Abstract
Netrin-1, an axon navigation cue was proposed to play a crucial role during colorectal tumorigenesis by regulating apoptosis. The netrin-1 receptors DCC and UNC5H were shown to belong to the family of dependence receptors that share the ability to induce apoptosis in the absence of their ligands. Such a trait confers on these receptors a tumor suppressor activity. Expression of one of these dependence receptors at the surface of a tumor cell is indeed speculated to render this cell dependent on ligand availability for its survival, hence inhibiting uncontrolled cell proliferation or metastasis. Consequently, it is a selective advantage for a tumor cell to lose this dependence receptor activity, as previously described with losses of DCC and UNC5H expression in human cancers. However, the model predicts that a similar advantage may be obtained by gaining autocrine expression of the ligand. We describe here that, unlike human nonmetastatic breast tumors, a large fraction of metastatic breast cancers overexpress netrin-1. Moreover, we show that netrin-1-expressing mammary metastatic tumor cell lines undergo apoptosis when netrin-1 expression is experimentally decreased or when decoy soluble receptor ectodomains are added. Such treatments prevent metastasis formation both in a syngenic mouse model of lung colonization of a mammary cancer cell line and in a model of spontaneous lung metastasis of xenografted human breast tumor. Thus, netrin-1 expression observed in a large fraction of human metastatic breast tumors confers a selective advantage for tumor cell survival and potentially represents a promising target for alternative anticancer therapeutic strategies.
Keywords: apoptosis, DCC, dependence receptor
Netrin-1, a diffusible laminin-related protein, has been shown to play a major role in the control of neuronal navigation during the development of the nervous system by interacting with its main receptors, DCC (Deleted in Colorectal Cancer) (1–3) and UNC5H (4, 5). However, more recently, netrin-1 has emerged as a completely different molecule that regulates cell survival. Indeed, the netrin-1 receptors DCC and UNC5H, i.e., UNC5H1, UNC5H2, and UNC5H3, belong to the so-called dependence receptor family (6, 7). Such receptors share the functional property of inducing cell death when disengaged from their ligands, whereas the presence of their ligands blocks this proapoptotic activity. Such receptors thus create cellular states of dependence on their respective ligands (8, 9).
This dependence effect has been suggested to act as a mechanism for eliminating tumor cells that would develop in settings of ligand unavailability: proliferation of tumor cells in a cell environment with constant and limited ligand presence or migration of metastatic tumor cells toward tissues where the ligand is not expressed. A selective advantage for a tumor cell would then be to lose the proapoptotic activity of its dependence receptors. Along this line, DCC was proposed in the early 1990s to be a tumor-suppressor gene, whose expression is lost in the vast majority of human cancers (10, 11). This hypothesis also fits with the observation that UNC5H genes are down-regulated in the vast majority of colorectal tumors, hence suggesting that the loss of UNC5H genes represents a selective advantage for tumor development (12–14). Interestingly, in mice, both inactivation of UNC5H3 and overexpression of netrin-1 in the gastrointestinal tract are associated with intestinal tumor progression (13, 15), hence demonstrating per se that loss of netrin-1 dependence receptors in the human pathology is a causal factor for tumor progression (16).
However, the model described above predicts that not only loss of netrin-1 receptors but also gain of ligand expression, i.e., autocrine expression, should be observed in human cancers, because they should represent similar selective advantages for tumor progression. This question is important not only for basic knowledge but also for putative therapy: Indeed, triggering tumor-cell death by inhibiting the extracellular interaction between netrin-1/receptors could represent an appealing anticancer strategy. We show here that the majority of breast tumors with metastatic propensity show increased netrin-1 expression, a feature that we used in different mouse models to prevent/inhibit metastatic development.
Results
Netrin-1 Is a Marker for Human Metastatic Breast Cancer.
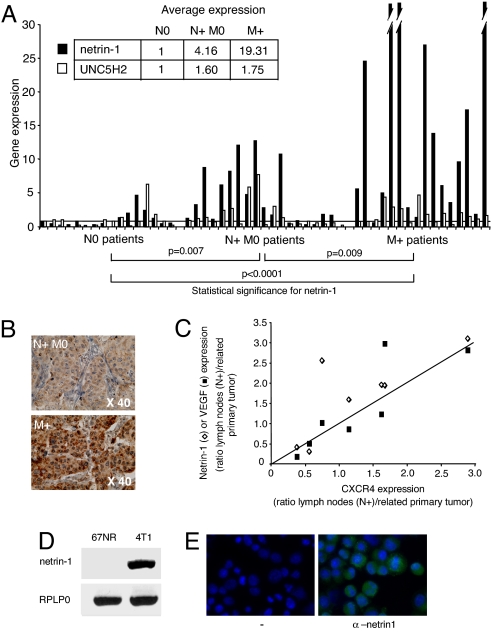
We first analyzed by quantitative (Q)RT-PCR, expression of netrin-1, its dependence receptors, i.e., DCC, UNC5H2, and UNC5H3, and, as positive control, a known marker of metastasis, CXCR4 (17) in a panel of 51 breast primary tumors. These tumors were from lymph node-negative patients (N0, 16 patients), lymph node-positive patients (N + M0, 19 patients) or metastatic patients at the time of diagnosis (M+, 16 patients). As already described, CXCR4 is significantly more expressed in N + M0 or M+ primary tumors compared with N0 tumors [supporting information (SI) Fig. 5A in SI Appendix]. Although DCC was barely detectable and UNC5H expression failed to display significant changes between the different types of tumors (data not shown, Fig. 1A and SI Fig. 5B in SI Appendix), netrin-1 is significantly more expressed in N + M0 tumors than in N0 tumors (median, 1.8 versus 0.5; P = 0.007) with a range of netrin-1 expression higher in N + M0 tumors (Fig. 1A and SI Fig. 5B in SI Appendix). Whereas 31.5% of the N + M0 tumors show at least a 5-fold increase in netrin-1, no such increase was detected in any tested N0 tumors (Fig. 1A and SI Fig. 5B in SI Appendix). An even more striking difference is observed when comparing netrin-1 expression in M+ versus N0 tumors (median, 7.8 versus 0.5; P < 0.0001). Along this line, 62.5% of M+ tumors show at least a 5-fold increase in netrin-1 expression. A significant difference in netrin-1 expression also exists between N + M0 and M+ tumors (median, 1.8 versus 7.8; P = 0.009). Moreover, netrin-1 overexpression is higher in M+ tumors than in N + M0 tumors, because 37.5% of M+ tumors display a >15-fold increase in netrin-1 levels, whereas such an increase is not detected in N + M0 tumors (Fig. 1A and SI Fig. 5B in SI Appendix). Interestingly, although netrin-1 is commonly spotted in the commercially available DNA microarrays, netrin-1 was not found associated with breast metastatic cancer in the different profiling experiments reported so far [(18), see www.oncomine.org]. Netrin-1 was, in most cases, detected only at background level, but, as shown in SI Fig. 5C in SI Appendix, this is probably due to a bias within the cDNA amplification procedure used in the profiling experiments. Immunohistochemistry performed on a panel of N + M0 or M+ breast primary tumors sections revealed netrin-1 protein expression (Fig. 1B and SI Fig. 5D in SI Appendix). To further analyze whether the high netrin-1 expression observed in N + M0 or M+ primary tumors is also observed in disseminating tumors cells, we next measured netrin-1 mRNA level in pairs including both the primary tumors and the related metastatic lymph nodes. The expression ratio between lymph nodes and primary tumors is higher for netrin-1 than for the other known genes involved in the metastatic process, i.e., CXCR4 and VEGFR, (Fig. 1C, six of seven pairs tested). Thus, netrin-1 up-regulation appears as a marker of lymph node involvement and distant metastatic disease in human breast cancer.
Fig. 1.
Netrin-1 is overexpressed in metastatic breast tumors. (A) Expression profile of netrin-1 and UNC5H2 examined with quantitative real-time RT-PCR. QRT-PCR was performed by using total RNA extracted from 51 tumor biopsies. They were obtained from patients with tumors localized to the breast (N0), with only axillary node involvement (N + M0), and with distant metastases at diagnosis (M+). Specific human netrin-1 primers (35) and primers corresponding to the human HMBS gene (hydroxymethylbilane synthase) were used. HMBS was used as a reference here because it shows a weak variability at the mRNA level between normal and breast tumoral tissues, as described (36). Another reference TBP was also used with similar results (data not shown). Netrin-1 expression is given as the ratio between netrin-1 expression in each sample and the average of netrin-1 expression in the N0 samples, shown by a horizontal bar. UNC5H2 expression is also given as the ratio between UNC5H2 expression in each sample and the average of UNC5H2 level in N0 samples. Nonparametric statistical significance tests (Mann–Whitney) were used; the P values for netrin-1 are indicated. The average of netrin-1 and UNC5H2 expression is indicated for each tumor stage (upper left). (B) Representative netrin-1 immunohistochemistries on sections from two human tumors presented in A. Enlargement is indicated. (C) Comparison between netrin-1 or VEGFR expression and CXCR4 expression in seven pairs of frozen human invaded lymph nodes/primary tumors. Ratio between the mRNA level measured by QRT-PCR in invaded lymph node to the one detected in the primary tumor is shown for each pair and is represented as the ratio of netrin-1 (VEGFR) to CXCR4. (D) cDNA amplification from 67NR or 4T1 cells loaded in an agarose gel. (E) Immunostaining on 4T1 cell line using netrin-1 antibody. A control without the primary antibody is indicated. Nuclei were visualized with Hoescht staining.
Disruption of the Netrin-1 Autocrine Loop Triggers Cancer Cell Death.
Because netrin-1 appears to be up-regulated in a large fraction of aggressive human breast cancers, we next investigated whether netrin-1 is also expressed in a panel of 50 breast cell lines. Interestingly netrin-1 is highly expressed in a sizeable fraction of human breast cancer cell lines (Table 1) and all cell lines showing high netrin-1 levels (greater than +++) have been derived from aggressive tumors or/and have been shown to form metastases in mice. We also studied two mouse cell lines, 4T1 and 67NR (Table 1 and Fig. 1D), developed by Miller and colleagues (19) as cell lines, derived from a BALB/c mouse spontaneous primary mammary tumor, showing different metastatic potentials when injected into syngenic mice. Although 67NR cells form primary mammary tumors but no metastasis, 4T1 cells form primary tumors and metastasis, especially in the lung, the liver, and the bone marrow. Interestingly, although netrin-1 receptors (i.e., DCC, UNC5H1, UNC5H2, and UNC5H3) failed to show significant difference between 67NR and 4T1 cell lines, netrin-1 mRNA was hardly detected in 67NR cells, whereas it was expressed in 4T1 cells (Fig. 1DE and SI Fig. 6A in SI Appendix). This presence of the netrin-1 mRNA in 4T1 cells is associated with the detection of netrin-1 protein after netrin-1 immunostaining performed in the absence of cell permeabilization (Fig. 1E). Therefore, metastatic 4T1 cells show autocrine production of netrin-1.
Table 1.
Expression of netrin-1 examined by QRT-PCR using total RNA extracted from 48 human breast and 2 mouse mammary cell lines
| Cell line | netrin-1 | Cell line | netrin-1 |
|---|---|---|---|
| Human | Human | ||
| 184-A1 | + | MDAMB 185 | +++ |
| 184-B5 | ++ | MDAMB 231 | − |
| BR-CA-MZ-01 | ++ | MDAMB 361 | ++ |
| BR-CA-MZ-02 | + | MDAMB 415 | + |
| BT-20 | ++ | MDAMB 435S | − |
| BT-474 | +++ | MDAMB 436 | +++ |
| BT-549 | ++ | MDAMB 453 | − |
| BY-549 | ++ | MDAMB 468 | ++ |
| CAMA-1 | ++ | S 68 | ++ |
| HBL-100 | ++ | SKBR3 | +++ |
| HCC38 | − | SKBR7 | +++++ |
| HCC202 | +++ | SUM 52 | ++ |
| HCC1395 | + | SUM 102 | ++ |
| HCC1500 | ++++ | SUM 149 | − |
| HCC1569 | ++ | SUM 159 | − |
| HCC1806 | + | SUM 185 | +++ |
| HCC1937 | ++ | SUM 190 | ++++ |
| HCC1954 | ++ | SUM 206 | ++ |
| HCC2218 | ++ | SUm 225 | − |
| HME-1 | ++ | SUM 229 | − |
| Hs 578T | + | T47D | ++++ |
| MCF7 | ++ | UACC812 | ++ |
| MCF-10A | − | ZR751 | − |
| MCF-10F | − | Mouse | − |
| MDAMB 134 | + | 67NR | +++++ |
| 4T1 |
Netrin-1 expression was analyzed compared with the housekeeping genes HMBS (Hydroxymethylbilane synthase) or RPLP0 expression in each sample. TBP was also used as a control here and gave similar results (data not shown). A range of netrin-1 expression is shown from +++++ (high) to − (barely detectable).
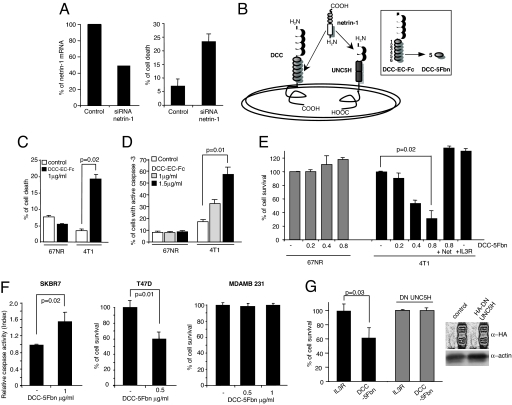
We next investigated whether this netrin-1 autocrine production confers a selective advantage to 4T1 cells by inhibiting DCC/UNC5H-induced cell death. We first down-regulated expression of netrin-1 in 4T1 cells using RNAi strategy. As shown in Fig. 2A, down-regulation of netrin-1 in 4T1 cells was associated with cell-death induction monitored by a trypan blue-exclusion assay. As a second approach, we looked for a compound that may inhibit the interaction between netrin-1 and its receptors. It was reported that a domain located in the N terminus of netrin-1 (the so-called laminin-VI domain) interacts with both DCC and UNC5H receptors [Fig. 2B; (20)]. We show that a soluble extracellular domain of DCC (DCC-EC-Fc) is able to inhibit both DCC/netrin-1 and UNC5H2/netrin-1 interaction, as measured by ELISA (SI Fig. 7A in SI Appendix). This soluble ectodomain is also able to inhibit netrin-1-induced DCC-mediated signaling pathways [e.g., MAPK activation (3)] (SI Fig. 7B in SI Appendix). DCC-EC-Fc was then added to a 4T1 cells culture—or as a control 67NR cells culture—and cell death/survival was monitored, either by a trypan blue-exclusion assay (Fig. 2C) or by a MTT assay (data not shown). In addition, apoptosis was more specifically monitored by measuring caspase activity (Fig. 2D). As shown in Fig. 2 C and D, addition of the competing soluble protein in the culture medium triggers death of 4T1 cells, whereas it has no effect on 67NR cell death.
Fig. 2.
Induction of metastatic cell death by inhibiting the netrin-1 autocrine loop. (A) Induction of 4T1 cell death by reducing netrin-1 level. The 4T1 cells were treated with scramble siRNA (control) or netrin-1 siRNA. Twenty-four hours later, the netrin-1 mRNA level was quantified by QRT-PCR (Left), and cell death was determined 1 day later by trypan blue-exclusion assay (Right). (B) Scheme representing netrin-1 and its receptors, DCC and UNC5H. (Inset) The extracellular part of the DCC receptor (DCC-EC-Fc) and the fifth fibronectin type III domain of DCC (DCC-5Fbn) used to induce cell death. (C–E) Quantitative analysis of cell death in 67NR and 4T1 cells treated with DCC-EC-Fc (C and D), with DCC-5Fbn (E) or with an unrelated soluble protein IL3R-EC-Fc. Cell death/survival was quantified by the trypan blue-exclusion assay (C), by caspase activity measurement by flow cytometry (D), or by MTT assay (E). In this latter test, netrin-1 was added in excess to reverse the effect of DCC-5Fbn. (F) Cell-death induction by DCC-5Fbn in SKBR7 and T47D cell lines but not in MDAMB231 cells. Cell survival was quantified by MTT assay (Right), and apoptosis was measured by caspase activity assay (Left). (G) Quantitative analysis of cell survival by MTT assay in the presence of either IL3R-Fc or DCC-5Fbn in 4T1 cells or 4T1 cells stably transfected with UNC5H2-IC D412N (DN UNC5H; i.e., the intracellular domain of UNCSH2 mutated in the caspase site acts as a dominant negative for UNC5H proapoptotic activity (SI Fig. 7D in SI Appendix). (Inset) Western blot using an anti-HA-UNC5H2 antibody was performed on the mock-transfected and DN UNC5H-expressing 4T1 cell lines. Standard deviations are indicated (n > 3). In C–G, Mann–Whitney tests were performed, and a P value is indicated.
Because the complete extracellular domain of DCC appears as only of modest interest for use in vivo and in therapy (DCC-EC-Fc is ≈1,100 aa large), we looked for an alternative polypeptide from the DCC extracellular domain that could trigger apoptosis in 4T1 cells but not in 67NR cells. We consequently produced a flag-tagged fifth fibronectin type III domain of DCC, DCC-5Fbn, a domain located in the ectodomain of DCC that is known to interact with netrin-1 (20) (Fig. 2B Inset). This 100-aa protein does not interfere with the binding of DCC/netrin-1 or UNC5H/netrin-1 but affects the ability of netrin-1 to trigger multimerization of these receptors, a multimerization required for the inhibitory activity of netrin-1 on DCC- or UNC5H-induced cell death (F. Mille, F. Llambi, C. Guix, C. Thibert, J.F., C. Guenebeaud, S. Castro-Obregon, D. E. Bredesen, and P.M., unpublished work). As shown in Fig. 2E, addition of DCC-5Fbn triggers, in a dose-dependent manner, death of 4T1 cells as measured by MTT assay. Similar results were obtained by using trypan blue-exclusion or caspase-activity assays (data not shown). This cell-death effect is directly associated with netrin-1 autocrine expression because (i) DCC-5Fbn has no effect on 67NR cells, (ii) addition of an excess netrin-1 blocks the death effect of DCC-5Fbn, and (iii) IL3R-EC-Fc (the extracellular domain of the IL3 receptor) fails to trigger 4T1 cell death (Fig. 2E).
The acquired survival advantage through netrin-1 autocrine expression is not restricted to murine tumor cells, because it is detected in human breast cancer cell lines. Indeed, addition of DCC-5Fbn to naturally netrin-1-expressing human breast adenocarcinoma T47D or SKBR7 cell cultures triggers cell-death induction measured either by caspase-3 activity assay or MTT assay, whereas it had no effect on netrin-1-negative cells like MDAMB-231 (Fig. 2F). Similar results were obtained by netrin-1 siRNA approach (SI Fig. 7C in SI Appendix and data not shown).
This cell-death induction upon inhibition of the netrin-1 autocrine loop, i.e., via inhibition of netrin-1 level, via inhibition of netrin-1/receptors interaction, or via inhibition of netrin-1 receptors multimerization, appears to occur through UNC5H receptors. Indeed, DCC is hardly detected in 4T1, T47D, or SKBR7 cells (SI Fig. 6A in SI Appendix and data not shown). Moreover, 4T1 cells stably expressing a dominant negative mutant of UNC5H receptors inhibiting UNC5H-induced apoptosis (SI Fig. 7D in SI Appendix) are no longer sensitive to DCC-5Fbn (Fig. 2G).
Disruption of the Netrin-1 Autocrine Loop Prevents Metastasis Formation.
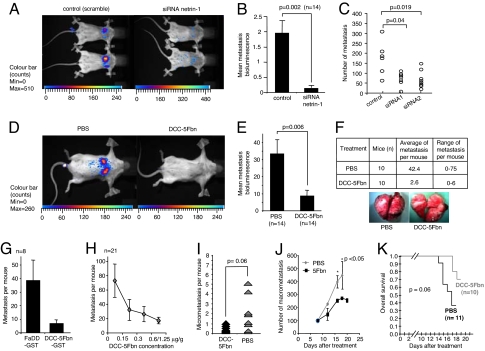
We next investigated whether the cell death effect observed in vitro may be extended in vivo. As a first approach, 4T1 cells stably transfected with a luciferase-based vector (4T1-luc) were i.v. injected into syngenic BALB/c mice. Metastasis formation was then analyzed by using luminescence recording. As shown in Fig. 3, when i.v. injected, 4T1-luc cells efficiently colonize lungs. To first assay the survival role of netrin-1 in promoting 4T1 cell-mediated lung metastasis, netrin-1 was down-regulated in vivo by daily i.p. injection of netrin-1 siRNA. I.p. injection allows the detection by QRT-PCR of netrin-1 siRNA molecules in different organs including lungs (SI Fig. 8A in SI Appendix). As shown in Fig. 3 A and B, after 9 days of injection, whereas scramble siRNA injected mice showed lung metastasis, netrin-1 siRNA injection is associated with a significant reduction of lung metastasis measured by luminescence recording. Similar results were obtained by direct anatomopathological examination of lungs after 14 days of injection (Fig. 3C). To bring the in vivo experiment closer to therapeutic relevance, we then performed a similar set of experiments using DCC-5Fbn injection rather than siRNA. Mice were then i.p. and i.v. injected (once i.v., once i.p.) from day 0 to day 13 with either PBS buffer or Flag-tagged-DCC-5Fbn (1.25 μg per gram of body weight per injection). Metastasis formation was again analyzed by using luminescence recording. As shown in Fig. 3 D and E, although in PBS-treated mice 4T1-luc cells efficiently colonize lungs, mice treated with DCC-5Fbn show a dramatic reduction of lung metastasis. This inhibition of metastasis formation was then confirmed by anatomopathological examination of lungs (data not shown and Fig. 3F). Similar results were obtained when we performed daily i.p. injection of GST-tagged-DCC-5Fbn instead of Flag-tagged-DCC-5Fbn and when an unrelated protein (GST-FaDD) was used as a negative control (Fig. 3G). A dose-dependence experiment with DCC-5Fbn was then performed, and, as shown in Fig. 3H, a 0.15 μg per gram of body weight is sufficient to decrease metastasis formation. To further analyze whether this treatment may not only prevent metastatic formation by triggering tumor-cell death before extravazation but also could erase metastatic lesions already established in the secondary organ, the DCC-5Fbn treatment was administered once micrometastases were detected in the lung. As shown in Fig. 3I, DCC-5Fbn treatment was associated with a decrease in metastasis compared with PBS treatment. To address whether DCC-5Fbn treatment could also decrease macrometastasis, DCC-5Fbn was administered once macrometastases were detected. As shown in Fig. 3J, DCC-5Fbn significantly decreases lung macrometastasis. The antimetastatic effect of DCC-5Fbn is, moreover, associated with increased mouse survival as shown in Fig. 3K.
Fig. 3.
Inhibition of metastasis formation in mice by disruption of the netrin-1 autocrine loop. The 4T1-luc cells were i.v. injected into BALB/c mice at day 0. (A–C) Scramble siRNA or netrin-1 siRNA was i.v. injected daily, and after 9 (A and B) or 14 (C) days, lung metastasis was studied by luminescence recording (A and B) or by examination of lungs under a microscope (C). (A) A representative image of luminescence recording of scramble siRNA- (Left) or netrin-1 siRNA- (Right) treated mice. (B) Quantification of the luminescent signal measured by the NightOwLB II system. A luminescent signal for each group (control vs. netrin-1 siRNA) is given as a mean of the different ratios between summed luminescent signal in the lung area (photons/sec) and a background area defined in the animal body. Number of mice is indicated (n = 14). (C) Number of lung metastatic nodules in individual mice were counted after day 14 under a dissection microscope in three treated populations [scramble (control), first set of siRNA also used in A and B, and second set of siRNA]. (D–F) As in A–C except than PBS or DCC-5Fbn were injected once daily i.v. or once i.p. starting at day 0. After 13 days, metastasis development was studied by luminescence recording (D and E) or by examination of lungs under a microscope (F). (F) A representative macroscopic photograph of lungs from PBS- (Left) or DCC-5Fbn- (Right) treated mice (Lower). Total number of lung metastatic nodules in individual mice were counted in the two treated populations (+PBS, +DCC-5Fbn) (Upper). (G) As in F except that GST-DCC-5Fbn (instead of Flag-DCC-5Fbn) or an unrelated protein GST-FaDD was injected daily. (H) Dose effect of DCC-5Fbn. As in F except that different concentrations of DCC-5Fbn were injected daily; n = 21. (I) The 4T1 cells (1 × 103) were i.v. injected at day 0. At day 7 (at this day from one to eight micrometastasis are visible, data not shown), the mice were treated daily with PBS or DCC-5Fbn, and enumeration of metastasis was performed at day 11. (J) The 4T1 cells (5 × 105) were i.v. injected at day 0. At day 8, when at least 90 macrometastasis are visible, the mice were treated daily with PBS or DCC-5Fbn, and enumeration of metastasis was performed from day 8 to day 18 for PBS-treated mice, as we were required ethically to euthanize them, to day 20 for DCC-5Fbn-treated mice (each point represents three mice). (K) Overall survival of mice, which were i.v. injected with 106 4T1 cells, treated daily with PBS (black) or DCC-5Fbn (gray). P values were calculated by using the Kaplan–Meier test. Standard deviations are indicated. In B, C, E, G, and I, Mann–Whitney tests were performed, and a P value is indicated.
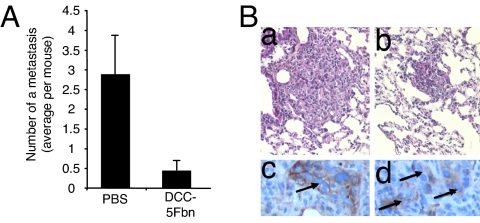
As a second approach to evaluate the antimetastatic effect of DCC-5Fbn, we investigated whether spontaneous development of metastases in lung from a primary breast cancer could also be inhibited by DCC-5Fbn. We took advantage of a fresh human breast tumor, which was characterized as expressing a high level of netrin-1, and that, when xenografted in nude mice, was giving rise to spontaneous development of metastases in the lung. Once tumors reached an optimal size after xenograft in nude mice, [i.e., lung colonization was detected at this stage (data not shown)], mice were treated daily with either PBS or DCC-5Fbn. As shown in Fig. 4, DCC-5Fbn treatment is associated with a significant decrease in the number of aggressive metastases in lungs. Interestingly, this reduction in the number of metastases is associated with the detection of metastatic lesions that are much smaller and associated with dense inflammatory reactions (Fig. 4 A and B), hence further supporting the view that, in mice, the inhibition by DCC-5Fbn of the prosurvival activity conferred by netrin-1 autocrine expression is associated with antimetastatic effect.
Fig. 4.
Disruption of the netrin-1 autocrine loop inhibits spontaneous lung metastasis from a xenografted human breast tumor. (A) Antitumor effect of DCC-5Fbn in nude mice xenografted with a fresh human breast tumor. A human breast tumor showing a high netrin-1 level was xenografted into nude mice, and when the tumor reached a palpable size, the mice were i.v. treated daily with PBS or DCC-5Fbn. Metastases were analyzed and enumerated by a pathologist. (B) Comparative low-power magnifications of lung metastases in control (a) and DCC-5Fbn-treated (b) animals; tumor cells, characterized by large size, abundant cytoplasm, and irregular nucleus, are arranged in small masses of variable diameter, scattered within the lung parenchyma. (c and d) Higher magnifications of lung metastases in a DCC-5Fbn-treated animal. Metastatic cells, revealed by an anti-human cytokeratin 7 antibody, form a small mass surrounded by a dense inflammatory reaction (c); at an advanced stage, tumor cells are dissociated by infiltrative inflammatory cells (d). (a and b) H&E staining, original magnifications ×200. (c and d) Immunoperoxidase, followed by nuclear counterstaining with Mayer's hematoxylin, original magnification ×400.
Discussion
Here, we show that netrin-1 expression may be considered as a marker of a breast tumor's ability to disseminate. Most of the breast primary tumors with metastasis abilities showed elevated netrin-1 expression. Both the human pathology and tumor cell line data and the mouse models described above support the view that this elevated netrin-1 level is a selective advantage acquired by the cancer cell to escape netrin-1-dependence receptor-induced apoptosis and, consequently, to survive independently of netrin-1 availability. From a mechanistic point of view, in human pathology, this autocrine expression of netrin-1 probably inhibits UNC5H-induced cell death. Along this line, the antimetastatic effect of netrin-1 siRNA in the 4T1 model is inhibited by injection of UNC5H1 and UNC5H2 siRNA (SI Fig. 8B in SI Appendix). Thus, DCC, which is barely detectable in the different groups (N0, N + M0, M+) of breast cancers studied, is probably of relatively modest interest in metastatic breast cancers. Thus, as predicted by the dependence receptor model, we have now shown that a tumor cell can escape receptor dependency in at least three ways. First, expression of the dependence receptor can be down-regulated, as extensively described for DCC and more recently for UNC5H (10, 12–14, 21). Second, the downstream death signaling can be shut down. We have recently shown that UNC5H2 proapoptotic activity relies on the binding of UNC5H2 to the serine/threonine DAPK (22), a protein that was demonstrated to be involved in metastasis regulation and down-regulated in human malignancy (23). Here, we show that a third selective advantage for the tumor cell is the self-production of the dependency ligand. One intriguing question remains as to why breast tumors with metastatic propensity seem to have preferably selected netrin-1 self-production rather than receptor loss, whereas colorectal tumors have mostly selected loss of the receptors rather than gain of netrin-1 expression—indeed, only 7% of colorectal cancers show an increase of netrin-1 expression (13, 15). A possible explanation is that netrin-1 expression not only confers a gain in survival to the disseminating cells, but also possibly a gain in the nonapoptotic/positive signaling of netrin-1 receptors. It is important to note that netrin-1 was originally described as a guidance cue (24), which, even though completely unproven, could play a role in the tropism of metastatic cells. Other proposed roles of netrin-1 include adhesion and morphogenesis regulation (25–27), both mechanisms that may be of importance for metastasis development. Similarly, netrin-1 was recently proposed to play a role during embryonic angiogenesis, and, even though conflicting results have been published (28–32), we cannot, at this stage, rule out the role of netrin-1 as an angiogenic factor that somehow could favor metastasis development at the secondary site. However, the gain of “positive” signaling by netrin-1 autocrine expression is probably not sufficient per se to promote metastasis. Indeed, when netrin-1 was forced in the nonmetastatic 67NR cells, we failed to observe any significant increase of lung metastasis after 67NR injection into mammary glands (SI Fig. 6 in SI Appendix). Thus, netrin-1 expression in tumor cells is probably not sufficient to enable metastasis formation from the primary site but mainly confers survival to metastatic cells.
These observations not only provide evidence for the importance of ligand/dependence receptor pairs in the regulation of tumor development, but also suggest a putative therapeutic strategy. Indeed, as of today, there is no efficient treatment for patients with metastatic breast cancer, a lack of treatment that leads to the death of 400,000 women worldwide per year (33). Here, we propose that a treatment based on inhibition of the interaction between netrin-1 and its dependence receptors could positively effect a large fraction of the patients suffering from metastatic breast cancer, i.e., patients who would show high netrin-1 expression in primary tumors. Future preclinical and clinical work needs now to demonstrate whether netrin-1 could represent an appealing target in two-thirds of metastatic breast cancers.
Methods
A detailed methods section is provided in SI Materials and Methods in SI Appendix.
Cell Line, Transfection, Immunoblotting, Immunoprecipitation, Plasmid Constructs, siRNA, and Reagents.
Human breast and mouse mammary cell lines listed were cultured by using standard procedures and were obtained from D. Birnbaum (Institut National de la Santé et de la Recherche Scientifque U 599, Centre de Recherche, Marseille, France) and F. Miller (Wayne State University, Detroit). Transfection, immunoblotting, and immunoprecipitation were performed as described in SI Materials and Methods in SI Appendix and essentially as described (22). The different constructs are described in SI Materials and Methods in SI Appendix. For cell culture use, scramble and netrin-1 siRNAs were designed by Santa Cruz Biotechnology as a pool of three target-specific 20- to 25-nt siRNAs. For the in vivo approach, two different single netrin-1, DCC, UNC5H1, or UNC5H2 siRNAs were produced (Sigma–Proligo). DCC-5Fbn was produced upon expression in BL21 lysate and upon affinity chromatography using Flag-Sepharose (Sigma). DCC-EC-Fc was obtained from R & D Systems and Netrin-1, UNC5H2-EC-Fc, GST-DCC-5Fbn, and GST-FaDD from Apotech/Axxora.
Human Breast Tumor Samples, QRT-PCR, and Immunohistology.
Fifty-one human breast cancer samples were provided by the tumor bank of the Centre Léon Bérard. Fresh tissue of the tumor was obtained during breast surgery before any systemic therapy and snap-frozen in liquid nitrogen. QRT-PCR was performed as described in SI Materials and Methods in SI Appendix and essentially as described (13). The sequences of the primers used are available upon request. Paraffin-embedded tumors tissues fixed in formalin were used for analysis. The pathologist selected representative areas from breast carcinomas (N + M0, M+) or liver or uterine metastases. Immunostaining was performed as described in SI Materials and Methods in SI Appendix and essentially as described (13).
Cell Death Assays.
The different cell death assays (trypan blue exclusion, MTT, caspase-3 activity assay) were performed as described in the supplementary materials and essentially as described (22, 34).
Tumor Mouse Models.
A complete description of the mouse models is provided in SI Materials and Methods in SI Appendix. Briefly, for mammary gland injection of 67NR cells, 106cells were injected. For i.v. injection, 105 (unless indicated) 4T1-luc cells were injected into a tail vein, and mice were either killed or analyzed by using luminescence recording. Lungs were removed, and metastatic nodules were counted and histological classification and grading of neoplastic lesions was performed in a blinded fashion and according to standard procedures. Daily i.p injection of scramble siRNA or netrin-1 siRNA (4 μg per mouse) (or DCC, UNC5H1, or UNC5H2) were performed according the procedure established by SeleXel Corp. DCC-5Fbn treatments were performed according the Fig. 3 legend. Xenografts of fresh human breast tumors in nude mice were performed according the protocol developed by Xentech. Among 20 fresh breast tumors, the tumor BC174 expressing high levels of netrin-1 and of UNC5H receptors was selected. For each xenograft, when tumors reached 62 mm3, PBS or 1.25 μg per gram of body weight DCC-5Fbn were administered i.v. every day.
Supplementary Material
Acknowledgments.
We thank O. Donze (Apotech, Lausanne, Switzerland) for advice and reagents; F. Cabon, I. Puisieux, and C. Delloye for technical advice; J. Blachier, A. Potemski, I. Durand, N. Gadot, and S. Goddard-Leon for excellent technical help; F. Miller, J. Adélaïde [Institut National de la Santé et de la Recherche Scientifique (INSERM) U 599, Marsielle, France] C. Caux, D. Birnbaum (INSERM U 590, Lyon, France), and G. Grelier (INSERM U 590, Lyon, France) for materials; H. Bilak for text correction; A. Puisieux for helpful discussion; Xentech for human breast tumor xenografts; and SeleXel for in vivo siRNA. This work was supported by an institutional grant from the Centre National de la Recherche Scientifique, Centre Léon Bérard, University of Lyon, and from the Ligue Contre le Cancer, Institut National du Cancer, Agence Nationale de la Recherche Blanche, and European Union Specific Targeted Research Project Hermione.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709810105/DC1.
References
- 1.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 2.Keino-Masu K, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 3.Forcet C, et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman SL, et al. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- 5.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 6.Mehlen P, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 7.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20:2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlen P, Thibert C. Dependence receptors: between life and death. Cell Mol Life Sci. 2004;61:1854–1866. doi: 10.1007/s00018-004-3467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell Death Differ. 2005;12:1031–1043. doi: 10.1038/sj.cdd.4401680. [DOI] [PubMed] [Google Scholar]
- 10.Fearon ER, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 11.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 12.Thiebault K, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci USA. 2003;100:4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernet A, et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin SK, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–1857. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazelin L, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 16.Grady WM. Making the case for DCC, UNC5C as tumor-suppressor genes in the colon. Gastroenterology. 2007;133:2045–2049. doi: 10.1053/j.gastro.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 18.van 't Veer LJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 19.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 20.Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC, UNC5 and mediates interactions between DCC, heparin. J Biol Chem. 2003;278:32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- 21.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22:3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Llambi F, et al. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. EMBO J. 2005;24:1192–1201. doi: 10.1038/sj.emboj.7600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inbal B, et al. DAP kinase links the control of apoptosis to metastasis. Nature. 1997;390:180–184. doi: 10.1038/36599. [DOI] [PubMed] [Google Scholar]
- 24.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 25.Yebra M, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park KW, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson BD, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006 doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larrivee B, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre F, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 34.Tauszig-Delamasure S, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci USA. 2007;104:13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latil A, et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer. 2003;103:306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 36.de Kok JB, et al. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–159. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.