Abstract
Heparan sulfate (HS) proteoglycans influence embryonic development and adult physiology through interactions with protein ligands. The interactions depend on HS structure, which is determined largely during biosynthesis by Golgi enzymes. How biosynthesis is regulated is more or less unknown. During polymerization of the HS chain, carried out by a complex of the exostosin proteins EXT1 and EXT2, the first modification enzyme, glucosaminyl N-deacetylase/N-sulfotransferase (NDST), introduces N-sulfate groups into the growing polymer. Unexpectedly, we found that the level of expression of EXT1 and EXT2 affected the amount of NDST1 present in the cell, which, in turn, greatly influenced HS structure. Whereas overexpression of EXT2 in HEK 293 cells enhanced NDST1 expression, increased NDST1 N-glycosylation, and resulted in elevated HS sulfation, overexpression of EXT1 had opposite effects. Accordingly, heart tissue from transgenic mice overexpressing EXT2 showed increased NDST activity. Immunoprecipitaion experiments suggested an interaction between EXT2 and NDST1. We speculate that NDST1 competes with EXT1 for binding to EXT2. Increased NDST activity in fibroblasts with a gene trap mutation in EXT1 supports this notion. These results support a model in which the enzymes of HS biosynthesis form a complex, or a GAGosome.
Heparan sulfate (HS) is a linear polysaccharide consisting of repeating units of N-acetylglucosamine (GlcNAc) and hexuronic acid (HexA) carrying sulfate groups in different positions. HS proteoglycans are ubiquitous components of cell surfaces and are also present in the extracellular matrix, predominantly in basement membranes (1). Through interactions with proteins they have important and vital functions, both during embryonic development (2) and in adult physiology (3).
The biosynthesis of HS takes place in the Golgi network (4), where the polysaccharide is polymerized on a linkage tetrasaccharide attached to a serine residue of a proteoglycan core protein. The linkage tetrasaccharide consists of xylose–galactose–galactose–glucuronic acid, with xylose covalently bound to the serine residue. The four monosaccharide residues are transferred by the action of four different enzymes. The committing step in HS biosynthesis is the tranfer of a GlcNAc residue to the linkage region. Subsequently, the HS polymerase complex composed of EXT1 and EXT2 adds alternating units of glucuronic acid (GlcA) and GlcNAc to the nonreducing end of the chain. As the chain is growing, the first modification enzyme GlcNAc N-deacetylase/N-sulfotransferase (NDST), removes N-acetyl groups from selected GlcNAc residues and replaces them with sulfate groups. After N-sulfation, the C5-epimerase converts GlcA residues to iduronic acid (IdoA). Sulfation at the 2-O position of IdoA residues and some GlcA acid is then carried out by a 2-O-sulfotransferase, followed by glucosamine 6-O-sulfation and, more rarely, 3-O-sulfation. The final HS biosynthesis product has a molecular design in which clusters of N- and O-sulfated sugar residues are separated by nonsulfated domains (4, 5). Recent results suggest that the overall organization of HS domains is of major importance for protein interaction (6).
The structure of HS differs between different organs and cell types (7). There is no template in HS biosynthesis, and how the fine structure of HS is determined is far from fully understood. Transcriptional regulation is, with a few exceptions (8), unexplored. In a study by Grobe and Esko (9), it was shown that the expression of NDSTs and other HS biosynthesis enzymes may be largely controlled at the translational level. In addition, it has been suggested that the biosynthesis enzymes may be gathered in a GAGosome where the enzymes work in close proximity of each other (1). Different composition of the GAGosome may result in different modification patterns of the HS chain.
The NDST enzymes are believed to have a key role in designing the sulfation pattern during biosynthesis because subsequent modifications occur in N-sulfated regions. Of the four vertebrate NDSTs, NDST1 and NDST2 have broad expression patterns and are found in most cell types and tissues during embryonic development and adult life, whereas NDST3 and NDST4 have a much more restricted expression pattern (10).
Several of the biosynthesis enzymes have been shown to interact, but so far only in pairs and not in bigger complexes. EXT1 and EXT2 have been shown to form a heterooligomeric complex that is accumulated in the Golgi apparatus (11, 12). The EXT1/EXT2 heterooligomer has a much higher glycosyltransferase activity than EXT1 alone, suggesting that this complex represents the biologically relevant form of the HS polymerization unit (12, 13). EXT1 alone, and the EXT1/EXT2 complex, can catalyze in vitro polymerization of the HS backbone structure on an oligosaccharide primer, whereas the activity of EXT2 is much weaker (14). Interactions have also been demonstrated between the C5-epimerase and the 2-O-sulfotransferase (15) and between the xylosyltransferase and galactosyltransferase-I (16, 17).
Here, we show that the relative amounts of the three enzymes (EXT1, EXT2, and NDST1) will determine NDST activity and influence HS structure. An interaction between NDST1 and EXT2 is also demonstrated, supporting the GAGosome concept.
Results
HEK 293 cells overexpressing NDST1 were stably transfected with EXT1 and EXT2 alone and in combination. Several clones were picked, expanded to cell lines, and checked for the presence of EXT1 and EXT2 containing constructs by PCR and mRNA expression by Northern blot analysis (data not shown). In addition to the two parental cell lines overexpressing NDST1, two cell lines of each with high expression of EXT1 together with NDST1, and EXT2 together with NDST1, and one cell line coexpressing all three enzymes were selected for further experimentation.
EXT Proteins Influence NDST Expression.
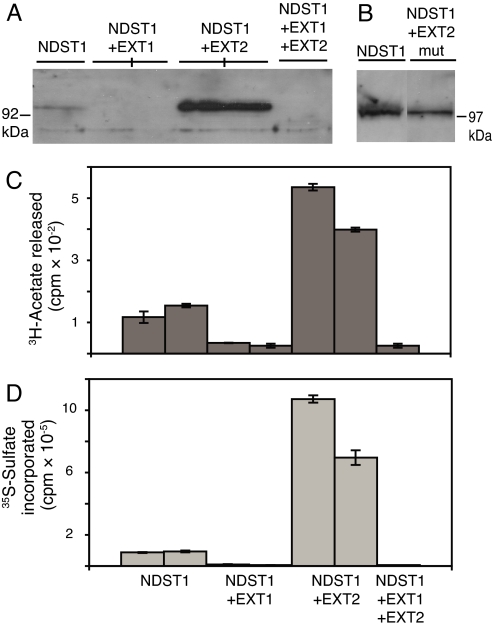
The NDST1-overexpressing cells had previously been characterized and shown by Western blotting to express detectable levels of NDST1 (18). When NDST1 expression was assayed in the EXT-transfected cells, we were surprised to find that coexpression of NDST1 and EXT2 resulted in a dramatic increase in translated NDST1 (Fig. 1A). In contrast, in NDST1-expressing cells transfected with EXT1 or with both EXT1 and EXT2, no NDST1 protein could be detected. The increased amount of NDST1 in EXT2-expressing cells and the lowered amount in the other cell lines could also be demonstrated by enzyme activity measurements. Cells coexpressing NDST1 and EXT2 showed an increase in both N-deacetylase and N-sulfotransferase activity compared with cells overexpressing NDST alone (Fig. 1 C and D), whereas cells coexpressing NDST1 and EXT1 and the cells overexpressing all three enzymes showed a decrease in enzyme activities (Fig. 1 C and D). As a control, mutations were also introduced in the EXT2 construct to abolish expression of the protein, and this construct (EXT2mut) was stably transfected into HEK 293 cells. With Western blotting, no EXT2 protein could be detected in the EXT2mut-expressing cells, whereas mRNA for the mutated EXT2 was indeed present in the cell clones as demonstrated by Northern blotting (data not shown). When these cells were tested for expression of NDST1 by Western blotting, it was apparent that no up-regulation of NDST1 expression had occurred (Fig. 1B). Also, N-deacetylase activity was the same in cells before and after EXT2mut transfection (data not shown). These results demonstrate that the observed effect on NDST1 expression is mediated by the EXT2 protein.
Fig. 1.
Protein expression and enzyme activity of NDST1. (A and B) NDST1 protein expression was analyzed by SDS/PAGE (10% gels) and Western blotting after solubilization of the cells in 1% Triton X-100-containing buffer. The separated proteins (20 μg) were blotted to a nitrocellulose membrane, and NDST1 was detected with anti-NDST1 peptide antibody 1A. (C and D) N-deacetylase (C) and N-sulfotransferase (D) activity of the cell lines examined in A were measured as described in Materials and Methods. Each bar in C and D represents the mean of two samples.
Altered HS Sulfation After EXT1 and EXT2 Overexpression.
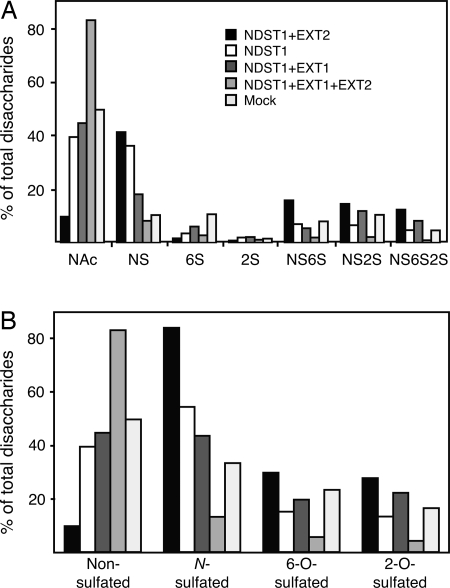
The structure of HS synthesized by the different HEK 293 cell lines was analyzed. Glycosaminoglycans were isolated from the cells and subjected to digestion with the chondroitinase ABC. After chromatography on DEAE Sephacel to remove the chondroitin sulfate degradation products, the remaining HS was treated with heparinase I, II, and III. The generated HS disaccharides were then analyzed by reversed-phase ion-pair (RPIP) HPLC. HS from cells overexpressing NDST1 together with EXT2 contained >80% N-sulfate groups compared with ≈50% in HS from cells expressing only NDST1 (Table 1). Cells coexpressing NDST1 and EXT1 showed a decrease in HS N-sulfation as did cells overexpressing all three enzymes (Table 1). Also O-sulfation was affected by EXT expression (Fig. 2). Cells coexpressing NDST1 and EXT2 synthesized HS with increased 2-O and 6-O sulfation, whereas HS from cells expressing both EXT1, EXT2, and NDST1 contained fewer O-sulfate groups. Unlike in EXT1/NDST1-overexpressing cells, 2-O and 6-O sulfation was increased.
Table 1.
Heparan sulfate N-sulfation in different cell lines
| Transfected cell line | Degree of N-sulfation, % |
|---|---|
| NDST11 | 54 |
| NDST1 | 59 |
| NDST1 + EXT1 | 43 |
| NDST1 + EXT1 | 40 |
| NDST1 + EXT2 | 84 |
| NDST1 + EXT2 | 85 |
| NDST1 + EXT1 + EXT2 | 13 |
The relative amount of N-sulfated disaccharides in HS synthesized by cells overexpressing NDST1, EXT1, and EXT2 in different combinations was determined by RPIP HPLC. Two different cell lines were analyzed except for the single line expressing all three enzymes. The values are means of two individual samples except for NDST11, which is the mean of four determinations.
Fig. 2.
Disaccharide analysis of HS from the different cell lines. HS disaccharide composition was determined by RPIP HPLC. (A) The relative amount of the different disaccharides shown is the mean value of two determinations of single cell lines. Nac, HexA-GlcNAc; NS, HexA-GlcNS; 6S, HexA-GlcNAc(6OS); 2S, HexA(2OS)-GlcNAc; NS6S, HexA-GlcNS(6OS); NS2S, HexA(2OS)-GlcNS; NS6S2S, HexA (2OS)-GlcNS(6OS). (B) Percentage of disaccharides with different modifications calculated from the data in A.
NDST1 Interacts with EXT2.
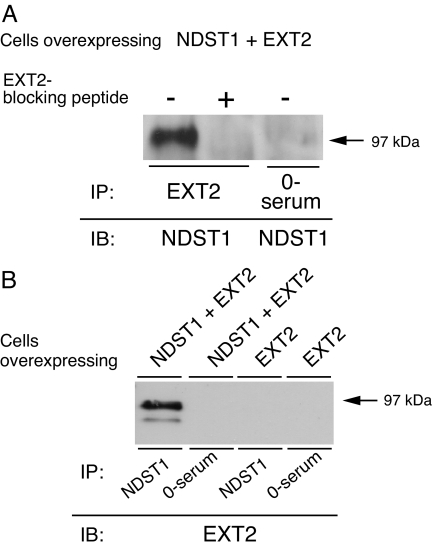
To look for an interaction between NDST1 and EXT2, solubilized cells coexpressing NDST1 and EXT2 were incubated with EXT2 antibodies followed by capturing of immunocomplexes on Protein A Sepharose. After washing, the Protein A Sepharose beads were boiled in SDS/PAGE loading buffer and analyzed by SDS/PAGE followed by Western blotting using an antibody recognizing NDST1. As shown in Fig. 3A, NDST1 protein was found in the immunoprecipitate when EXT2 antibodies were used in the immunoprecipitation (IP), but was absent when preimmune serum or EXT2 antibodies preincubated with an EXT2 blocking peptide were used (Fig. 3A). The interaction between NDST1 and EXT2 could also be established when NDST1/EXT2-coexpressing cells were incubated with NDST1 antibodies, and the presence of EXT2 protein in the immunoprecipitates was analyzed. Western blotting demonstrated that EXT2 protein was found in the immunocomplexes formed with the NDST1 antibodies but was absent when preimmune serum was used for the IP (Fig. 3B). In cells overexpressing only EXT2, no EXT2 protein could be detected after IP with the NDST1 antibodies (Fig. 3B).
Fig. 3.
Coprecipitation of NDST1 and EXT2. (A) Lysates from NDST1/EXT2-overexpressing cells were incubated with preimmune serum or EXT2 antibodies (N15) with and without prior preincubation with EXT2 blocking peptide. NDST1 was detected after separation of immune complexes by SDS/PAGE (10% gels) and immunoblotting using α-trunc-NDST1 antibodies. (B) Lysates from NDST1/EXT2- or EXT2-overexpressing cells were incubated with α-trunc-NDST1 antibodies and preimmune serum, respectively. EXT2 was detected after separation of immune complexes by SDS/PAGE (10% gels) and immunoblotting using EXT2 antibodies (N15).
Increased NDST1 Glycosylation in EXT2-Overexpressing Cells.
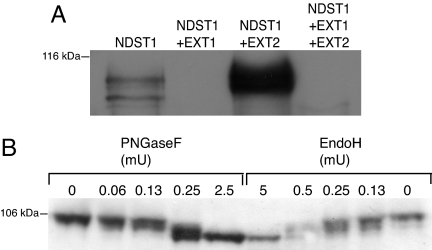
NDST1 has four potential N-glycosylation sites. Of these at least three are occupied, as shown by PNGaseF treatment (data not shown). In cells overexpressing NDST1, unglycosylated and differently N-glycosylated forms of the protein can be detected (Fig. 4A). In cells transfected with both NDST1 and EXT2, no unglycosylated NDST1 is found. Instead, NDST1 with two or three N-glycans accumulate (Fig. 4A). The N-glycans of NDST1 were completely removed by both PNGase F and endoglycosidase H (endo H) (Fig. 4B). The susceptibility to endo H indicates that the NDST1 glycans are of the high mannose type, which are present on glycoproteins in the endoplasmic reticulum (ER) and in the cis/medial compartments of Golgi but not in trans-Golgi and the trans-Golgi network (TGN).
Fig. 4.
Glycosylation of NDST1 protein. (A) Cell lysates were analyzed by 10% SDS/PAGE and Western blotting as described in the legend to Fig. 1, apart from a longer separation time. (B) Lysates from NDST1/EXT2-overexpressing cells were incubated with the indicated amounts of PNGaseF or endo H followed by SDS/PAGE (7.5% gel) and Western blotting.
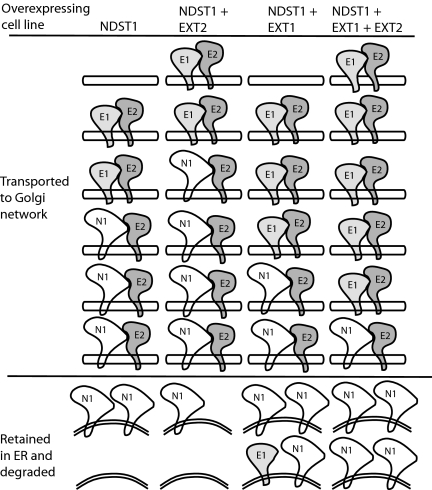
GAGosome Model.
A model to explain our results in light of the demonstrated interaction between NDST1 and EXT2 and the increased N-glycosylation of NDST1 when coexpressed with EXT2 is presented in Fig. 5. In NDST1-overexpressing cells, endogenous EXT2 transports endogenous EXT1 and a fraction of the translated NDST1, whereas nonbound excess NDST1 is destined for degradation. When both NDST1 and EXT2 are overexpressed, a larger fraction of the excess NDST1 is bound to EXT2 and is transported to its correct location in the Golgi compartment. If instead EXT1 is coexpressed together with NDST1, EXT1 will occupy most of the EXT2 proteins, resulting in massive NDST1 degradation. Finally, when all three proteins are overexpressed together, EXT1 wins over NDST1 and is exported to the Golgi compartment at the expense of NDST1. This model thus predicts that the relative concentrations of the three proteins will determine the GAGosome composition, which in turn will determine the structure of the HS chain synthesized.
Fig. 5.
GAGosome model of EXT–NDST1 interactions. NDST1, N1; EXT1, E1; EXT2, E2.
NDST Activities in Mice Overexpressing EXT2.
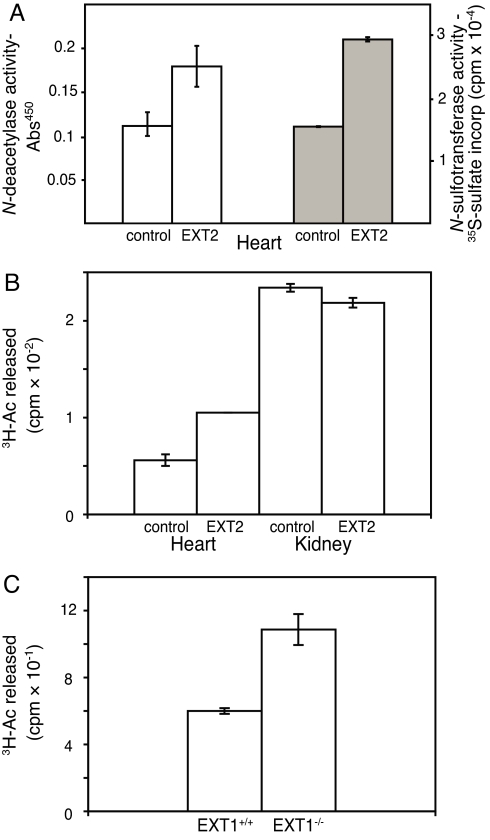
Because the results presented were obtained in vitro we wanted to test the model in an in vivo system. We therefore analyzed NDST activities in tissue extracts from EXT2-overexpressing mice. For unknown reasons the protein was expressed only in some tissues, but not detected in others. Heart tissue showed a high expression of EXT2, whereas kidney extract was devoid of the protein (as determined by Western blotting; data not shown). Indeed, both N-deacetylase and N-sulfotransferase activities were increased in heart tissue from the transgenic mice compared with WT mice (Fig. 6A), whereas enzyme activity in kidney extracts from control and transgenic mice were similar (Fig. 6B).
Fig. 6.
Validation of the GAGosome model. (A) N-deacetylase activity (open bars, as measured by an ELISA method) and N-sulfotransferase activity (filled bars) in heart tissue from two control and two EXT2 transgenic mice. (B) N-deacetylase activity in heart and kidney tissue from one control and one transgenic mouse, measured in duplicate. (C) N-deacetylase activity in fibroblasts from EXT1Gt/Gt and control mice. Each sample was measured in triplicate.
The model would also predict that lowering the concentration of the EXT proteins also would affect NDST1 levels. To investigate whether this was the case, we took advantage of EXT1Gt/Gt fibroblasts, which through a gene trap mutation in EXT1 express drastically reduced levels of the protein (19). As predicted, the N-deacetylase activity in EXT1Gt/Gt fibroblasts was higher compared with WT cells (Fig. 6C).
Discussion
The GAGosome concept was introduced in a review by Esko and Selleck in 2002 (1), where the GAGosome was defined as a physical complex of enzymes committed to the assembly of HS. It was also speculated that the stochiometry and composition of the complexes could affect the fine structure of the chains. Colocalization and complex formation of HS biosynthesis enzymes have previously been shown for EXT1 and EXT2 and for the 2-O-sulfotransferase and the C5-epimerase (11, 12, 15). An interaction between the xylosyltransferase and galactosyltransferase 1 has also been reported (16, 17). Here, we demonstrate that the relative amounts of NDST1, EXT1, and EXT2 determine the outcome of HS biosynthesis. The results obtained in the EXT2 transgenic mice (Fig. 6 A and B) demonstrate that the relative expression of the enzymes will influence enzyme activity also in vivo. In addition, the increased N-deacetylase activity in EXT1Gt/Gt fibroblasts supports a reverse relationship between these two enzymes.
The massive increase in NDST activity and protein content in EXT2-coexpressing cells (Fig. 1) can be explained if NDST1 depends on EXT2 for transport to the Golgi compartment. The reduction of NDST1 protein and enzyme activity in the presence of EXT1 may indicate that NDST1 and EXT1 compete for binding to EXT2, with unbound NDST1 possibly retained in the ER and degraded. Also, in HEK293 cells transfected with EXT1 but not with NDST1, a reduction in the endogenous NDST activity could be recorded, whereas no or little effect on NDST activity was seen in EXT2-transfected cells (data not shown). As recently discussed (26), EXT2 appears to be present in a molar excess over EXT1 and the lack of effect of EXT2 overexpression on endogenous NDST1 may simply be caused by the limited availability of NDST1 in HEK 293 cells.
Most interestingly, the altered levels of NDST1 expression have prominent effects on HS structure. In HS from EXT2/NDST1-overexpressing cells, the content of N-sulfate groups was increased to >80% compared with 54% in NDST1-overexpressing cells (Table 1), and also O-sulfation was increased (Fig. 2). The polysaccharide synthesized in these cells actually resembles heparin. In EXT1/NDST1-overexpressing cells HS N-sulfation had dropped to ≈40%, whereas the N-sulfate content in HS from the cells overexpressing all three enzymes was as low as 13% (Table 1). Although we would like to think that the large decrease in HS N-sulfation in these cells is caused by a higher affinity of the EXT1–EXT2 than the NDST1–EXT2 interaction, it is possible that ER stress may have caused an increased protein degradation (20).
Because no fraction of the analyzed NDST1 proteins contained endo H-resistant glycans (Fig. 4B), it appears likely that the mature protein is localized to cis/medial Golgi and less likely that the endo H-susceptible NDST1 molecules all represent newly synthesized unprocessed ER components. Also, other HS biosynthesis enzymes have previously been demonstrated to colocalize with markers for medial Golgi (15, 21, 22), and studies with brefeldin A have suggested that HS biosynthesis occurs proximal to trans-Golgi/TGN where CS biosynthesis takes place (23, 24). So, from our present data we cannot determine whether the interaction between NDST1 and EXT2 occurs in the ER or the Golgi compartment or if it is already established in the ER and persists to the Golgi compartment.
However, what we can conclude is that the relative concentration of EXT1, EXT2, and NDST1 will greatly influence the sulfation pattern of HS synthesized just as predicted in the GAGosome model (1).
Materials and Methods
Cloning, Transfection, and Cell Lines.
Mouse EXT1 and EXT2 cDNA (GenBank accession nos. NM_010162 and NM_010163, respectively) alone or in combination, were cloned into the pBudCE4.1 vector (Invitrogen), which contains two multiple cloning sites (MCS) and is designed for simultaneous expression of two proteins. EXT1 was cloned into the MCS with the cytomegalovirus (CMV) immediate-early promotor and EXT2 into the MCS with the human elongation factor 1α (EF1α) promotor. The three constructs and empty pBudCE4.1 vector alone were transfected into human embryonic kidney (HEK) 293 cells stably expressing NDST1 from the pCDNA3 vector (25), by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Stable clones were selected by using 0.4 mg/ml Zeocin and 0.8 mg/ml G-418 and were grown in DMEM + l-glutamine, containing 10% FCS, 2.5 units/ml fungizone, and 100 units/ml per 100 μg/ml penicillin/streptomycin. Two clones expressing EXT1 or EXT2 together with NDST1 and mock-transfected NDST1-expressing cells, and one clone coexpressing EXT1, EXT2, and NDST1 were selected and maintained. HEK 293 cells overexpressing EXT2 have been described (26).
Two point mutations were introduced into EXT2 cDNA to prevent its translation. The QuikChange site-directed mutagenesis kit (Stratagene) was used for this purpose. The first mutation was made by using the primer 5′-CTCATCCCAAGGATGTAGACCAAGCACCGAATC-3′ and its complementary sequence, with the altered nucleotide in bold. This alteration introduces a stop codon at position 55–57 bp of the coding sequence. The second mutation was made by using primer 5′-CATACGTGTTTCGATGTCTAGCGCTGCGGCTTC-3′and its complementary sequence, with the altered nucleotide in bold, which will introduce a stop codon at position 280–282 bp. The mutated EXT2 (EXT2mut) cDNA cloned into the pBudCE4.1 vector was transfected into HEK 293 cells overexpressing NDST1, and stable clones were selected as described above. Immortalized fibroblasts obtained from WT and homozygous EXT1Gt/Gt mice (19), were cultured in DMEM as described above, but without G418 and zeocin.
PCR of Cell Clones.
Cells from a T 25 flask were trypsinized and washed with cold PBS. Approximately 5% of the cells were solubilized in 0.03 M NaOH and 0.05% SDS. After incubation at 95°C the lysates, diluted 25 times, were used for PCR. Primers for verification of EXT1 transfections were forward primer 5′-TAATACGACTCACTATAGGG, specific for the CMV promotor, and reverse primer 5′-TCAGTGTAGTCAGGCC, specific for EXT1. Primers for verification of EXT2 transfection were forward primer 5′-TCAAGCCTCAGACAGTGGTTC, specific for the EF1α promotor, and reverse primer 5′-GAGCTCCAGGCAACATA, specific for EXT2.
NDST Assays of Cell Clones.
Cells in a T 25 flask were solubilized for 30 min at 4°C in 0.15 ml of solubilization buffer [1% Triton X-100, 50 mM Tris·HCl (pH 7.4), 2 mM EDTA, 1 mM PMSF, and 10 μg/ml pepstatin]. Protein concentration was determined by using the Bio-Rad protein assay. N-deacetylase and N-sulfotransferase activities were analyzed as described (27). Shortly, N-deacetylase activity was measured by incubating solubilized cells (80 μg of protein) and 10,000 cpm of N-[3H]acetyl-labeled K5 capsular polysaccharide for 30 min at 37°C in 0.2 ml, containing 50 mM Mes (pH 6.3), 10 mM MnCl2, and 1% Triton X-100. The released [3H]acetate was detected in a biphasic scintillation counting system.
N-sulfotransferase activity was analyzed by measuring incorporation of 35S from the sulfate donor [35S]PAPS into N-deacetylated Escherichia coli capsular K5 polysaccharide as a substrate. Solubilized cells (80 μg of protein) were incubated with substrate and 2 μCi [35S]PAPS in 50 mM Hepes (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, 5 mM CaCl2, 3.5 μM NaF, and 1% Triton X-100 in 0.1 ml for 30 min at 37°C. The polysaccharide was precipitated with ethanol for 4 h, separated from excess [35S]PAPS by Sephadex G25 superfine (GE Healthcare) gel filtration, and quantified by scintillation counting.
Generation of Transgenic Mice Overexpressing EXT2.
EXT2 cDNA was also inserted into the pCAGGS expression vector (gift from J. Minyazaki, Osaka University, Osaka, Japan). The expression is under the control of the CMV immediate-early enhancer and the chicken β-actin promoter (CAG) for constitutively high tissue expression from fertilized eggs and early embryonic stage through adulthood. The cDNA construct was cloned into the unique EcoR1 site between the CAG promoter and the rabbit β-globin sequence. Verification of 5′ to 3′ direction of the construct was made by PCR, using forward primer 5′-CATGCCTTCTTCTTTTTCCT-3′, specific for the CAG promoter of the vector, and reverse primer 5′-GGCTTCTAGTTCCTCTCTGTACTC-3′, specific for EXT2 cDNA. The vector was linearized with BamH1 and SalI and injected into B6CBAF1 oocytes. Genomic integration was verified by PCR of genomic DNA obtained by extractions of tail biopsies. PCR was performed with the primers specific for the CAG promoter of the vector and for EXT2 cDNA as described above. Transgene progeny (founder mice) from implanted foster mice were mated with WT B6CBAF1 mice to generate F1 generation. Mating of F1 siblings was performed to generate F2 transgenic mice.
NDST Assays on Heart and Kidney Tissue from EXT2-Overexpressing Mice.
Heart and kidney tissue from control and EXT2-overexpressing adult mice were homogenized in solubilization buffer containing 1% Triton-X100, 50 mM Tris·HCl (pH 7.5), 2 mM EDTA, 1 mM Pefablock, and 10 μg/ml pepstatin A. N-deacetylase activity was analyzed by using an ELISA-based assay. Shortly, 50 μg/ml of E. coli K5 capsular polysaccharide in PBS was used to coat a F96 Maxisorp Nunc-immuno plate (Nunc) overnight. After washing with TBS-Tween, N-deacetylase assay mix (as described above) was added to the wells together with the samples (0.1 mg of solubilized protein) and incubated for 30 min at 37°C. Another washing step with TBS-Tween was performed, and the wells were then blocked with 1% gelatin in TBS for 2 h in room temperature. Incubation with mAb JM 403 (28), diluted 1:20,000 in TBS-Tween for 1 h was followed by washing and incubation with anti-mouse IgM-peroxidase, diluted 1:800 in TBS-Tween for 1 h at room temperature. The ELISA was developed by incubating 15 min with peroxidase substrate containing 0.11 M NaAc, 80 μg/ml 3,5,3′,5′ tetramethylbenzidine, and 0.03% H2O2. The reaction was stopped by the addition of 2 M H2SO4 and absorbance was measured at 450 nm. N-sulfotransferase assay was performed on 0.1 mg solubilized protein as described above, using 1.25 μCi [35S]PAPS.
SDS/PAGE and Western Blotting.
Trypsinized cells were solubilized and protein concentration was determined by using the Bio-Rad assay. After SDS/PAGE, the separated proteins were electroblotted (Bio-Rad) to Hybond ECL nitrocellulose membranes (GE Healthcare). The membranes were blocked in 7% milk in TBS-Tween buffer, followed by incubation with the indicated antibodies in blocking buffer. As secondary antibody, anti-rabbit HRP-conjugated antibody (GE Healthcare), or anti-goat IgG (HRP; Santa Cruz) diluted 1:5,000 were used. The filters were developed in an ECL system (RPN 2106; Amersham Biosciences) and exposed to Fuji film.
Structural Analysis of HS Using RPIP HPLC.
Unlabeled glycosaminoglycans were isolated from one T175 flask for each of the different cell lines as described (7). The isolated polysaccharide was degraded to disaccharides by enzymatic cleavage. The generated HS disaccharides were subjected to RPIP HPLC analysis followed by post column derivatization with cyanoacetamide and detection in a fluorescence detector (7).
Preparation of Rabbit α-Trunc-NDST1 Antibody.
Recombinant mNDST1 (amino acids 48–882) with an added N-terminal His tag was produced in HEK 293 cells. The recombinant enzyme lacks the cytoplasmic tail and transmembrane region and is secreted into the culture medium. After affinity-chromatography on Ni-NTA agarose (Qiagen), the purified protein was used to produce antibodies in a rabbit (α-trunc-NDST1).
Immunoprecipitation.
Cells coexpressing NDST1 and EXT2 and cells overexpressing only EXT2 (see above) were harvested from T75 cell flasks, washed in PBS, and solubilized in 750 μl of solubilization buffer. For EXT2 IP, cells coexpressing NDST1 and EXT2 from three T75 flasks were pooled, solubilized, and divided into three equal parts. NaCl was added to the supernatants to a final concentration of 0.15 M followed by preincubation end over end for 1 h at 4°C with 40 μl/sample of Protein A Sepharose CL-4B (50/50 slurry) (GE Healthcare). The Protein A Sepharose was discarded after centrifugation at 16,000 × g for 20 s, and to the supernatants was added either 5 μl of rabbit α-trunc-NDST1 antibody or 5 μl of goat anti-EXT2 antibody N15 (Santa Cruz) followed by end-over-end incubation for 1 h at 4°C. One sample was incubated with EXT2 antibody that had been preincubated 30 min with the corresponding blocking peptide (Santa Cruz) in a 1:200 molar ratio. As a negative control, one sample was incubated with preimmune serum from the rabbit injected with trunc-NDST1. Samples containing the EXT2 antibody were incubated an extra hour with rabbit anti-goat IgG (Jackson ImmunoResearch). Next, 40 μl of Protein A Sepharose (50/50 slurry) was added to each sample followed by incubation for 1 h as before. The Protein A Sepharose beads were washed once with 0.5 M NaCl, 0.1% Triton X-100 in 50 mM Tris·HCl, pH 7.4. After a final centrifugation at 16,000 × g, 18 μl of washing buffer and 6 μl of 4× SDS/PAGE loading dye were added to each pellet followed by denaturation for 5 min at 96°C. SDS/PAGE and Western blotting were performed as described above by using 1:1,000 dilutions of both primary antibodies.
Deglycosylation.
One hundred micrograms of protein from lysates of cells overexpressing NDST1 alone or NDST1 and EXT2 in combination was treated for 2 h at 37°C with either PNGaseF (Calbiochem; no. 362185) or endo H (Calbiochem; no. 324717) according to the manufacturer's protocol. After incubation, one-third of the samples were analyzed by SDS/PAGE followed by Western blotting with NDST1 antibody 1A (18).
Acknowledgments.
We thank Eva Hjertson of Uppsala University for excellent technical assistance and the Uppsala University Transgenic Facility for generating the EXT2 transgenic mice. This work was funded by the Swedish Research Council, the program Glycoconjugates in Biological Systems sponsored by the Swedish Foundation for Strategic Research, Polysackaridforskning AB, and Gustav V:s 80-årsfond.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Esko JD, Selleck SB. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 2.Bulow HE, Hobert O. Annu Rev Cell Dev Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 4.Esko JD, Lindahl U. J Clin Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugahara K, Kitagawa H. IUBMB Life. 2002;54:163–175. doi: 10.1080/15216540214928. [DOI] [PubMed] [Google Scholar]
- 6.Kreuger J, Spillmann D, Li JP, Lindahl U. J Cell Biol. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledin J, Staatz W, Li JP, Gotte M, Selleck S, Kjellen L, Spillmann D. J Biol Chem. 2004;279:42732–42741. doi: 10.1074/jbc.M405382200. [DOI] [PubMed] [Google Scholar]
- 8.Morii E, Ogihara H, Oboki K, Sawa C, Sakuma T, Nomura S, Esko JD, Handa H, Kitamura Y. Blood. 2001;97:3032–3039. doi: 10.1182/blood.v97.10.3032. [DOI] [PubMed] [Google Scholar]
- 9.Grobe K, Esko JD. J Biol Chem. 2002;277:30699–30706. doi: 10.1074/jbc.M111904200. [DOI] [PubMed] [Google Scholar]
- 10.Aikawa J, Grobe K, Tsujimoto M, Esko JD. J Biol Chem. 2001;276:5876–5882. doi: 10.1074/jbc.M009606200. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Morimoto K, Shimizu T, Takahashi M, Kurosawa H, Shirasawa T. Biochem Biophys Res Commun. 2000;268:860–867. doi: 10.1006/bbrc.2000.2219. [DOI] [PubMed] [Google Scholar]
- 12.McCormick C, Duncan G, Goutsos KT, Tufaro F. Proc Natl Acad Sci USA. 2000;97:668–673. doi: 10.1073/pnas.97.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senay C, Lind T, Muguruma K, Tone Y, Kitagawa H, Sugahara K, Lidholt K, Lindahl U, Kusche-Gullberg M. EMBO Rep. 2000;1:282–286. doi: 10.1093/embo-reports/kvd045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busse M, Kusche-Gullberg M. J Biol Chem. 2003;278:41333–41337. doi: 10.1074/jbc.M308314200. [DOI] [PubMed] [Google Scholar]
- 15.Pinhal MA, Smith B, Olson S, Aikawa J, Kimata K, Esko JD. Proc Natl Acad Sci USA. 2001;98:12984–12989. doi: 10.1073/pnas.241175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz NB, Roden L, Dorfman A. Biochem Biophys Res Commun. 1974;56:717–724. doi: 10.1016/0006-291x(74)90664-0. [DOI] [PubMed] [Google Scholar]
- 17.Silbert JE, Sugumaran G. IUBMB Life. 2002;54:177–186. doi: 10.1080/15216540214923. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson J, Eriksson I, Kjellen L. Biochemistry. 2003;42:2110–2115. doi: 10.1021/bi026928g. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Busse M, Ueno M, Kelly OG, Skarnes WC, Sugahara K, Kusche-Gullberg M. J Biol Chem. 2004;279:32134–32141. doi: 10.1074/jbc.M312624200. [DOI] [PubMed] [Google Scholar]
- 20.Schroder M, Kaufman RJ. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 21.Crawford BE, Olson SK, Esko JD, Pinhal MA. J Biol Chem. 2001;276:21538–21543. doi: 10.1074/jbc.M100880200. [DOI] [PubMed] [Google Scholar]
- 22.Nagai N, Habuchi H, Esko JD, Kimata K. J Cell Sci. 2004;117:3331–3341. doi: 10.1242/jcs.01191. [DOI] [PubMed] [Google Scholar]
- 23.Kolset SO, Prydz K, Fjeldstad K, Safaiyan F, Vuong TT, Gottfridsson E, Salmivirta M. Biochem J. 2002;362:359–366. doi: 10.1042/0264-6021:3620359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlin-Hansen L, Yanagishita M. J Biol Chem. 1993;268:17370–17376. [PubMed] [Google Scholar]
- 25.Pikas DS, Eriksson I, Kjellen L. Biochemistry. 2000;39:4552–4558. doi: 10.1021/bi992524l. [DOI] [PubMed] [Google Scholar]
- 26.Busse M, Feta A, Presto J, Wilen M, Gronning M, Kjellen L, Kusche-Gullberg M. J Biol Chem. 2007;282:32802–32810. doi: 10.1074/jbc.M703560200. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson I, Kusche M, Unger E, Wlad H, Nylund L, Lindahl U, Kjellen L. J Biol Chem. 1991;266:8044–8049. [PubMed] [Google Scholar]
- 28.van den Born J, Gunnarsson K, Bakker MAH, Kjellén L, Kusche-Gullberg M, Maccarana M, Berden JHM, Lindahl U. J Biol Chem. 1995;270:31303–31309. doi: 10.1074/jbc.270.52.31303. [DOI] [PubMed] [Google Scholar]