Abstract
The prospect of finding macroevolutionary trends and rules in the history of life is tremendously appealing, but very few pervasive trends have been found. Here, we demonstrate a parallel increase in the morphological complexity of most of the deep lineages within a major clade. We focus on the Crustacea, measuring the morphological differentiation of limbs. First, we show a clear trend of increasing complexity among 66 free-living, ordinal-level taxa from the Phanerozoic fossil record. We next demonstrate that this trend is pervasive, occurring in 10 or 11 of 12 matched-pair comparisons (across five morphological diversity indices) between extinct Paleozoic and related Recent taxa. This clearly differentiates the pattern from the effects of lineage sorting. Furthermore, newly appearing taxa tend to have had more types of limbs and a higher degree of limb differentiation than the contemporaneous average, whereas those going extinct showed higher-than-average limb redundancy. Patterns of contemporary species diversity partially reflect the paleontological trend. These results provide a rare demonstration of a large-scale and probably driven trend occurring across multiple independent lineages and influencing both the form and number of species through deep time and in the present day.
Keywords: Arthropoda, correlates of diversity, disparity, macroevolutionary trend, tagmosis
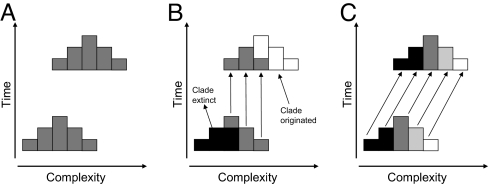
Most of the natural sciences operate by documenting patterns and trends and thereby formulating general rules. Evolution, however, is an essentially contingent process, meaning that evolutionary trajectories can rarely be predicted. Proposed evolutionary trends, such as Cope's rule for evolutionary size increase within lineages, have generally turned out to be only weakly predictive, either resulting from passive diffusion away from some barrier (1–4) or applying only at local temporal and taxonomic scales (5–9). Here, we demonstrate a remarkable and pervasive trend for increasing morphological complexity in multiple parallel lineages of the Crustacea [the major arthropod group with the longest and most disparate fossil record (10)] throughout the Phanerozoic. Previous studies by Cisne (11) and Wills et al. (10) have shown that average complexity, as indexed by the serial differentiation and specialization of limbs along the body axis (a process known as tagmatization or tagmosis), increased from the Cambrian to the present. However, this pattern could have resulted from nothing more than random diffusion from a minimum boundary (1, 12, 13) or from the differing fortunes of a small number of clades that happen coincidentally to differ in complexity, rather than from a pervasive and driven trend within most clades (see Fig. 1). This work uses recently available phylogenetic evidence that allows us to distinguish between these (and other) possibilities.
Fig. 1.
When is a shift in the mean a trend? (A) A large clade displays an overall increase in the complexity of its constituent subclades. Over time, the distribution of complexity values is shifted to the right, and an apparent trend for increasing complexity results. However, this pattern can derive from two very different although not exclusive processes. (B) Increasing mean complexity of the clade can result from the fortuitous extinction and origination of a small number of constituent major subclades, which coincidentally, have very different mean complexity values. (C) A driven trend results from a parallel increase in complexity within all or most of the constituent subclades. This mechanism is the dominant one in our crustacean data. Determining the relative contributions of this process and the process in the Center requires a phylogenetic approach, which has hitherto been lacking.
To understand the mechanisms by which the average complexity of crustaceans increased throughout the Phanerozoic, this article addresses a range of related questions. Did many parallel lineages evolve from a condition of serial limb homonomy toward increasing serial differentiation, or were less morphologically complex clades replaced wholesale by more complex ones (Fig. 1)? Did the degree of tagmosis differentially affect diversification or extinction rates? How do limb number and the numbers of limb types contribute to the trend? Is the extent of tagmosis related to present-day diversity? We conclude that increasing complexity of appendage composition represents a rare demonstration of a pervasive and long-term trend that has helped to shape patterns of diversification since the Cambrian.
Results
Complexity Increases Throughout the Phanerozoic.
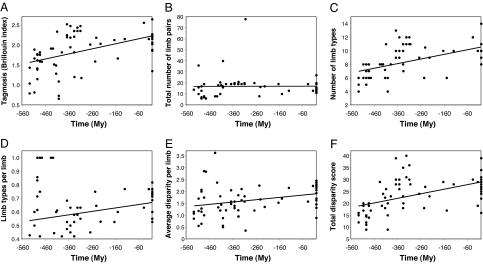
The degree of tagmosis (appendage complexity), as measured by using the Brillouin index [see supporting information (SI) Appendix A], has increased significantly from the Cambrian to the present among free-living crustaceans (Fig. 2 and SI Appendix B), with linear regressions explaining 25.0% of the variability over time. Among the six morphological indices used, the strongest temporal pattern was for the number of limb types (R2 = 0.322), whereas average disparity per limb (i.e., degree of differentiation from neighboring limbs) and total disparity of limb form also revealed significant, positive trends. The average number of types per limb displayed a positive but marginally nonsignificant trend. By contrast, there was no trend in the total number of limbs over time.
Fig. 2.
Regression analysis of limb diversity through the Phanerozoic, based on fossil origination times of 66 free-living crustacean orders. (A) Brillouin tagmosis value (R2 = 0.250, P < 0.0001). (B) Total limb number (R2 = 0.00046, P = 0.865). (C) Number of limb types (R2 = 0.322, P < 0.0001). (D) Number of limb types per limb (R2 = 0.049, P = 0.074). (E) Average disparity per limb (R2 = 0.091, P = 0.014). (F) Total disparity across all limbs (R2 = 0.291, P < 0.0001). For full regression statistics, see SI Appendix B.
The variables tested for trends are not independent of one another (SI Appendix C). The number of limb types is the strongest correlate of Brillouin tagmosis values (r = 0.862; P < 0.0001), whereas total limb number was the only index negatively and also nonsignificantly related to Brillouin tagmosis (r = −0.196; P = 0.115). The degree of tagmosis was not correlated with fossilization potential among living crustaceans (SI Appendix D), and results were similar when including only those taxa having a fossil record.
Comparing the tagmosis values from the first third of the taxa to originate (mean = 1.542 ± 0.37 SD; n = 22) with those of the most recent third (mean = 2.104 ± 0.26 SD; n = 22) by using Welch's two-sample t tests confirmed the trend toward increasing Brillouin tagmosis (t = −5.795, df = 37.979, P < 0.001). Significant increases were also found in the number of limb types and in the total disparity score, but nonsignificant increases were observed in the number of limb types per limb and average disparity per limb. Total limb number averaged 12.88 pairs (±7.015 SD) among the taxa originating earlier and 15.14 (±4.00) among the more recent originations (t = −1.307, df = 33.369, P = 0.20). Thus, there was no sustained tendency for limb number to increase substantially during the course of crustacean evolution, even though the faunas at a select few of the earliest stages possessed smaller numbers of limbs on average (≈10–12 limbs) than those of more recent stages (≈17–19 limbs).
Complexity Increases Within Many Independent Lineages.
Comparing ancestors with their descendants can illuminate the dynamics of evolutionary trends (6, 12, 13). Unfortunately, the fossil record is rarely complete enough to identify ancestors with any confidence. However, our phylogenetically independent comparisons of early fossils with their closest extant relatives are useful proxies. Five of the six parameters (again, the sole exception is the total number of appendages) showed a significant positive trend, with the extant groups exhibiting greater limb diversity than their earlier relatives in 10 or 11 of 12 cases (Table 1 and Fig. 3; see SI Appendix E). Thus, the overall increase in mean tagmosis values resulted from a parallel trend in many lineages (as depicted in Fig. 1C), rather than from wholesale replacement of a few less tagmatized clades by more highly tagmatized ones (as depicted in Fig. 1B). Moreover, increases were observed in lineages starting from intermediate and low tagmosis values, suggesting that diffusion from a minimum boundary cannot fully explain the trend.
Table 1.
Matched-pairs analysis of patterns of crustacean tagmosis evolution
| Trait | Sign test |
Wilcoxon test (vs. H0: median = 0) |
|||||
|---|---|---|---|---|---|---|---|
| No. of differences |
P value | Median rate, billion years−1 | V | P value | |||
| Increases | Decreases | No change | |||||
| Brillouin tagmosis value | 10 | 2 | 0 | 0.0386 | 0.682 | 68 | 0.0210 |
| Total no. of limbs | 5 | 4 | 3 | 1.00 | 0 | 19 | 0.722 |
| No. of limb types | 10 | 1 | 1 | 0.0117 | 4.466 | 59 | 0.0234 |
| No. of types per limb | 10 | 1 | 1 | 0.0117 | 0.301 | 61 | 0.0145 |
| Average disparity per limb | 10 | 2 | 0 | 0.0386 | 1.044 | 75 | 0.00244* |
| Total disparity across limbs | 11 | 1 | 0 | 0.00635* | 11.633 | 70 | 0.0122 |
Twelve fossil crustaceans were matched with Recent taxa (see Methods and SI Appendix E) to investigate whether the trend in tagmosis is repeated across independent lineages.
*Results remaining significant after sequential Bonferroni correction.
Fig. 3.
Changes in complexity over time for 12 phylogenetically matched pairs of fossil and living crustaceans, measured in Brillouin units. Numbers refer to the matched pairs listed in SI Appendix E.
Relative Complexity Differs at Origination and Extinction.
Newly originated taxa tended to have higher tagmosis values (by a median difference of 0.17 unit), more limb types (0.66 more), and a higher total disparity score (1.9 points higher) than average for their contemporaries, in more than two-thirds of cases for all of these traits (see Table 2). These results were significant in both sign and Wilcoxon tests (all P < 0.004). By contrast, at extinction, taxa showed no significant difference from average in these traits but tended to have a lower number of limb types per limb and lower disparity per limb (i.e., higher limb redundancy) (P < 0.015 for both Wilcoxon and sign tests).
Table 2.
Relative tagmosis values of crustacean orders (compared with the contemporaneous average) at their time of origin and extinction
| Trait | Sign test |
Wilcoxon test |
|||||
|---|---|---|---|---|---|---|---|
| No. of differences |
P value | Median difference (H0 = 0) | V | P value | |||
| Positive | Negative | Zero | |||||
| Relative value at origination; n = 66 (51) | |||||||
| Brillouin tagmosis value | 46 (35) | 20 (16) | 0 (0) | 0.0019* (0.0110*) | 0.172 (0.184) | 1564 (935) | 0.0034* (0.0109*) |
| Total no. of limbs | 33 (29) | 30 (19) | 3 (3) | 0.801 (0.193) | 0.667 (1.882) | 1231 (800) | 0.128 (0.0300) |
| No. of limb types | 47 (37) | 18 (13) | 1 (1) | 0.00042* (0.00094*) | 0.660 (0.989) | 1655.5 (990.5) | 0.00014* (0.00067*) |
| Mean no. of limb types per limb | 32 (21) | 33 (29) | 1 (1) | 1.00 (0.322) | −0.00790 (−0.0420) | 948 (506) | 0.418 (0.206) |
| Average disparity per limb | 28 (19) | 38 (32) | 0 (0) | 0.268 (0.0919∼) | −0.146 (−0.163) | 843 (452) | 0.0942 ∼ (0.0485) |
| Total disparity score | 46 (36) | 20 (15) | 0 (0) | 0.0019* (0.0046*) | 1.910 (2.50) | 1569 (976) | 0.0031* (0.0034*) |
| elative value at extinction; n = 25 | |||||||
| Brillouin tagmosis value | 12 | 13 | 0 | 1.00 | −0.0283 | 159 | 0.937 |
| Total limb number | 16 | 9 | 0 | 0.230 | 1.609 | 1015 | 0.565 |
| No. of limb types | 13 | 12 | 0 | 1.00 | 0.125 | 166 | 0.936 |
| Mean no. limb types per limb | 6 | 19 | 0 | 0.0146 | −0.115 | 67 | 0.00882* |
| Average disparity per limb | 4 | 21 | 0 | 0.00091* | −0.454 | 40 | 0.00103* |
| Total disparity score | 10 | 15 | 0 | 0.424 | −1.438 | 129 | 0.375 |
For the origination analyses, the first values are for results including all taxa (with living taxa having no fossil record being recorded as originating in the Holocene), and the numbers in parentheses are the results excluding taxa lacking a fossil record. Significant (P < 0.05) results are highlighted in bold.
*Results remaining significant after sequential Bonferroni correction (performed across the six traits within each category of analysis).
Contemporary Species Richness Is Correlated with Complexity.
Brillouin tagmosis scores showed a significant positive association with present-day species richness and explained 16% of the variation in diversity among clades (regression analysis: F1,36 = 7.08, P = 0.012; see SI Appendix F). Overall, 23 Brillouin tagmosis contrasts were positive, whereas 14 were negative (sign test: P = 0.188). The number of limb types per limb (F1,36 = 4.52, R2 = 0.112, P = 0.040) and average disparity per limb (F1,36 = 4.42, R2 = 0.109, P = 0.043) were also both positively associated with diversity (SI Appendix F). The slope of the relationship between species diversity and the total number of limbs was near zero (−0.01) and nonsignificant (F1,36 = 1.15, R2 = 0.03, P = 0.29), indicating that limb number is not associated with diversification (SI Appendix F).
Discussion
Increasing Complexity as a Major Trend Through the Phanerozoic.
The fossil record of the Crustacea, from the Cambrian to the present, documents a clear trend of increasing appendage diversity within individuals. Moreover, we have demonstrated that this trend has been instrumental in patterning both the form and diversity of species over time. Because the extant biota is a well studied but unrepresentative sample of the history of life, trends detected in the fossil record are rarely reflected in contemporary diversity patterns. The significant relationship between tagmosis values and modern diversity is therefore all the more striking.
Our results allow three principal inferences about the trend of complexity in the Crustacea. First, parallel increases in tagmosis values across separate evolutionary lineages contributed to the overall trend, indicating that the temporal pattern does not simply result from limited faunal turnover. As such, tagmatization may constitute a constraint-driven trend (see ref. 14). Given that increases were observed even in lineages whose tagmosis values were far above theoretical or observed minimum values, and given the evidence for increases in minimum tagmosis values in arthropods over time (10, 11, 15), this pattern may be considered an active trend, at least in part (see refs. 12 and 16). Another possibility is that the trend resulted from a random walk, but we consider it unlikely. In particular, our sampling scheme masks the huge number of crustacean species that existed throughout much of the Phanerozoic. If thousands of lineages of crustaceans were free to wander randomly up and down the complexity gradient, we would expect at least a small number of free-living extant species to have secondarily revisited the less-complex patterns of the Paleozoic. The fact that none have done so and that almost all nonparasitic crustaceans retain (at very least) strongly differentiated cephalic and anterior trunk appendages suggests strongly that the trend in complexity is driven. Moreover, complex forms, once originated, appear to be maintained by some mechanism (17), which might be some combination of developmental canalization (18) and ecological competition. With reference to this last possibility, we note that many species in marginal habitats (e.g., cave forms) often have reduced complexity.
Second, increasing complexity is primarily a function of the increasing number of limb types that accrue at the rate of ≈4.5 per billion years, according to the phylogenetic analysis, rather than any trend in total limb number. This finding concurs with a broader but less detailed study that sampled Cambrian and extant taxa from across all major arthropod groups (11).
Third, patterns in both origination and extinction of orders contribute to the trend. The diversity of limb types in newly originated taxa is significantly greater than average for any given time. It is possible that some new limb types constituted key innovations that opened up new adaptive zones (19, 20). By contrast, taxa going extinct tend to display a lower than average number of limb types per limb; i.e., limb redundancy may be disadvantageous and is associated with those taxa going extinct. The fact that tagmatization is implicated as a correlate of both origination and extinction rates suggests that the trend is at least partly caused by clade selection among species and higher clades (21) as well as within populations of individuals.
Complexity and Diversification.
Differences in limb complexity explained ≈16% of the variation in contemporary species diversity among clades, making the degree of tagmosis a relatively strong single correlate of diversity compared with others so far investigated (e.g., see refs. 22–25). Our results regarding species-level diversity therefore parallel Cisne's (11) conclusions, based on taxonomic analysis only, that increasing tagmosis was associated with mounting ordinal-level diversity through the fossil record. Although causation is difficult to establish, it is possible that the evolution of new limb types may have been instrumental in promoting diversification (the key innovation hypothesis) (e.g., ref. 26; for review, see ref. 20). Perhaps greater intraindividual limb diversity could contribute to the further “evolvability” or “versatility” (27) of a lineage, allowing new and different functions to arise more readily and promoting niche diversification. New limb types may also increase the scope for complex sexual display and copulation, potentially amplifying any role of sexual selection, which has been linked with diversification in vertebrates (28, 29). However, it may be that particular habitats limit diversification in some groups. For example, the Remipedia have one of the lowest tagmosis values among extant free-living crustaceans, but their confinement to deep caves connected to the sea (30) may have been the main factor determining their diversity.
Future Directions.
Although the Phanerozoic trend for increasing limb complexity in the Crustacea is well supported, there are several outstanding questions.
Is complexity still increasing?
The maximum contemporaneous tagmosis value has been quite consistent since the Devonian (when it reached ≈2.52 Brillouin units with the appearance of the decapods, climbing only slightly to 2.56 with the origin of the amphipods in the mid-Tertiary, and again to 2.64 in the Recent Amphionidacea). Thus, tagmosis values have not approached their theoretical maximum (e.g., 3.05–3.35 for individuals with 20–25 limbs that are all different). Although some stasis in the maximum is expected toward the present (as we examined this trait at a high taxonomic level), the observed degree of stasis is not inevitable; many new orders have appeared since the Devonian. It is unclear whether further increases are precluded by inflexible developmental programs, whether all of the major types of viable body plans have already been explored, or whether further increases in complexity would not confer any additional functional advantages. An analysis of trends at lower taxonomic levels would help to address this question.
What are the mechanisms of tagmosis increase?
Do new lineages have an intrinsic tendency to be more serially differentiated, or do those that happen to be so have a better chance of becoming established? Insights from developmental biology may help to address this issue. Oligomerization specifically refers to the tendency for repeated structures (such as segments within appendages) or armature (such as spines and setae) to evolve by loss, fusion, or reduction (31, 32). This trend has been recognized for some time, although maximum likelihood analysis in the Crustacea has demonstrated that the trend is relatively weak, with many lineages evolving in the opposite direction (33). Nonetheless, oligomerization may be one of the main mechanisms by which limbs evolve novel morphologies and diversify. However, the strength of this link and the developmental underpinnings of both of these trends require further attention.
Is the trend for increasing complexity present in other taxa?
Increases in modularity and in numbers of independently evolving parameters have apparently occurred in a variety of traits and lineages (16), including molluscan shells (27), cichlid jaws (34), and parrot cranial morphology (35). Increasing tagmosis, specifically, may also be a more general phenomenon than documented here; for example, differentiated trunk regions evolved multiple times independently within the trilobites (36). However, further investigation is required because previous treatments of tagmosis across arthropod taxa (10, 11) were not broken down into independent lineages (16), as here; nor has the relationship between tagmosis values and contemporary diversity been explored. In addition to other arthropods, annelids would be an excellent subject for study because they also have a serial body plan and tagmosis (31, 37). Moreover, “Williston's law” (the differentiation of structure and function by reduction and specialization) may apply to the evolution of vertebrate limbs as well (38, 39). Indeed, the duplication and evolutionary differentiation of sister copies are pervasive processes at all levels of organization: from genes to segments, somites and limbs, and up to genomes and entire body plans. Exploring these mechanisms will be a key endeavor in understanding the evolution of the diversity of biological forms.
Materials and Methods
Measures of Complexity.
Our measures of complexity quantify limb diversity within individuals. Because all of the limbs constitute a complete sample for that individual, following Cisne (11), we first used the Brillouin index, which has its origin in information theory (see 40, 41):
where N is the total number of limb pairs, and n is the number of limb pairs of the ith type. For fossil and extant free-living crustaceans in our sample, this index ranges from 0.656 to 2.641. Values for parasitic taxa are sometimes lower, and some parasites entirely lack limbs.
The Brillouin index combines several aspects of limb diversity into a single measure (10, 11). To understand the trends in detail, we considered three more specific measures: the total number of limbs, the number of limb types, and the average number of types per limb. By convention, the “number of limbs” and “number of types,” etc., always refer to the number of pairs of limbs.
Coding the number of limb types is inevitably subjective. As implemented by Cisne (11), successive limbs were compared down the length of the body, and at some critical level of difference, they were deemed to be different. The critical level of difference was quite small, and no attempt was made to define it. We have refined this approach significantly here, coding the magnitude of the differences between successive limb pairs on a subjective scale of 0–5, by using the following approximate definitions. The codes therefore provide an ordinal scale of differences: 0, no difference (and grouped together into the same type for the Brillouin index); 1, a small difference in form and/or numbers of endites; 2, a difference in the number of podomeres; 3, a difference in the number/identity of rami and number of podomeres; 4, a difference in the number/identity of rami, number of podomeres, and/or a moderate difference in gross form; and 5, a large difference in gross form.
All of the scoring was performed by one investigator (M.A.W.), and the coding was checked by recoding after an interval of 1 month.
Two simple indices of limb diversity have been devised to summarize these limb disparity data. The first is simply the average disparity per limb, an index that is analogous to one of the simplest and best measures of diversity turnover in ecological studies (41, 42). The second is the sum of disparity scores across all pairs of adjacent limbs along the body axis, providing an index of “total disparity” for each taxon (for all limb formulae and indices, see SI Appendix A).
Correlation coefficients (Pearson's product–moment correlations) were calculated between all pairs of variables to assess their interrelationships (see SI Appendix C).
Taxon Sampling and Stratigraphic Ranges.
A largely ordinal-level approach to sampling crustaceans was adopted because previous studies found little variation in tagmosis values within orders (10, 43). The taxonomic systems of Schram (30) and Martin and Davis (44) were primarily used for fossil and living crustaceans, respectively, whereas further details regarding sources and sampling strategy can be found in SI Appendices A and G. Sixty-six nonparasitic taxa were included in most analyses. (Parasites were retained in our dataset in SI Appendix A for completeness and to facilitate future research.)
Stratigraphic ranges of orders were mainly from Benton (45), with additions from Wills (46) and others (see SI Appendix A). We used the midpoint stage ages from Gradstein et al. (47). Records designated by question marks were included here because they mainly involved only slight extensions to the more solid ranges. Crustaceans range from the Caerfi in the Lower Cambrian to the Holocene, spanning 77 epochs and stages. (For further details regarding our inclusion of a few recently described fossil taxa, see SI Appendix A.)
Characterizing the Overall Trend.
We quantified the slope of the overall trend by plotting tagmosis values against the midpoint age of the series or stage in which the taxon first appeared in the fossil record. Least-squares linear regression analysis was performed for all six limb indices. Because multiple traits were examined, sequential Bonferroni correction (48) was performed. Regressions were repeated omitting any outliers, those points whose Studentized residuals were >3 SD away from zero.
Approximately one-third of all living higher taxa have no fossil record. Because ordinal-level taxa are highly unlikely to have arisen in the Holocene, the origination dates for taxa lacking fossils are almost certainly wrong. Therefore, we tested for a bias in fossilization potential in association with tagmosis values by comparing living taxa having a fossil record (n = 26) with those lacking a record (n = 15). None of the indices showed a significant difference (Welch's t tests; SI Appendix D). We repeated the taxonomic regression analyses omitting taxa with no fossils for comparison with the more comprehensive analyses, but the results were very similar (data not shown).
To test for overall differences between the earliest and most recent crustaceans, we used t tests to compare trait values of the earliest third and most recent third of taxa.
Matched-Pairs Analysis.
Any trend detected above could either result from the replacement of less serially differentiated lineages by more complex ones, or it could reflect parallel evolution in every lineage (13). To discriminate between these scenarios, we tested for a trend in phylogenetically matched pairs of extinct and extant crustaceans (for the list of pairs, see SI Appendix E).
Our analysis used the phylogeny of Wills (46), which includes the largest number of fossil and living taxa. We used a method derived from that of Hone et al. (8) to pair extinct taxa with extant counterparts originating later in the fossil record. No minimum temporal separation was imposed because all pairs were >300 million years apart. Extinct and extant taxa in each pair were compared to determine the change in Brillouin tagmosis (and other indices). The direction of change was tested against the null expectation (increases as common as decreases) by using a sign test. We also determined the slope, dividing the difference in each parameter by the age of the fossil group. (The origination ages of the living taxa were not subtracted from the numerator because our data are for extant representatives.) We compared the median slope across pairs to a null expectation of zero by using a Wilcoxon test.
Origination and Extinction.
Because trends in tagmosis values are tested at a high taxonomic level and over long time scales, it is possible that clade-level patterns of origination versus extinction (rather than, or in addition to, evolution within lineages) are involved in any trends. Thus, we tested whether relative tagmosis values are associated with the probability of origination or extinction of higher taxa. From the tagmosis value for each taxon, we first subtracted the contemporaneous mean at its time of origination to create a set of differences. The numbers of positive and negative differences were then compared with a null expectation of equal numbers by using a sign test. We also used a Wilcoxon test to determine whether the median differences deviated significantly from zero. We repeated these steps for periods of extinction. For the origination analyses, we repeated the tests excluding taxa without a fossil record.
Complexity and Species Diversity.
To assess any potential association between the degree of tagmosis of lineages and their contemporary species diversity, it is necessary to account for phylogeny because taxa of the same rank are often of different evolutionary ages. Moreover, pseudoreplication can be a problem when trait differences occur at internal nodes and closely related lineages are similar (7). Therefore, we used phylogenetically independent contrasts, in which the differences in diversity values within each sister-clade pair in a phylogeny are compared with the corresponding differences in the value of the trait (49). We used the total-evidence cladogram of Wheeler et al. (50) (their Fig. 17.6A), with some taxa placed according to other studies (for details and the phylogeny, see SI Appendix G). Species richness values were taken primarily from Bowman and Abele (51) and Schram (30) (see SI Appendix G). Sensitivity analyses employing alternative phylogenies and set of species richness data produced results very similar to those reported here (data not shown).
Independent Contrasts Analysis.
We used MacroCAIC (49) to generate the contrasts in both diversity and tagmosis values. Because branch length estimates were not available for our composite phylogeny, branch lengths were set to unity, and the proportional dominance index (PDI) was used to measure diversity differences among clades, as recommended by Isaac et al. (52) for such cases. The resulting PDI values were calculated such that positive contrasts are obtained when the more diverse clade has the higher trait value compared with its sister clade, and negative contrasts are produced when the more diverse clade has the lower trait value. Although the sign of PDI depends on the direction of the tagmosis value relationship, the magnitude depends on the difference in diversity.
We analyzed the contrast data in R by using three statistical tests with increasingly stringent assumptions: a sign test on the direction of PDI contrasts, a Wilcoxon test of median PDI against a null expectation of zero, and regression through the origin (53) on the diversity and tagmosis value contrasts. We had no predictions about the direction of the tagmosis value–diversity relationship. Cisne (11) documented a concordant increase in both ordinal diversity and average tagmosis value over time since the Cambrian, which he discussed in light of increasing complexity of ecological roles and global communities. However, this large-scale pattern may have merely been the result of a passive trend arising from increased variance in tagmosis values over time. Therefore, we used two-tailed tests in all cases.
Supplementary Material
Acknowledgments.
We thank Timothy Barraclough, Doug Erwin, Richard Fortey, Laurence Hurst, Armand Leroi, Todd Oakley, and Stuart Reynolds for helpful discussions and suggestions at various stages during the long gestation of this project. This work was supported by a Beit Scientific Research Fellowship, a Natural Sciences and Engineering Research Council of Canada Scholarship, an Overseas Research Scholarship (to S.J.A.), and a Biotechnology and Biological Sciences Research Council grant (to M.A.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709378105/DC1.
References
- 1.Stanley SM. Explanation for Cope's rule. Evolution (Lawrence, Kans) 1973;27:1–26. doi: 10.1111/j.1558-5646.1973.tb05912.x. [DOI] [PubMed] [Google Scholar]
- 2.Gould SJ. New York: Harmony; 1996. Full House: The Spread of Excellence from Plato to Darwin. [Google Scholar]
- 3.Gould SJ. Cope's rule as psychological artefact. Nature. 1997;385:199–200. [Google Scholar]
- 4.Jablonski D. Body-size evolution in Cretaceous molluscs and the status of Cope's rule. Nature. 1997;385:250–252. [Google Scholar]
- 5.Arnold AJ, Kelly DC, Parker WC. Causality and Cope's rule: Evidence from planktonic Foraminifera. J Paleontol. 1995;69:203–210. [Google Scholar]
- 6.Alroy J. Cope's rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280:731–734. doi: 10.1126/science.280.5364.731. [DOI] [PubMed] [Google Scholar]
- 7.Purvis A, Orme CDL, Dolphin K. Why are most species small-bodied? A phylogenetic view. In: Blackburn TM, Gaston KJ, editors. Macroecology: Concepts and Consequences. Malden: Blackwell; 2003. pp. 155–173. [Google Scholar]
- 8.Hone DWE, Keesey TM, Pisani D, Purvis A. Macroevolutionary trends in the Dinosauria: Cope's rule. J Evol Biol. 2005;18:587–595. doi: 10.1111/j.1420-9101.2004.00870.x. [DOI] [PubMed] [Google Scholar]
- 9.Hunt G, Roy K. Climate change, body size evolution, and Cope's rule in deep-sea ostracodes. Proc Natl Acad Sci USA. 2006;103:1347–1352. doi: 10.1073/pnas.0510550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills MA, Briggs DEG, Fortey RA. Evolutionary correlates of arthropod tagmosis: Scrambled legs. In: Fortey RA, Thomas RH, editors. Arthropod Relationships. London: Chapman & Hall; 1997. pp. 57–65. [Google Scholar]
- 11.Cisne JL. Evolution of world fauna of aquatic free-living arthropods. Evolution (Lawrence, Kans) 1974;28:337–366. doi: 10.1111/j.1558-5646.1974.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 12.McShea DW. Mechanisms of large-scale evolutionary trends. Evolution (Lawrence, Kans) 1994;48:1747–1763. doi: 10.1111/j.1558-5646.1994.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 13.Alroy J. Understanding the dynamics of trends within evolving lineages. Paleobiology. 2000;26:319–329. [Google Scholar]
- 14.McShea DW. The evolution of complexity without natural selection, a possible large-scale trend of the fourth kind. Paleobiology. 2005;31(suppl):146–156. [Google Scholar]
- 15.Lankester ER. The structure and classification of the Arthropoda. Q J Microsc Sci. 1904;47:523–582. [Google Scholar]
- 16.Carroll SB. Chance and necessity: The evolution of morphological complexity and diversity. Nature. 2001;409:1102–1109. doi: 10.1038/35059227. [DOI] [PubMed] [Google Scholar]
- 17.Valentine JW, Collins AG, Meyer CP. Morphological complexity increase in metazoans. Paleobiology. 1994;20:131–142. [Google Scholar]
- 18.Flatt T. The evolutionary genetics of canalization. Q Rev Biol. 2005;80:287–316. doi: 10.1086/432265. [DOI] [PubMed] [Google Scholar]
- 19.Simpson GG. The Major Features of Evolution. New York: Columbia Univ Press; 1953. [Google Scholar]
- 20.Hunter JP. Key innovations and the ecology of macroevolution. Trends Ecol Evol. 1998;13:31–36. doi: 10.1016/s0169-5347(97)01273-1. [DOI] [PubMed] [Google Scholar]
- 21.Williams GC. New York: Oxford Univ Press; 1992. Natural Selection: Domains, Levels, and Challenges. [Google Scholar]
- 22.Orme CDL, Isaac NJB, Purvis A. Are most species small? Not within species-level phylogenies. Proc R Soc London Ser B. 2002;269:1279–1287. doi: 10.1098/rspb.2002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme CDL, Quicke DL, Cook JM, Purvis A. Body size does not predict species richness among the metazoan phyla. J Evol Biol. 2002;15:235–247. [Google Scholar]
- 24.Isaac NJB, Jones KE, Gittleman JL, Purvis A. Correlates of species richness in mammals: Body size, life history, and ecology. Am Nat. 2005;165:600–607. doi: 10.1086/429148. [DOI] [PubMed] [Google Scholar]
- 25.Phillimore AB, Freckleton RP, Orme CDL, Owens IPF. Ecology predicts large-scale patterns of phylogenetic diversification in birds. Am Nat. 2006;168:220–229. doi: 10.1086/505763. [DOI] [PubMed] [Google Scholar]
- 26.Heard SB, Hauser DL. Key evolutionary innovations and their ecological mechanisms. Hist Biol. 1995;10:151–173. [Google Scholar]
- 27.Vermeij GJ. Biological versatility and Earth history. Proc Natl Acad Sci USA. 1973;70:1936–1938. doi: 10.1073/pnas.70.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barraclough TG, Harvey PH, Nee S. Sexual selection and taxonomic diversity in passerine birds. Proc R Soc London Ser B. 1995;259:211–215. [Google Scholar]
- 29.Streelman JT, Danley PD. The stages of vertebrate evolutionary radiation. Trends Ecol Evol. 2003;18:126–131. [Google Scholar]
- 30.Schram FR. Crustacea. New York: Oxford Univ Press; 1986. [Google Scholar]
- 31.Dogiel VA. Oligomerization of the Homologous Organs as One of the Main Paths in Animal Evolution. Leningrad: Leningrad Univ Press; 1954. [Google Scholar]
- 32.Huys R, Boxshall G. Copepod Evolution. London: Ray Society; 1991. pp. 315–370. [Google Scholar]
- 33.Adamowicz SJ, Purvis A. From more to fewer? Testing an allegedly pervasive trend in the evolution of morphological structure. Evolution (Lawrence, Kans) 2006;60:1402–1416. [PubMed] [Google Scholar]
- 34.Hulsey CD, de Leon FJG, Rodiles-Hernandez R. Micro- and macroevolutionary decoupling of cichlid jaws: A test of Liem's key innovation hypothesis. Evolution (Lawrence, Kans) 2006;60:2096–2109. [PubMed] [Google Scholar]
- 35.Tokita M, Kiyoshi T, Armstrong KN. Evolution of craniofacial novelty in parrots through developmental modularity and heterochrony. Evol Dev. 2007;9:590–601. doi: 10.1111/j.1525-142X.2007.00199.x. [DOI] [PubMed] [Google Scholar]
- 36.Hughes NC. Trilobite body patterning and the evolution of arthropod tagmosis. BioEssays. 2003;25:386–395. doi: 10.1002/bies.10270. [DOI] [PubMed] [Google Scholar]
- 37.Sveshnikov VA. Ecological radiation of the polychaetes. Ophelia. 1991;(Suppl 5):271–274. [Google Scholar]
- 38.Williston SW. Water Reptiles of the Past and Present. Chicago: Univ Chicago Press; 1914. electronic reprint (2000) (Arment Biol Press) [Google Scholar]
- 39.Carroll SB, Grenier JK, Weatherbee SD. 2nd Ed. Malden: Blackwell; 2005. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. [Google Scholar]
- 40.Pielou E. Ecological Diversity. New York: Wiley; 1975. pp. 9–11. [Google Scholar]
- 41.Magurran AE. Measuring Biological Diversity. Malden, MA: Blackwell; 2004. [Google Scholar]
- 42.Whittaker RH. Vegetation of the Siskiyou Mountains. Ore Calif Ecol Monogr. 1960;30:279–338. [Google Scholar]
- 43.Flessa KW, Powers KV, Cisne JL. Specialization and evolutionary longevity in the Arthropoda. Paleobiology. 1975;1:71–81. [Google Scholar]
- 44.Martin JW, Davis GE. An updated classification of the Recent Crustacea. Nat Hist Mus Los Angeles County Sci Ser. 2001;39:1–124. [Google Scholar]
- 45.Benton MJ. The Fossil Record 2. London: Chapman & Hall; 1993. pp. 321–356. [Google Scholar]
- 46.Wills MA. Crustacean disparity through the Phanerozoic: Comparing morphological and stratigraphic data. Biol J Linn Soc. 1998;65:455–500. [Google Scholar]
- 47.Gradstein FM, Ogg JG, Smith AG, editors. A Geologic Time Scale 2004. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]
- 48.Rice WR. Analyzing tables of statistical tests. Evolution (Lawrence, Kans) 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 49.Agapow P-M, Isaac NJB. MacroCAIC: Revealing correlates of species richness by comparative analysis. Divers Distrib. 2002;8:41–43. [Google Scholar]
- 50.Wheeler WC, Giribet G, Edgecombe GD. Arthropod systematics: The comparative study of genomic, anatomical, and paleontological information. In: Cracraft J, Donoghue MJ, editors. Assembling the Tree of Life. New York: Oxford Univ Press; 2004. pp. 281–295. [Google Scholar]
- 51.Bowman TE, Abele LG. Classification of the Recent Crustacea. In: Abele LG, editor. The Biology of Crustacea. I. Systematics, the Fossil Record, and Biogeography. New York: Academic; 1982. pp. 1–27. [Google Scholar]
- 52.Isaac NJB, Agapow P-M, Harvey PH, Purvis A. Phylogenetically nested comparisons for testing correlates of species richness: A simulation study of continuous variables. Evolution (Lawrence, Kans) 2003;57:18–26. doi: 10.1111/j.0014-3820.2003.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 53.Garland T, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.