Abstract
The Mediterranean Sea is losing its biological distinctiveness, and the same phenomenon is occurring in other seas. It gives urgency to a better understanding of the factors that affect marine biological invasions. A chemoecological approach is proposed here to define biotic conditions that promote biological invasions in terms of enemy escape and resource opportunities. Research has focused on the secondary metabolite composition of three exotic sea slugs found in Greece that have most probably entered the Mediterranean basin by Lessepsian migration, an exchange that contributes significantly to Mediterranean biodiversity. We have found toxic compounds with significant activity as feeding deterrents both in the cephalaspidean Haminoea cyanomarginata and in the nudibranch Melibe viridis. These findings led us to propose aposematism in the former and dietary autonomy in producing defensive metabolites in the latter case, as predisposing factors to the migration. In the third mollusk investigated, the anaspidean Syphonota geographica, the topic of marine invasions has been approached through a study of its feeding biology. The identification of the same compounds from both the viscera of each individual, separately analyzed, and their food, the seagrass Halophila stipulacea, implies a dietary dependency. The survival of S. geographica in the Mediterranean seems to be related to the presence of H. stipulacea. The initial invasion of this exotic pest would seem to have paved the way for the subsequent invasion of a trophic specialist that takes advantage of niche opportunities.
Keywords: Lessepsian migration, defense, Opisthobranchia, resource opportunities
A vast literature on so-called “sea slugs” (Mollusca: Gastropoda: Opisthobranchia) indicates that these mollusks have developed a variety of defensive strategies, including the use of “chemical weapons” (refs. 1–5 and references therein). Chemical defense is now understood as the driving force behind the evolution of the group, preceding the regression of the shell and the abandonment of mechanical defense (6–9). Adaptive radiation, with switches from one chemically defended food source to another, has been documented by means of comparative techniques. Opisthobranchs are known to contain a wide range of secondary metabolites with peculiar structural characteristics and activities. In most cases the compounds have dietary origin, although they are often modified by the slugs. Several lineages are remarkable in having evolved the ability to biosynthesize metabolites that were originally obtained from food de novo (9). This evolutionary innovation emancipates the dietary specialists from dependency on their original food and presents them with a variety of ecological opportunities.
This article suggests that a synthesis of what is known about these aspects of the biology of opisthobranchs and ideas derived from community ecology (10) could allow us to analyze how predator avoidance and the exploitation of resource opportunities influence the migration of exotic sea slug species to new environments. We used a chemical approach to study three nonindigenous opisthobranch populations recorded in the eastern Mediterranean Sea in the context of the ecological phenomenon known as Lessepsian migration (11).
Among the European seas the Mediterranean seems to be the major recipient of exotic species, many of which have reached its eastern basin from the Red Sea through the Suez Canal (12). This biotic exchange contributes significantly to the biodiversity of the Mediterranean. Consequently, the International Commission for the Scientific Exploration of the Mediterranean (CIESM), with 23 member states, is continuously reviewing reliable evidence of new or confirmed records (13–15). Unfortunately, not much information is available on biotic factors determining the ability of alien species to invade. We believe that this is in part due to the “compartmentalization” of disciplines, such as chemistry and biology, having their own publication outlets, whereas an integration of the results from different scientific areas could allow one to address issues that reach beyond the disciplinary skills of individual scientists.
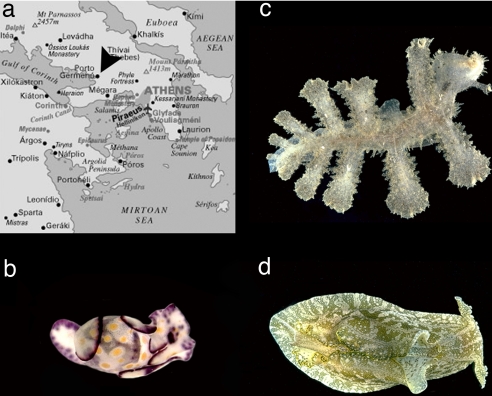
We initiated this investigation in November 2001, when along the coasts of the Gulf of Corinth in Greece (Fig. 1a) one of us (E.M.) quite unexpectedly encountered populations of both the cephalaspidean Haminoea cyanomarginata Heller & Thompson, 1983 (Fig. 1b), and the nudibranch Melibe viridis Kelaart, 1858 [ = Melibe fimbriata Alder & Hancock, 1864 (16)] (Fig. 1c). The former is a Red Sea species previously unknown in the Mediterranean Sea. The latter is a widespread tropical Indo-West Pacific species previously reported in the Mediterranean as an immigrant species (15). During two additional campaigns at the same site in 2002 and 2003, a population of the exotic aplysiid anaspidean Syphonota geographica (Adams & Reeve, 1850) (Fig. 1d) was also encountered.
Fig. 1.
Exotic sea slugs found in Greece. (a) Sampling site. (b) Haminoea cyanomarginata. (c) Melibe viridis. (d) Syphonota geographica. (Photos in c and d are courtesy of W. B. Rudman and are for illustrative purposes only and not of the specimens examined here.)
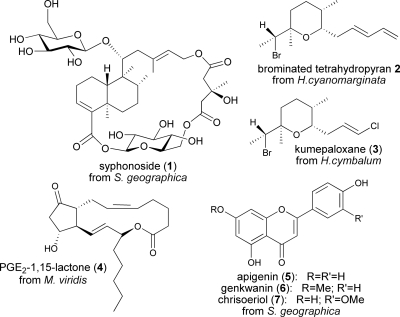
The first two species have never been chemically investigated so far, whereas two previous articles reported chemical studies carried out on the initial collection of S. geographica in Greece (2002). The first study led to the isolation of degraded sterols for which a defensive role was implied by the fact that they were selectively localized in the skin of the animals (17), exhibiting strong structural analogies with bioactive molecules found in other anaspideans from different geographical areas (18–20). In the same work, preliminary analysis of the extract obtained from the S. geographica viscera showed the presence of other components, for which a dietary origin from the seagrass Halophila stipulacea was indicated by the identification of plant fragments in the stomach content of one individual. A second investigation confirmed the dietary relationship between the mollusk and the seagrass by the identification of the unusual novel compound syphonoside (1) (Fig. 2), as the main metabolite both in the animal and in the plant (21). Here, we report a further chemical analysis of S. geographica, performed on a subsequent collection of biological material in Greece. In this case, viscera of each individual mollusk were separately extracted and compared with H. stipulacea extracts to investigate the degree of alimentary specialization of S. geographica.
Fig. 2.
Structures of compounds 1–7.
Results
Chemical Study of H. cyanomarginata.
In H. cyanomarginata, we identified the brominated tetrahydropyran 2 (Fig. 2) previously isolated from an Australian sponge (22), also found in the congeneric species Haminoea cymbalum from Indian coasts (23), and structurally related to kumepaloxane (3) found in H. cymbalum from Guam (24). 1H NMR analysis revealed the almost exclusive presence of 2 in the lipophilic extract of the mucus secreted by the animals when molested, strongly suggesting its involvement in the chemical defense. Compound 2 was also present in the skin of H. cyanomarginata. The natural volumetric concentration of 2 in whole individuals was 2.26 mg/ml, whereas it was absent in the extract obtained from the internal parts.
Chemical Study of M. viridis.
In the M. viridis mucous secretion, which is extruded from the dorsal appendages during autotomy, we found the ichthyotoxic prostaglandin E2-1,15-lactone 4 (Fig. 2) previously isolated from the Mediterranean Tethys fimbria (25, 26). This compound was also present in the M. viridis dorsal cerata extract, but it was not detected in the digestive apparatus of the nudibranch. Unfortunately, transformations occurring during HPLC separations prevented assessing the natural concentration of 4 in the cerata.
Chemical Study of S. geographica and H. stipulacea.
From the viscera of S. geographica we isolated the known bioactive flavonoids apigenin (5), genkwanin (6), and chrisoeriol (7) (Fig. 2) (27–30). Syphonoside (1) (21) was also detected as a polar component. Remarkably, the viscera of each individually studied mollusk showed similar chemical patterns. The same compounds were also detected in the extract obtained from the seagrass H. stipulacea.
Toxicity Assays.
Compound 2 showed high toxicity to the mosquito fish Gambusia affinis at the concentration of 1 ppm, while the ichthyotoxicity of compound 4 at 10 ppm, previously reported by Marin et al. (26), was also confirmed. Even though such bioassay with freshwater organisms is not ecologically relevant, we believe that it proves the toxic potential of compounds 2 and 4.
Feeding Deterrence Assay.
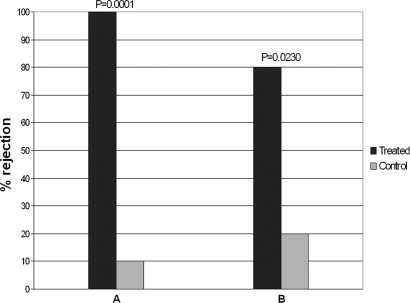
Food treated with compounds 2 and 4, was unpalatable to the generalist marine shrimp Palaemon elegans, showing significant differences in rejection rate vs. control food (Fig. 3). In particular, compound 2 at its natural volumetric concentration in H. cyanomarginata (2.3 mg/ml) produced a food rejection with a highly significant difference in respect to the control (P = 0.0001). The rejection rate of food treated with compound 4 from M. viridis also turned out to be significantly different with respect to the control (P = 0.0230), at the concentration of 1.0 mg/ml. A sample size of 10 shrimps was used for each of the four separate series of individual replicates.
Fig. 3.
P. elegans alimentary response to food pellets treated with compound 2 (A) and 4 (B) at the volumetric concentration of 2.3 mg/ml and 1.0 mg/ml, respectively. Two-tailed Fisher's exact test: P < 0.05 vs. control; n = 10 for each bar.
Discussion
How a species responds to resources, natural enemies, and the physical environment determines its ability to invade (10). The results presented here demonstrate that a chemoecological approach can be efficiently applied to identify enemy escape and resource opportunities that provide alien organisms with the required biotic conditions for a successful invasion of new habitats.
As is true of many other groups of animals, powerful chemical defense combined with aposematic (warning) coloration tends to be most pronounced in opisthobranchs that live in communities where the climate is stable and biodiversity levels are high. It may prove very enlightening to see what happens when organisms from regions that are, relatively speaking, ecologically saturated (such as the Red Sea) move to undersaturated regions (such as the Mediterranean). In H. cyanomarginata, a mollusk included in the CIESM list of exotic mollusks in the Mediterranean (15), we found the compound 2, previously isolated from an Australian sponge and in H. cymbalum from Indian coasts. The finding of a typical sponge metabolite in this herbivorous mollusk raises intriguing speculations regarding its origin. The absence of compound 2 in the viscera of this mollusk suggests a de novo biosynthesis, as does the finding of this metabolite and the related compound 3 in a congeneric species, H. cymbalum, from India and Guam, respectively. Compound 3 already showed deterrent properties toward generalist carnivorous fishes (24). Here, we observed toxicity of 2 and its high significant activity as feeding deterrent at the natural concentration in whole H. cyanomarginata individuals. As in the case of the congeneric Indo-Pacific H. cymbalum, the bright coloration of H. cyanomarginata should give potential predators the opportunity to learn from negative experience. In fact, the color pattern that distinguishes H. cyanomarginata from the cryptically colored Mediterranean Haminoea species is linked to the presence of a chemical weapon and implies aposematism. We did not observe any predatory attempts on H. cyanomarginata in our field studies, nor on H. cymbalum during our previous diving campaigns in India and the Philippines. Further experiments on the ability of model predators to discriminate different color patterns could lead to determination of the efficiency of such coloration in avoidance learning.
Interestingly, previously studied indigenous Mediterranean Haminoea species were found to contain alkyl-pyridines named haminols. Even though haminols turn out to be neither toxic nor deterrent, they induce alarm responses in trail-following conspecifics (31–34). Obviously, the use of such alarm pheromones, released in case of danger and acting as intraspecific warning messengers, implies the sacrifice of a number of slow moving individuals that need a long time to escape predation. Therefore, alarm pheromones would work only with established abundant populations. Here, we propose that the toxic and unpalatable allomone 2 of H. cyanomarginata represents the chemical basis of a defensive mechanism that was more effective during the first phase of colonization of the Mediterranean by this species, when the sacrifice of a few colonizing individuals could also have prevented the establishment of an active population. In apparent contrast with this, the North Pacific Haminoea species recorded in the Mediterranean, H. japonica Pilsbry, 1895 [ = Haminoea callidegenita Gibson & Chia, 1989 (35)], is known to contain alkylphenols for which a potential alarm pheromone activity has been proposed (34). Such discrepancy is explained by the fact that the cryptically colored H. japonica, encountered in the Mediterranean only in Venice Lagoon, seems to have been transported by humans along with cultures of Ruditapes philippinarum, a mollusk of commercial interest (15).
The second species studied, M. viridis, also included in the CIESM list (15), shares nonselective dietary requirements with the previously studied Tethys fimbria. Both species, belonging to the same family, Tethydidae, mainly feed by swallowing small crustaceans dwelling near the sea bottom. Both use the autotomy of dorsal appendages, called cerata, as defensive behavior. In T. fimbria, it was suggested that prostaglandin E2-1,15-lactone 4 acts as chemical mediator in the defense (26). It was also shown that it has a de novo origin from arachidonic acid (36). In the case of the Lessepsian M. viridis, the ichthyotoxic compound 4 was not detected in the extract obtained from the animal bodies, which include the digestive apparatus, also supporting a nondietary origin of this metabolite, whereas its presence both in the cerata and in the slime released by the mollusk during autotomy suggested its involvement in chemical defense. In the present study the toxicity of 4 has been confirmed. Moreover, we assayed the compound for feeding deterrence on Palaemon. Even though chemical degradations occurring during HPLC separation prevented assaying 4 at its natural volumetric concentration in the dorsal appendages, the compound resulted in significant deterrence at a relatively low concentration. This led us to propose that the successful establishment of M. viridis in the Mediterranean should have been favored by both its nonspecialized alimentary habits and its autonomy from diet in producing defensive metabolites. Compound 4, released with the mucus secreted by contracting cerata after autotomy, is contained in the “toxic cloud” that envelops predators attempting to eat the nudibranch. As in the case of H. cyanomarginata, the presence of a “repulsive smell” in the mucous secretion provides M. viridis with a first line of defense, while unpalatable compounds in the skin should work after the animal or part of it is in the predator's mouth.
Finally, a special case of trophic association between two nonindigenous species has been approached. We found the same lipophilic compounds (1 and 5–7) both in the viscera of each studied S. geographica individual and in the seagrass H. stipulacea. Their presence only in the viscera led us to rule out a defensive role of this metabolites in the animals. Flavonoids are widely distributed in plants and are known to be potent antioxidants, while in a previous article (21), compound 1 did not show cytotoxic activity against cell lines but was able to inhibit high density induced apoptosis. However, our findings imply a dietary dependency. S. geographica is unique among Anaspidea in no longer feeding on algae. Even though the diet of this mollusk in its native habitat is unknown, its survival in the Mediterranean seems to be linked to the presence of H. stipulacea. The establishment of this seagrass in the Mediterranean Sea could have enabled the subsequent migration of its grazer, supporting its invasion in terms of alimentary resources. This may be a case of “facilitation,” defined as an interaction in which one species has a positive effect on the persistence or population growth of another species (37). Inversely, the recent migration of S. geographica could be evaluated as a limiting factor to the expansion rate of H. stipulacea, the exotic seagrass recorded in the Mediterranean since 1895 (38) that is one of the nine macrophyte species that are considered invasive, replacing keystone species and/or causing environmental and economic damage (39).
At present, it is not possible to predict the long-term fate of these recently introduced opisthobranch populations and their impact on the native community structure. However, the chemistry is proven here a powerful tool for the study of the biotic conditions that promote their successful establishment in early stages of colonization, favoring the increase in their population density.
In a combination of chemical and biological information, our results on Lessepsian sea slugs seem to provide also a predictive framework, where the likelihood of future establishment of opisthobranch populations in new regions depends on the efficiency of their chemical defense and on the availability of specific food sources. For example, sacoglossan mollusks that feed on Caulerpa racemosa in their native habitats and derive defensive compounds from that alga (7) could reasonably follow their prey in the nonnative environments recently and dramatically colonized by this invasive marine macrophyte (40, 41).
Moreover, because of the great potential of marine benthic animals and plants to provide bioactive compounds, a chemoecological approach offers a potential tool in biotechnology with interesting recycling options in possible eradication programs. Just as an additional example, an attempt to eradicate the exotic pest H. stipulacea could take advantage of the opportunity to recover bioactive flavones, such as the tumor growth inhibitor 5 (42, 43), from the extirpated biomaterial.
Methods
Animals.
Sea slugs were collected off Porto Germeno coasts (Gulf of Corinth, Greece) by SCUBA diving. Populations of H. cyanomarginata and M. viridis were found yearly at the same diving site during the triennium 2001–2003, and S. geographica was recorded during 2002 and 2003. Thirty-one individuals of H. cyanomarginata (average size 8 mm) were collected during November 2001 on sandy bottom at 15 m depth. Twenty-nine individuals were molested by agitation in a Petri disk to obtain 2.5 ml of a viscous mucous secretion. Twelve individuals of M. viridis (average size 150 mm) were collected on sandy bottom at 15–30 m depth in December 2003. Ten individuals were molested by agitation in beakers until the detachment of the dorsal cerata. Animals, detached appendages, and the slime secreted during autotomy (25.0 ml) were separately stored. Eight individuals of S. geographica (average size 50 mm) were collected on sandy bottom at 10–15 m depth in December 2003.
Plant.
H. stipulacea was also collected in the same sampling site during December 2003 at 5–10 m depth.
All samples were stored at −20°C until their chemical analysis.
Chemical Study of H. cyanomarginata.
Frozen animals (22 individuals) were extracted by treatment with ultrasound vibration in acetone at room temperature to obtain selectively the crude extract of their skin. A further treatment with acetone in a mortar led to the extraction of metabolites also present in the inner organs, including the digestive tract. After concentration, the two extracts were diluted with water and extracted with diethyl ether affording 11.7 and 41.5 mg, respectively. Exhaustive acetone extraction of the other seven whole individuals (0.8 ml total volume) followed by diethyl ether extraction gave 12.7 mg of crude extract. Mucous secretion (2.5 ml) was directly treated with diethyl ether giving 6.9 mg of crude extract.
The TLC comparison showed a main UV-visible band in the extract from the external part, mucus, and whole individuals, whereas it was absent in the internal part. The compound corresponding to the TLC spot was purified from the skin extract by fractionation on a C18 HPLC column (gradient from 80% acetonitrile/water to 100% acetonitrile over 30 min; UV detector), giving 5.3 mg of pure known compound 2, showing [α]D, 1H, and 13C NMR data identical to those described in the literature (22). The natural volumetric concentration of 2 was deduced by comparison of the HPLC profile of the crude extract obtained from seven whole individuals with the HPLC calibration curve of pure 2, by using the same chromatographic conditions. The 1H NMR spectrum of the crude extract from the slime showed the almost exclusive presence of compound 2.
Chemical Study of M. viridis.
Frozen cerata and bodies were extracted by treatment with acetone in a mortar and ultrasound vibration at room temperature. The aqueous residue was diluted with water and extracted with diethyl ether. The mucous secretion (25.0 ml) was directly extracted with diethyl ether. All extracts were separately fractionated on a C18 HPLC column (gradient from 30% acetonitrile/water to 100% acetonitrile over 65 min; UV detector). One of the peaks obtained was present in the cerata and mucus extracts, but it was not detected in the HPLC profile of the extract from the animal bodies including the digestive apparatus.
Unfortunately, chemical degradations occurring during HPLC allowed us to obtain only 2.0 mg of the pure compound corresponding to the described HPLC peak, preventing assay of the metabolite at natural concentration in the nudibranch. However, the purified compound showed 1H and 13C NMR data identical to those described in the literature for compound 4 (25).
Chemical Study of S. geographica.
The viscera of each frozen mollusk (eight individuals), obtained by dissection, were separately extracted by acetone at room temperature by both using ultrasound vibration and crumbling with a pestle. The filtered acetone solutions were concentrated and aqueous residues were extracted with diethyl ether and subsequently with 1-butanol. The extracts were, primarily, analyzed by TLC using two different mobile phases (light petroleum ether/diethyl ether and chloroform/methanol in different ratio) showing identical chromatographic patterns. The diethyl ether extracts were then combined to give 290.1 mg of crude residue, which was fractionated by Sephadex LH-20 chromatography eluted with chloroform/methanol in ratio 1:1 to give a fraction that was further purified on preparative silica gel TLC (90% chloroform/methanol) affording the known flavones apigenin (5, 2.7 mg), genkwanin (6, 1.0 mg), and chrisoeriol (7, 1.6 mg), all identified by comparison with 1H and 13C NMR data described in the literature (27–30). In each n-butanolic extract, we identified syphonoside (1) by TLC comparison with the standard compound we recently isolated and reported from both S. geographica and H. stipulacea (21).
Chemical Study of H. stipulacea.
Part of the plant sample (264.6 g wet weight) was extracted by acetone at room temperature both by ultrasound vibration and grinding in a blender. The aqueous residue was diluted with water and extracted with diethyl ether and then with 1-butanol. The ether extract (273.7 mg) was analyzed by TLC and purified by using the same procedure of S. geographica giving apigenin (5, 0.8 mg), genkwanin (6, 0.8 mg), and chrisoeriol (7, 1.7 mg), also identified by 1H and 13C NMR data analysis. The presence of compound 1 in the 1-butanol extract was confirmed by TLC comparison with the standard compound (21).
Toxicity Assay.
Ichthyotoxicity assays on the mosquito fish Gambusia affinis were conducted following the procedure by Gunthorpe and Cameron (44) and using the toxicity ranking according to Coll et al. (45). Compounds were added to fresh water (70 ml) in pure acetone (0.5 ml) at 1 and 10 ppm, whereas controls used pure solvent.
Feeding Deterrence Assay.
To assay the effects of compounds 2 and 4 on food palatability, we performed a bioassay with the marine generalist shrimp Palaemon elegans. This crustacean is very common in the Mediterranean, having a broad range of alimentary habits, including small mollusks. Food was prepared by slightly modifying a method proposed by Pawlik et al. (46). Each pure compound dissolved in 0.5 ml of acetone was added to a mixture composed of alginic acid (30 mg), ground freeze-dried squid mantle (50 mg), and purified sea sand (30 mg; granular size 0.1–0.3 mm). Sand was included in the mixture to prevent the floating of the pellets on the surface of the water during the experiments. After evaporation of the solvent, one drop of food coloring (E124 and E110) and distilled water was added to 1-ml volume. Food coloring was added for an easy detection of the ingested food in the digestive tube of the shrimps. The mixture was stirred, loaded into a 5-ml syringe, and extruded into a 0.25 M calcium chloride solution for 2 min to harden. The resulting spaghetti-like red strand was cut into 10-mm-long pellets. Control foods were made in the same manner, with the addition of 0.5 ml of acetone but without the purified metabolites.
Shrimps (average size 30 mm), collected along the coast of Pozzuoli (Naples, Italy), were kept in an aquarium for 1 week to get them accustomed to the daily proposed artificial food. After 3 days of total fasting, they were individually placed in 500-ml beakers filled with 300 ml of sea water.
Control or treated pellets were presented to shrimps in series of 10 independent replicates. After 30 min, the presence of an evident red spot in the digestive tube of the shrimps was assumed as proof of acceptance and, conversely, its absence was the sign of a rejection response (Fig. 4).
Fig. 4.
P. elegans accepts (Left) and rejects (Right) the proposed food.
The significance of differences in the consumption of treated vs. control pellets was evaluated by two-tailed Fisher's exact test. P values <0.05 were considered statistically significant.
Acknowledgments.
We thank C. Vagias for precious assistance during the field work, T. M. Gosliner for help in the identification of H. cyanomarginata and M. viridis, and W. B. Rudman for providing photos of M. viridis and S. geographica.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Karuso P. In: Bioorganic Marine Chemistry. Scheuer PJ, editor. Vol 1. Berlin: Springer; 1987. pp. 31–60. [Google Scholar]
- 2.Faulkner DJ. In: Ecological Roles of Marine Natural Products. Paul VJ, editor. Ithaca, New York: Comstock Publishing Associates; 1992. pp. 119–163. [Google Scholar]
- 3.Cimino G, Fontana A, Gavagnin M. Curr Org Chem. 1999;3:327–372. [Google Scholar]
- 4.Cimino G, Ciavatta ML, Fontana A, Gavagnin M. In: Bioactive Compounds from Natural Sources. Tringali C, editor. London: Taylor & Francis; 2001. pp. 578–637. [Google Scholar]
- 5.Mollo E, Gavagnin M, Carbone M, Guo Y-W, Cimino G. Chemoecology. 2005;15:31–36. [Google Scholar]
- 6.Faulkner DJ, Ghiselin MT. Mar Ecol Prog Ser. 1983;13:295–301. [Google Scholar]
- 7.Cimino G, Ghiselin MT. Chemoecology. 1998;8:51–60. [Google Scholar]
- 8.Cimino G, Ghiselin MT. Chemoecology. 1999;9:187–207. [Google Scholar]
- 9.Cimino G, Ghiselin MT. In: Marine Chemical Ecology. McClintock JB, Baker BJ, editors. FL: CRC, Boca Raton; 2001. pp. 115–154. [Google Scholar]
- 10.Shea K, Chesson P. Trends Ecol Evol. 2002;17:170–176. [Google Scholar]
- 11.Por F. Ecol Stud. 1978;23:1–228. [Google Scholar]
- 12.Streftaris N, Zenetos A, Papathanassiou E. Oceanogr Mar Biol Annu Rev. 2005;45:419–453. [Google Scholar]
- 13.Golani D, Orsi-Relini L, Massuti E, Quingnard JP. Fishes, CIESM Atlas of Exotic Species in the Mediterranean. In: Briand F, editor. Vol 1. Monaco: CIESM; 2002. [Google Scholar]
- 14.Galil B, Froglia C, Noël P. Crustacean Decapods and Stomatopods, CIESM Atlas of Exotic Species in the Mediterranean. In: Briand F, editor. Vol 2. Monaco: CIESM; 2002. [Google Scholar]
- 15.Zenetos A, Gofas S, Russo G, Templado J. Molluscs, CIESM Atlas of Exotic Species in the Mediterranean. In: Briand F, editor. Vol 3. Monaco: CIESM; 2003. [Google Scholar]
- 16.Gosliner TM, Smith VG. Proc Cal Acad Sci. 2003;54:302–355. [Google Scholar]
- 17.Gavagnin M, Carbone M, Nappo M, Mollo E, Roussis V, Cimino G. Tetrahedron. 2005;61:617–621. [Google Scholar]
- 18.Miyamoto T, Higuchi R, Komori T, Fujioka T, Mihashi K. Tetrahedron Lett. 1986;27:1153–1156. [Google Scholar]
- 19.Spinella A, Gavagnin M, Crispino A, Cimino G, Martinez E, Ortea J, Sodano G. J Nat Prod. 1992;55:989–993. doi: 10.1021/np50085a027. [DOI] [PubMed] [Google Scholar]
- 20.Ortega MJ, Zubia E, Salvà J. J Nat Prod. 1997;60:488–489. doi: 10.1021/np9607464. [DOI] [PubMed] [Google Scholar]
- 21.Gavagnin M, Carbone M, Amodeo P, Mollo E, Vitale RR, Roussis V, Cimino G. J Org Chem. 2007;72:5625–5630. doi: 10.1021/jo0704917. [DOI] [PubMed] [Google Scholar]
- 22.Capon RJ, Ghisalberti EL, Jefferies PR. Tetrahedron. 1982;38:1699–1703. [Google Scholar]
- 23.Fontana A, Ciavatta ML, D'Souza L, Mollo E, Naik CG, Parameswaran PS, Wahidulla S, Cimino G. J Indian Inst Sci. 2001;81:403–415. [Google Scholar]
- 24.Poiner A, Paul VJ, Scheuer PJ. Tetrahedron. 1989;45:617–622. [Google Scholar]
- 25.Cimino G, Spinella A, Sodano G. Tetrahedron Lett. 1989;30:3587–3592. [Google Scholar]
- 26.Marin A, Di Marzo V, Cimino G. Mar Biol. 1991;111:353–358. [Google Scholar]
- 27.Agrawal PK, Thakur RS, Bansal MC. In: Carbon-13 NMR of Flavonoids. Agrawal PK, editor. Amsterdam: Elsevier; 1989. pp. 95–182. [Google Scholar]
- 28.Corticchiato M, Bernardini A, Costa J, Bayet C, Saunois A, Voirin B. Phytochemistry. 1995;40:115–120. [Google Scholar]
- 29.Rabe B, Steenkam JA, Joubert E, Burger JFW, Ferreira D. Phytochemistry. 1995;35:1559–1565. [Google Scholar]
- 30.Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York: Springer; 1970. [Google Scholar]
- 31.Marin A, Alvarez LA, Cimino G, Spinella A. J Moll Stud. 1999;65:121–131. [Google Scholar]
- 32.Cimino G, Passeggio A, Sodano G, Spinella A, Villani G. Experientia. 1991;47:61–63. doi: 10.1007/BF02041252. [DOI] [PubMed] [Google Scholar]
- 33.Spinella A, Alvarez LA, Passeggio A, Cimino G. Tetrahedron. 1993;49:1307–1314. [Google Scholar]
- 34.Spinella A, Alvarez LA, Cimino G. Tetrahedron Lett. 1998;39:2005–2008. [Google Scholar]
- 35.Gosliner TM, Behrens DW. Proc Cal Acad Sci. 2006;57:1003–1010. [Google Scholar]
- 36.Di Marzo V, Cimino G, Crispino A, Minardi C, Sodano G, Spinella A. Biochem J. 1991;273:593–600. doi: 10.1042/bj2730593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricciardi A. In: Invasive Alien Species, A New Synthesis. Mooney HA, Mack RN, McNeely JA, Neville LE, Schei PJ, Waage JK, editors. Washington, DC: Island; 2005. pp. 162–178. [Google Scholar]
- 38.Lipkin Y. Israel J Bot. 1975;24:198–200. [Google Scholar]
- 39.Boudouresque CF, Verlaque M. Mar Poll Bull. 2002;44:32–38. doi: 10.1016/s0025-326x(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 40.Verlaque M, Boudouresque CF, Meinesz A, Gravez V. Bot Mar. 2000;43:49–68. [Google Scholar]
- 41.Raniello R, Mollo E, Lorenti M, Gavagnin M, Buia MC. Biol Invasions. 2007;9:361–368. [Google Scholar]
- 42.Ling-Zhi L, Jing F, Qiong Z, Xiaowen H, Xianglin S, Bing-Hua J. Mol Pharmacol. 2005;68:635–643. [Google Scholar]
- 43.Czyz J, Madera Z, Irmer U, Korohoda W, Huelser DF. Int J Cancer. 2005;114:12–18. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]
- 44.Gunthorpe L, Cameron AM. Mar Biol. 1987;94:39–43. [Google Scholar]
- 45.Coll JC, La Barre S, Sammarco PW, Williams WT, Bakus G. Mar Ecol Prog Ser. 1982;8:271–278. [Google Scholar]
- 46.Pawlik JR, Chanas B, Toonen RJ, Fenical W. Mar Ecol Prog Ser. 1995;127:183–194. [Google Scholar]