The research field of plant–microbe interactions experiences a surge of fundamental insights thanks to the adoption of a restricted number of model systems. However, to see the broader picture and understand the complexity of evolutionary relationships, it is equally important to study relevant non-model interactions. This task is (still) difficult because of the lack of genomics and genetics tools, but rewarding when the outcome proves some long-standing assumptions and suggests new ways to address old questions. A nice example is the work of Gherbi et al. (1) presented in this issue of PNAS. Their article deals with a non-model plant, Casuarina glauca, whose roots interact with both symbiotic fungi and nitrogen-fixing bacteria, and evidences the central and ancestral role of the plant receptor SYMRK in the establishment of the three major types of root endosymbioses.
Microbial associations with plant roots form underground networks of diverse and intimate interactions. Three major groups of endophytic root symbioses are of crucial importance for the geobiochemical cycle and the ecological equilibrium of our planet. Fungi of the order of the Glomeromycota form arbuscular myccorhiza (AM) in a ubiquitous ancestral symbiosis with roots of the majority of land plants leading to nutrient exchange at an extended fungus–plant membrane interface (arbuscules) inside cortical root cells (2). More recently in evolution, two types of root symbioses with nitrogen-fixing bacteria arose in the Eurosid I clade of dicots (3). Molecular dinitrogen can only be enzymatically reduced by prokaryotes. In symbiotic interactions, the bacteria directly deliver the fixed nitrogen to the host and provide nitrogen input in the ecosystem using the sun energy captured by the host via photosynthesis. Legume roots interact with Gram-negative rhizobia, whereas non-legume actinorhizal plants interrelate with Frankia bacteria that are Gram-positive filamentous actinomycetes (4, 5). Although based on different schemes, in both cases, the roots develop special structures, nodules, that house nitrogen-fixing bacteria installed inside cortical cells.
The legume endosymbioses are the most amenable to molecular dissection. Rhizobial functions for nodulation have been studied by forward and reverse genetics and numerous legume genes with a key role in the interaction have been identified with forward genetics and map-based cloning in the model legumes Medicago truncatula and Lotus japonicus (4). Bacterial lipochitooligosaccharide nodulation factors (NFs) trigger the programs for organ formation and entry. Entry is (mostly) intracellular in the epidermis via deviation of root hair tip growth to form a closed compartment for bacterial colonization (root hair curling) followed by inverted tip growth and intracellular infection thread formation. Several legume complementation groups are known that are essential for nodule initiation. At the top of the signal perception transduction hierarchy are the NF receptors, LysM-type receptor kinases, whose inactivation eliminates all responses to bacteria or purified NFs (4). Purified NFs trigger many events in root hair cells, including an early calcium influx, rhythmic calcium oscillations (spiking) around the nucleus, root hair deformations (for curling), gene expression, and cell division. Also essential for nodulation and for most (but not all) of the early responses are genes encoding cation channels located in the nuclear membrane, a calcium–calmodulin-dependent kinase (CCamK) that presumably decodes the calcium spiking and a plasma membrane-located receptor-like kinase with extracellular leucine-rich repeat domains, SYMRK (synonym for MsNork = MtDmi3 = LjSymRK = PsSym19 = SrSymRK = CgSymRK), which is needed for nodule initiation as well as for bacterial internalization in cortex cells during symbiosome formation (6–10) (Fig. 1). These functions are also required for AM establishment in the legume hosts and must have been recruited from this ancestral interaction to function in the nodulation program (11).
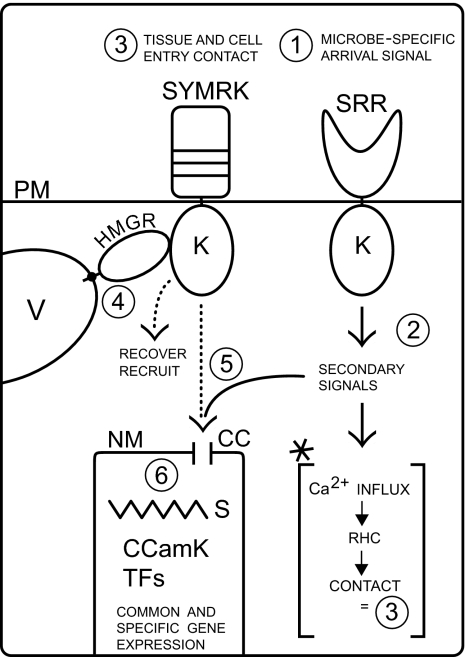
Fig. 1.
Model for the role of SYMRK in endosymbiosis. Microbial symbionts emit signals that are perceived by a signal-specific receptor (SRR) to initiate primary responses (1). Activation of the SRR kinase domain elicits secondary signals (2). In bacterial endosymbioses that cross the epidermis, calcium influxes cause root hair curling to form a closed invasion pocket, a process that leads to wall interactions and hence contact (asterisk, 3). AM fungi form appressoria that contact the host wall (3). Contact or wall disturbance causes touch/defense responses (not shown) that are perceived (3) and modulated by SYMRK interference (4). Activated SYMRK might recruit vesicles for polar responses to counteract defense or/and promote local membrane synthesis for invasion or internalization (4) and intervenes in activation of calcium spiking events around the nucleus (5). Calcium spiking is interpreted by CCamK and leads to transcription factor activation and gene expression. CC, cation channel; CCamK, calcium- and calmodulin-dependent kinase; HMGR, 3-hydroxyglutaryl CoA reductase; K, kinase domain; NM, nuclear membrane; RHC, root hair curling; PM, plasma membrane; S, calcium spiking; SYMRK, leucine-rich repeat-type receptor kinase; TF, transcription factor; SRR, signal recognition receptor; V, vesicle.
Now, Gherbi et al. (1) demonstrate for the first time that one of these genes, SYMRK, is required in a non-legume interaction. C. glauca is a pioneer plant that grows in marginal soils and establishes AM with fungi and actinorhizal nodules with Frankia strains. Frankia bacteria have been recalcitrant to genetic analysis, but the recent release of sequenced genomes promises approaches to find nodulation-related genes (12). At present, the signals that trigger actinorhizal nodule development are unknown. The analysis of the function of CgSymRK by RNA interference (RNAi) in transgenic C. glauca roots shows that a reduction in CgSymRK expression severely impairs nodulation and symbiotic nitrogen fixation, with formation of pseudo-nodules and accumulation of phenolic compounds, and affects early steps of AM invasion, especially the fungal penetration of the root cortex. Hence, SYMRK is important for all three modes of root endosymbioses (1).
Despite the obvious differences, AM, Frankia, and rhizobia interactions with plant roots share several features. The microbes are allowed inside plant tissues and, therefore, have (in most cases) to cross the strongly protected epidermal barrier. Plants possess an elaborate innate immune system (13): they perceive microbe-associated microbial patterns (MAMPs), are sensitive to touch and wounding, and guard the integrity of the wall. Moreover, the host invests extensively in local membrane synthesis for microbial entry and establishment of a functional interphase in the cytoplasm, be it in the form of arbuscules, vesicles, or symbiosomes.
When reflecting about possible functions for SYMRK, let us summarize the available data. SYMRK is an essential component of early epidermal responses to AM fungi and bacteria. In legumes, knockout of the gene interferes with NF-induced calcium spiking and gene expression, but not with calcium influx (14). Down-regulation of SYMRK expression by RNAi results in infection threads that fail to release rhizobia from infection droplets and are structurally abnormal, suggestive of defense responses (9, 10). C. glauca roots with reduced CgSymRK transcript levels have severe nodulation problems with accumulation of phenolic compounds (1). SYMRK is expressed in the legume root epidermis and in the nodule infection zone, and the protein is located in plasma and infection thread membranes (9, 10). Whereas SYMRK kinase domains are much conserved, the extracellular domains are rather divergent; nevertheless, CgSymRK complements the L. japonicus symrk mutation, suggesting a not very specific ligand or signal perception (1).
Two additional sets of data have to be considered. First, Kevei et al. (15) recently reported on a putative mevalonate synthase (MtHMGR1), the catalytic domain of which interacts with the kinase domain of SYMRK in a yeast two-hybrid assay. The M. truncatula protein seems to be associated with uncharacterized vesicles and plays a crucial role in nodule organogenesis as shown by pharmacology and RNAi. The mevalonate pathway mediates the synthesis of isoprenoid compounds. Modulation of MtHMGR activity by SYMRK after contact perception could interfere with steroid synthesis to control defense responses or polar recruitment for membrane synthesis.
Last, but not least, a nonsymbiotic symrk legume mutant phenotype, described by Esseling et al. (16), provides a most relevant hint as to the triggers that might activate the receptor kinase. Legume symrk mutants have a strongly diminished ability to recover from touch responses. In the presence of rhizobia, symrk mutant root hairs curl in two dimensions but without forming the three-dimensionally closed pocket, presumably because of touch oversensitivity. The mutants have aberrant calcium spiking and lack nodulin gene expression, but they still show a calcium influx that could be responsible for the root hair growth changes (14, 16).
Integration of these data, fragmentary as they are, leads to the model illustrated in Fig. 1. To announce their arrival, future endosymbionts send a diffusible signal that is perceived by specific receptors to trigger primary host responses. For their invasion strategy, AM fungi form appressoria that cause mechanical stress (see supplemental video 1 in ref. 17) and presumably trigger touch or defense responses. SYMRK is required to counteract or divert these responses to allow primary tissue entry and subsequent intracellular installment, probably via polar recruitment of vesicles and vesicle-associated proteins and cargo. In the bacterial symbioses that make use of root hair curl invasion, the microbial signal triggers root hair curling (or related processes), creating a contact situation and a need for SYMRK in a strategy similar to that for AM.
In conclusion, keeping in mind the symbiosis defects of symrk knockout mutants or knockdown transgenic roots, the expression pattern of the gene, the ancestral role of SYMRK in AM symbioses, and its recruitment in the bacterial interactions, SYMRK might plausibly play a crucial role at the symbiotic interfaces in guarding and converting touch (defense) responses for the benefit of microbial accommodation.
Footnotes
The author declares no conflict of interest.
See companion article on page 4928.
References
- 1.Gherbi H, et al. SymRK defines a common genetic basis for plant root endosymbioses with AM fungi, rhizobia and Frankia bacteria. Proc Natl Acad Sci USA. 2008;105:4928–4932. doi: 10.1073/pnas.0710618105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parniske M. Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol. 2004;7:414–421. doi: 10.1016/j.pbi.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Soltis DE, et al. Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc Natl Acad Sci USA. 1995;92:2647–2651. doi: 10.1073/pnas.92.7.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: The Sinorhizobium–Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlowski K, Bisseling T. Rhizobial and actinorhizal symbioses: What are the shared features? Plant Cell. 1996;8:1899–1913. doi: 10.1105/tpc.8.10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endre G, et al. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- 7.Stracke S, et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature. 2002;417:959–962. doi: 10.1038/nature00841. [DOI] [PubMed] [Google Scholar]
- 8.Oldroyd GED, Downie JA. Nuclear calcium changes at the core of symbiosis signalling. Curr Opin Plant Biol. 2006;9:351–357. doi: 10.1016/j.pbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Limpens E, et al. Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc Natl Acad Sci USA. 2005;102:10375–10380. doi: 10.1073/pnas.0504284102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capoen W, Goormachtig S, De Rycke R, Schroeyers K, Holsters M. SrSymRK, a plant receptor essential for symbiosome formation. Proc Natl Acad Sci USA. 2005;102:10369–10374. doi: 10.1073/pnas.0504250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kistner C, Parniske M. Evolution of signal transduction in intracellular symbiosis. Trends Plants Sci. 2002;7:511–518. doi: 10.1016/s1360-1385(02)02356-7. [DOI] [PubMed] [Google Scholar]
- 12.Normand P, et al. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res. 2007;17:7–15. doi: 10.1101/gr.5798407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 14.Shaw SL, Long SR. Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 2003;131:976–984. doi: 10.1104/pp.005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kevei Z, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell. 2007;19:3974–3989. doi: 10.1105/tpc.107.053975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esseling JJ, Lhuissier FGP, Emons AMC. A nonsymbiotic root hair tip growth phenotype in NORK-mutated legumes: Implications for nodulation factor-induced signaling and formation of a multifaceted root hair pocket for bacteria. Plant Cell. 2004;16:933–944. doi: 10.1105/tpc.019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell. 2005;17:3489–3499. doi: 10.1105/tpc.105.035410. [DOI] [PMC free article] [PubMed] [Google Scholar]