Abstract
When cells progressing in mid-S phase are damaged with a base-modifying chemical, they arrest in S phase long after the CHK1 checkpoint signal fades out, partly because of p53-mediated long-lasting induction of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. We have recently found that enforced expression of Cdc6, the assembler of prereplicative complexes, markedly advances recovery from the prolonged S-phase arrest and reactivation of Cdk2 despite the presence of a high level of induced p21. Here, we report that Cdc6 protein can activate p21-associated Cdk2 in an ATP-dependent manner in vitro. Consistently, Cdc6 mutated for ATPase or a putative cyclin binding motif is no longer able to activate the Cdk2 in vitro or promote reinitiation of S-phase progression and reactivation of Cdk2 in vivo. These results reveal the never anticipated function of Cdc6 and redefine its role in the control of S-phase progression in mammalian cells.
Keywords: Cy motif, DNA damage, MMS, Walker B domain, intra-S checkpoint
The replication of chromosomal DNA is initiated by the assembly of prereplicative complexes (PreRCs) on replication origins that takes place in late M–G1 phases (1–5). In the assembly of PreRCs, Cdc6 plays the crucial role, loading the minichromosome maintenance (MCM) helicase complex on the origin recognition complex (ORC)-bound early and late replication origins by using the energy of ATP hydrolysis (4, 6, 7). Cdt1 cooperates with Cdc6, whereas geminin antagonizes Cdt1. After a PreRC is assembled on both origins, several factors, some of which require activation by Cdk2 that is negatively regulated by phosphorylation at Tyr-15 and association with cyclin-dependent kinase inhibitors such as p21WAF1/Cip1, are further loaded on early origins. In parallel, Cdc6 protein undergoes phosphorylation at Ser-54 by active Cdk2 and is exported into the cytoplasm (8). Finally, DNA polymerases are recruited to those origins to initiate DNA replication after activation of MCM helicase by Cdc7. Thus, S phase begins.
When cells progressing in G1 phase are damaged with ionizing radiation or UV light, the sensors ATR/ATM detect DNA lesion and activate CHK1/CHK2 kinases by phosphorylating at Ser-345 and Thr-68, respectively (9, 10). Activated CHK1/CHK2 inactivates and destabilizes Cdc25A phosphatase. This rapid reaction causes initial inactivation of Cdk2 as a consequence of accumulation of the Tyr-15 phosphorylated form. In parallel, both ATR/ATM and activated CHK1 stabilize and activate p53, which in turn induces a few factors, including p21, which is responsible for long-lasting inactivation of Cdk2 and prolonged G1 phase arrest (11) because of the blockade of firing both early and late replication origins.
By contrast, when cells progressing in S phase are similarly damaged, the same sensors detect DNA damage and activate CHK1/CHK2, but the p53–p21 pathway is not effectively used because of at least in part relatively poor induction of p21 (12–14). Consequently, S-phase arrest is short and inactivation of Cdk2 is transient. The lack of effective utilization of the p53–p21 pathway, however, is not an intrinsic property of S-phase cells. Recently, we have found that when S-phase-progressing cells are damaged with a base-modifying chemical, such as methyl methanesulfonate (MMS) and cisplatin, the p53–p21 pathway is effectively activated and induces a prolonged S-phase arrest with similarly extended inactivation of Cdk2 by binding of p21 (15). We have further found that Cdc6 protein, which is destabilized upon treatment with such a chemical, profoundly influences the timing of recovery from S-phase arrest and reactivation of Cdk2. Thorough investigation of the mechanistic basis for this phenomenon has led us to discover the unexpected function of Cdc6.
Results
Enforced Expression of Cdc6 Advances Recovery from MMS-Induced S-Phase Arrest and Reactivation of Cdk2.
During studies on G1–S transition control, we came across the finding that the level of Cdc6 protein critically determined the timing of recovery from MMS-induced S-phase arrest and reactivation of Cdk2 despite a high level of induced p21. When mid-S phase-traversing rat fibroblast normal rat kidney (NRK)-49F (16) was damaged with MMS, it arrested in S phase with inactivation of Cdk2 long after Ser-345-phosphorylation of CHK1 faded out. This prolonged arrest (>20 h) was accompanied by induction of p21 and a marked reduction in the level of Cdc6 (Fig. 1A Left). We noticed that recovery from the arrest always coincided not only with activation of Cdk2 but also with recovery of the Cdc6 level and therefore we examined the possible effects of enforced expression and repression of Cdc6 on the timing of recovery and reactivation of Cdk2.
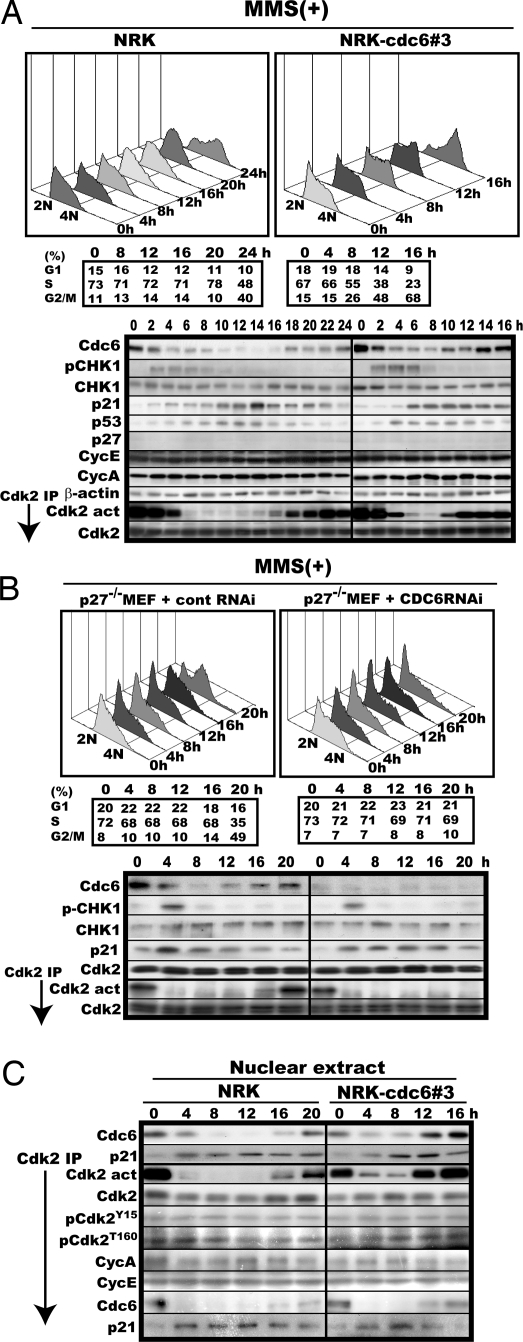
Fig. 1.
Enforced expression of Cdc6 advances both reinitiation of S-phase progression and reactivation of Cdk2, whereas RNAi-mediated repression of endogenous Cdc6 expression retards recovery from S-phase arrest and reactivation of Cdk2. (A) Mid-S phase-synchronized original NRK and NRK-Cdc6#3 cells were treated with MMS and cultured with every 2-h cell harvests. The harvested cells were analyzed by flow cytometry (Upper) and assayed for Cdc6, S345-phosphorylated CHK1 (pCHK1), CHK1, p21, p53, p27KIP1, cyclin E (CycE), cyclin A (CycA), Cdk2, Cdk4, Cdk6, Mcm2, Mcm3, Mcm4, Mcm5, Mcm6, Mcm7 by immuno-blotting and for the activity and amount of Cdk2 (Lower). The cell populations in each cell-cycle phase of the harvested cells were calculated from the cytometric patterns with the bundled software and are shown below the cytometric patterns. The factors not shown were unchanged during the time course and similar in levels between original NRK and the Cdc6 overexpresser. β-actin was used as loading control. (B) Logarithmically proliferating p27−/− MEF cells were transfected with the universal negative control RNA duplex or the Cdc6-specific duplex, 72 h later synchronized to mid-S phase, treated with MMS, and analyzed as in A. Cdk2 served as a loading control. (C) The nuclear extracts in A were analyzed for Cdc6 and p21 and assayed for the activity, amount, and phosphorylation states of Cdk2 and its associated proteins.
When the same fibroblast, but engineered to constitutively overexpress Cdc6 at 2-fold higher a level (17), was similarly analyzed, recovery from S-phase arrest was found to begin much earlier (8–12 h) with similarly advanced reactivation of Cdk2 despite the presence of a high level of induced p21, but without a noticeable shortening of the fade-out time of S345-phosphorylated Chk1 (Fig. 1A Right), which suggested that DNA repair per se was virtually uninfluenced by the enforced expression of Cdc6. This finding was not specific to a particular base-modifying agent or cell. The same results were obtained with cisplatin, a frequently used DNA adduct, and the mouse fibroblast line C3H10T1/2 [supporting information (SI) Fig. S1].
By contrast, RNAi-mediated repression of endogenous Cdc6 prolonged both S-phase arrest and Cdk2 inactivation. In this experiment, p27−/− mouse embryonic fibroblast (MEF) was used instead of NRK because RNAi did not work well for this cell line. When Cdc6 expression was repressed roughly to one-fifth of the original level during MMS treatment and thereafter via RNAi, p27−/− MEF remained arrested in S phase with inactive Cdk2 even at 20 h when a bulk of control RNAi-treated p27−/− MEF was already in G2/M with fully activated Cdk2 (Fig. 1B). In a similar experiment without MMS treatment, Cdc6-repressed cells traversed S phase and reached G2/M within 4 h (data not shown).
Cdc6 Binds Cdk2 with Concurrent Release of Bound p21 During Reactivation of Cdk2.
The experimental results shown above revealed a close link between Cdc6 expression and Cdk2 activation although it was not known whether or not the advanced reactivation of Cdk2 was solely responsible for the Cdc6-invoked facilitation of recovery from arrest. On the other hand, the Cdk2 inactivation and the prolonged S-phase arrest induced by such a base-modifying chemical were caused primarily by p53-dependently induced p21 because unlike WT MEF, p21−/− MEF suffered neither obvious inactivation of Cdk2 nor a prolonged S-phase arrest after MMS treatment (15). Given these two findings, we came up with the idea that Cdc6 might be able to directly activate p21-associated Cdk2 by removing the bound p21 because Cdc6 contains a cyclin-binding motif and forms a complex at least with Cdk2-cyclin A (18). We first explored this idea by investigating the possible binding of Cdc6 to the Cdk2 with a simultaneous release of p21 during reactivation. Consistent with the idea, increasing amounts of Cdc6 and inversely decreasing amounts of p21 coimmunoprecipitated with nuclear Cdk2 during its advanced reactivation (Fig. 1C). There were no significant changes in the amount of associated cyclins or the phosphorylation state of Cdk2 during this process.
Cdc6 Activates p21-Associated Cdk2 in Vitro.
We next extended exploration of this idea by examining whether Cdc6 protein could activate p21-associated Cdk2 in vitro. A cDNA encoding C-terminally His-tagged Cdc6 was constructed and expressed in an in vitro transcription/translation system. His-tagged Cdc6 was then affinity-purified. In parallel, p21-bound inactive Cdk2 was immunoprecipitated from lysates of the NRK cells arrested in mid-S phase by treatment with MMS. The purified Cdc6 was free from contaminations by Cdk2, cyclin A, cyclin E, p21 and p27KIP1, another Cdk2 inhibitor, whereas the immunoprecipitated Cdk2 contained p21, cyclin A, and cyclin E, but no detectable levels of Cdc6 or p27KIP1 (Fig. 2A).
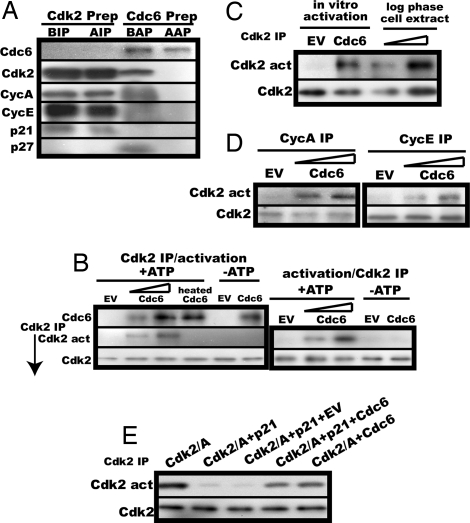
Fig. 2.
Cdc6 activates p21-associated Cdk2 in an ATP-dependent manner in vitro. (A) p21-bound Cdk2 in MMS-treated S phase-arrested NRK cell lysates and in vitro synthesized His-tagged Cdc6 preparation were assayed for Cdc6, Cdk2, cyclin A, cyclin E, p21, and p27KIP1 by immunoblot before and after anti-Cdk2 immunoprecipitation (BIP and AIP) or affinity-purification (BAP and AAP). (B) (Left) The immunoprecipitated p21-bound Cdk2 (equivalent to the Cdk2 amount in 30 μl of the S-phase-arrested cell lysate) was incubated in the activation buffer with 5 μl (equivalent to the Cdc6 mount in 25 μl of the S-phase cell lysate) or 10 μl of the purified His-tagged Cdc6 preparation, 10 μl of either heat-inactivated purified His-tagged Cdc6 or similarly affinity-purified EV product in the presence or absence of ATP, followed by assays for the activity and amount of Cdk2 as described in Materials and Methods. (Right) p21-bound Cdk2 before immunoprecipitation (50 μl of the S-phase-arrested cell lysate) was incubated in an 80-μl activation buffer with 5 or 10 μl of the purified Cdc6, immunoprecipitated with the anti-Cdk2 antibody, and assayed for the activity and amount of Cdk2. In parallel, the same amount of p21-associated Cdk2 as in Left was incubated with 10 μl of the purified Cdc6 or the EV product in the absence of ATP, immunoprecipitated, and assayed. (C) The relative Cdk2 activity obtained by in vitro activation as in B was compared with that in a lysate of logarithmically proliferating NRK cells. (D) p21-bound Cdk2 was immunoprecipitated with the anti-cyclin A or the anti-cyclin E antibody from the same NRK cell lysate as in A and subjected to the activation assay as in B. (E) Baculovirus-expressed affinity-purified Cdk2–cyclin A complexes were preincubated at 30°C for 30 min in 50 mM Tris·HCl (pH 7.5) containing 150 mM NaCl and 10 mM MgCl2 with or without E. coli-expressed double affinity-purified p21 (the same amount as in MMS-treated S-phase cells, relative to Cdk2), immunoprecipitated with the anti-Cdk2 antibody, incubated with double affinity-purified FLAG- and His-tagged Cdc6 (≈2 fold excess) or its control vector product, and assayed for Cdk2 activity as in B.
The roughly same amounts of the purified Cdc6 and the immunoprecipitated Cdk2 as present in S phase cells or a 2-fold excess of the purified Cdc6 over the Cdk2 were then incubated in a buffer containing Mg2+ and ATP. Cdc6, but not heat-inactivated Cdc6, could indeed activate p21-inactivated Cdk2 in a dose-dependent manner but only in the presence of ATP, as assayed with a truncated Rb as a substrate (Fig. 2B Left). This activation was not an experimental artifact potentially caused by use of antibody-bound Cdk2 because Cdc6 could activate antibody-unbound, p21-associated Cdk2 as well (Fig. 2B Right). In this reaction, Cdk2 appeared to be nearly fully activated because its relative activity was comparable with that of the Cdk2 prepared from logarithmically proliferating NRK cells (Fig. 2C).
Cdc6 showed no strict preference for the associated cyclin and effectively activated both cyclin A-associated Cdk2-p21 (Cdc2 present as a minor population) and cyclin E-associated Cdk2-p21 (Fig. 2D). In this activation, no other factors appeared to be involved because full activation was obtained with reticulocyte-expressed double affinity-purified FLAG- and His-tagged Cdc6 and baculovirus-expressed affinity-purified Cdk2-cyclin A complexes inactivated by Escherichia coli-expressed double affinity-purified p21 (Fig. 2E).
Both ATPase and Cyclin-Binding Motifs in Cdc6 Are Essential for in Vitro Activation of p21-Associated Cdk2.
Cdk2 uses ATP, raising the possibility that Cdc6-mediated Cdk2 activation might require its own kinase activity. However, this possibility was excluded. Roscovitine (19), a potent reversible inhibitor of Cdk2 kinase reaction, had virtually no effect on activation when added to the activation mixture, whereas it almost completely inactivated Cdk2 when added to the kinase assay mixture (Fig. 3A).
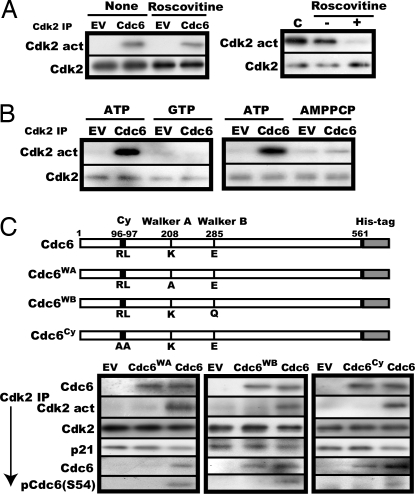
Fig. 3.
Both ATPase and cyclin-binding motifs in Cdc6 are essential for activation of p21-associated Cdk2 in vitro. (A) (Left) The same activation assay was performed as in Fig. 2B with the addition of 100 μM roscovitine followed by removal of roscovitine and assay of the activity and amount of Cdk2. (Right) Active Cdk2 immunoprecipitated from a logarithmically growing NRK cell lysate was incubated with 100 μM roscovitine under the same condition as for the Cdk2 activation assay, washed (−) or unwashed (+), and assayed for the activity and amount of Cdk2. C stands for direct assay of the Cdk2 without incubation with roscovitine. (B) The same activation as in Fig. 2B was performed with 10 mM ATP, GTP, or AMPPCP. (C) Immunoprecipitated p21-bound Cdk2 was incubated in the activation reaction mixture with 10 μl of purified Cdc6, Cdc6WA, Cdc6WB, or Cdc6Cy, whose structures are illustrated, and determined for the activity and the amount of Cdk2 and coimmunoprecipitated p21, Cdc6, and Ser-54-phosphorylated Cdc6. In parallel, the relative amounts of the Cdc6s added to the activation reaction were compared by immunoblot.
Next, the requirement of ATP for the activation of Cdk2 was more thoroughly investigated. Cdc6 uses the energy of ATP hydrolysis to assemble PreRC (4, 6, 7). Therefore, GTP and AMPPCP (20), a nonhydrolysable ATP analogue, are ineffective. Similarly, these ATP analogues were ineffective for activation of the Cdk2 (Fig. 3B). To verify the direct utilization of ATP by Cdc6 itself, two Cdc6 mutants deficient for ATP binding and ATP hydrolysis were prepared by substituting Lys-208 in the Walker A domain with Ala (Cdc6WA) and Glu-285 in the Walker B domain with Gln (Cdc6WB) (6), respectively, and tested for their ability of activation. Both mutants failed to activate the Cdk2 (Fig. 3C).
Like many other Cdk2 substrates, Cdc6 contains the cyclin-binding motif called “Cy” or “RXL,” which mediates its interaction with Cdk2 at least partially (8, 21, 22). Rat Cdc6 has such a motif (RL) at 96–97. This motif was found essential for activation of Cdk2 and Cy-less Cdc6 (Cdc6Cy), in which the RL was substituted with AA, could not activate the Cdk2 (Fig. 3C). In this assay, WT Cdc6 efficiently bound Cdk2 and induced a marginal, but reproducible, reduction of the associated p21 and concomitant Ser-54 phosphorylation of the bound Cdc6. Thus, Cdc6 served as substrate for Cdk2 as well during activation. By contrast, Cdc6WA could not bind Cdk2, whereas both Cdc6WB and Cdc6Cy bound, but none of them induced any detectable decrease of associated p21, consistent with their inability to activate Cdk2.
Cdc6 Mutated for ATPase or Cy Is Unable to Facilitate Recovery from MMS-Induced S-Phase Arrest or Reactivation of Cdk2 in Vivo.
To confirm the in vitro results, we finally investigated the ability of the Cdc6 mutants to reactivate Cdk2 in vivo. Because constitutive overexpression of these mutants might harm proliferation of the cells, Tet-Off induction was used to express the Cdc6 mutants in p27−/− MEF (23), which should be better suited for this study because use of this cell would eliminate potential complications by p27KIP1.
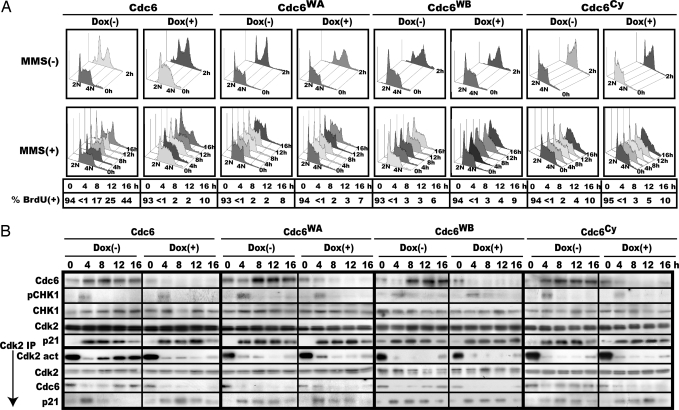
The p27−/− MEFs inducible for WT or mutant Cdc6 were synchronized to mid-S, treated with MMS, and monitored for their S-phase progression and reactivation of Cdk2. Meanwhile, doxycycline was removed to induce these proteins at around the time of analysis, whereas in control this drug continued to be added. Without MMS treatment, there was no significant influence of mutant Cdc6 expression on S-phase progression, and the majority of these cells traversed S phase and entered G2/M within 2–3 h. Upon MMS treatment, all of these cells arrested in S phase, but unlike WT Cdc6, none of them facilitated reinitiation of S-phase progression or reactivation of Cdk2 (Fig. 4). Furthermore, Cdc6WA neither bound Cdk2 nor released p21, whereas both Cdc6WB and Cdc6Cy effectively bound Cdk2 but could not liberate p21 (Fig. 4B), thus confirming the in vitro results.
Fig. 4.
Cdc6 mutated for ATPase or Cy is unable to promote recovery from S arrest or reactivation of Cdk2 in vivo. (A) p27−/− MEF cells inducible for Cdc6, Cdc6WA, Cdc6WB, or Cdc6Cy were synchronized to mid-S phase with or without doxycycline, treated with MMS, and monitored for S-phase progression by flow cytometry and pulse BrdU incorporation. Percentage of BrdU incorporation at 0 h was measured without MMS treatment and therefore represents the population of S phase-progressing cells at the start of the experiments. (B) Each cell harvested in A was analyzed for the levels of Cdc6, S345-phosphorylated CHK1, CHK1, Cdk2, and p21 and the activity and amount of Cdk2, coimmunoprecipitated Cdc6 and p21. Cdk2 served as a loading control in this experiment.
Although essential for Cdk2 activation, the Cy motif in Cdc6 appeared to be dispensable for PreRC assembly because, unlike Cdc6WB, overexpression of Cdc6Cy showed no apparent dominant-negative effects on cell growth unless under suffering DNA damage. When p27−/− MEFs induced or uninduced for Cdc6Cy or Cdc6WB to 2- to 3-fold higher a level over endogenous Cdc6 were examined for their proliferation, Cdc6Cy-induced MEF was virtually indistinguishable from uninduced MEF whereas Cdc6WB-induced MEF was significantly slower than the uninduced, consistent with the dominant-negative effect of this mutation on PreRC assembly as reported (6) (SI Fig. S2). Under the growth-promoting conditions, overexpression of WT Cdc6 had no effects on proliferation. By contrast, the Cy mutant showed the dominant-negative effect on Cdk2 activation. When examined in culture medium containing a low level of MMS (1/10 the concentration generally used), Cdc6Cy-induced MEF was slower, whereas WT Cdc6-induced MEF was slightly faster than the corresponding uninduced MEFs, consistent with the above data.
Discussion
The experimental data presented here demonstrate that Cdc6 activates p21-associated Cdk2 in vivo as well as in vitro, likely by using ATP hydrolysis energy, revealing the never-anticipated function for the initiator of chromosomal replication. Although Cdc6 is essential for this activation, the in vitro data do not rule out some role for the proteins that might have tightly associated with Cdc6 or Cdk2-cyclin A and therefore, copurified from the reticulocyte lysates or the baculovirus preparations. In this reaction, Cdc6 serves as not only activator but also substrate of Cdk2 as indicated by its Ser-54 phosphorylation in vitro. Activation appears to take place by ATP-driven removal of bound p21 by Cdc6, which is evident in the in vivo experiment. Consistently, both Cdc6WA and Cdc6WB, mutated for ATP binding and ATP hydrolysis, respectively, were no longer able to activate the Cdk2 in vitro as well as in vivo. However, contrary to expectation, Cdc6WA lost the ability to bind the p21-Cdk2-cyclin A/E complexes despite the presence of the cyclin-binding motif, whereas both Cdc6WB and Cdc6Cy, the latter mutated for a cyclin-binding motif, retained the ability to bind but not to activate the complexes. Together with the ineffectiveness of AMPPCP, a nonhydrolysable ATP analogue, these results suggest that the initial binding of Cdc6 to the ternary complexes is largely independent of the Cy motif but absolutely depends on its association with ATP, whereas the subsequent removal of p21 requires the Cy motif and presumed hydrolysis of ATP.
The ATP binding-dependent but apparently Cy-independent association of Cdc6 with the ternary complexes may be exerted via the same mechanism as the one used for the loading of Cdc6 to ORC-bound replication origins because both depend on ATP binding, which induces conformational changes to the Cdc6 molecule (6, 7). On the other hand, the mechanistic basis for the requirement of the Cy for Cdk2 activation is less clear at present. However, given the fact that p21 contains two Cy motifs, each to be essential for inactivation of Cdk2-cyclin A or Cdk2-cyclin E (24, 25), it would be conceivable that the Cy might be required for Cdc6 to displace the p21 bound to the Cy/RXL-recognition groove on the cyclin during removal of p21, therefore without the Cy, activation being forced to abort. The occurrence of Ser-54 phosphorylation of the bound Cdc6 after activation (Fig. 3C) is consistent with this scenario because Cy-containing Cdk2 substrates ought to bind the Cy/RXL-recognition groove on the cyclin to undergo efficient phosphorylation (26–28). Presumed ATP hydrolysis-coupled p21 replacement by Cdc6 is unlikely to be a result of a simple competition for Cy-mediated Cdk2 binding between p21 and Cdc6 as suggested for Cdc25A and p21 (29) because Cdc6WB could not activate p21-associated Cdk2 in vitro and in vivo despite possessing both Cy and Cdk2-binding abilities.
Although release of 21 was closely coupled with activation of Cdk2 in vivo, in vitro reactivation of Cdk2 by Cdc6 was accompanied by only a marginal decrease of bound p21 despite nearly full activation. Nevertheless, this apparent lack of linear inverse relation between activity and bound p21 in vitro is consistent with the previous finding by Zhang et al. (30) showing that in an in vitro inactivation assay a 2-fold less amount of p21 failed to inactivate Cdk2–cyclin A despite only a slight reduction in the amount of bound p21, on the basis of which they proposed that the binding of multiple p21 molecules was required for inactivation of each Cdk2–cyclin complex. This model predicts that in the in vitro reactivation assay removal of only a half of the bound p21 would be sufficient for full activation, given the assumption that both situations are comparable. The actual data (Fig. 3C) were not far off this prediction. Further studies are needed to understand the activation mechanism at a molecular level and the minimal structure of Cdc6 required for its full ability of activation.
Given the presented findings, one important question that would immediately arise is: does Cdc6 activate Cdk2 inactivated by other inhibitors or other Cdks inactivated by p21? Unlike for the partner cyclins, there may be a strict preference for the inhibitor protein that can be removed by Cdc6. In a preliminary experiment, Cdc6 failed to activate p27KIP1-associated Cdk2 in vitro.
Finally, we would like to discuss the biological implications of this function of Cdc6. First, Cdc6 is not merely a factor essential for PreRC assembly, but the one that could play a crucial role in the control of S-phase progression, particularly under cellular stress or differentiation where p21 is up-regulated in a p53-dependent and -independent manner (31). Our latest finding indicates that Cdc6 actually determines utilization of p21-dependent damage checkpoint at least in S phase under physiological conditions. Unlike MMS treatment, which destabilizes Cdc6 via Huwe1-dependent ubiquitination (32), a low-dose UV irradiation does not destabilize Cdc6 and activates only Cdc25A-dependent damage checkpoint. However, when endogenous Cdc6 is repressed by RNAi, p21-dependent checkpoint becomes coactivated and induces prolonged S-phase arrest and p21-mediated inactivation of Cdk2 just like after MMS treatment (unpublished work). The cell nucleus appears to be the primary site for the regulation of Cdk2 by Cdc6 because during the S-phase arrest and recovery the majority of Cdc6 protein was localized in the nucleoplasm/chromatin-bound fraction regardless of whether Cdk2 was activated or not. Second, Cdc6 is an ATP-dependent remodeling factor targeting at least two dissimilar molecules: MCM in PreRC assembly and p21 in Cdk2 activation. Such target diversity raises the possibility that Cdc6 might have more targets for remodeling that play a role in S phase.
Materials and Methods
Chemicals.
MMS was purchased from Sigma; cis-diamminedichloroplatinum (cisplatin) was from Wako. The antibodies αCdc6 (DCS-180) and αMcm7 Ab-2 (47DC141) were obtained from NeoMarkers; αCdk2 (M2), αCdk2(M2)-G, αCdk4 (C-22), αCdk6 (C-21), αCyclin E (C-19), αCyclin A (C-19), αMcm2 (N-19), αMcm3 (N-19), αMcm4 (C-20), αMcm5 (C-18), αMcm6 (N-19), αE2F1 (C-20), αp21WAF1 (C-19), αCHK1 (G-4), αp53 (Pab240), and αpCdc6(S54) (Sc-12920) were from Santa Cruz Biotechnology; αp-Y15Cdk2, αp-T160Cdk2, αp-Rb(S807/S811), and αp-CHK1(S345) were from Cell Signaling; and active recombinant Cdk2-cyclin A (14–448) was from Upstate.
Cell Culture, Synchronization, and MMS Treatment.
NRK-Cdc6#3 was constructed and maintained as described (17). C3H10T1/2-Cdc6 and C3H10T1/2-EV were constructed by retrovirus vector-mediated gene transduction of a CMV-promoter-based expression unit with or without a rat Cdc6 cDNA insert, respectively, and maintained in DMEM with 10% FCS.
NRK-49F, C3H10T1/2, and their Cdc6 overexpressers were synchronized to mid-S phase by 16 h incubation after G0 arrest and release, treated with 120–150 μg of MMS per ml in growth medium for 2–3 h, and cultured in fresh medium containing 25 ng of colcemid per ml. WT, p21−/−, and Tet-Off p27−/− MEF were synchronized to early S phase by a double thymidine block (33), fed with fresh medium for 1–2 h to progress into mid-S phase, treated with 120–180 μg of MMS per ml for 2–3 h, and cultured in colcemid-containing growth medium with cell harvest at the specified intervals.
Cytometric Analysis.
Cytometric analysis was performed with the FACScan flow cytometer (Beckman Coulter ECPIS XL) equipped with a computer-programmed analysis of cell population in each cell cycle phase according to the manufacturer's manual.
Pulse BrdU Labeling.
Cells were incubated in culture medium containing 10 μM BrU for 2 h and determined for those under chromosomal replication by anti-BrdU antibody staining.
Preparation of Whole-Cell Extract, Immunoblot, and Cdk2 Kinase Assay.
Harvested cells were lysed as described (17). Lysates were divided into halves. One half was used for immunoblot analysis, and the other half was used for immunoprecipitation of Cdk2. Cdk2 kinase activity was assayed with a truncated Rb protein (QED Bioscience) as a substrate followed by immunoblot detection of Ser-807-phosphorylated Rb as described (17).
Preparation of Nuclear Extracts.
Nuclear extracts were obtained by extraction with immunoprecipitation buffer containing 0.35 M NaCl after removal of the cytosol after permeabilization of the plasma membrane with digitonin (34).
RNAi.
Cells were transfected with the Cdc6-specific 27-mer RNA duplex (Integrated DNA Technologies) or the universal negative control duplex at 10 nM according to the vender's instruction. Analyses were performed 72 h posttransfection.
Production and Purification of WT and Mutant Cdc6 Proteins.
cDNAs encoding C-terminally His (6 histidines)-tagged rat Cdc6, Cdc6WA, Cdc6WB, and Cdc6Cy (see Fig. 3B for structure) were constructed, inserted into the pEU3-NII vector (Toyobo), in-vitro transcribed, and translated in a rabbit reticulocyte lysate containing 1 mM PMSF. The Cdc6 products were affinity-purified with Ni-NTA beads (Invitrogen). Alternatively, Cdc6 protein tagged with three FLAGs and six histidines in tandem at its C terminus was expressed in a reticulocyte lysate and double affinity-purified with Ni-NTA beads and anti-FLAG M2 gels (Sigma).
In Vitro Cdk2 Activation.
p21-bound, inactive Cdk2 was immunoprecipitated with the anti-Cdk2 antibody and protein G-Sepharose beads from a lysate of NRK cells arrested in S phase by MMS treatment as in Fig. 1 and incubated at 30°C for 30 min in a total 20 μl of 50 mM Tris·HCl (pH 7.5) buffer containing 10 mM MgCl2 with the addition of 5 or 10 μl of the final solution of purified His-tagged Cdc6 or similarly purified empty vector (EV) product as a negative control in the presence or absence of 10 mM ATP (or GTP, AMPPCP). The Cdk2-bound beads were then washed three times with glycerol-free immunoprecipitation buffer, once with Cdk2 kinase assay buffer, and determined for the activity with a truncated Rb protein (QED Bioscience) as a substrate (17) for its amount and coimmunoprecipitated proteins when specified.
Alternatively, inactive recombinant p21–Cdk2–cyclin A complexes were used for activation reaction, which were prepared by preincubation with baculovirus-expressed affinity-purified Cdk2–cyclin A complexes (46% purity) (Upstate) and recombinant p21, the latter expressed in E. coli as a protein fused with GST at its N terminus and tagged with six histidines at its C terminus, then affinity-purified with glutathione beads, digested with factor Xa to remove GST, and further affinity-purified with Ni-NTA beads.
Construction of p27−/− MEF Inducible for Cdc6, Cdc6WA, or Cdc6Cy.
The cDNA encoding His-tagged Cdc6, Cdc6WA, or Cdc6Cy was inserted into the pRevTRE response vector (Clontech). The pRevTet-Off vector and the pRevTRE response vector with a Cdc6 cDNA insert were separately transfected into the EcoPack packaging cell line. The resulting pRevTet-Off virus was infected into p27−/− MEF (23) to produce a stable Tet-Off cell clone. This Tet-Off clone was then infected with pRevTRE-Cdc6 or corresponding mutants to obtain a pool of cell clones inducible for Cdc6 or its mutant proteins. Cells were maintained in DMEM containing 10% FCS and 1 μg of doxycycline per ml. Expression was induced by withdrawal of doxycycline.
Acknowledgments.
We thank P. Berg for critical comments and K. Nakayama (Kyushu University, Japan) for p27−/− MEF. This work was supported by research grants, including one for Global Center of Excellence, from the Ministry of Education, Science, and Culture of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at at www.pnas.org/cgi/content/full/0706392105/DCSupplemental.
References
- 1.Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook JG, et al. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc Natl Acad Sci USA. 2002;99:1347–1352. doi: 10.1073/pnas.032677499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 4.Kelly TJ, Brown GW. Regulation of chromosome replication. Annu Rev Biochem. 2000;69:829–880. doi: 10.1146/annurev.biochem.69.1.829. [DOI] [PubMed] [Google Scholar]
- 5.Lei M, Tye BK. Initiating DNA synthesis: From recruiting to activating the MCM complex. J Cell Sci. 2001;114:1447–1454. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- 6.Herbig U, Marlar CA, Fanning E. The Cdc6 nucleotide-binding site regulates its activity in DNA replication in human cells. Mol Cell Biol. 1999;10:2631–2645. doi: 10.1091/mbc.10.8.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DG, Bell SP. ATPase switches controlling DNA replication initiation. Curr Opin Cell Biol. 2000;12:280–285. doi: 10.1016/s0955-0674(00)00089-2. [DOI] [PubMed] [Google Scholar]
- 8.Petersen BO, Lukas J, Sørensen CS, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–401. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka S, et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 11.Di Leonard A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 12.Gottifredi V, Prives C. The S phase checkpoint: When the crowd meets at the fork. Semin Cell Dev Biol. 2005;16:355–368. doi: 10.1016/j.semcdb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Gottifredi V, Shieh S, Taya Y, Prives C. p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc Natl Acad Sci USA. 2001;98:1036–1041. doi: 10.1073/pnas.021282898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattia M, Gottifredi V, McKinney K, Prives C. p53-dependent p21 mRNA elongation is impaired when DNA replication is stalled. Mol Cell Biol. 2007;27:1309–1320. doi: 10.1128/MCB.01520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan Q, Jinno S, Yamamoto H, Okayama H. Chemical DNA damage activates p21(WAF1/CIP1)-dependent intra-S checkpoint. FEBS Lett. 2007;581:5879–5884. doi: 10.1016/j.febslet.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 16.Terada Y, Tatsuka M, Jinno S, Okayama H. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature. 1995;367:358–362. doi: 10.1038/376358a0. [DOI] [PubMed] [Google Scholar]
- 17.Jinno S, Yageta M, Nagata A, Okayama H. Cdc6 requires anchorage for its expression. Oncogene. 2002;21:1777–1784. doi: 10.1038/sj.onc.1205249. [DOI] [PubMed] [Google Scholar]
- 18.Saha P, et al. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol Cell Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Azevedo WF, et al. Eur J Biochem. 1997;243:518–528. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 20.Erzberger JP, Mott ML, Berger JM. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol. 2006;13:676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- 21.Furstenthal L, Kaiser BK, Swanson C, Jackson PK. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J Cell Biol. 2001;152:1267–1278. doi: 10.1083/jcb.152.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilmes GM, et al. Interaction of the S-phase cyclin Clb5 with an “RXL” docking sequence in the initiator protein Orc6 provides an origin-localized replication control switch. Gene Dev. 2004;18:981–991. doi: 10.1101/gad.1202304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama K, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotedar R, et al. p21 contains independent binding sites for cyclin and cdk2: both sites are required to inhibit cdk2 kinase activity. Oncogene. 1996;12:2155–2164. [PubMed] [Google Scholar]
- 26.Zhu L, Harlow E, Dynlacht BD. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995;9:1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]
- 27.Adams PD, et al. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda R, et al. The structure of cyclin E1/CDK2: Implications for CDK2 activation and CDK2-independent roles. EMBO J. 2005;24:452–463. doi: 10.1038/sj.emboj.7600554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha P, Eichbaum Q, Silberman ED, Mayer BJ, Dutta A. p21CIP1 and Cdc25A: Competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338–4345. doi: 10.1128/mcb.17.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 31.Parker SB, et al. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 32.Hall JR, et al. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell. 2007;18:3340–3350. doi: 10.1091/mbc.E07-02-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kume K, et al. Oncogenic signal-induced ability to enter S phase in the absence of anchorage is the mechanism for the growth of transformed NRK cells in soft agar. New Biol. 1992;4:504–511. [PubMed] [Google Scholar]
- 34.Adam SA, Sterne-Marr R, Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]