Abstract
Multiple signaling pathways are engaged by the type I and II IFN receptors, but their specific roles and possible coordination in the generation of IFN-mediated biological responses remain unknown. We provide evidence that activation of Akt kinases is required for IFN-inducible engagement of the mTOR/p70 S6 kinase pathway. Our data establish that Akt activity is essential for up-regulation of key IFN-α- and IFN-γ-inducible proteins, which have important functional consequences in the induction of IFN responses. Such effects of the Akt pathway are unrelated to regulatory activities on IFN-dependent STAT phosphorylation/activation or transcriptional regulation. By contrast, they reflect regulatory activities on mRNA translation via direct control of the mTOR pathway. In studies using Akt1 and Akt2 double knockout cells, we found that the absence of Akt kinases results in dramatic reduction in IFN-induced antiviral responses, establishing a critical role of the Akt pathway in IFN signaling. Thus, activation of the Akt pathway by the IFN receptors complements the function of IFN-activated JAK–STAT pathways, by allowing mRNA translation of IFN-stimulated genes and, ultimately, the induction of the biological effects of IFNs.
The type I (α, β, ω, τ, ζ) and II (γ) interferons (IFNs) exhibit a wide spectrum of biological activities in target cells, including antiviral, immunomodulatory, antiangiogenic, and growth inhibitory effects (1–6). Because of such effects, certain IFN subtypes have been extensively used over the years in clinical medicine for the treatment of various malignancies, viral infections, and neurologic disorders (7–9). Such extensive use of these cytokines for the treatment of human diseases underscores their importance and emphasizes the need to better understand the mechanisms by which they generate their pleiotropic biological effects.
Beyond the classic JAK–STAT pathways, a plethora of evidence has emerged over recent years implicating other signaling cascades in the transmission of signals generated by the type I and II IFN receptors. Among them, MAPK pathways, and in particular the p38 MAPK signaling cascade, appear to play key roles in the optimal transcriptional regulation in response to IFNs, in the absence of direct effects on the activation or nuclear translocation of STAT proteins (10, 11). Such functions of p38 and its effectors have important biological implications and are required for generation of both antiviral and growth inhibitory responses in response to IFNs (12, 13). Another signaling cascade, the mTOR/p70 S6 kinase pathway is also regulated by both the type I (14) and II (15) IFN receptors, whereas the downstream target of mTOR, translational repressor 4E-BP1, exhibits a negative regulatory role in the generation of the antiviral effects of IFN-α (16), raising the possibility that mTOR-mediated signals participate in the optimal generation of IFN-induced signals and biological effects.
Previous work had shown that the catalytic subunit of the PI3-kinase is induced during the IFN-dependent interaction of its p85 regulatory subunit with insulin receptor substrate proteins (17, 18). Subsequent studies demonstrated that Akt, a known downstream effector of the PI3-kinase (19, 20), is activated by IFNs in different cell types (21–24), but the functional relevance of this pathway and its downstream effectors in IFN signaling remains unknown. In fact, there has been conflicting evidence in the literature on the role of Akt in type I IFN signaling. Some studies have suggested that this kinase plays a negative regulatory role in the generation of IFN responses, by blocking IFN-dependent apoptosis (21) and/or promoting cell survival (22). On the other hand, other work has suggested that Akt is a positive regulator of IFN-stimulated adhesion of monocytes (23) and a positive regulator of IFN-β-dependent induction of the β-R1 (SCYB11) gene (24). Such discrepancies in defining the role of Akt kinase in IFN signaling apparently reflects the inherent difficulties of working with pharmacological inhibitors that exhibit relative lack of specificity.
In the current study, we used cells with targeted disruption of the Akt1 and Akt2 genes to definitively establish their roles in IFN signaling. Our data demonstrate that IFN-dependent engagement of Akt is required for downstream activation of mTOR and the p70 S6 kinase (p70 S6K) pathway and phosphorylation/deactivation of 4E-BP1. Importantly, we found that Akt activation is required for mRNA translation of IFN-stimulated genes (ISGs) and generation of the antiviral effects of IFNs. Altogether, these studies establish an important complementary role for Akt1 and Akt2 to the function of the classic IFN-regulated STAT pathway, involving control of mRNA translation of ISGs via activation of mTOR and its effectors.
Results
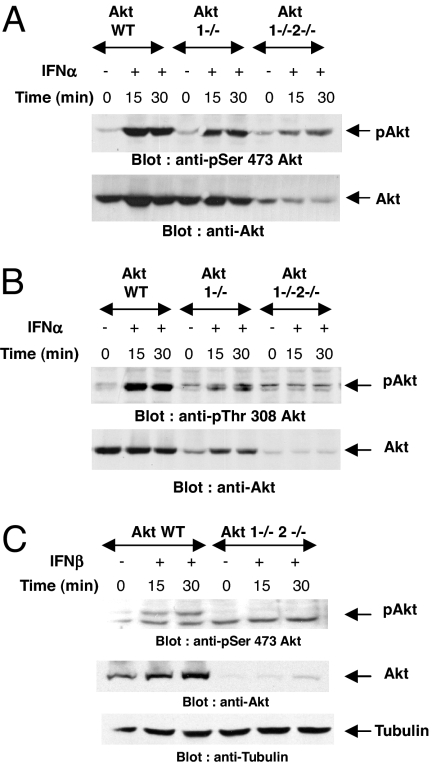
We examined the effects of different type I IFNs on the phosphorylation/activation of Akt. We used immortalized mouse embryonic fibroblasts (MEFs) obtained from parental Akt1−/− (25) and Akt1−/−Akt2−/− mice (26). Treatment of wild-type MEFs with mouse IFN-α resulted in phosphorylation of Akt on Ser-473 (Fig. 1A), whereas a weaker signal was still detectable in the single knockout Akt1−/− MEFs, apparently reflecting phosphorylation of Akt2 (Fig. 1A). Such phosphorylation was defective in double Akt1−/−2−/− cells (Fig. 1A). The weak residual phospho activity noted in some experiments in these cells likely reflects the presence of the Akt3 isoform. Treatment of parental cells with IFN-α also resulted in Akt phosphorylation on Thr-308 (Fig. 1B), the PDK1 phosphorylation site (20). Treatment of parental MEFs with another type I IFN, IFN-β, also resulted in phosphorylation of Akt in wild-type cells (Fig. 1C), but no signal was detectable in Akt1−/−2−/− cells (Fig. 1C). Altogether, these studies established that Akt is phosphorylated in a type I IFN-dependent manner on key sites required for its activation.
Fig. 1.
Type I IFN-dependent phosphorylation/activation of Akt. (A and B) Akt1+/+2+/+, Akt1−/−2+/+, and Akt1−/−2−/− MEFs were treated with mouse IFN-α for the indicated times. Equal protein aliquots were processed for immunoblotting with antibodies against phosphorylated forms of Akt on Ser-473 (A) or Thr-308 (B). Respective blots were stripped and reprobed with a pan anti-Akt antibody as indicated. (C) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were treated with mouse IFN-β for the indicated times. (Top) Equal protein aliquots were immunoblotted with an antibody against the phosphorylated form of Akt on Ser-473. (Middle) Equal protein aliquots from the same experiment were resolved on separate gels and probed with an anti-Akt antibody. (Bottom) The blot shown in Top was reprobed with an anti-tubulin antibody to control for protein loading.
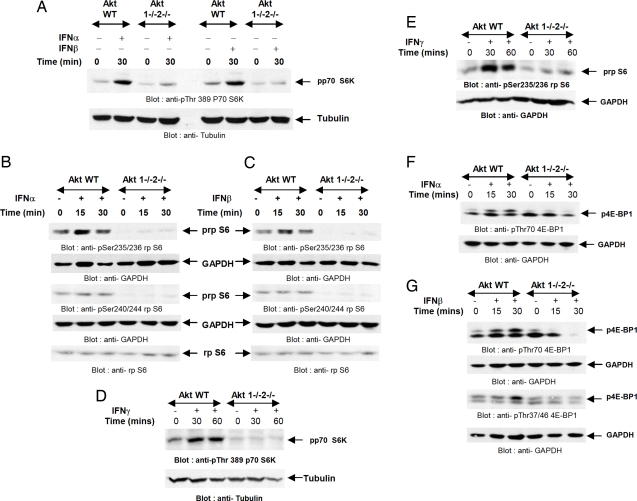
We subsequently proceeded to determine whether type I IFN-inducible phosphorylation/activation of p70 S6K (14) is Akt-dependent. Both IFN-α and IFN-β induced strong phosphorylation of p70 S6K in wild-type MEFs, but this phosphorylation was defective in Ak1/Akt2 double knockout MEFs (Fig. 2A). In addition, the phosphorylation of the downstream effector of p70 S6K, S6 ribosomal protein (rpS6), was defective in the absence of Akt1/Akt2 (Fig. 2 B and C). Such defects were seen in the phosphorylation of the protein on both Ser-235/236 and Ser-240/244 (Fig. 2 B and C) whether IFN-α (Fig. 2B) or IFN-β was used (Fig. 2C).
Fig. 2.
Requirement of Akt1 and Akt2 for type I and II IFN-dependent engagement of mTOR-regulated signaling cascades. (A) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were treated with mouse IFN-α or mouse IFN-β for the indicated times. Equal protein aliquots were processed for immunoblotting with an antibody against the phosphorylated form of p70 S6 kinase on Thr-389. The same blot was reprobed with an anti-tubulin antibody. (B and C) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were treated with mouse IFN-α (B) or mouse IFN-β (C) for the indicated times. Equal protein aliquots were processed for immunoblotting with antibodies against the phosphorylated forms of the S6 ribosomal protein on Ser-235/236 or Ser-240/244. The respective blots were reprobed with anti-GAPDH antibody as indicated. Equal protein aliquots from the same experiments were resolved on separate gels and immunoblotted with an antibody against rpS6. (D and E) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were treated with mouse IFN-γ, and equal protein aliquots were processed for immunoblotting with an antibody against the phosphorylated form of p70 S6 kinase on Thr-389 (D Upper) or with an antibody against the phosphorylated form of rp S6 on Ser-235/236 (E Upper). Respective blots were reprobed with an anti-tubulin antibody (D Lower) or an anti-GAPDH antibody (E Lower). (F and G) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were treated with mouse IFN-α (F) or mouse IFN-β (G) for the indicated times. Equal protein aliquots were processed for immunoblotting with antibodies against the phosphorylated forms of 4EBP1 on Thr-70 or Thr-37/46, as indicated. The same blots were reprobed with GAPDH to control for protein loading.
Beyond type I IFNs, activation of the mTOR pathway occurs during the engagement of the type II (IFN-γ) receptor (15). In similar experiments using IFN-γ, we also found defective phosphorylation of p70 S6K (Fig. 2D) and downstream phosphorylation of rpS6 (Fig. 2E), establishing that Akt activity is a common requirement for the activation of the mTOR/p70 S6K pathway, in response to both type I and II IFNs. In other experiments, we assessed the requirement of Akt activity in type I IFN-inducible phosphorylation of the translational repressor 4E-BP1 (14), an event that is known to be mTOR-dependent, but p70 S6K-independent (19). Although IFN-α- or IFN-β-induced phosphorylation of 4E-BP1 on Thr-70 and Thr-37/46 was clearly detectable in Akt1+/+2+/+ MEFs, such phosphorylation/deactivation (16) of the protein was not observed in the Akt1/Akt2 double knockout cells (Fig. 2 F and G), establishing that engagement of Akt is required for regulation of both major pathways downstream of mTOR.
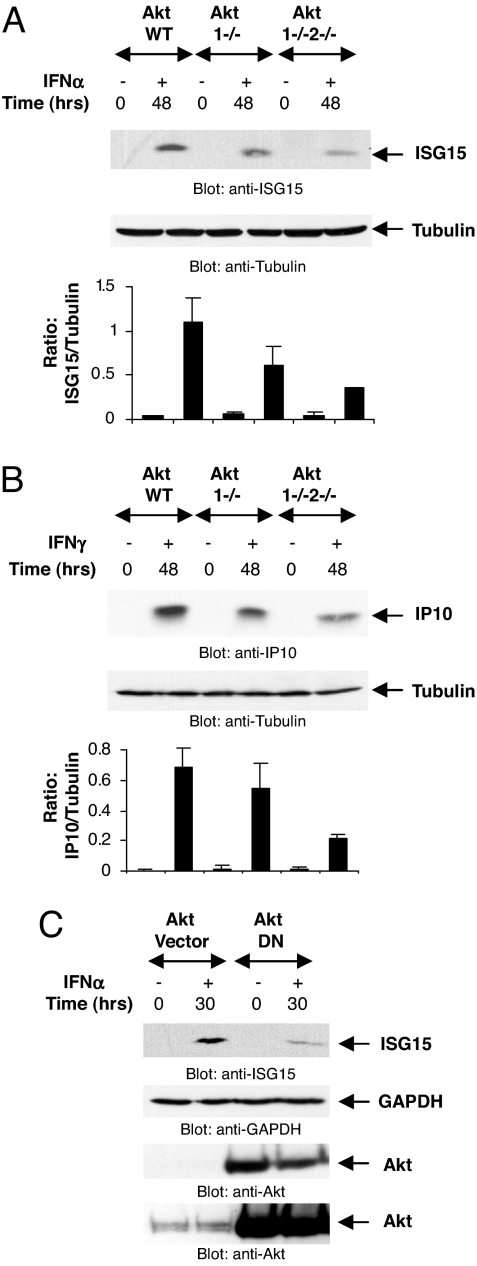
In subsequent experiments, we sought to determine whether the function of Akt is required for expression of protein products known to participate in the generation of IFN-dependent biological effects. Initially, we determined the effects of targeted disruption of the Akt1/Akt2 genes on the expression of ISG15, a type I IFN-induced protein product that participates in the generation of IFN responses by regulating ISGylation (27, 28). In addition, we assessed the requirement of Akt activity on the expression of CXCL10 (IP10), a type II IFN-induced chemokine known to regulate IFN-γ-dependent proapoptotic responses (29). Akt1+/+2+/+, Akt1−/−2+/+, and Akt1−/−Akt2−/− MEFs were incubated in the presence or absence of mouse IFN-α, and cell lysates were resolved by SDS/PAGE and immunoblotted with an anti-ISG15 antibody. There was strong induction of ISG15 protein expression in parental MEFs, but such expression was decreased in Akt1−/− cells (Fig. 3A). Moreover, induction of ISG15 protein expression was even more suppressed and only barely detectable in the double knockout Akt1/Ak2 knockout cells (Fig. 3A). Similar to what we observed in the case of ISG15 expression by IFN-α, there was defective IFN-γ-inducible expression of the CXCL10 protein in cells with targeted disruption the Akt1 and Akt2 genes (Fig. 3B), suggesting that Akt kinases are common elements in the signaling pathways for both type I and II IFNs. To definitively establish the requirement of Akt kinase activity on IFN-dependent ISG15 expression, we performed experiments in which a kinase-defective/dominant-negative Akt mutant was overexpressed in U2OS cells, and the effects of such overexpression on IFN-dependent ISG15 induction were examined. As shown in Fig. 3C, overexpression of the Akt dominant negative mutant suppressed ISG15 protein expression, further demonstrating the importance of Akt kinases in the process.
Fig. 3.
Akt is required for IFN-dependent ISG15 and CXCL10 protein expression. (A) Akt1+/+2+/+, Akt1−/−2+/+, and Akt1−/−2−/− MEFs were treated with mouse IFN-α as indicated. Equal protein aliquots were processed for immunoblotting with an anti-mouse ISG15 antibody. The same blot was reprobed with an anti-tubulin antibody as indicated. The signals for ISG15 and tubulin from three independent experiments (including the one shown) were quantitated by densitometry, and the intensity of ISG15 relative to tubulin was calculated. Data are expressed as means of ratios of ISG15/tubulin ± SE for each experimental condition. (B) Akt MEFs were treated with mouse IFN-γ as indicated. Equal protein aliquots were processed for immunoblotting with an anti-mouse IP10 antibody. Same blot was reprobed with anti-tubulin antibody as indicated. The signals for IP10 and tubulin from two independent experiments (including the one shown) were quantitated by densitometry, and the intensity of IP10 relative to tubulin was calculated. Data are expressed as means of ratios of IP10/tubulin ± SE for each experimental condition. (C) U20S cells were transfected with either the control empty vector or a dominant-negative Akt mutant and treated with human IFN-α as indicated. Equal protein aliquots were processed for immunoblotting with anti-ISG15 antibody. The blot was stripped and probed with anti-GAPDH antibody. Lysates from the same experiment were resolved separately and immunoblotted with an anti-Akt antibody. Also shown is longer exposure of the same blot to demonstrate the presence of endogenous Akt protein.
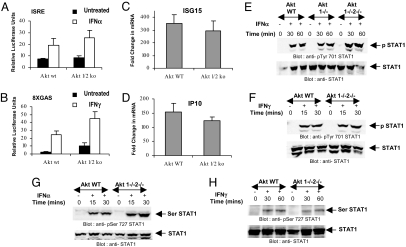
Our data suggested that the Akt pathway is required for type I and II IFN-inducible protein expression for genes that are known to be regulated by the classic JAK–STAT pathway (2, 3, 5). To determine whether such effects reflect regulatory activities of Akt on events that control IFN-dependent gene transcription, luciferase reporter assays were carried out to determine the effects of Akt on type I IFN-dependent transcription via IFN-stimulated response element (ISRE) elements or type II IFN-dependent transcription via IFN-γ activation site (GAS) elements. There were no defects in IFN-α-inducible transcription via ISRE elements (Fig. 4A) or IFN-γ-inducible transcription via GAS elements (Fig. 4B) in Akt1−/−2−/− cells, indicating that Akt activity is not essential for transcriptional regulation of IFN-α- or IFN-γ-inducible genes. Consistent with these results, when IFN-dependent mRNA expression for the Isg15 and Cxcl10 genes was directly assessed, there were no significant differences in the induction seen in Akt1−/−2−/− MEFs compared with Akt1+/+2+/+ MEFs (Fig. 4 C and D), suggesting that decreased expression of these proteins in Akt1−/−2−/− cells does not reflect effects on the transcription of respective genes. Furthermore, the lack of Akt1/Akt2 expression did not result in defective phosphorylation of Stat1 on Tyr-701 or Ser-727 in response to either IFN-α (Fig. 4 E and G) or IFN-γ (Fig. 4 F and H). Thus, although induction of ISG15 and CXCL10 protein expression by type I and II IFNs is defective in the absence of Akt1/Akt2, targeted disruption of the genes for these kinases does not alter IFN-inducible transcription, suggesting that IFN-dependent engagement of Akt selectively regulates mRNA translation of IFN-target genes.
Fig. 4.
IFN-dependent gene transcription and STAT activation is intact in the absence of Akt. (A and B) Akt1+/+2+/+ or Akt1−/−2−/− MEFs were transfected with a β-galactosidase expression vector and either ISRE (A) or GAS (B) luciferase plasmids. Triplicate cultures were either left untreated or treated with IFN-α (A) or IFN-γ (B) for 6 h, and luciferase reporter assays were performed. The data are expressed as relative luciferase units for each condition, normalized for β-galactosidase activities, and represent means ± SE. Values of five independent experiments are shown for A, and values of four independent experiments are shown for B. (C and D) Akt1+/+2+/+ or Akt1−/−2−/− MEFs were incubated in presence or absence of IFN-α (C) or IFN-γ (D). Expression of mRNA for Isg15 or Cxcl10 genes was evaluated by quantitative RT-PCR (Taqman). GAPDH was used for normalization. Data are expressed as fold increase over untreated sample and represent means ± SE of five experiments. (E and F) Akt MEFs were treated with mouse IFN-α (E) or IFN-γ (F) as indicated. Equal protein aliquots were processed for immunoblotting with an antibody against the phosphorylated form of Stat1 on Tyr-701 (Upper), and blots were stripped and reprobed with an anti-Stat1 antibody (Lower). (G and H) Akt MEFs were treated with mouse IFN-α (G) or IFN-γ (H) for indicated times. Equal protein aliquots were processed for immunoblotting with an antibody against the phosphorylated form of Stat1 on Ser-727 (Upper). The same blots were stripped and reprobed with an anti-Stat1 antibody (Lower).
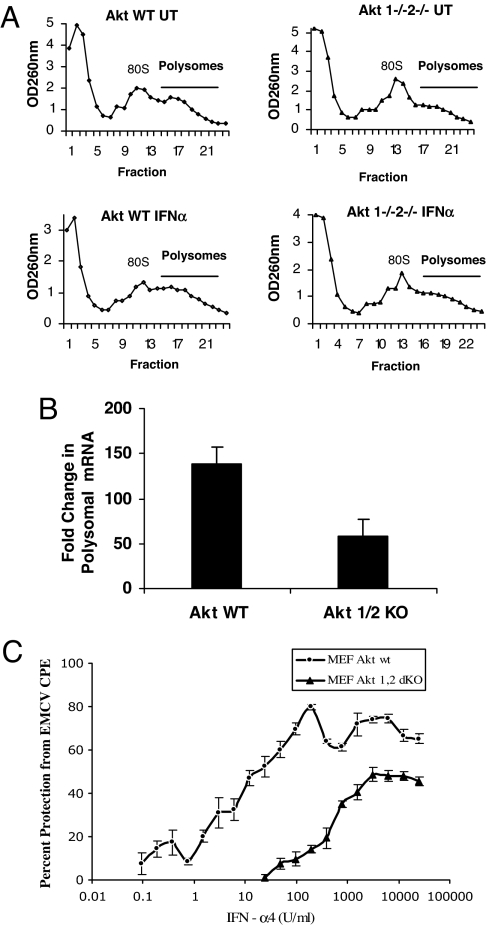
In further studies we directly examined the role of the Akt pathway in the type I IFN-dependent mRNA translation of the Isg15 gene. Akt1+/+2+/+ and Akt1−/−2−/− cells were treated with IFN-α, polysomal mRNA was isolated (Fig. 5A), and the induction of Isg15 mRNA in the polysomal fractions was determined. As shown in Fig. 5, mRNA translation for the Isg15 gene was defective in Akt1/2 knockout cells (Fig. 5B), directly establishing that the Akt1/2 activity ultimately regulates type I IFN-inducible mRNA translation, apparently via regulatory control on the activation of the mTOR pathway.
Fig. 5.
Engagement of Akt is essential for IFN-dependent mRNA translation and generation of antiviral responses. (A) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were either left untreated or treated with mIFN-α. Cell lysates were separated on 10–50% sucrose gradient, and OD at 260 nm was recorded. The OD260 is shown as a function of gradient depth for each treatment. (B) Polysomal fractions were collected as indicated in A, and RNA was isolated. Subsequently, quantitative real-time RT-PCR assays to determine Isg15 mRNA expression in the polysomal fractions was carried out, using GAPDH for normalization. Data are expressed as fold increase over IFN-α-untreated samples and represent means ± SE of three independent experiments. Paired t test analysis comparing induction of Isg15 mRNA in polysomal fractions of Akt1−/−2−/− MEFs versus Akt1+/+2+/+ MEFs showed a paired P value = 0.0087. (C) Akt1+/+2+/+ and Akt1−/−2−/− MEFs were incubated in triplicate with the indicated doses of mouse IFN-α. The cells were subsequently challenged with EMCV, and the cytopathic effects (CPE) were quantified 4 days later. Data are expressed as percent protection from CPE of EMCV.
We subsequently sought to evaluate the functional relevance of the IFN-activated Akt pathway in the generation of IFN responses. A common and universal characteristic of all different IFNs is their ability to protect cells from viral infections. We examined the antiviral properties of mouse IFN-α4 against encephalomyocarditis virus (EMCV) infection in wild-type and Akt1/Akt2 knockout MEFs as compared with parental wild-type MEFs. Cells expressing Akt1/Akt2 exhibited the expected sensitivity to the antiviral effects of IFN-α4, with small amounts of the cytokine resulting in significant protection from the cytopathic effects of EMCV (Fig. 5C). However, Akt1−/−2−/− MEFs were far less sensitive to the antiviral effects of IFN-α4, establishing that engagement of Akt kinases is required for IFN-induced antiviral responses on target cells.
Discussion
It is now well established that the function of STAT proteins is required for IFN-dependent gene transcription of ISGs, and that tyrosine phosphorylation of STATs by activated JAKs is required for the nuclear translocation of STATs and binding to ISRE or GAS elements in the promoters of ISGs (1–6). In addition, phosphorylation of Stats on Ser-727 is also required for its full transcriptional activity (2–6). Such IFN-dependent serine phosphorylation of Stat1 appears to be mediated primarily by the serine kinase activities of members of the protein kinase C family of proteins, including PKCδ (30–33) and PKCε (34).
Although the signaling pathways that mediate transcription of IFN-targeted genes have been studied extensively, the events that account for mRNA translation and protein expression of specific gene products remain largely unknown. We have recently provided evidence that type I and II IFNs induce activation of mTOR and the p70 S6 kinase (14–16), raising the possibility that this signaling cascade accounts for IFN-dependent mRNA translation. However, the precise mechanisms of activation of this pathway have remained unknown. A confounding issue is the fact that by inducing antiproliferative effects, IFNs affect global suppression of mRNA translation, whereas a major mechanism by which IFNs generate antiviral responses involves suppression of viral RNA translation (35–37).
In the present study, we provide evidence that establishes that the major role of the Akt pathway in type I or II IFN signaling is downstream engagement of mTOR-regulated pathways and control of mRNA translation. Using cells with targeted disruption of the genes for the two major Akt isoforms, Akt1 and Akt2, we directly demonstrate an important role of Akt kinases in IFN-dependent mRNA translation and induction of antiviral responses. These findings establish that Akt plays a positive role in IFN signaling and address an outstanding issue of how signals generated at the type I or II IFN receptor ultimately lead to generation of protein products that mediate the biological effects of IFNs. Our findings support a model in which, in parallel to the activation of the classic JAK–STAT pathway, the IFN receptors engage a signaling cascade involving sequential engagement of Akt, mTOR, and its effectors. Such engagement of the Akt/mTOR pathway subsequently leads to selective and targeted mRNA translation of specific ISGs, such as Isg15, the transcription of which is controlled by IFN-activated STAT complexes. Our data strongly suggest that the role of Akt in IFN signaling is selective and specific in the control of mRNA translation. A previous study had demonstrated that activation of the PI3-kinase pathway plays a key role in phosphorylation of Ser-727 of Stat1 (38). Such data had raised the possibility that Akt may be the downstream regulator of PI3- kinase that mediates Stat1 serine phosphorylation (38, 39). Consistent with these results, our previous studies show that PI3-kinase activity is required for IFN-γ-dependent serine phosphorylation of Stat1 (31). However, our studies using Akt1−/−2−/− MEFs demonstrate that Akt1/Akt2 activity is not required for Stat1–Ser-727 phosphorylation and suggest that different kinase regulates Stat1 serine phosphorylation downstream of the PI3-kinase. The lack of Akt involvement in the process is consistent with PKCδ acting as the primary serine kinase for Stat1, as its activation by IFN-γ is PI3-kinase-dependent (30–33).
Akt is activated in response to various growth factors and cell survival signals and phosphorylates and regulates the activity of several downstream targets, including Tsc2, GSK3, FoxO transcription factors, PRAS40, and Bad (39–41). In general, the primary functions of Akt appear to be promotion of cell survival, growth, and proliferation, whereas Akt isoforms also play key roles in the promotion of angiogenesis and control of cellular metabolism (41). Our findings that the function of Akt is required for the generation of IFN-dependent antiviral responses and complements the function of the IFN-activated STAT pathway indicate that Akt plays a novel and important role in IFN signaling. In addition, they suggest that Akt exhibits divergent biological effects in a cytokine-dependent manner, depending on the context of its activation. It is of particular interest that several viruses activate and use the PI3-kinase/Akt pathway to promote cell survival and virus replication (42, 43). The fact that the same pathway is used by IFNs to generate antiviral responses raises the intriguing possibility that IFN receptors compete with viruses for the use of Akt kinases and potentially deprive them from a pathway essential for their replication. Although the validity of such a hypothesis remains to be established, our data provide definitive evidence that the Akt pathway is essential for the induction of IFN responses and complements the function of the STAT pathway.
Materials and Methods
Cell Lines and Reagents.
Immortalized MEFs from Akt knockout mice (25, 26) were cultured in DMEM containing 10% FCS and antibiotics. The dominant negative K179M Akt mutant has been described (44, 45).
Cell Lysis and Immunoblotting.
Cells were treated, lysed, and prepared for immunoblotting as described (16, 46). For short time treatments (up to 60 min), MEFs were serum-starved overnight before treatment with 104 units/ml of mouse IFN-α4 or IFN-β or 5 × 103 units/ml of IFN-γ.
Luciferase Reporter Assays.
Luciferase reporter assays were performed as described (10, 16).
Quantitative RT-PCR.
Cells were treated with 5 × 103 units/ml of IFN-α or 2.5 × 103 units/ml of IFN-γ for 6 h, and quantitative RT-PCR was carried out as described (16). Real-time RT-PCR to determine expression of Isg15 and Ip10 mRNAs was carried out by using commercially available FAM-labeled probes and primers (Applied Biosystems). GAPDH was used for normalization.
Isolation of Polysomal RNA.
Akt WT and Akt 1−/−2−/− MEFS were treated with mIFN-α for 40 h, and polysomal fractionation was performed by using slight modifications of previously described protocols (47, 48). The polysomal fractions were pooled, and total RNA from polysomal fractions was isolated with a Rnaqeous-micro kit from Ambion.
Antiviral Assays.
The antiviral effects of mouse IFN-α in MEFs were determined in assays using EMCV as the challenge virus as described (16).
Further experimental details are listed in supporting information (SI) Text.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants CA77816, CA100579, and CA121192 (to L.C.P.), a grant from the Department of Veterans Affairs (to L.C.P.), and Canadian Institutes of Health Research Grant MOP 15094 (to E.N.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710907105/DC1.
References
- 1.Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1420. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 3.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Platanias LC, Fish EN. Signaling pathways activated by interferons. Exp Hematol. 1999;27:1583–1592. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 5.Platanias LC. Mechanisms of type I and type II interferon-mediated signaling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 6.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Parmar S, Platanias LC. Interferons: Mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Javed A, Reder AT. Therapeutic role of β-interferons in multiple sclerosis. Pharmacol Ther. 2006;110:35–56. doi: 10.1016/j.pharmthera.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Loutfy MR, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary study. J Am Med Assoc. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 10.Uddin S, et al. Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem. 1999;274:30127–30131. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, et al. Role of p38alpha MAP kinase in type I interferon signaling. J Biol Chem. 2004;279:970–979. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- 12.Mayer IA, et al. The p38 MAPK pathway mediates the growth inhibitory effects of interferon α in BCR-ABL-expressing cells. J Biol Chem. 2001;276:28570–28577. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- 13.Katsoulidis E, et al. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–9037. doi: 10.1158/0008-5472.CAN-04-4555. [DOI] [PubMed] [Google Scholar]
- 14.Lekmine F, et al. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- 15.Lekmine F, et al. Interferon γ engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp Cell Res. 2004;295:173–182. doi: 10.1016/j.yexcr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Kaur S, et al. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J Biol Chem. 2007;282:1757–1768. doi: 10.1074/jbc.M607365200. [DOI] [PubMed] [Google Scholar]
- 17.Uddin S, et al. Interferon α engages the insulin receptor substrate-1 to associate with the phosphatidylinositol 3′-kinase. J Biol Chem. 1995;270:15938–15941. doi: 10.1074/jbc.270.27.15938. [DOI] [PubMed] [Google Scholar]
- 18.Uddin S, et al. Activation of the phosphatidylinositol 3-kinase serine kinase by IFN-α. J Immunol. 1997;158:2390–2397. [PubMed] [Google Scholar]
- 19.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 20.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Lei H, et al. AKT activation and response to interferon β in human cancer cells. Cancer Biol Ther. 2005;4:709–715. doi: 10.4161/cbt.4.7.1767. [DOI] [PubMed] [Google Scholar]
- 22.Yang CH, et al. Interferon α/β promotes cell survival by activating nuclear factor κB through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 23.Navarro A, Anand-Apte B, Tanabe Y, Feldman G, Larner AC. A PI-3 kinase-dependent, Stat1-independent signaling pathway regulates interferon-stimulated monocyte adhesion. J Leukocyte Biol. 2003;73:540–545. doi: 10.1189/jlb.1002508. [DOI] [PubMed] [Google Scholar]
- 24.Rani MR, Hibbert L, Sizemore N, Stark GR, Ransohoff RM. Requirement of phosphoinositide 3-kinase and Akt for interferon-β-mediated induction of the β-R1 (SCYB11) gene. J Biol Chem. 2002;277:38456–38461. doi: 10.1074/jbc.M203204200. [DOI] [PubMed] [Google Scholar]
- 25.Chen WS, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng XD, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;1:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie KJ, et al. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 28.Ritchie KJ, Zhang DE. ISG15: The immunological kin of ubiquitin. Semin Cell Dev Biol. 2004;15:237–246. doi: 10.1016/j.semcdb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HM, et al. Gamma interferon-inducible protein 10 induces HeLa cell apoptosis through a p53-dependent pathway initiated by suppression of human papillomavirus type 18 E6 and E7 expression. Mol Cell Biol. 2005;25:6247–6258. doi: 10.1128/MCB.25.14.6247-6258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin S, et al. Protein kinase C-δ (PKC-δ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem. 2002;277:14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- 31.Deb DK, et al. Activation of protein kinase Cδ by IFN-γ. J Immunol. 2003;171:267–273. doi: 10.4049/jimmunol.171.1.267. [DOI] [PubMed] [Google Scholar]
- 32.Zhao KW, et al. Interferon-α-induced expression of phospholipid scramblase 1 through STAT1 requires the sequential activation of protein kinase Cδ and JNK. J Biol Chem. 2005;280:42707–42714. doi: 10.1074/jbc.M506178200. [DOI] [PubMed] [Google Scholar]
- 33.Kwon MJ, Yao Y, Walter MJ, Holtzman MJ, Chang CH. Role of PKCδ in IFN-γ-inducible CIITA gene expression. Mol Immunol. 2007;44:2841–2849. doi: 10.1016/j.molimm.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhury GG. A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cepsilon, and MAPK in mesangial cells regulates interferon-γ-induced STAT1α transcriptional activation. J Biol Chem. 2004;279:27399–27409. doi: 10.1074/jbc.M403530200. [DOI] [PubMed] [Google Scholar]
- 35.Samuel CE. Antiviral actions of interferon: Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 36.Hazari S, et al. Alpha interferon inhibits translation mediated by the internal ribosome entry site of six different hepatitis C virus genotypes. J Gen Virol. 2005;86:3047–3053. doi: 10.1099/vir.0.81132-0. [DOI] [PubMed] [Google Scholar]
- 37.Guo J, Hui DJ, Merrick WC, Sen GC. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19:6891–6899. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-γ-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–33368. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 39.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Sulis ML, Parsons R. PTEN: From pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 41.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrhardt C, et al. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. 2007;81:3058–3067. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun M, et al. Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J Virol. 2008;82:105–114. doi: 10.1128/JVI.01520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt (PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke TF, et al. The protein kinase encoded by the Akt protooncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 46.Kaur S, et al. Role of protein kinase C-δ (PKC-δ) in the generation of the effects of IFN-α in chronic myelogenous leukemia cells. Exp Hematol. 2005;33:550–557. doi: 10.1016/j.exphem.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Koritzinsky M, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arava Y. Isolation of polysomal RNA for microarray analysis. Methods Mol Biol. 2003;224:79–87. doi: 10.1385/1-59259-364-X:79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.