SH. PCBs and PBBs: biologic and toxic effects on C57BL/6J and DBA/2J inbred mice. Toxicology. 1984;31:191–206. doi: 10.1016/0300-483x(84)90101-x. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Parkinson A, Campbell MA, Safe S. Polybrominated biphenyls as aryl hydrocarbon hydroxylase inducers: structure-activity correlations. Chem Biol Interact. 1982;42:53–66. doi: 10.1016/0009-2797(82)90141-7. [DOI] [PubMed] [Google Scholar]
- Safe S. Limitations of the toxic equivalency factor approach for risk assessment of TCDD and related compounds. Teratog Carcinog Mutagen. 1997;17:285–304. [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Safe SH. Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds. J Anim Sci. 1998;76:134–141. doi: 10.2527/1998.761134x. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol Sci. 2005;88:127–133. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y, Bawardi O, Schlenk D. Pesticides and PCBs in sediments and fish from the Salton Sea, California, USA. Chemosphere. 2004;55:797–809. doi: 10.1016/j.chemosphere.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Schecter A, Stanley J, Boggess K, Masuda Y, Mes J, Wolff M, Furst P, Furst C, Wilson-Yang K, Chisholm B. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ Health Perspect. 1994;102 (Suppl 1):149–158. doi: 10.1289/ehp.94102s1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D, Buchmann A, Dietz K, Lipp HP, Brunner H, Sirma H, Munzel P, Hagenmaier H, Gebhardt R, Bock KW. Promotion of preneoplastic foci in rat liver with 2,3,7,8-tetrachlorodibenzo-p-dioxin, 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin and a defined mixture of 49 polychlorinated dibenzo-p-dioxins. Carcinogenesis. 1994;15:509–515. doi: 10.1093/carcin/15.3.509. [DOI] [PubMed] [Google Scholar]
- Schrenk D, Lipp HP, Wiesmuller T, Hagenmaier H, Bock KW. Assessment of biological activities of mixtures of polychlorinated dibenzo-p-dioxins: comparison between defined mixtures and their constituents. Arch Toxicol. 1991;65:114–118. doi: 10.1007/BF02034936. [DOI] [PubMed] [Google Scholar]
- Schwab BW. The TEF approach for hexachlorobenzene. Environ Health Perspect. 1999;107:A183–184. doi: 10.1289/ehp.107-1566534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanainen U, Tuomisto JT, Tuomisto J, Viluksela M. Structure-activity relationships and dose responses of polychlorinated dibenzo-p-dioxins for short-term effects in 2,3,7,8-tetrachlorodibenzo-p-dioxin-resistant and -sensitive rat strains. Toxicol Appl Pharmacol. 2002;181:38–47. doi: 10.1006/taap.2002.9386. [DOI] [PubMed] [Google Scholar]
- Smialowicz RJ, DeVito MJ, Riddle MM, Williams WC, Birnbaum LS. Opposite effects of 2,2’,4,4’,5,5’-hexachlorobiphenyl and 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antibody response to sheep erythrocytes in mice. Fundam Appl Toxicol. 1997;37:141–149. doi: 10.1006/faat.1997.2323. [DOI] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Hirabayashi Y, Kaneko T, Ema M, Kanno J. Assessment of the cleft palate induction by seven PCDD/Fcongeners in the mouse fetus. Organohalogen Compounds. 2003;64:336–338. [Google Scholar]
- Takigami H, Sakai S, Brouwer A. Bio/chemical analysis of dioxin-like compounds in sediment samples from Osaka Bay, Japan. Environ Technol. 2005;26:459–469. doi: 10.1080/09593332608618556. [DOI] [PubMed] [Google Scholar]
- Toyoshiba H, Walker NJ, Bailer AJ, Portier CJ. Evaluation of toxic equivalency factors for induction of cytochromes P450 CYP1A1 and CYP1A2 enzyme activity by dioxin-like compounds. Toxicol Appl Pharmacol. 2004;194:156–168. doi: 10.1016/j.taap.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Tysklind M, Tillitt D, Eriksson L, Lundgren K, Rappe C. A toxic equivalency factor scale for polychlorinated dibenzofurans. Fundam Appl Toxicol. 1994;22:277–285. doi: 10.1006/faat.1994.1031. [DOI] [PubMed] [Google Scholar]
- van Birgelen AP. Hexachlorobenzene as a possible major contributor to the dioxin activity of human milk. Environ Health Perspect. 1998;106:683–688. doi: 10.1289/ehp.106-1533492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Birgelen AP, DeVito MJ, Akins JM, Ross DG, Diliberto JJ, Birnbaum LS. Relative potencies of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls derived from hepatic porphyrin accumulation in mice. Toxicol Appl Pharmacol. 1996a;138:98–109. doi: 10.1006/taap.1996.0103. [DOI] [PubMed] [Google Scholar]
- van Birgelen AP, Fase KM, van der Kolk J, Poiger H, Brouwer A, Seinen W, van den Berg M. Synergistic effect of 2,2’,4,4’,5,5’-hexachlorobiphenyl and 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic porphyrin levels in the rat. Environ Health Perspect. 1996b;104:550–557. doi: 10.1289/ehp.96104550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors TEFs for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, De Jongh J, Poiger H, Olson JR. The toxicokinetics and metabolism of polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) and their relevance for toxicity. Crit Rev Toxicol. 1994;24:1–74. doi: 10.3109/10408449409017919. [DOI] [PubMed] [Google Scholar]
- van der Burght AS, Clijsters PJ, Horbach GJ, Andersson PL, Tysklind M, van den Berg M. Structure-dependent induction of CYP1A by polychlorinated biphenyls in hepatocytes of cynomolgus monkeys (Macaca fascicularis) Toxicol Appl Pharmacol. 1999;155:13–23. doi: 10.1006/taap.1998.8606. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, van der Bruggen M, van der Linden J, van den Berg M. Effects of several dioxin-like compounds on estrogen metabolism in the malignant MCF-7 and nontumorigenic MCF-10A human mammary epithelial cell lines. Toxicol Appl Pharmacol. 2003;190:241–250. doi: 10.1016/s0041-008x(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Kannan K, Khim JS, Falandysz J, Nikiforov VA, Blankenship AL, Giesy JP. Relative potencies of individual polychlorinated naphthalenes to induce dioxin-like responses in fish and mammalian in vitro bioassays. Arch Environ Contam Toxicol. 2000;39:273–281. doi: 10.1007/s002440010105. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Stahl BU, Birnbaum LS, Rozman KK. Subchronic/chronic toxicity of 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD) in rats. Part II. Biochemical effects. Toxicol Appl Pharmacol. 1997a;146:217–226. doi: 10.1006/taap.1997.8240. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Stahl BU, Birnbaum LS, Schramm KW, Kettrup A, Rozman KK. Subchronic/chronic toxicity of 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD) in rats. Part I. Design, general observations, hematology, and liver concentrations. Toxicol Appl Pharmacol. 1997b;146:207–216. doi: 10.1006/taap.1997.8239. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Stahl BU, Rozman KK. Subchronic (13-week) toxicity of heptachlorodibenzo-p-dioxin in male Sprague-Dawley rats. Chemosphere. 1994:2381–2393. doi: 10.1016/0045-6535(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Vos JG. Health Effects of Hexachlorobenzene and the TEF Approach. Environ Health Perspect. 2000;108:A58. doi: 10.1289/ehp.108-a58a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waern F. Studies on polychlorinated dibenzo-p-dioxins and dibenzofurans with emphasis on their relative potency and interactions in the rat. Karolinska Institute; Stockholm, Sweden: 1995. [Google Scholar]
- Waern F, Flodstrom S, Busk L, Kronevi T, Nordgren I, Ahlborg UG. Relative liver tumour promoting activity and toxicity of some polychlorinated dibenzo-p-dioxin- and dibenzofuran-congeners in female Sprague-Dawley rats. Pharmacol Toxicol. 1991;69:450–458. doi: 10.1111/j.1600-0773.1991.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Walker NJ, Crockett PW, Nyska A, Brix AE, Jokinen MP, Sells DM, Hailey JR, Easterling M, Haseman JK, Yin M, Wyde ME, Bucher JR, Portier CJ. Dose-additive carcinogenicity of a defined mixture of “dioxin-like compounds”. Environ Health Perspect. 2005;113:43–48. doi: 10.1289/ehp.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Senthilkumar K, Masunaga S, Takasuga T, Iseki N, Morita M. Brominated Organic Contaminants in the Liver and Egg of the Common Cormorants Phalacrocorax carbo from Japan. Environ Sci Technol. 2004;38:4071–4077. doi: 10.1021/es0307221. [DOI] [PubMed] [Google Scholar]
- Weber LW, Greim H. The toxicity of brominated and mixed-halogenated dibenzo-p-dioxins and dibenzofurans: an overview. J Toxicol Environ Health. 1997;50:195–215. doi: 10.1080/009841097160456. [DOI] [PubMed] [Google Scholar]
- Weistrand C, Noren K. Polychlorinated naphthalenes and other organochlorine contaminants in human adipose and liver tissue. J Toxicol Environ Health A. 1998;53:293–311. doi: 10.1080/009841098159295. [DOI] [PubMed] [Google Scholar]
- Wermelinger M, Poiger H, Schlatter C. Results of a 9-month feeding study with OCDD and OCDF in rats. Organohalogen Compounds. 1990;1:221–224. [Google Scholar]
- Wilker C, Johnson L, Safe S. Effects of developmental exposure to indole-3-carbinol or 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive potential of male rat offspring. Toxicol Appl Pharmacol. 1996;141:68–75. doi: 10.1006/taap.1996.0261. [DOI] [PubMed] [Google Scholar]
- Williams DT, Kennedy B, LeBel GL. Chlorinated naphthalenes in human adipose tissue from ontario municipalities. Chemosphere. 1993;27:795–806. [Google Scholar]
- Yoshimura H, Yoshihara S, Ozawa N, Miki M. Possible correlation between induction modes of hepatic enzymes by PCBs and their toxicity in rats . New York Academy of Sciences; New York: 1979. [DOI] [PubMed] [Google Scholar]
- Zabel EW, Walker MK, Hornung MW, Clayton MK, Peterson RE. Interactions of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners for producing rainbow trout early life stage mortality. Toxicol Appl Pharmacol. 1995;134:204–213. doi: 10.1006/taap.1995.1185. [DOI] [PubMed] [Google Scholar]
- Zeiger M, Haag R, Hockel J, Schrenk D, Schmitz HJ. Inducing effects of dioxin-like polychlorinated biphenyls on CYP1A in the human hepatoblastoma cell line HepG2, the rat hepatoma cell line H4IIE, and rat primary hepatocytes: comparison of relative potencies. Toxicol Sci. 2001;63:65–73. doi: 10.1093/toxsci/63.1.65. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qin C, Safe S. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]


