Abstract
Wandering albatrosses (Diomedea exulans) forage over thousands of square kilometers of open ocean for patchily distributed live prey and carrion. These birds have large olfactory bulbs and respond to fishy-scented odors in at-sea trials, suggesting that olfaction plays a role in natural foraging behavior. With the advent of new, fine-scale tracking technologies, we are beginning to explore how birds track prey in the pelagic environment, and we relate these observations to models of odor transport in natural situations. These models suggest that odors emanating from prey will tend to disperse laterally and downwind of the odor source and acquire an irregular and patchy concentration distribution due to turbulent transport. For a seabird foraging over the ocean, this scenario suggests that olfactory search would be facilitated by crosswind flight to optimize the probability of encountering a plume emanating from a prey item, followed by upwind, zigzag flight to localize the prey. By contrast, birds approaching prey by sight would be expected to fly directly to a prey item, irrespective of wind direction. Using high-precision global positioning system (GPS) loggers in conjunction with stomach temperature recorders to simultaneously monitor feeding events, we confirm these predictions in freely ranging wandering albatrosses. We found that initial olfactory detection was implicated in nearly half (46.8%) of all flown approaches preceding prey-capture events, accounting for 45.5% of total prey mass captured by in-flight foraging. These results offer insights into the sensory basis for area-restricted search at the large spatial scales of the open ocean.
Keywords: area-restricted search, foraging, olfaction, subantarctic, plume tracking
Wandering albatrosses (Diomedea exulans) routinely forage over thousands of kilometers of open ocean by using sensory strategies that are not well understood. While a considerable body of research has focused on understanding diet, flight energetics, foraging range, and potential for fisheries interaction (1), relatively little work to date has dealt with questions related to navigation (2, 3) or the sensory mechanisms birds use to detect and capture prey (4, 5). Like other procellariiforms, the wandering albatross has a well developed olfactory system. These birds have among the largest olfactory bulbs of any extant bird (6), and results from behavioral experiments performed at sea suggest an attraction to fishy-smelling odorants (7). These results are consistent with their foraging habits in that wandering albatrosses tend to be widely ranging hunters and scavengers, foraging primarily on various species of squid (8). Our current understanding is that procellariiforms use a combination of visual and olfactory cues to assist them in several different aspects of foraging, including identifying productive areas of ocean (5), prey capture (9), and network foraging by cueing off the behavior of hetero- or conspecifics to locate prey (7, 10). While previous studies have shown that wandering albatrosses forage over both oceanic (depths of 2,000 m or more) and neritic (depths of 2,000 m or less) zones by using both foraging-in-flight and sit-and-wait strategies (11), no study has ever detailed fine-scale movement of any procellariiform at sea with a view toward investigating the sensory basis of foraging, particularly with respect to odor tracking. How animals track odors in water and air is, however, a topic of considerable broader interest with respect to developing algorithms for plume-following behavior at various spatial scales (12, 13).
The dynamics of odor dispersal is complicated in the environment where these birds forage (14). Over the ocean, scents will be dispersed from a source (for example, a potential prey item) laterally and downwind in turbulent plumes. At large spatial scales, turbulent transport works faster than molecular diffusion. The structure of the scented plume will thus be complex, consisting of scented eddies resembling discontinuous, filamentous patches. Odor filaments can also be transported considerable distances downwind from the source. This situation leads to an irregular, patchy concentration distribution within the plume rather than smooth gradients. Thus, odor plumes are characterized by intermittency, and the task at hand for the foraging seabird is one of tracking the distribution of high-concentration, scented eddies to a source where visual cues could assist in prey capture.
How other animals accomplish this task has been the focus of considerable research (14–17). Most data come from observing the behavior of insects and marine invertebrates in laboratory flume experiments, and several trends emerge from these investigations (17). First, for animals operating in either water or air, a common behavioral adaptation to initiate olfactory search is to orient direction of movement orthogonally or obliquely to the direction of flow. This orientation will maximize the chances of encountering an odor plume. Once a scented eddy is encountered, the animal will move up-current or upwind to stay within that odor filament, or alternatively, within the boundary of the odor plume (14, 16, 18, 19). Because the filaments are intermittent, the animal will, however, eventually lose contact with the scent, and this situation should trigger cross-current or crosswind casting or zigzag movement until the animal reencounters the scent. Casting behavior is commonly observed in animals that track odor plumes (18) because it maximizes the animal's likelihood that it will reencounter an odor filament. While the behavioral algorithms that lead to upwind movement are still a matter of controversy (13, 19), casting behavior coupled to up-current or upwind movement is characteristic of olfactory search across a variety of species, including various types of moths, crabs, and lobsters (15).
Because of technical limitations of working in the open ocean, little work has been done investigating odor tracking behavior in the procellariiforms (20) or, for that matter, any freely ranging animal at large (hundreds of meters to kilometers) spatial scales (21). Systematic shipboard observations as well as data collected from satellite telemetry studies suggest that albatrosses tend to fly obliquely to the wind to optimize dynamic soaring (11, 22–25), but fine-scale movement patterns associated with prey capture have not yet been examined. With respect to olfactory search, Nevitt et al. (20) have shown differences in turning behavior in response to scented plumes, but responses tend to be more pronounced in burrow-nesting species than in larger albatrosses (4, 7). Nevitt and other researchers have also reported observations of characteristic upwind, zigzag turning in response to scented aerosols (20) or buoys (26, 27), but these reports tend to be descriptive and are thus of limited value in testing predictions about the sensory basis of foraging.
Weimerskirch et al. (28) recently used global positioning system (GPS) monitoring coupled with stomach temperature recorders to examine predictions related to area-restricted search (ARS) in freely ranging wandering albatross. This instrumentation provides high-precision location data [GPS, 10-s sampling rate (29)] in tandem with recordings of both the size and the timing of prey ingestion (30). Here we extended this approach to test predictions related to different sensory strategies birds use to find their prey (17). First, we confirmed crosswind flight behavior, which has already been documented in the literature for wandering albatross by using both satellite tracking data (1-h sampling intervals) (22) and direct observation at sea (24, 25). Next, we categorized tracks preceding prey-capture events with respect to whether there was evidence for turn or zigzag flight before touchdown, which is diagnostic of olfactory search in other systems. We predicted that in-flight approach types in response to olfactory cues would occur upwind. By contrast, we predicted that in-flight approach types mediated predominantly by visual cues should occur irrespective of wind direction. Finally, we examined tracks for differences in day- or nighttime activity with respect to prey capture. Specifically, we predicted that strategies that rely purely on vision should be less used at night, whereas olfactory strategies should not be as affected.
Results
The first prediction for olfactory search is that birds should prefer crosswind flight because this behavior increases the chances of encountering a plume. We analyzed overall flight direction relative to wind direction for 55 track segments flown between touchdown events. The relative bearings were significantly bimodally distributed, perpendicular to the wind (r = 0.502, Z = 13.878, P < 0.001, Rayleigh test). This analysis confirmed that wandering albatrosses favor an overall crosswind flight path throughout the foraging trip, and adapt their search behavior to maintain crosswind flight [see supporting information (SI) Fig. 5 for a distribution histogram and an example track].
Wandering Albatrosses Use Different Approach Types.
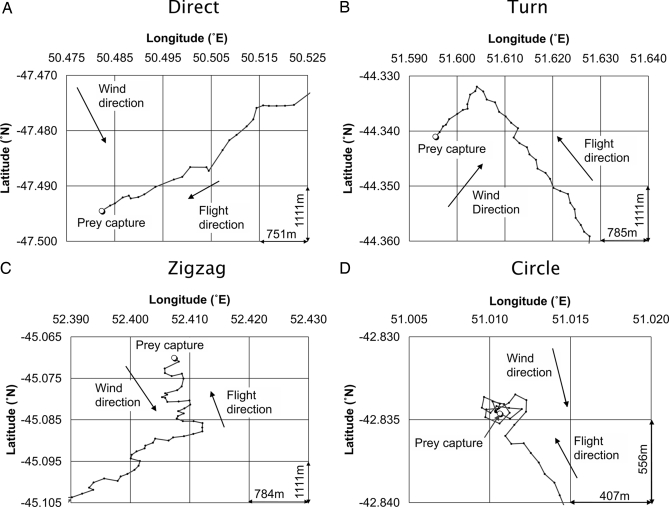
We next analyzed tracks for differences in approach type indicative of visual or olfactory-mediated search. Tracks were analyzed blind with respect to wind direction in a radius of 10 km from the touchdown point. We used this distance because it offered a convenient reference point for track analysis that was also beyond the visual range at which a bird was likely to be able to see either a prey item or a conspecific on the water (31). We identified four different approach types on the basis of their different shapes (Fig. 1): direct (no change in the overall flight direction), turn/zigzag (one or more turns of >45°), circle (bird circles the point of prey capture), and water (bird remains on the water between prey captures, not shown in Fig. 1). The approach shapes did not include turns due to dynamic soaring (i.e., the undulating flight style of albatrosses) because this motion was filtered out by the 10-s position sampling interval (see Methods in SI Text). Approach types occurred with equal frequency over neritic and pelagic waters (see Results in SI Text for details).
Fig. 1.
Examples of tracks for each of the flown approach types for direct (A), turn (B), zigzag (C), and circle (D) approach types. Each diagram shows wind and flight direction as well as the horizontal and vertical distances represented by the smallest grid rectangle.
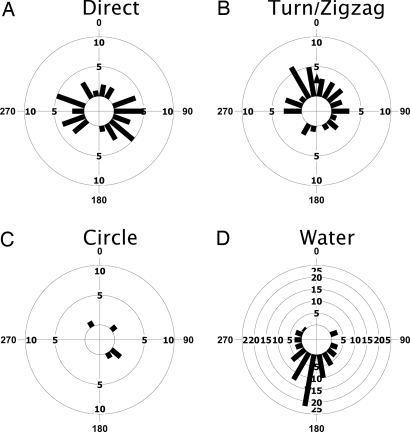
Olfactory foraging predicts that the direction of approach should be predominantly upwind. We tested this prediction by plotting approach direction relative to wind direction for each of the approach types (Fig. 2). Turn/zigzag approaches were preferentially oriented upwind (n = 39, mean vector = 0.6°, r = 0.384, Z = 5.74, P = 0.003, Rayleigh test), whereas direct approaches were bimodally distributed perpendicular to the wind (n = 43, r = 0.314, Z = 4.227, P = 0.014). Circle approaches were randomly distributed (n = 5, r = 0.477, Z = 1.14, P = 0.338), and water approaches were preferentially oriented downwind (n = 78, mean vector = 190°, r = 0.72, Z = 40.401, P < 0.001). As has previously been reported, this last result is consistent with predictions of the sit-and-wait strategy, where wandering albatrosses forage by sitting on the water and drifting downwind on wind-induced surface currents (28). Taken together, the turn/zigzag approach type satisfied the predictions of olfactory search, whereas direct and circle approaches seemed to more closely fall in line with expectations for visually mediated search.
Fig. 2.
Circular histogram plots of approach bearing relative to wind direction for direct (A), turn/zigzag (B), circle (C), and water (D) approaches. Plots are normalized such that upwind is zero degrees for each graph. Turn and zigzag approaches were significantly oriented upwind whereas, water approaches [sit-and-wait approaches (28)] were significantly oriented downwind. Direct approaches were bimodally distributed, and circle approaches were uniformly distributed with respect to wind direction (see text).
Wind speed did not influence approach type, and flight speeds were similar among approach types (see Results, Wind Speed and Approach Flight Speed in SI Text for details).
In other systems (most notably, moths), olfactory searches are often characterized by the linearity index of the track, ranging from 0 (minimally straight) to 1.0 (maximally straight) (19). We therefore calculated the linearity of each approach type between the start of the approach and the point of prey capture. For direct approach types, linearity values averaged 0.779 ± 0.028 (range: 0.23–1.00). Linearity for turn approaches was similar, averaging 0.74 ± 0.037 (range: 0.38–1.00). Average linearity was lower for zigzag approach types at 0.63 ± 0.064 (range: 0.1–0.92), and circle was the lowest, averaging 0.09 ± 0.02 (range: 0.05–0.15). Thus, measures of linearity confirmed the qualitative patterns we observed in the tracks, in that direct and turn approach types tended to be straighter than zigzag approaches.
Linearity and Wind.
Although upwind zigzag or turning is a well established characteristic of olfactory search in water and air, the path birds take when flying upwind could also be more sinuous (or less linear) because of constraints imposed by the mechanics of upwind flight. We therefore tested whether linearity of the approach was correlated with wind direction for turn/zigzag and direct approaches (see SI Fig. 6). We found no correlation for turn/zigzag approaches (n = 39, r = 0.25, P = 0.105, circular–linear correlation test); however, for direct approaches, birds tended to fly in a straighter line when they flew crosswind (n = 43, r = 0.392, P = 0.002, bimodal circular–linear correlation). There was no correlation between linearity and approach bearing relative to wind direction for circular approaches (n = 5, r = 0.548, P = 0.539, circular–linear correlation test).
These results (see also Results in SI Text) suggest that wandering albatrosses do not necessarily reduce linearity in response to flying upwind at this spatial scale, and our analysis emphasizes that upwind zigzagging is not a mechanical constraint linked to flight in this context.
Daytime Versus Nighttime Foraging.
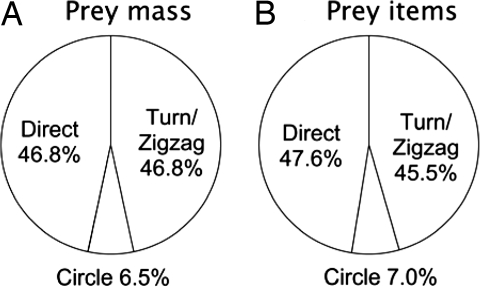
In terms of the total mass of prey captured (day and night combined), the most productive approach types were direct (36%, 16,015 g/44,102 g) and turn/zigzag (35%, 15,312 g/44,102 g), followed by water (24%, 10,424 g/44,102 g) and circle (5%, 2,351 g/44,102 g). The proportion of prey captured by using turn/zigzag approaches represented 17% (1,967 g/11,921 g) of all daytime captures or 37% (1,967 g/5,324 g) of daytime in-flight captures and 41% (13,344 g/32,180 g) of all nighttime captures or 47% (13,344 g/28,354 g) of nighttime, in-flight captures. As is illustrated in SI Fig. 7A, the turn/zigzag approach types caught heavier prey during the day versus night (n = 36, S = 127.5, Z = −2.593, P = 0.0095, Mann–Whitney test), as did water approaches (n = 76, S = 763.5, Z = −2.195, P = 0.0282). There was no difference between day versus night prey item mass for direct or circle approaches (direct: n = 36, S = 159, Z = −1.511, P = 0.1308; circle: n = 5, S = 4, Z = 0.3536, P = 0.7237, Mann–Whitney test).
In terms of the number of prey items caught (SI Fig. 7B), direct approaches were used in 32% (25/153) of all daytime captures (equivalent to 46% (25/54) of daytime in-flight captures), but in only 15% (11/74) of all nighttime captures (equivalent to 48% (11/23) of nighttime in-flight captures). Direct and turn/zigzag approaches were more frequent during the day than at night (direct approaches: n = 36, df = 1, χ2 = 5.59, P = 0.018; turn/zigzag approaches: n = 36, df = 1, χ2 = 5.59, P = 0.018, identical results are by coincidence). By contrast, water approaches provided 32% (25/79) of daytime and 69% (51/74) of nighttime prey captures (water approaches: n = 76, df = 1, χ2 = 9.08, P = 0.0026). Thus, as has been previously reported (28), birds tended to favor foraging-in-flight approach types by day and sit-and-wait approach types by night (11). In contrast to our expectations, however, upwind and turn approaches captured more than twice as many prey items during the day as at night.
Because these results suggest that visual cues are likely to be important in prey capture, we also examined the relationship between nighttime foraging-in-flight behavior and the potential availability of moonlight. We found that nighttime, in-flight foraging favored phases of the moon when more moonlight was potentially available (see SI Fig. 8 and associated Results in SI Text for details). Only one nighttime, in-flight prey-capture event was recorded during a new moon, and this event was characterized as a zigzag approach type, indicative of olfactory search. Wind speed and prey mass caught were not correlated (see Results in SI Text for details).
Approach Distances and Long-Range Olfaction.
The distance between the start of the approach and the location of prey capture provides a conservative estimate of detection distance. Turn approaches (n = 24) appeared to be initiated at a distance of 1,315 ± 320 m from the point of prey capture, whereas zigzag approaches (n = 12) started 2,401 ± 346 m from the prey-capture site. In both cases, the maximum detection distance we observed was >5 km (turn: 5,809 m; zigzag: 5,022 m). For circle approaches (n = 5), the average approach distance was 1,008 ± 574 m and the maximum distance we observed was 3,154 m. These analyses included both daytime and nighttime approaches. Because flow velocity influences plume dynamics under laboratory flume conditions (19), we were interested in whether wind speed impacted detection distance, but we found no systematic effect of wind speed on approach distance for turn/zigzag approaches (n = 36, ρ = 0.248, P = 0.146, Spearman rank correlation). Direct approaches were excluded because, by definition, the approach start point could not be determined. The effect of wind speed on circle approaches was not calculable because of the small sample size (n = 5).
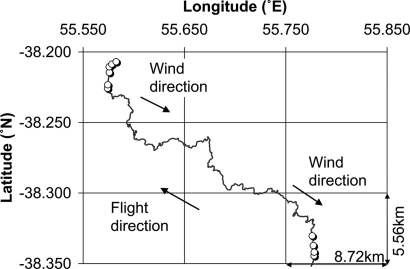
Finally, we explored the possibility that upwind turns were occurring beyond the 10-km limit that we used to classify the approaches. We initially used the limit of 10 km because, for typical heights above the water where albatrosses fly [an average of 8 m (25)], 10 km approaches the theoretical visual horizon. However, olfactory cues potentially travel beyond the horizon and approaches that appear direct at the scale of 5–10 km may, in fact, be the final leg in a long upwind turn. Although this analysis cannot address whether upwind turns occur in response to cues emanating from the captured prey, we occasionally observed tracks consisting of a turn followed by upwind flight (Fig. 3), which suggested to us that birds could be responding to olfactory cues at distances in excess of 20 km. These observations suggest that the distance thresholds for olfactory detection may be farther than we originally presumed.
Fig. 3.
Evidence for long-range olfactory detection. Sample track illustrating upwind flight after a series of sit-and-wait feeding events. The bird took off and meandered upwind for ≈20 km before touching down again to feed repeatedly on the water at a hotspot. Open circles indicate feeding events. Arrows refer to flight and wind direction as indicated. Note that wind direction shifted by ≈5° over the course of the bird's flight. The horizontal and vertical distances represented by the smallest grid rectangle are shown in the lower right corner.
Discussion
The results presented here suggest that freely ranging wandering albatrosses approach prey items by using different identifiable behaviors, which, in turn, suggest different sensory modes of prey detection (17). For example, a prediction of visual search is that once the prey item is detected, the approach should take the most direct route, particularly as the ocean presents a dynamic, moving landscape where a small prey item might be obscured by waves, or pass out of a bird's field of view (31). Visually mediated search should not necessarily be linked to wind direction, and would likely be more effective for targeting large prey items during the day than for sighting small prey items at night, unless prey items are bioluminescent (8). By comparison, olfactory search should be oriented upwind or involve zigzag plume tracking behavior before prey capture (14, 19). Unless size correlates to the potency of the scent, prey size should be more variable than for visual search, and olfactory search should be favored at night when visual cues from the prey or other birds are obscured. Characteristics of approach behavior may also reflect a multimodal strategy where an olfactory cue alerts the bird to a productive area or leads the bird to the vicinity of the prey item until it is visually spotted. This approach type would predict that the size of prey might impact detectability even though the initial search is olfactory (32).
Examining approach types in light of these predictions, our interpretation is that both the direct and circle approach types are likely to be visually mediated. Direct approaches tended to occur crosswind, and one of the surprising results of this study was that birds tended to capture prey as they flew crosswind, along a direct route, giving the impression that prey were detected at very close range and could be captured opportunistically without requiring the bird to deviate from the set flight direction. Birds used the direct approach for roughly half their in-flight approaches (Fig. 4), and similar numbers of prey were captured in oceanic versus neritic waters. Birds caught more than twice as many prey items during the day as at night by using this method, and prey items were about twice as large as those caught at night. Although circle approach types appeared to be used much less frequently, they occurred haphazardly with respect to wind direction, suggesting that search was not olfactorily driven and may have been induced by sighting other birds. Circle approaches were used more often during the day than at night and oceanic versus neritic differences were not discernable.
Fig. 4.
Summary of relative use of different in-flight approach types with respect to total prey mass (A) and total number of prey items captured (B). Total prey mass = 44,102 g; total number of prey items captured = 153 for all tracks.
By contrast, our interpretation is that turn and zigzag approach types are more likely to reflect variations on olfactory search, suggesting that nearly half of the foraging in-flight approaches we observed involved detecting the scent of the prey item before touchdown (Fig. 4). In support of our predictions for olfactory search, this approach type tended to occur upwind. Upwind flight is three times as energetically expensive as crosswind or downwind flight (22), which would suggest that visually mediated detection would favor following prey items that were detected downwind. Yet birds tended to turn upwind. In contrast to our predictions, this type of approach occurred more frequently during the day than at night, and birds also tended to capture larger prey items during the day than at night by using this approach mode. These observations are, however, consistent with an olfactory-triggered search that relies on multimodal information for target location (32). For example, many species of insect pollinators use scent cues to locate particular flowers, but will fly past an odor source unless the visual stimulus of the flower is also present. Therefore, the most parsimonious explanation for these results is that wandering albatrosses are able to take advantage of olfactory cues for initial detection and localization of a potential prey item, whereas prey capture is facilitated by seeing the prey item directly. Visual references provided by ocean surface features (e.g., wind streaks, or ripples) may also provide birds with directional feedback for upwind orientation during olfactory search. Although we do not know the weather conditions under which birds foraged, this idea is supported by our observation that nighttime, in-flight foraging tended to coincide with the potential availability of moonlight.
Taken together, these data suggest that wandering albatrosses can spot prey directly, or use a combination of olfactory and visual cues for foraging in-flight. Wandering albatrosses have large eyes for their body size among the procellariiforms (33), which leads to a common assumption that visual acuity in albatrosses is comparable or greater than in humans, but this is likely not the case. In albatross species that have been studied, ganglion cell density, an indirect measure of visual acuity, tends to be highest along a visual streak rather than a fovea, and peak cell density is between one-third and one-fourth of what it is in humans, and roughly one-seventh of that in raptor species, such as sparrow hawks, which are highly adapted for visual foraging (34). This observation suggests that albatross vision is tuned to different features in the environment than we typically assume. The visual streak may serve as a horizon detector and thus contribute to flight stabilization or steering; however, visual streaks also tend to occur in animals that are adapted to scan the horizon for the motion of predators or prey [e.g., rabbits (35) and wolves and dogs (36)]. Although the retinal anatomy is not known for wandering albatross, it is worth considering that their eyes may also be better tuned to monitoring motion on the horizon, such as other bird activity in the context of network foraging (10), rather than spotting prey items from a distance.
Thus, it is interesting to note that, for direct approaches, birds tended to fly crosswind, and their foraging path rarely deviated before prey capture or even between touchdown events. These observations suggest that birds spotted prey items only at close visual range as they flew over them. Previous research has suggested that crosswind flight is the most energetically efficient mode of flight (22). By taking advantage of the wind, this strategy is energetically inexpensive while allowing high-speed travel over extremely large foraging areas. This mode of flight is also optimal for opportunistic olfactory search, which could assist in extending the bird's detection range to exploit additional prey items that may not fall directly in its flight path. With respect to nighttime foraging, in-flight approach types were also successfully used at night, suggesting that wandering albatrosses probably have good night vision and can effectively take advantage of moonlight, if weather conditions permit. The size of prey captured by using the direct approach was lower at night, which we assume reflects a shift in target species rather than an inability to see floating carrion at night. However, little is known about visual sensitivity (or the ability to see contrast) among the procellariiforms (see Discussion in SI Text).
The sit-and-wait strategy was also predominantly used at night and offers insights about the sensory ecology of foraging. This foraging strategy involves sitting on the water, presumably in an area where prey are likely to surface. Although birds do not track prey by using this strategy, it is possible that they recognize potentially productive areas based on visual cues such as the presence of other birds milling in the area, changes in water color, or scents (e.g., dimethyl sulfide, DMS) associated with transiently productive areas where prey are likely to surface later on (5, 28). Thus, vision, olfaction, or some combination of cues is likely to alert birds to the presence of potential prey, which may assist them in recognizing a potentially fruitful location to sit-and-wait. Interestingly, our analysis also revealed instances where birds flew upwind from a sitting position (e.g., Fig. 3), which is an olfactory search behavior that has been documented in insects (see discussion in ref. 19). In the case of insects, flight is stimulated by detecting an odor, the source of which can be considerable distances upwind. Weimerskirch et al. (22) have reported transient increases in heart rate of wandering albatrosses before liftoff, and they have suggested that this physiological response is in some way anticipatory. Increases in heart rate are routinely used as a physiological assay for olfactory response in birds, including procellariiforms (37), suggesting that odors might be useful in stimulating birds to leave one sit-and-wait area for another in this context.
With a better appreciation for the sensory ecology that might be driving approach and prey capture, we can begin to address questions about how sensory abilities contribute to foraging behavior in more general terms. As marine predators and scavengers, wandering albatrosses are expected to respond to prey-capture events in ways that optimize their ability to encounter additional prey items. For example, working with insects, Kareiva and Odell (38) proposed that animals foraging on patchily distributed prey should increase their turning behavior in response to prey capture because this behavior increases the probability of encountering additional prey items while minimizing energetic costs (39). This behavior is usually referred to as area-restricted search (ARS). Without considering the sensory mechanisms for detection, ARS predicts an increased turning after successful prey capture, punctuated by movement in a straight line as animals move between patches.
It has recently been shown, however, that wandering albatrosses, which operate on enormous spatial scales, do not tend to increase their turning behavior in response to prey capture as has been observed in other species (most notably, insects). Using first passage time, Weimerskirch et al. (28) found that scales for ARS ranged from 5 to 90 km. They found that birds increased sinuosity only after large prey were captured, and that this occurred only at small scales (≈5 km). One interpretation is that, in these instances where ARS increased, the response of the bird was to employ a visual search (circling), which increases sinuosity. However, in most cases, they found that birds responded to prey capture by flying crosswind in a straight line, a behavior that is also a mode of olfactory search. Flying in a straight line perpendicular to wind direction optimizes the chances that the bird will reencounter an odor plume, and is thus an appropriate search strategy for the problem at hand (14, 17). While this search strategy is energetically inexpensive (22) and highly adapted to the range of spatial scales where these birds operate, it is not predicted by current nonsensory based models for ARS. Moreover, while this mode of search may be particularly adaptive for scavenging prey on the surface, other smaller albatross species that specialize in feeding on live prey are likely to take advantage of sensory features in different ways (40). Our study demonstrates that examining foraging in the context of the sensory ecology driving prey capture is a critical step for further study into this area.
Methods
A detailed account of the methodology for the instrumentation of the birds used in this study has recently been published elsewhere (28). Here, we provide a brief overview; further details for each section are provided in the Methods in SI Text. Work was performed in accordance with Institut Polaire Français Paul Emile Victor guidelines for the ethical treatment of animals.
Field Instrumentation.
Data were obtained from 19 wandering albatrosses (Diomedea exulans) nesting on Possession Island (46°S 51°E), in the Crozet Archipelago in the southwestern Indian ocean. Data were recorded early in the brooding period (2002–2004) when chicks were 10–20 days old.
Once their partner had relieved them at the nest, birds were outfitted with GPS and stomach temperature transmitters and associated receiver-recorders by using previously established methods (28).
Data Analysis.
Categorization of approach types.
Tracks were displayed by using ArcGIS software (ESRI Version 8), and projected by using WGS84 at scales ranging from 1:1,000 to 1:30,000. This projection provided a detailed view in a 10-km radius around the point of prey capture. To prevent bias, the approach tracks were initially categorized by eye, blind to wind direction or speed. No filtering of the GPS data was applied.
Wind direction and speed.
Wind direction and wind speed data were obtained for all touchdown points from the National Aeronautics and Space Administration/Jet Propulsion Laboratory QuikSCAT Daily Level 3 Gridded Ocean Wind Vectors (data product 109, http://poet.jpl.nasa.gov).
Bathymetric data and day/night times.
Bathymetric data were obtained from the General Bathymetric Chart of the Oceans (GEBCO) 1-min global bathymetric grid (www.ngdc.noaa.gov/mgg/gebco/grid/1mingrid.html) for the rectangular area surrounding all touchdown points (−30°N,30°E to −60°N,60°E). The neritic feeding events were identified by selecting those events within the polygons bounding the 2,000-m depth contours. All other events were defined as oceanic feeding events. Day/night times were obtained from the U.S. Naval Observatory (http://aa.usno.navy.mil/data/docs/RS_OneYear.html) and using civil twilight times (night begins when the sun is >6° below the horizon) for the point 48°S, 50°E. Nighttime thus excluded dusk and dawn.
Linearity.
Linearity of the approach tracks was calculated as the direct distance between two points divided by the cumulative distance flown between them. See Methods in SI Text for details. Direct approaches had no obvious starting point by definition and so linearity was measured at a distance of ≈5 km from prey capture. Approaches with missing data points were excluded.
Statistical analysis.
Statistical analyses were typically performed by using JMP version 7 (SAS Institute). Nonparametric methods were applied in cases where data were nonnormally distributed. Circular methods were performed according to Batschelet (41) with Oriana Software. Data are expressed as mean ± standard error throughout the manuscript.
Supplementary Material
Acknowledgments.
We thank Dr. Thomas Eisner for inviting us to contribute to this issue. We thank Marie Hélène Burle, Géraldine Mabille, and Pierrick Blanchard for deploying equipment in the field. We are grateful to Dr. Mark Willis for thoughtful discussion and to Dr. Neil Willits for statistical consulting services. Dr. Terence O'Dwyer and Jennifer DeBose generously commented on early drafts of the manuscript. This work was supported by l'Institut Polaire Français Paul Emile Victor Grant 109 (to H.W.) and U.S. National Science Foundation Grant IBN-0212467 (to G.A.N.). This is contribution 2401 from the Bodega Marine Laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709047105/DC1.
References
- 1.Warham J. The behaviour, population biology and physiology of the petrels. London: Academic; 1996. [Google Scholar]
- 2.Papi F. Navigation of marine, freshwater and coastal animals: concepts and current problems. Mar Freshw Behav Physiol. 2006;39:3–12. [Google Scholar]
- 3.Akesson S, Weimerskirch H. Albatross long-distance navigation: comparing adults and juveniles. J Navigation. 2005;58:365–373. [Google Scholar]
- 4.Nevitt GA, Bonadonna F. Seeing the world through the nose of a bird: new developments in the sensory ecology of procellariiform seabirds. Mar Ecol Prog Ser. 2005;287:292–295. [Google Scholar]
- 5.Nevitt GA. Olfactory foraging by Antarctic procellariiform seabirds: Life at high Reynolds numbers. Biol Bull. 2000;198:245–253. doi: 10.2307/1542527. [DOI] [PubMed] [Google Scholar]
- 6.Bang BG, Cobb S. The size of the olfactory bulb in 180 species of birds. Auk. 1968;85:55–61. [Google Scholar]
- 7.Nevitt GA, Reid K, Trathan P. Testing olfactory foraging strategies in an Antarctic seabird assemblage. J Exp Biol. 2004;207:3537–3544. doi: 10.1242/jeb.01198. [DOI] [PubMed] [Google Scholar]
- 8.Croxall JP, Prince PA. Dead or alive, night or day—How do albatrosses catch squid? Antarct Sci. 1994;6:155–162. [Google Scholar]
- 9.Martin GR, Prince PA. Visual fields and foraging in procellariiform seabirds: Sensory aspects of dietary segregation. Brain Behav Evol. 2001;57:33–38. doi: 10.1159/000047224. [DOI] [PubMed] [Google Scholar]
- 10.Silverman E, Veit RR, Nevitt GA. Nearest neighbors as foraging cues: information transfer in a patchy environment. Mar Ecol Prog Ser. 2004;277:25–36. [Google Scholar]
- 11.Weimerskirch H, Cherel Y, Cuenot-Chaillet F, Ridoux V. Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology. 1997;78:2051–2063. [Google Scholar]
- 12.Lytridis C, Virk GS, Rebour Y, Kadar EE. Odor-based navigational strategies for mobile agents. Adapt Behav. 2001;9:171–187. [Google Scholar]
- 13.Willis MA. Odor-modulated navigation in insects and artificial systems. Chem Senses. 2005;30:I287–I288. doi: 10.1093/chemse/bjh227. [DOI] [PubMed] [Google Scholar]
- 14.Vickers NJ. Mechanisms of animal navigation in odor plumes. Biol Bull. 2000;198:203–212. doi: 10.2307/1542524. [DOI] [PubMed] [Google Scholar]
- 15.Webster DR, Weissburg MJ. Chemosensory guidance cues in a turbulent chemical odor plume. Limnol Oceanogr. 2001;46:1034–1047. [Google Scholar]
- 16.Zimmer-Faust RK, Finelli CM, Pentcheff ND, Wethey DS. Odor plumes and animal navigation in turbulent water flow: A field study. Biol Bull. 1995;188:111–116. doi: 10.2307/1542075. [DOI] [PubMed] [Google Scholar]
- 17.Dusenbery DB. Sensory Ecology. New York: Freeman; 1992. [Google Scholar]
- 18.Carde RT. Odour plumes and odour-mediated flight in insects. Ciba Found Symp. 1996;200:54–70. doi: 10.1002/9780470514948.ch6. [DOI] [PubMed] [Google Scholar]
- 19.Belanger JH, Willis MA. Adaptive control of odor-guided locomotion: Behavioral flexibility as an antidote to environmental unpredictability. Adapt Behav. 1996;4:217–253. [Google Scholar]
- 20.Nevitt GA, Veit RR, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature. 1995;376:681–682. [Google Scholar]
- 21.Turchin P. Fractal analyses of animal movement: A critique. Ecology. 1996;77:2086–2090. [Google Scholar]
- 22.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Philos Trans R Soc London Ser B. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear LB, Ainley DG. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis. 1997;139:221–233. [Google Scholar]
- 24.Alerstam T, Gudmundsson GM, Larsson B. Flight tracks and speeds of Antarctic and Atlantic seabirds: radar and optical measurements. Philos Trans R Soc London Ser B. 1993;340:55–67. [Google Scholar]
- 25.Pennycuick CJ. The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Philos Trans R Soc London Ser B. 1982;300:75–106. [Google Scholar]
- 26.Grubb TC. Smell and foraging in shearwaters and petrels. Nature. 1972;237:404–405. [Google Scholar]
- 27.Hutchison LV, Wenzel BM. Olfactory guidance in foraging by procellariiforms. Condor. 1980;82:314–319. [Google Scholar]
- 28.Weimerskirch H, Pinaud D, Pawlowski F, Bost CA. Does prey capture induce area-restricted search? A fine scale study using GPS in a marine predator, the wandering albatross. Am Nat. 2007;170:734–743. doi: 10.1086/522059. [DOI] [PubMed] [Google Scholar]
- 29.Weimerskirch H, Bonadonna F, Bailleul F, Mabille G, Dell'Omo G, Lipp H-P. GPS tracking of foraging albatrosses. Science. 2002;295:1259. doi: 10.1126/science.1068034. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RP, et al. Reliability of stomach temperature changes in determining feeding characteristics of seabirds. J Exp Biol. 1995;198:1115–1135. doi: 10.1242/jeb.198.5.1115. [DOI] [PubMed] [Google Scholar]
- 31.Haney JC, Fristrup KM, Lee DS. Geometry of visual recruitment by seabirds to ephemeral foraging flocks. Ornis Scandinavica. 1992;23:49–62. [Google Scholar]
- 32.Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Anim Behav. 2005;69:407–418. [Google Scholar]
- 33.Brooke MDL, Hanley S, Laughlin SB. The scaling of eye size with body mass in birds. Proc R Soc London Ser B. 1999;266:405–412. [Google Scholar]
- 34.Hayes BP, Brooke MDL. Retinal ganglion-cell distribution and behavior in procellariiform seabirds. Vision Res. 1990;30:1277–1289. doi: 10.1016/0042-6989(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 35.Famiglietti EV. “Small-tufted” ganglion cells and two visual systems for the detection of object motion in rabbit retina. Visual Neurosci. 2005;22:509–534. doi: 10.1017/S0952523805224124. [DOI] [PubMed] [Google Scholar]
- 36.Peichl L. Topography of ganglion cells in the dog and wolf retina. J Comp Neurol. 1992;324:603–620. doi: 10.1002/cne.903240412. [DOI] [PubMed] [Google Scholar]
- 37.Nevitt GA, Bonadonna F. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol Lett. 2005;1:303–305. doi: 10.1098/rsbl.2005.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kareiva P, Odell G. Swarms of predators exhibit preytaxis if individual predators use area-restricted search. Am Nat. 1987;130:233–270. [Google Scholar]
- 39.Charnov EL. Optimal foraging, marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 40.Finelli CM, Pentcheff ND, Zimmer RK, Wethey DS. Physical constraints on ecological processes: a field test of odor-mediated foraging. Ecology. 2000;81:784–797. [Google Scholar]
- 41.Batschelet E. Circular Statistics in Biology. New York: Academic; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.