Abstract
In allogeneic hematopoietic cell transplantation (HCT), donor T cell-mediated graft versus host leukemia (GVL) and graft versus autoimmune (GVA) activity play critical roles in treatment of hematological malignancies and refractory autoimmune diseases. However, graft versus host disease (GVHD), which sometimes can be fatal, remains a major obstacle in classical HCT, where recipients are conditioned with total body irradiation or high-dose chemotherapy. We previously reported that anti-CD3 conditioning allows donor CD8+ T cells to facilitate engraftment and mediate GVL without causing GVHD. However, the clinical application of this radiation-free and GVHD preventative conditioning regimen is hindered by the cytokine storm syndrome triggered by anti-CD3 and the high-dose donor bone marrow (BM) cells required for induction of chimerism. Histone deacetylase (HDAC) inhibitors such as suberoylanilide hydroxamic acid (SAHA) are known to induce apoptosis of cancer cells and reduce production of proinflammatory cytokines by nonmalignant cells. Here, we report that SAHA inhibits the proliferative and cytotoxic activity of anti-CD3-activated T cells. Administration of low-dose SAHA reduces cytokine production and ameliorates the cytokine storm syndrome triggered by anti-CD3. Conditioning with anti-CD3 and SAHA allows induction of chimerism with lower doses of donor BM cells in old nonautoimmune and autoimmune lupus mice. In addition, conditioning with anti-CD3 and SAHA allows donor CD8+ T cell-mediated GVA activity to reverse lupus glomerulonephritis without causing GVHD. These results indicate that conditioning with anti-CD3 and HDAC inhibitors represent a radiation-free and GVHD-preventative regimen with clinical application potential.
Keywords: anti-CD3 mAb, graft versus host disease, hematopoietic cell transplantation, systemic lupus erythematosus, suberoylanilide hydroxamic acid
Allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for hematological malignancies and refractory autoimmune diseases such as systemic lupus erythematosus (SLE) (1–3). However, in classical HCT, recipients are usually conditioned with total body irradiation (TBI) and/or high-dose chemotherapy. The toxicity of conditioning, even with reduced intensity, and the potential for graft versus host disease (GVHD) are preventing the application of allogeneic HCT to the treatment of refractory autoimmune diseases, although it is considered justified for the treatment of life-threatening hematological malignancies (1–3).
It has been demonstrated that TBI and high-dose chemotherapy conditioning play a critical role in initiating GVHD (4). The conditioning procedures cause tissue damage, release of proinflammatory cytokines and chemokines, and activation of host antigen-presenting cells (APCs), resulting in a proinflammatory cascade and donor alloreactive T cell infiltration of GVHD target tissues (5–7). Therefore, we and others have been searching for a radiation-free and GVHD-preventive conditioning regimen for allogeneic HCT. It was reported that transplantation of a large dose of donor bone marrow (BM) cells and administration of costimulatory blockade (i.e., anti-CD40L) induced mixed chimerism in nonautoimmune mice (8), but this regimen has not been shown to work in autoimmune mice.
We recently reported that infusion of donor CD8+ T or CD4+ T-depleted spleen cells and BM cells induced chimerism without GVHD in nonautoimmune and autoimmune NOD mice (9–11). The mechanisms of GVHD prevention in the anti-CD3-conditioned recipients include confining donor CD8+ T cells to host lymphohematological tissues and tolerizing the alloreactive T cells (10). The anti-CD3-conditioning regimen allows donor CD8+ T cells to mediate graft versus host leukemia (GVL) activity and graft versus autoimmune (GVA) activity without causing GVHD (9–11). In addition, the chimeric NOD recipients with chimerism established by anti-CD3 conditioning showed regeneration of islet β-cells and reversal of diabetes (11). Therefore, the anti-CD3-conditioning regimen has the potential to promote allogeneic HCT for treating autoimmune diseases. However, anti-CD3 conditioning often causes a cytokine storm syndrome, which includes hypothermia and hypoglycemia. The cytokine storm syndrome and the high-dose donor BM cells required for the induction of chimerism hamper the clinical application of this anti-CD3-based conditioning regimen.
Chromatin remodeling by acetylation or deacetylation of histones play an critical role in the regulation of gene expression (12, 13). Histone acetylation is controlled by two classes of enzymes: histone acetyltransferases (HATs) add acetyl groups to lysine residues, whereas histone deacetylases (HDACs) remove the acetyl groups (13). Acetylation of histones relaxes chromatin structure, allowing the binding of transcription factors and promoting transcription. In contrast, deacetylation of histones condenses chromatin structure and represses gene transcription (12, 13). Inhibiting the deacetylation of histones results in hyperacetylation and modifies gene expression either positively or negatively in a cell type-specific manner (13).
Suberoylanilide hydroxamic acid (SAHA), also known as vorinostat, contains a hydroxamic acid moiety that binds to the zinc-containing pocket in the catalytic site of the HDAC 6 and thus causes their reversible inhibition (14). It has been reported that SAHA selectively increases the expression of many genes in tumor cells, causing them to undergo cell-cycle arrest and apoptosis (15). Although micromolar concentrations of SAHA are required for anti-tumor effects, nanomolar concentrations of SAHA can reduce the secretion of inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, and IL-12 by nonmalignant cells (16). It was reported that administration of low-dose SAHA reduced serum levels of inflammatory cytokines and ameliorated GVHD without inhibiting donor T cell function and their GVL activity (17, 18).
In the current study, we also found that high-concentration SAHA augmented apoptosis and low-concentration SAHA inhibited the proliferative and cytotoxic activity of the anti-CD3-activated T cells. Administration of low-dose SAHA during anti-CD3 conditioning ameliorated cytokine storm syndrome and augmented the induction of chimerism without causing GVHD. In addition, conditioning with anti-CD3 and SAHA allowed induction of complete chimerism without causing GVHD, which “cured” systemic lupus in NZB/W F1 mice with severe glomerulonephritis. This radiation-free and GVHD-preventative conditioning regimen has great potential for clinical application.
Results
SAHA Augmented Apoptosis and Inhibited Proliferation of Anti-CD3-Activated T Cells in a Dose-Dependent Manner.
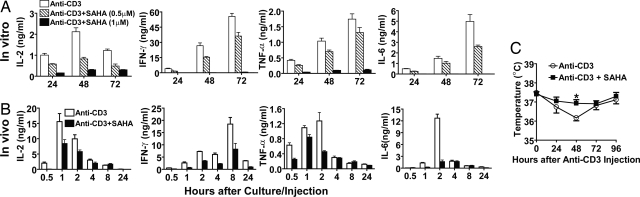
SAHA was shown to mediate apoptosis of growing tumor cells (15) and inhibit proliferation of autoreactive T cells (19). Therefore, we tested the effect of SAHA on T cells that were activated by anti-CD3 mAb. BALB/c spleen cells were stimulated with plate-bound anti-CD3 in the culture medium with titrated concentration of SAHA (0.5–4 μM) or solvent DMSO for 72 h. We found that SAHA augmented T cell apoptosis in a dose-dependent manner. Although 0.5 μM SAHA showed little augmentation of T cell apoptosis compared with cells stimulated with anti-CD3 alone, 1 μM SAHA augmented the apoptosis by ≈2-fold; 2 μM SAHA augmented the apoptosis by ≈3-fold; and 4 μM SAHA led to apoptosis of nearly all T cells (P < 0.01, Fig. 1A). Cells cultured with medium alone and cells cultured with SAHA alone showed little apoptosis (data not shown). It was of interest that, although 0.5 μM SAHA did not significantly change the apoptosis or survival rate of the anti-CD3-activated T cells (Fig. 1 A and B), it inhibited the proliferation of the T cells by 2-fold (P < 0.01, Fig. 1C). Although 40% of the residual T cells in the culture of 1 μM SAHA were annexin V− live cells, they showed no proliferation at all (P < 0.01, Fig. 1C). In addition, consistent with the previous reports (17, 18), 0.5 μM SAHA showed no inhibition of the donor T cells stimulated with allo-APCs, but 1 μM SAHA markedly inhibited the T cell proliferation (Fig. 1D). These results indicate that high concentrations (>1 μM) of SAHA augment apoptosis and low concentrations (<1 μM) of SAHA inhibit the proliferation of the anti-CD3 activated T cells.
Fig. 1.
SAHA augmented apoptosis and inhibited proliferation of anti-CD3-activated T cells in a dose-dependent manner. (A) BALB/c spleen cells (0.5 × 106) were stimulated with plate-bound anti-CD3 in culture medium with titrated concentrations of SAHA (0 to 4 μM) for 72 h. Thereafter, cells were stained with anti-TCRαβ, DAPI, and annexin V. TCRαβ+ cells were gated and shown in a histogram of annexin V. The percentages of annexin V+ cells are shown for each culture. One representative FACS pattern of four replicated experiments is shown. The mean ± SE of the percentage of annexin V+ cells under different SAHA concentration (0 to 4 μM) were 38.1 ± 6.5%, 41.5 ± 7.2%, 64.4 ± 6.6%, 85.5 ± 2.7%, and 97.1 ± 1.5%. (B) Percentage of viable T cells among total T cells in the culture with SAHA at concentration of 0 to 1 μM. (C) T cell proliferation by anti-CD3 stimulation with SAHA at concentration of 0 to 1 μM. (D) T cell proliferation by allo-APC stimulation with SAHA at concentration of 0 to ≈1 μM. B–D show mean ± SE of four replicated experiments.
Administration of Low-Dose SAHA Reduced Cytokine Storm Triggered by Anti-CD3.
One of the side effects of anti-CD3 conditioning was the cytokine storm triggered by anti-CD3 activation of T cells. The elevated levels of TNF-α during the cytokine storm caused hypothermia, and the mice appeared to be inactive (20). Next, we tested whether SAHA could reduce cytokine production triggered by anti-CD3. First, BALB/c spleen cells (0.5 × 106) were stimulated with plate-bound anti-CD3 in culture medium with 0.5 or 1 μM SAHA. Supernatants were harvested 24, 48, and 72 h after culture, and IL-2, IFN-γ, TNF-α, and IL-6 concentrations were measured with ELISA. We observed that, after anti-CD3-stimulation, IL-2 concentration peaked at 48 h after culture, and IFN-γ, TNF-α, and IL-6 all peaked at 72 h after culture. Compared with the culture with anti-CD3 stimulation only, addition of 0.5 μM SAHA to the culture reduced cytokine production significantly at all time points (P < 0.05, Fig. 2A), and addition of 1 μM SAHA to the culture made all four cytokines nearly undetectable (P < 0.01, Fig. 2A).
Fig. 2.
SAHA reduced in vitro and in vivo cytokine production triggered by anti-CD3. (A) BALB/c spleen cells (0.5 × 106) were stimulated with plate-bound anti-CD3 in the presence of SAHA at concentrations of 0, 0.5, and 1 μM. IL-2, IFN-γ, TNF-α, and IL-6 in culture supernatant were measured at 24, 48, and 72 h after culture. Mean ± SE of four replicated experiments are shown. (B) BALB/c mice were i.v. injected with anti-CD3 (5 μg/g) with or without coinjection of SAHA (40 μg/g) 12 and 1 h before anti-CD3 injection. Serum cytokines were measured kinetically (0.5 to ≈24 h) after anti-CD3 injection. Mean ± SE of four mice at each time point is shown. (C) Kinetic body temperature change of mice injected with anti-CD3 alone or anti-CD3 and SAHA. There were seven mice in each group.
Second, old BALB/c mice (>16 weeks) were injected with anti-CD3 (5 μg/g) alone or anti-CD3 and SAHA at 40, 100, or 200 μg/g. Sera were harvested kinetically at 0.5, 1, 2, 4, 8, and 24 h after injection for the measurement of cytokines. The mice were also measured for body temperature at the same time. We observed that, after injection of anti-CD3 alone, serum IL-2 levels peaked at 1 h after injection; serum IFN-γ levels peaked at 8 h; serum TNF-α and IL-6 levels peaked at 2 h; and all of the serum cytokine levels fell back to background levels 24 h after injection (Fig. 2B). Interestingly, the mice started to look sick 24 h after injection and appeared to be most inactive 48 h after injection, and then they recovered and looked fairly normal again 96 h after injection. The body temperature of the mice reached its lowest level 48 h after injection and then recovered to nearly normal 96 h after injection (Fig. 2C).
However, coinjection of SAHA at a dose of 40 μg/g reduced serum levels of IL-2, IFN-γ, TNF-α, and IL-6 by >2-fold at the peak time points compared with that of the mice injected with anti-CD3 alone (P < 0.01, Fig. 2B). The coinjection of SAHA also significantly improved hypothermia (P < 0.01, Fig. 2C), and the treated mice appeared much more active. Coinjection of SAHA at a dose of 100 μg/g resulted in similar serum cytokine reduction and improvement in hypothermia compared with the mice coinjected with SAHA at 40 μg/g (data not shown). However, coinjection of SAHA at a dose of 200 μg/g caused the mice to develop severe diarrhea, and 30% of the mice died 5 days after anti-CD3 conditioning, although they all had similar reductions in serum cytokine levels compared with the mice coinjected with SAHA at 40 μg/g (data not shown). Together, our results indicate that administration of a low dose (≤100 μg/g) of SAHA ameliorates cytokine storm syndrome triggered by mitogenic anti-CD3.
Coinjection of Low-Dose SAHA Augmented Induction of Chimerism in Old BALB/c Recipients Conditioned with Anti-CD3.
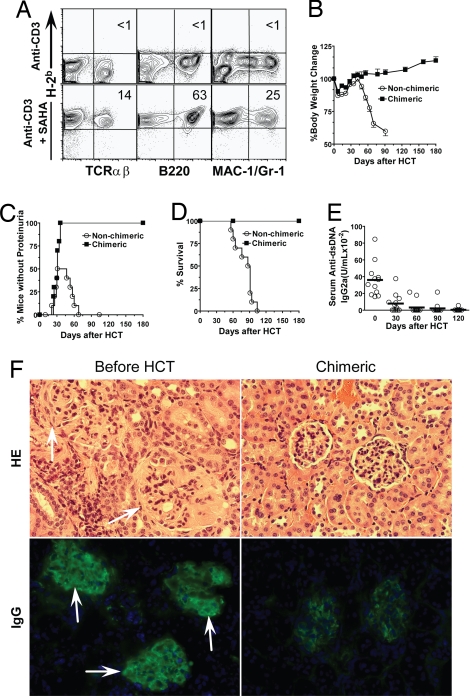
We observed that conditioning with anti-CD3 alone was not sufficient for induction of chimerism in old (>12-week-old) recipients, although it was sufficient in young (<8-week-old) recipients (11). Next, we tested whether the coinjection of low-dose SAHA could augment induction of chimerism in old recipients. Accordingly, old (>16-week-old) BALB/c mice were conditioned with anti-CD3 alone or with a combination of anti-CD3 and SAHA (40 μg/g). Nine days after conditioning, mice were injected with BM (2 × 106/g, ≈50 × 106 per mouse) and CD4+ T-depleted spleen cells (4 × 106/g) from C57BL/6 donors. The recipients were monitored for clinical signs of GVHD and checked for chimerism 8 weeks after HCT. We found that 0/12 of the recipients conditioned with anti-CD3 alone developed chimerism, but 12/12 of the recipients conditioned with anti-CD3 and SAHA developed complete chimerism, in which almost all of the T, B, macrophage, and granulocyte cells were donor type (Table 1 and Fig. 3A). We observed no clinical signs of GVHD in the chimeric recipients, and their body weight change was the same as that of the nonchimeric mice given conditioning only (Fig. 3B). These results indicate that SAHA can augment the induction of chimerism without augmenting the induction of GVHD when coinjected with anti-CD3 for conditioning allogeneic recipients.
Table 1.
Induction of chimerism in recipients conditioned with anti-CD3 + SAHA
| Recipient | Conditioning | % chimerism |
|---|---|---|
| Old BALB/c | Anti-CD3 | 0/12 |
| Anti-CD3 + SAHA | 12/12 | |
| Lupus NZBW F1 | Anti-CD3 | 0/10 |
| Anti-CD3 + SAHA | 10/10 |
Fig. 3.
SAHA augmented induction of chimerism in old BALB/c mice. Old (>16 weeks) BALB/c mice were conditioned with anti-CD3 alone or anti-CD3 and SAHA and transplanted with C57BL/6 donor BM (2 × 106) and CD4+ T-depleted spleen cells (4 × 106). The recipients were monitored for clinical signs of GVHD daily and body weight weekly. (A) At 8 weeks after HCT, blood mononuclear cells were stained for H-2b (donor marker), TCRαβ, B220, and Mac-1/Gr-1. The percentages of donor T, B, and macrophage cells are shown. One representative sample of 12 mice in each group is shown. (B) Body weight change curves of HCT recipients conditioned with anti-CD3 alone (nonchimeric) or anti-CD3 plus SAHA (chimeric). There were 12 mice in each group. Mean ± SE is shown at each time point. (C) Yield of residual DAPI− live T cells in spleen of mice conditioned with anti-CD3 or anti-CD3 plus SAHA 9 days after anti-CD3 injection at the day of HCT. (D) Sorted T cells (0.2 × 106) from the spleen of conditioned BALB/c mice 9 days after anti-CD3 injection were stimulated with C57BL/6 dendritic cells (0.1 × 106) for 5 days. The proliferation was measured with 3H-TdR incorporation, and the stimulation index was calculated by formula: {[cpm of responder × stimulator] − [cpm of responder alone]} ÷ [cpm of responder alone]. Mean ± SE of four mice in each group is shown. (E) At 5 days after HCT, donor-type spleen cells from congenic C57BL/6 (CD45.1) and CFSE-labeled host-type spleen cells from BALB/c were injected into recipients conditioned with anti-CD3 alone or anti-CD3 plus SAHA. Then, 18 h later, spleen cells were harvested and stained with anti-CD45.1. Staining is shown in CD45.1 versus CFSE. One representative FACS pattern of four mice in each group is shown. The mean ± SE of CD45.1+ or CFSE+ cells in recipients conditioned with anti-CD3 alone versus recipients conditioned with anti-CD3 plus SAHA were 1.21 ± 0.07% versus 1.80 ± 0.08% or 0.35 ± 0.03% versus 0.20 ± 0.01%. (F) The ratio of residual CD45.1+ donor-type cells versus CFSE+ host-type cells were calculated, and mean ± SE of 4 recipients in each group is shown.
Next, we explored the mechanisms whereby SAHA augmented engraftment when used with anti-CD3 in the conditioning of recipients. Because host T cells play a major role in graft rejection, we focused on the effect of SAHA on host T cells. We found that coinjection of low-dose (40 or 100 μg/g) SAHA neither significantly increased the apoptosis of host T cells nor reduced the yield of residual live T cells in the spleen of treated mice (Fig. 3C). Because low-concentration SAHA inhibited proliferation of anti-CD3 activated T cells, although it did not augment apoptosis of the T cells (Fig. 1), we compared the proliferative capacity of T cells from the BALB/c recipients conditioned with anti-CD3 alone or anti-CD3 and SAHA in response to stimulation by C57BL/6 donor dendritic cells. We found that the proliferation of the T cells from the former was 2-fold more compared with the latter (P < 0.01, Fig. 3D). Furthermore, we compared the cytotoxic activity of the residual host T cells in recipients conditioned with anti-CD3 alone or anti-CD3 plus SAHA 5 days after HCT by injection of spleen cells from naïve donor and host mice. We found that residual host T cells in anti-CD3-conditioned recipients had stronger cytotoxic activity compared with those in the recipients conditioned with anti-CD3 and SAHA, so that the ratio of the residual CD45.1+ donor-type targets versus carboxyfluoroscein succinimidyl ester (CFSE)-labeled host-type targets in the recipients conditioned with anti-CD3 was 2-fold lower than that in the recipients conditioned with anti-CD3 plus SAHA (P < 0.01, Fig. 3 E and F). Together, augmentation of engraftment by conditioning with low-dose SAHA and anti-CD3 is at least in part via inhibition of the rejecting function of the residual host T cells.
Conditioning with Anti-CD3 and Low-Dose SAHA Allowed Induction of Complete Chimerism That Reversed Overt Lupus.
Sorted allogeneic stem cells recently have been shown to establish mixed chimerism in sublethally irradiated NZB/W F1 recipients and reverse overt lupus (21). However, 50% of the chimeric recipients continued to have high levels of serum autoantibodies, and ≈30% of the chimeric recipients continued to have proteinuria and eventually died of lupus (21). This observation indicates that the pathogenic memory T and B cells continue to exist in the lupus recipients with mixed chimerism and continue to mediate lupus, and induction of complete chimerism may be required for the cure of lupus.
We tested whether conditioning with anti-CD3 and SAHA could allow induction of complete chimerism without GVHD in old NZB/W F1 mice with severe glomerulonephritis and proteinuria. First, we observed that one injection of anti-CD3 with multiple injections of low-dose SAHA (40 μg/g) was not sufficient for induction of chimerism in old NZB/W F1 mice with severe proteinuria (data not shown), which might be attributable to the loss of antibody and SAHA in urine, which could have led to the insufficient depletion of host T cells. Therefore, we repeated the injection of anti-CD3 and SAHA 5 days after the first injection. Five days after the second injection, the mice were infused with the same dose of donor BM cells (2 × 106/g) and CD4+ T-depleted spleen cells (4 × 106/g) as used for the old BALB/c mice. We found that, although none (0/10) of the recipients conditioned with anti-CD3 alone developed chimerism, all (10/10) of the recipients conditioned with anti-CD3 and SAHA developed complete chimerism (Table 1 and Fig. 4A). In addition, the chimeric recipients showed no clinical signs of GVHD over a 180-day period after HCT, and the recipients showed a steady body weight increase as they aged. In contrast, the nonchimeric recipients conditioned with anti-CD3 alone showed severe body weight loss caused by the progression of lupus (Fig. 4B).
Fig. 4.
Conditioning with anti-CD3 and SAHA allowed induction of complete chimerism and reversed severe glomerulonephritis. Old NZB/W F1 mice (> 7 months) with severe proteinuria were conditioned with anti-CD3 and SAHA and transplanted with C57BL/6 donor BM (2 × 106/g) and CD4+ T-depleted spleen cells (4 × 106/g). The recipients were monitored for clinical signs of GVHD daily and body weight and proteinuria twice a week. The recipients were checked for chimerism 8 weeks after HCT. (A) Blood mononuclear cells of the anti-CD3 and SAHA-conditioned mice with or without HCT were stained for H-2b (donor marker) versus TCRαβ, B220, or Mac-1/Gr-1. The percentage of donor-type T, B, and macrophage cells is shown. One representative of 10 mice in each group is shown. (B) Body weight change curves of the mice given conditioning alone or conditioning and HCT over a 180-day period after HCT. Mean ± SE of 10 mice in each group is shown. (C and D), Proteinuria change curve and survival curve of the lupus mice given conditioning alone or conditioning and HCT. (E) Kinetic changes of serum levels of anti-dsDNA IgG2a antibodies in lupus mice given conditioning and HCT. (F) Hematoxylin/eosin staining of kidney tissues and immunofluorescent staining of IgG deposition in glomeruli of the lupus mice before treatment and 180 days after HCT. One representative sample of four examined mice in each group is shown.
We also longitudinally monitored the proteinuria, serum autoantibody levels, and survival of the lupus mice with or without chimerism. We found that the nonchimeric recipients conditioned with anti-CD3 alone temporarily reversed lupus in ≈50% of the mice, but they all showed severe proteinuria again 60 days after treatment and all died 120 days after treatment (Fig. 4 C and D). In contrast, all of the chimeric recipients became proteinuria-free ≈50 days after HCT and all survived for >180 days after HCT (Fig. 4 C and D). The serum anti-dsDNA levels of the chimeric recipients gradually decreased and became almost undetectable by 60 days after HCT (Fig. 4E). The histopathology of the kidneys of the chimeric recipients were compared with that of the lupus mice before HCT. We found that, although the glomeruli of the untreated lupus mice appeared swollen and had severe IgG deposition, the glomeruli of the chimeric recipients appeared to be normal and had very little IgG deposition (Fig. 4F). These results indicate that conditioning with anti-CD3 and SAHA allows donor CD8+ T cells to eliminate host memory pathogenic T cells and autoantibody-secreting B cells without causing GVHD and to cure lupus glomerulonephritis.
Discussion
We have demonstrated that low-dose SAHA reduced serum cytokine levels and ameliorated the cytokine storm syndrome triggered by anti-CD3 during conditioning; the low-dose SAHA also reduced the proliferative and cytotoxic activity of the residual host T cells, resulting in augmentation of donor hematopoietic cell engraftment without causing GVHD. In addition, we demonstrated that conditioning with SAHA and anti-CD3 allowed induction of complete chimerism and cure of severe lupus glomerulonephritis.
We observed that low-dose SAHA reduced in vitro and in vivo cytokine production (IL-2, IFN-γ, TNF-α, and IL-6) triggered by mitogenic anti-CD3. It is not yet clear how SAHA inhibits the cytokine cascade triggered by anti-CD3 activation of T cells. It is likely that SAHA inhibits the transcription and translation of IL-2 in T cells and subsequently inhibits cytokine secretion by T cells and other cells such as macrophages. It was reported that an HDAC inhibitor (trichostatin A) represses IL-2 gene expression in anti-CD3 activated T cells (22). Low-dose SAHA treatment resulted in increased acetylation of histone H3 and decreased mRNA levels of IFN-γ and TNF-α in activated spleen cells (17). SAHA treatment also inhibited phosphorylation of STAT1 and STAT3 in response to LPS and alloactivation in vitro (18), and STAT1 and STAT3 play a critical role in the production of proinflammtory cytokines such as IFN-γ and IL-17 (23, 24). Administration of low-dose SAHA also ameliorated cytokine storm symptoms such as hypothermia triggered by anti-CD3. This finding might result from the marked reduction in the serum levels of TNF-α after administration of SAHA. It was reported previously that cytokine storm syndrome caused by injection of mitogenic anti-CD3 was ameliorated by neutralizing TNF-α (20).
We observed that SAHA augmented induction of chimerism when used with anti-CD3 for conditioning of allogeneic recipients, which was consistent with our observation that high-concentration SAHA augmented the apoptosis and low-concentration SAHA reduced the proliferative and cytotoxic activity of T cells activated by anti-CD3. Thus, the residual T cells in the recipients conditioned with anti-CD3 and low-dose SAHA had a reduced capacity to reject the donor cells. It is not yet clear how SAHA renders the anti-CD3-activated T cells apoptotic or less proliferative/cytotoxic. High concentrations of SAHA have been shown to induce tumor cell apoptosis by activating extrinsic Fas–FasL and TNFαR–TNF-α apoptotic pathways (15) and cytochrome-caspase-9 intrinsic pathways (15); low concentration of SAHA predominantly induced cell-cycle arrest at G1 phase in both normal and transformed cells (15). In addition, we observed that SAHA markedly reduced the IL-2 production of anti-CD3 activated T cells, which may contribute to the reduction of T cell proliferation because IL-2 plays a critical role in T cell proliferation. Therefore, SAHA can augment depletion or inhibition of the anti-CD3-activated host T cells, resulting in facilitation of donor hematopoietic cell engraftment.
Donor T cells usually induced GVHD in recipients conditioned with chemotherapy (1, 25). However, we observed no clinical signs of GVHD in the recipients conditioned with anti-CD3 and SAHA after infusion of a high dose of CD4+ T-depleted spleen cells. The GVHD prevention mechanisms in recipients conditioned with anti-CD3 alone, which include confining of donor T cells to host lymphohematological tissues and tolerizing the residual alloreactive donor T cells, was reported in our previous publication (10). It is not surprising that the addition of SAHA to the anti-CD3-conditioning regimen did not lead to induction of GVHD, even though SAHA is a chemoreagent, because unlike traditional chemoreagents that cause host tissue damage and release of proinflammatory cytokines and chemokines (1, 4), SAHA inhibits the tissue release of the proinflammatory cytokines and chemokines (16–18). In addition, HDAC inhibitors similar to SAHA have been shown to augment the generation and function of FoxP3+ regulatory T (Treg) cells (26) and modulate APC function to down regulate Th1 differentiation (27). Treg cells were shown to prevent GVHD (28), and Th1 cells were shown to mediate GVHD (1).
Induction of mixed chimerism with sorted donor stem cells was able to ameliorate systemic lupus but not able to cure the disease (21), indicating that GVA activity mediated by donor T cells may be required. However, GVA activity is usually associated with GVHD in recipients conditioned with TBI or high-dose chemotherapy (2, 3). In our current study, NZB/W F1 mice with severe glomerularnephritis and proteinuria were conditioned with SAHA and anti-CD3 and were induced to develop complete chimerism with no clinical signs of GVHD. The chimeric recipients showed gradual disappearance of serum autoantibodies, proteinuria, and glomerularnephritis. These results indicate that conditioning with anti-CD3 and SAHA allows donor CD8+ T cells to mediate GVA activity without causing GVHD.
In conclusion, addition of a HDAC inhibitor such as SAHA to the anti-CD3-based conditioning regimen ameliorates the cytokine storm syndrome triggered by the mitogenic anti-CD3 and reduces the required donor BM dose for induction of chimerism but does not cause GVHD. This is a demonstration that an anti-tumor chemoreagent can be used for conditioning of allogeneic recipients without induction of GVHD. This radiation-free and GVHD-preventive conditioning regimen may promote the application of allogeneic HCT for treating refractory autoimmune disease in addition to the treatment of hematological malignancies.
Materials and Methods
Mice.
C57BL/6 (H-2b, CD45.2), congenic C57BL/6 (H-2b, CD45.1), BALB/c (H-2d),and female NZB/W F1 (H-2d/z) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained in a pathogen-free room at City of Hope Animal Research Facilities (Duarte, CA). Animal use protocols were approved by the institutional review committee.
Flow Cytometric Analysis and Cell Sorting.
The following anti-mouse mAbs were purchased from BD Biosciences PharMingen (San Diego, CA), eBioscience (San Diego, CA), and R&D Systems (Minneapolis, MN): TCR (H57–597), CD4 (RM4–5), CD8 (53–6.7), B220 (RA3–6B2), CD11b/Mac-1 (M1/70), Gr-1 (RB6–8C5), CD45.1(A20), H-2b(AF6–88.5), and H-2d (34–2-12). FACS was performed with a four-laser MoFlo Immunocytometry System (Dako Cytomation, Fort Collins, CO), and data were analyzed with FlowJo software (Tree Star, San Carlos, CA), as previously described (9–11). The apoptosis measuring kit (anti-annexin V Ab) was purchased from BD PharMingen. CD4−-SPL cells from donor spleen were negatively selected with anti-CD4-FITC and anti-FITC micromagnetic beads purchased from Miltenyi Biotec (Auburn,CA), as previously described (9–11).
Chemoreagents.
SAHA (vorinostat) was provided by Aton Merck Pharmaceutical and the National Cancer Institute. SAHA was dissolved in DMSO at 1 M and aliquots were kept at −20°C. For in vitro assays, SAHA was diluted with culture media. For in vivo assays, SAHA was diluted in 45% PEG 400 (Sigma) and 55% water.
Conditioning of Recipients and HCT.
Production of anti-CD3 mAb (145–2C11) was described in our previous publication (10). Recipient mice were injected i.v. with anti-CD3 (5 μg/g) on day −9 and were injected i.p. with SAHA (40–200 μg/g) 12 and 1 h before anti-CD3 injection, then SAHA was injected daily for 7 days after anti-CD3 injection. On day 0, the conditioned recipients were transplanted with donor BM cells (2 × 106/g, ≈50 × 106 per mouse) and CD4+ T-depleted spleen cells (4 × 106/g). The BM and spleen cells were injected again 7 days after the first injection. The recipients were monitored for clinical signs of GVHD and checked for chimerism as previously described (9–11). Proteinuria in NZB/W F1 mice was measured on a scale of 1–4+ using a colorimetric assay for albumin (Albustix, Bayer). Mice were considered to have proteinuria if three consecutive urine samples were >2+, according to the scale (100 mg/dl), as described in our previous publications (29, 30).
CFSE-Labeling of Spleen Cells.
Splenocytes were suspended at 1–3 × 107 cells per ml, and CFSE (Invitrogen) was added at a final concentration of 2.5 μM. Cells were incubated at 37°C for 10 min, as previously described (31).
In Vivo Cytotoxicity Assay and Mixed Lymphocyte Reaction (MLR).
At day 5 after HCT, recipients were injected with 20 × 106 unlabeled splenocytes from congenic donor-type C57BL/6 (H-2b, CD45.1) and 20 × 106 CFSE-labeled splenocytes from host-type BALB/c (H-2d). Eighteen hours later, splenocytes were harvested and stained with PE-conjugated anti-CD45.1. The percentages of remaining CD45.1+ and CFSE-labeled cells were determined by FACS analysis. The in vivo cytotoxic activity of residual host T cells was reflected by the ratio of residual CD45.1+ donor-type cells versus residual CFSE-labeled host-type cells. This assay system was also previously described (31). The MLR assay was performed as previously described (10).
Histopathology of Kidney.
Kidney tissues were fixed in formalin before embedding in paraffin blocks. Tissue sections were stained with hematoxylin and eosin as described previously (9–11). The immunofluorescent staining was performed with frozen tissue slides. Staining and image preparation procedures were previously described (9–11).
Measurement of Cytokines and Antibodies in Serum and Culture Supernatants.
Serum cytokines (IL-2, IFN-γ, TNF-α, and IL-6) were measured with ELISA kits (BD Biosciences PharMingen) as previously described (10). Anti-dsDNA IgG was measured with ELISA as previously described (29, 30). Anti-dsDNA titers are expressed in units per milliliter, using a reference-positive standard of pooled serum from 6- to 7-month-old NZB/W F1 mice. A 1:100 dilution of this standard serum was arbitrarily assigned a value of 100 units/ml.
Statistical Analysis.
Comparison of survival groups was analyzed by using the log-rank test with Prism version 4.0 (GraphPad, San Diego, CA). Comparison of two means was analyzed by using the unpaired two-tailed Student t test.
Acknowledgments.
We thank James Young for critical reading of the manuscript. We thank Lucy Brown and her staff at City of Hope Flow Cytometry Facility and Sofia Loera and her staff at City of Hope Anatomic Pathology Laboratory for their excellent technical assistance, and we thank Dr. Richard Ermel and his staff at City of Hope Research Animal Facility for providing excellent animal care. The studies were supported by a pilot grant and a career development grant from Lymphoma Special Program Grant of Research Excellence (to S.F.), the Marcus Foundation, and a generous gift from Mr. Lynn Davis.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sullivan KM. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 635–664. [Google Scholar]
- 2.Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–627. doi: 10.1038/nature03728. [DOI] [PubMed] [Google Scholar]
- 3.Shizuru J. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 324–343. [Google Scholar]
- 4.Ferrara J, Antin J. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 353–368. [Google Scholar]
- 5.Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 6.Teshima T, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 7.Chakraverty R, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wekerle T, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, et al. Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb. Blood. 2005;105:2180–2188. doi: 10.1182/blood-2004-06-2411. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol. 2007;178:838–850. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, et al. Elimination of insulitis and augmentation of islet β cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci USA. 2007;104:2337–2342. doi: 10.1073/pnas.0611101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 13.Marks P, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 14.Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3:344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 15.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 16.Leoni F, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy P, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng C, et al. Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1. Exp Hematol. 2006;34:776–787. doi: 10.1016/j.exphem.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alegre M, et al. Hypothermia and hypoglycemia induced by anti-CD3 monoclonal antibody in mice: role of tumor necrosis factor. Eur J Immunol. 1990;20:707–710. doi: 10.1002/eji.1830200337. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Berdan S, Gille D, Weissman IL, Christensen JL. Reversal of autoimmune disease in lupus-prone New Zealand black/New Zealand white mice by nonmyeloablative transplantation of purified allogeneic hematopoietic stem cells. Blood. 2007;110:1370–1378. doi: 10.1182/blood-2007-03-081497. [DOI] [PubMed] [Google Scholar]
- 22.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong C. Diversification of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez R, et al. Cyclosporine and mycophenolate mofetil prophylaxis with fludarabine and melphalan conditioning for unrelated donor transplantation: a prospective study of 22 patients with hematologic malignancies. Bone Marrow Transplant. 2004;33:1123–1129. doi: 10.1038/sj.bmt.1704493. [DOI] [PubMed] [Google Scholar]
- 26.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 27.Brogdon JL, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: A role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 30.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–1222. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris ES, et al. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. J Clin Invest. 2005;115:3093–3103. doi: 10.1172/JCI25249. [DOI] [PMC free article] [PubMed] [Google Scholar]