Abstract
The highly pathogenic avian influenza (HPAI) H5N1 virus that emerged in southern China in the mid-1990s has in recent years evolved into the first HPAI panzootic. In many countries where the virus was detected, the virus was successfully controlled, whereas other countries face periodic reoccurrence despite significant control efforts. A central question is to understand the factors favoring the continuing reoccurrence of the virus. The abundance of domestic ducks, in particular free-grazing ducks feeding in intensive rice cropping areas, has been identified as one such risk factor based on separate studies carried out in Thailand and Vietnam. In addition, recent extensive progress was made in the spatial prediction of rice cropping intensity obtained through satellite imagery processing. This article analyses the statistical association between the recorded HPAI H5N1 virus presence and a set of five key environmental variables comprising elevation, human population, chicken numbers, duck numbers, and rice cropping intensity for three synchronous epidemic waves in Thailand and Vietnam. A consistent pattern emerges suggesting risk to be associated with duck abundance, human population, and rice cropping intensity in contrast to a relatively low association with chicken numbers. A statistical risk model based on the second epidemic wave data in Thailand is found to maintain its predictive power when extrapolated to Vietnam, which supports its application to other countries with similar agro-ecological conditions such as Laos or Cambodia. The model's potential application to mapping HPAI H5N1 disease risk in Indonesia is discussed.
Keywords: animal husbandry, epidemiology, remote sensing, spatial modeling
Highly pathogenic avian influenza (HPAI) H5N1 virus emerged in Southern China in the mid-1990s (1), but the first large-scale epizootic took place in the winter of 2003/2004 in East and Southeast Asia (2). The virus persisted in the region until the winter of 2005/2006 when it spread westward across the Palearctic zoogeographical region (as of August 6, 2007, 60 countries have reported the virus; ref. 3). The impact in the affected countries comprises human disease and death (194 people of 321 cases as of August 16, 2007; ref. 4 and www.who.int/csr/disease/avian_influenza/country/en), mortality in poultry and birds culled to halt the spread of disease, and the loss of local and international trade of poultry and poultry products. In Southeast Asia alone, it has been estimated that HPAI H5N1 virus outbreaks caused the death of 140 million domestic birds with economic losses at ≈$10 billion (5). Importantly, reducing virus circulation in the poultry sector is the best way to prevent human infections and a possible mutation in a form that could pass between humans (6).
The rapid spread of HPAI H5N1 virus results from its ability to transmit through both human and bird host contact systems (7). However, even if HPAI H5N1 virus has been introduced, local development vary greatly as the virus does not become established, spread, and persist everywhere equally. Virus establishment is influenced by the extent of surveillance and early detection, and therefore it is subject to an unknown degree of underreporting bias. Once established, HPAI H5N1 virus spread is believed to be influenced primarily by local trade patterns, density of wet markets, poultry production structure, and disease control and preventive efforts. Persistence is thought to be mediated by domestic ducks because of their potential role as virus reservoir (8–10), but the large live poultry markets also probably contribute (11). However, even when the main risk factors associated with local introduction, spread, and persistence are broadly known, it remains challenging to quantify their relative importance and contribution and to define HPAI H5N1 virus outbreak risk in space and time (location-sensitive “hot spots” and time-sensitive “hot times”).
With the panzootic originating and persisting in East and Southeast Asia, it is of interest to concentrate on this region to quantify how established risk factors are associated with disease patterns. Vietnam and Thailand are interesting in that regard because they were both subject to several epidemic waves, applied differential control strategies, succeeded in temporarily controlling the virus, and were faced with periodic reoccurrences (Fig. 1). Vietnam undertook massive repeated vaccination campaigns in combination with other control measures. Thailand has not implemented vaccination and placed emphasis on early detection, prevention of movements in high-risk areas, including premovement testing, and transformation of the free-ranging duck production sector (12). It is difficult to assess whether the reoccurrences resulted from local persistence of the virus or new introductions, but recent results suggest that Southeast Asia may constitute a regional “evolutionary sink” for HPAI H5N1 virus (13), supporting the idea that the region faces periodic reintroductions.
Fig. 1.
Temporal distribution of daily HPAI H5N1 virus records reported in Thailand (Upper) and Vietnam (Lower).
In previous work, it was demonstrated that the risk of HPAI H5N1 virus presence was associated with free-ranging duck numbers in Thailand (10) and the local abundance of both duck and geese in Vietnam (14), in addition to other risk factors such as chicken numbers, human population, and topographical features. Both studies also found evidences of a relationship between HPAI H5N1 virus presence and rice production. Free-ranging duck production in Thailand is largely confined within areas with double or triple rice cultivation per year because the rice grain left in the field after harvest provides a low-cost source of feed for duck production (15). Similar coupled production systems associating free-ranging ducks and multiple-rice agriculture are observed across Southeast Asia, including Vietnam (e.g., ref. 16) and Indonesia. Rice paddy fields are an important habitat for free-ranging ducks, but also for wild waterfowl exploiting the same food resource in the wintering season, and thus they may form a critical risk factor in HPAI H5N1 virus introduction, persistence, and spread. In recent years, substantial progress has been achieved in methods for predicting rice crop distributions. It is now possible to routinely map and monitor rice paddy agriculture (17, 18) and cropping intensity in Asia, using images from the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor onboard the National Aeronautics and Space Administration Terra satellite. The satellite image-based algorithms permit the production of maps of cropping intensity, cropping calendar (planting and harvesting dates), and irrigation practices at moderate spatial resolution (250–500 m) and in near-real-time fashion.
This article has three main objectives: first, to compare risk factors associated with HPAI H5N1 virus presence in Thailand and Vietnam during the three epidemic waves that took place between early 2004 and end 2005 by using the same set of risk factors (elevation, human population, and chicken and duck density); second, to evaluate and compare the added value of rice cropping variables for predicting HPAI H5N1 virus presence in the two countries; and third, to evaluate the predictive power of an HPAI H5N1 virus risk map for Southeast Asia, by developing a model for Thailand, testing it for Vietnam, and applying it to the region.
Results
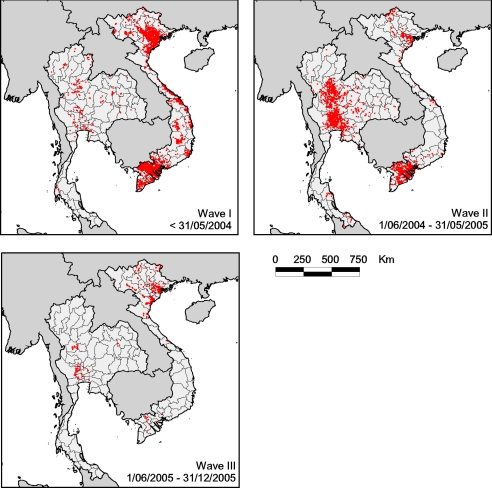
The three H5N1 epidemic waves that have affected Thailand and Vietnam show distinct temporal and spatial distributions (Figs. 1 and 2). The spatial pattern of wave II in Thailand is explored in further detail in Gilbert et al. (10) and Tiensin et al. (19), and the temporal and spatial patterns of waves I–III in Vietnam are examined in Pfeiffer et al. (14). However, the results obtained from the statistical analysis of outbreak distributions in relation to risk factors are more consistent than one would expect on the basis of the contrasted spatial patterns between waves (Table 1). The variables showing the highest level of consistency in their relationship with HPAI H5N1 virus presence are human population, rice cropping intensity, and, to a somewhat lesser extent, duck numbers, all of which were found to be significantly associated with virus presence in both countries. Chicken numbers failed to show as a significant predictor of disease presence, except in wave III in Vietnam. The latter is probably because wave III primarily affected the Red River delta where chickens are far more abundant than in the rest of the country. If one considers only the northern part of Vietnam in the analysis [supporting information (SI) Table S1], chicken number is no longer a highly significant predictor of HPAI H5N1 virus presence. The number of ducks was positively associated with HPAI H5N1 virus presence in Thailand waves I and II and Vietnam wave II. Elevation was negatively related to virus presence only in Thailand during waves I and II, although the significance of the change in −2 log likelihood (LL) upon removal is close to 0.05 for Vietnam wave I. All significant variables showed consistency in similar sign in all models where they were significantly related to HPAI H5N1 virus presence. The predictive power of the models, as measured by the area under the curve (AUC) (Table 1), receiver-operating characteristic (ROC) curves (Fig. S1) and Cohen's kappa is moderate, with the AUC value ranging from 0.66 to 0.88.
Fig. 2.
Spatial distribution of HPAI H5N1 virus records reported during the three main epidemic waves in Thailand and Vietnam.
Table 1.
Summary results of the autologistic regression models for the three main HPAI H5N1 virus epidemic waves in Thailand and Vietnam between 2004 and 2005
| Wave | Cst | Alt | Hpop | Ch | Du | CropMean | ArT | AUC, ± SD | kappa, ± SD | Pseudo-R2, ± SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Thailand I | −1.05 | 0.00103* | 2.25 10−5 | 3.39 10−6 | 2.03 10−5 | 0.556 | 247 | 0.66 ± 0.023 | 0.26 ± 0.043 | 0.062 ± 0.019 |
| 2.31† | 5.46 | 2.98 | 7.14 | 4.13 | 22.4 | |||||
| P = 0.129‡ | P = 0.0194 | P = 0.08411 | P = 0.00753 | P = 0.0422 | P < 0.001 | |||||
| Thailand II | −1.143 | −0.00219 | 1.06 10−5 | 1.88 10−7 | 3.04 10−5 | 0.964 | 88.6 | 0.79 ± 0.0073 | 0.49 ± 0.018 | 0.19 ± 0.012 |
| 25.7 | 5.92 | 1.07 | 44.7 | 46.8 | 65.8 | |||||
| P < 0.001 | P = 0.0150 | P = 0.301 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Thailand III | 0.0634 | −0.0128 | 5.99 10−5 | 1.69 10−6 | 5.51 10−5 | 0.777 | 1486 | 0.88 ± 0.034 | 0.67 ± 0.073 | 0.40 ± 0.079 |
| 19.3 | 2.99 | 1.49 | 5.04 | 2.03 | 5.58 | |||||
| P < 0.001 | P = 0.084 | P = 0.222 | P = 0.024 | P = 0.154 | P = 0.0181 | |||||
| Vietnam I | −1.74 | −0.00021 | 9.19 10−5 | 4.71 10−6 | 6.09 10−6 | 0.188 | 63.1 | 0.69 ± 0.0049 | 0.27 ± 0.009 | 0.078 ± 0.0042 |
| 3.83 | 90.0 | 3.56 | 2.49 | 5.73 | 808 | |||||
| P = 0.0503 | P < 0.001 | P = 0.0591 | P = 0.115 | P = 0.0167 | P < 0.001 | |||||
| Vietnam II | −2.67 | 0.000328 | 9.22 10−5 | −2.11 10−6 | 2.85 10−5 | 0.730 | 160 | 0.77 ± 0.0085 | 0.41 ± 0.018 | 0.17 ± 0.013 |
| 1.39 | 19.8 | 1.45 | 12.1 | 19.3 | 312 | |||||
| P = 0.238 | P < 0.001 | P = 0.229 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Vietnam III | −1.58 | 0.000408 | −3.90 10−5 | 3.43 10−5 | 6.4 10−6 | 0.497 | 245 | 0.66 ± 0.018 | 0.25 ± 0.031 | 0.055 ± 0.014 |
| 1.31 | 3.55 | 14.1 | 1.20 | 4.36 | 198 | |||||
| P = 0.253 | P = 0.0595 | P < 0.001 | P = 0.274 | P = 0.0367 | P < 0.001 |
All values result from 500 models including all positive and an equivalent number of bootstrap-selected negatives. Alt, Hpop, Ch, Du and CropMean are the average altitude, human population, chicken number, duck number, and mean number of rice crop harvested 500-m pixels, respectively, in each subdistrict (Thailand) or commune (Vietnam). ArT is the autoregressive term.
*Average coefficient.
†Average change in −2LL upon variable removal.
‡Significance of the change.
In complement with the results presented in Table 1, we verified that the significance of cropping intensity did not result from the fact that free-grazing ducks and farmed ducks were pooled into a single category. In Thailand, the data on free-grazing ducks and farmed ducks were available separately and allowed testing models where they replaced the duck/geese variables. The results showed that (i) the free-grazing duck variable is significantly associated with HPAI H5N1 virus presence (change in −2LL upon removal is 8.56, 58.49, and 3.90 for waves, I, II, and III, respectively), and (ii) cropping intensity maintains its significance in waves I and II in the presence of the free-grazing duck variable (change in −2LL upon removal is 3.28, 40.83, and 1.85 for waves, I, II, and III, respectively). Farmed ducks were in no instance found to be significantly associated with virus presence.
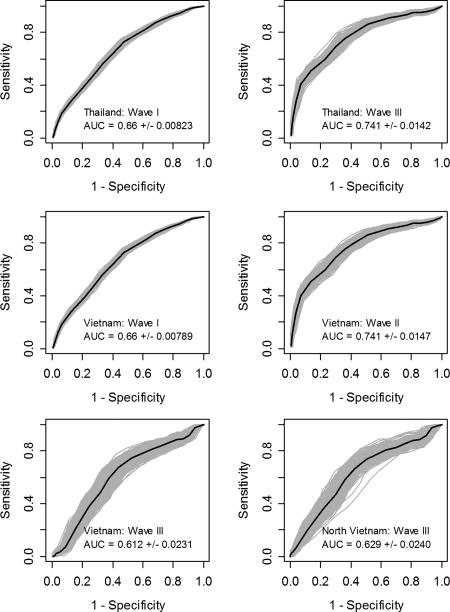
Despite their moderate predictive power, these five variables appear to provide relatively robust risk prediction. The model with best-fit parameters based on Thailand wave II data applied to predicting HPAI H5N1 virus presence in other epidemic waves provides very close predictive power to that of each wave's best-fit model (Fig. 3). In Thailand, the wave II model predicts wave I and wave III risk with an AUC of 0.66 and 0.74, respectively, which are comparable with the 0.66 and 0.88 of each best-fit model. More remarkably, the Thailand wave II model predicts Vietnam waves I, II, and III with AUC values of 0.66, 0.74, and 0.61, respectively, which are comparable with 0.69, 0.77, and 0.66 of each best-fit model. This result implies that spatial and temporal extrapolation of the model from Thailand wave II to other areas and other years is possible at relatively low cost in terms of loss of predictive power. On average, the AUC of the Thailand wave II model accounted for 93.5% of the AUC of the best-fit models.
Fig. 3.
ROC curves of the predictive power of the Thailand wave II multiple logistic regression model on the presence/absence of HPAI H5N1 virus at the subdistrict (Thailand) or commune (Vietnam) level (gray areas, 500bootstrap ROC curves; black lines, average ROC curve).
Discussion
The results presented here highlight three main findings: (i) although large differences may have resulted from the contrasting and dynamic disease management in Thailand and Vietnam, there is a common, consistent risk pattern reemerging for the three waves observed in Thailand and Vietnam; (ii) cropping intensity is consistently associated with HPAI H5N1 virus presence in Thailand and Vietnam; and (iii) the model of HPAI H5N1 virus risk developed in Thailand with the data from the second epidemic wave maintains its predictive power when applied to other epidemic waves or other regions, indicating that the model predictions can be extrapolated in space and time.
Disease management in the two countries changed markedly over the 3 years, and the results of the risk factor analysis need to be considered by taking account of these changes. During the first wave, there was undoubtedly a problem with disease reporting because the highest priority for the two countries was to curb the epidemics in an emergency situation, involving logistically demanding culling and depopulation. These operations took most of the resources, distracting attention from disease detection and notification. At the same time, the public and farmers were not yet well informed on risky practices of HPAI transmission, which may have assisted the spread of the disease and could explain some of the findings obtained for wave I. In Thailand, outbreaks were scattered and less numerous than during Vietnam wave I, and underreporting may explain part of this difference. With human population density as the top predictor of wave I HPAI H5N1 presence in Vietnam this suggests a relatively large contribution of human-related transmission during that wave. The relatively lower importance of duck and cropping intensity, both variables interpreted here as defining the ecological niche of the virus, is then not surprising as it may have been hidden by human-mediated transmission or may also result from reporting bias.
By the time the second wave emerged, disease surveillance systems were fully in place. Thailand implemented its x-ray surveys involving the participation of several hundred thousand inspectors searching door to door for evidence of HPAI presence (19). The second wave epidemic started earlier in Thailand and was concentrated to areas where free-grazing ducks are raised. In Vietnam, the epidemic wave was concentrated in the Mekong delta where domestic ducks are most abundant. At the time of the second wave, people and farmers were much better informed about the risk of human-mediated transmission of HPAI. The second-wave epidemic was significant for both countries, highlighting that control efforts were still insufficient. In both countries, the number of ducks and cropping intensity constitute the most important predictors during the second wave, followed by human population. Although the role of ducks as a reservoir of virus may already have played a role during wave I in Thailand, the results suggest it played a more prominent role during the second wave.
Thailand and Vietnam both undertook major efforts to step up control efforts. Thailand targeted domestic ducks by implementing systematic annual testing, imposing premovement testing, and initiating the transformation of the free-ranging duck sector into farms. Vietnam undertook massive vaccination campaigns, targeted also at domestic duck populations. Both strategies were apparently very effective in that the third wave that started in July 2005 involved a very low number of outbreaks in Thailand and was confined mostly to the northern part of the country in Vietnam. With control measures targeted at ducks, which decouples the linkage between free-grazing ducks and paddy rice fields, ducks and rice cropping intensity became less strong predictors of the HPAI H5N1 presence in wave III.
The statistical relationship between ducks and HPAI H5N1 virus presence and the role of ducks as reservoir has been discussed elsewhere (10, 14, 20–22). However, we found a strong and consistently positive association with rice cropping intensity across epidemic waves and countries. Rice descriptors may form a better risk predictor than free-grazing duck number numbers for causal reasons. For example, the x-ray survey that provided the duck data was carried out at the village level, and flocks were assigned to the village where the duck raisers lived. However, free-ranging duck flocks are moved around to multiple, adjacent, and nonadjacent districts during their life span (21). The added value of a rice variable over a free-ranging duck variable may therefore ensue from the fact that the rice descriptor defines more precisely where the ducks are moved, fed, and kept during high-risk periods with extensive opportunities for virus release and exposure. Moreover it is possible that rice paddy fields form a temporary habitat of other bird species also feeding on leftover rice grains that may become infected and initiate a local outbreak. Finally, rice fields are frequently flooded, and water presence improves viral persistence in the environment as opposed to dry soils.
Given the role of free-grazing duck as a strong driver of the spatial distribution of HPAI risk in Thailand and Vietnam, demographics and seasonality of the free-grazing duck production sector may also help in the understanding of the temporal variability in HPAI H5N1 virus prevalence. Exploratory results obtained from Thailand suggest that this may indeed be the case. Most duck restocking in the form of hatching and subsequent release in nurseries takes place in July/August, so that the rapidly growing young ducks benefit from the forage at the peak of the monsoon-associated rice harvest in November/December/January. Juvenile ducks are known to be of particular importance in avian influenza ecology (23, 24), and because of these demographics, the peak in the proportion of juveniles in the flocks occurs in September/October; at the time when the peak of the Thailand second wave outbreak took place. So far this evidence is circumstantial, but it suggests the need for the collection of comprehensive data quantifying duck production in space and time in all duck–rice areas where HPAI H5N1 virus persistence risk is to be quantified.
A traditional problem with risk distribution maps predicted by statistical models, based on linking the presence/absence of a disease or species to a series of predictors, is that they often lose much of their predictive power when extrapolated outside of the spatial range of their training data, which makes external validation difficult. It is thus quite remarkable that this model based on Thailand data loses so little predictive power when validated in Vietnam, which gives confidence to extrapolating the model to adjacent areas. Of course, the model has only moderate predictive power, but it is largely compensated for by its extrapolation ability.
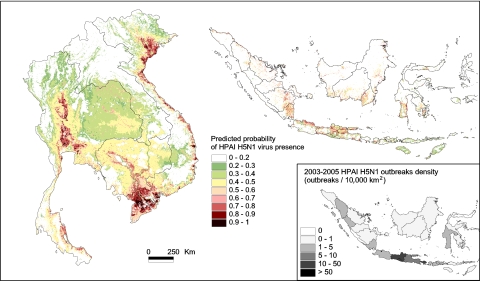
It thus appeared justified to produce risk maps for adjacent regions with similar farming conditions, such as Vietnam, Laos, or Cambodia (Fig. 4). In the latter two countries, the model predicts only restricted HPAI H5N1 virus spread in Laos, a higher risk in the areas surrounding the Tonle Sap Lake in Cambodia, and, even more so, in the transfrontier area of the Mekong delta. A risk map based on the same model was also produced for Indonesia (Fig. 4), but it needs to be interpreted cautiously, for several reasons. First, comparatively higher disease circulation is taking place in Indonesia as compared with Thailand and Vietnam. This assumption is based on regular reports of human cases (4) reflecting disease circulation in poultry and results from participatory disease surveys. Observations in the field suggest a much higher relative contribution of people and chickens to disease spread and persistence, and with it, a relatively lower importance of duck–rice contribution to the spatial definition of HPAI risk. At present, these observations are particularly difficult to evaluate because of the scarce and patchy data on HPAI H5N1 virus distribution in Indonesia. Second, eco-climatic conditions differ from those in the Mekong countries. Precipitation is less seasonal and more evenly distributed across the monthly calendar. As a result, the divide between rain-fed rice crop areas with a single rice crop per year and areas with irrigated rice and two or three rice crops per annum is less clear. The mapping of rice cropping intensity is also complicated by the local relief of volcano landscapes that results in small paddy fields asynchronously planted at any time of the year (25). Hence, although free-grazing ducks are known to be associated with rice production, the scale at which this relationship applies, and its spatial and temporal characteristics may differ from those in the Mekong countries. For the above reasons, HPAI H5N1 data for Indonesia for 2003–2005 were not used to evaluate the model quantitatively, but are provided for illustrative purpose (Fig. 4).
Fig. 4.
Predictions of HPAI H5N1 virus relative risk of presence in Thailand, Laos, Cambodia, and Vietnam based on the Thailand wave II model using the following parameters: Logit(p) = −0.903 − 3.11 10−3 Alt − 1.62 10−4 HpopDn − 2.47 10−5 ChDn + 5.40 10−4 DuDn + 0.968 CropI, where Alt, HpopDn, ChDn, DuDn, and CropI are the elevation, human population density, chicken density, duck density, and annual number of rice crop harvest, respectively, in each 500-m pixel. (Inset) Shown is the density of HPAI H5N1 outbreaks recorded in Indonesia in 2003–2005 expressed as number of outbreaks per 10,000 km2.
An applied result of this study is that the distribution of rice cropping intensity can readily be established at any time and be used to complement traditional duck census data. Remote sensing data are available at a much higher spatial and temporal resolution than traditional censuses, thus allowing fine-scale risk mapping. So the prospect of fine-scale spatiotemporal prediction of duck production and the associated HPAI risk is to be considered as a potential medium-term output still requiring significant development and understanding of spatiotemporal relationships between duck and crop farming. Areas of first interest include Thailand and Vietnam, where detailed data are available for performing follow-up analyses, in particular to contrast the Mekong and Red River deltas. But this recommendation extends to other countries, even outside of Asia, where duck production may also be an important driver of HPAI H5N1 virus persistence. For example, preliminary unpublished reports indicate that several tens of millions of ducks and geese are concentrated in the Nile Delta of Egypt; similarly, the Hadejia-Jama'are river system in Kano (Nigeria) is an important area for duck production.
One should also acknowledge that considerable variability remains unexplained by our model, given that duck distribution is only one of the drivers of HPAI disease risk and should be considered in conjunction with other factors such as the diverse production systems for terrestrial poultry, role of wet markets, contacts with migratory and resident avifauna, and environmental conditions affecting persistence of the virus outside the host in the environment (26).
Materials and Methods
Data.
Data on HPAI H5N1 virus outbreaks in Thailand included laboratory-confirmed cases recorded between January 23, 2004 and December 31, 2005, compiled by the Department of Livestock Development, Ministry of Agriculture and Cooperatives, Bangkok, Thailand. Data on HPAI H5N1 virus outbreaks in Vietnam were collated by the Vietnam government's Department of Animal Health between January 10, 2004 and December 31, 2005. Data on Indonesia were provided by the Directorate General of Livestock Services in the Ministry of Agriculture, Jakarta, Indonesia, but these data were used only for illustrative purpose.
All epidemiological data were grouped into three main epidemic waves (Fig. 1). HPAI H5N1 virus struck the two countries for the first time during the winter of 2003/2004, with the first cases being officially notified in December (Vietnam) and January (Thailand). Both countries established surveillance and control systems, and this first wave of outbreaks was followed by a period with virtually no new cases during May and June. New cases started to emerge again in July. The countries were better prepared at the onset of this second wave that reached its highest incidence in October 2004 (Thailand) and January 2005 (Vietnam). In 2005, both countries succeeded in controlling the disease, again with no outbreaks during May and June, but the virus again re-emerged in July. When this third wave started, Thailand and Vietnam had been investing significant effort into halting the disease, with early detection, control of duck movements, and premovement testing in Thailand and massive vaccination campaigns combined with trade restrictions in Vietnam. Therefore, it appears justified to group the epidemiological data into three epidemic waves. The first wave (termed wave I) pools the data from January 2004 to May 2004, the second wave (wave II) pools the data from June 2004 to May 2005, and the third wave (wave III) pools the data from June 2005 to December 2005.
This work aimed to limit the number of risk factors to a few carefully chosen variables scrutinized for their quantitative association with HPAI H5N1 virus presence risk in space and time for each epidemic wave and country. Some variables available for Thailand were not available for Vietnam, and vice versa, prompting us to restrict the study to the variables available in both countries. All data were collated at the subdistrict level in Thailand (median area: 40.4 km2) and commune level in Vietnam (median area: 14.4 km2).
Poultry census data for Thailand were collected from October to mid-November 2004 during the x-ray survey organized by the Department of Livestock Development (Bangkok, Thailand), involving the participation of several hundreds of thousands inspectors searching door to door for evidence of HPAI presence. These inspectors collected detailed information on domestic poultry numbers and species in each and every farm and household. For the present study, five poultry variables were extracted from the x-ray survey database: farm duck numbers, which includes meat and layer ducks raised in farms, free-grazing ducks, geese, native chickens, and chickens used in industrial production (broilers and layers). Poultry data for Vietnam were extracted from the 2001 Agricultural Census database (General Statistics Office of Vietnam), where two poultry categories could be extracted at the commune level: (i) chickens and (ii) ducks plus geese. To obtain an identical variable applicable to both countries, native and farm chicken data from Thailand were pooled into a single chicken variable. Similarly, ducks, free-grazing ducks, and geese were pooled into a single duck and geese variable. We chose to test chicken numbers in the model because most recorded outbreaks were observed in chicken flocks. The duck and geese number was taken as a variable because previous findings identified ducks as an important risk factors, and recent results highlighted domestic waterfowl as the foremost host species among poultry, with a much higher virus prevalence on a year-round basis than in chickens (22).
Additional variables obtained at the subdistrict (Thailand) and commune (Vietnam) levels comprise: human population (27, 28) and average elevation [90-m resolution Digital Terrain Model from the Shuttle Radar Topography Mission data, STRM V3 (ref. 29 and http://srtm.csi.cgiar.org)]. Human population was chosen because it is an indicator of trade-related viral traffic and, possibly, because it creates a bias in reporting. Elevation was included because it was found to be a significant risk predictor in both Thailand (10) and Vietnam (14), is easily obtained for any country, and is considered to be a surrogate indicator of other unmeasured variables related to HPAI risk. High-elevation areas have higher slopes and land cover dominated by forests and permanent vegetation. In contrast, flat plains, deltas, and coastal areas are dominated by agriculture and a mixture of intensive uses of the land. In addition, wetlands, rivers, canals, ponds, and irrigation networks are concentrated in those lowlands.
Satellite-based mapping algorithm using data from the MODIS of the Terra satellite allows identification and tracking of image pixels that experienced flooding and rice transplanting over time, based on temporal profile analysis of the Normalized Different Vegetation Index, Enhanced Vegetation Index, and Land Surface Water Index (17, 18). The method permits an estimation of the cropping intensity (number of rice cropping in a year) within individual 500-m pixels. This variable estimated for 2004 was aggregated by averaging the pixel values within each subdistrict and commune of Thailand and Vietnam, respectively. This variable was incorporated in the analysis because of preliminary results indicating that the presence of rice paddy fields is associated with HPAI H5N1 virus presence risk (10, 14), and that free-grazing ducks are found in much higher numbers where cropping intensity is high (15).
Analyses.
We used a multiple logistic regression framework to relate HPAI H5N1 presence to the predictors by pooling the data by epidemic wave and country, converting the number of outbreaks into presence or absence. The model was built by using altitude, human population, chicken, ducks and geese, and mean rice cropping intensity as predictors of HPAI H5N1 presence for each of the three synchronous epidemic waves in Thailand and Vietnam at the subdistrict and commune levels, respectively.
An autologistic approach was used to account for spatial autocorrelation in the response variable, by forcing an additional covariate, termed autoregressive term, into the multiple logistic regression model (30). The autoregressive term accounts for spatial dependency in the response variable and is estimated by averaging the presence/absence among a set of neighbors defined by the limit of autocorrelation, weighted by the inverse of the Euclidean distance (30). The extent of the autocorrelation in the response variable is obtained from the range of the spatial correlogram ρ(h) of the response variable, here HPAI H5N1 virus presence or absence.
Another problem that arises when applying logistic regression models to disease data are that low prevalence values for the response variable (<10%) tend to bias model performance metrics (31). For each model, we selected all HPAI H5N1 virus-present subdistricts or communes and a randomly selected equivalent number of negative samples. We then bootstrapped this operation 500 times. For each model and each set of negatives, we estimated the coefficient of each variable, the change in −2LL of each variable upon removal, the AUC of the ROC plots, Cohen's κ index, and pseudo-R2 as indicators of model performance. We then averaged these estimates over the 500 bootstraps and estimated the significance of the average change in −2LL of each variable upon removal. The individual variable coefficient and change in −2LL upon removal were estimated based on an autologistic model, and the AUC, ROC curve, Cohen's κ, and pseudo-R2 were estimated based on the same model without the autoregressive term.
Our second objective was to test the performance of a model built for a particular time period and region for predicting HPAI H5N1 virus presence for a different time period and region, i.e., to measure the ability of a model to extrapolate risk predictions in space and time. The parameters of the best-fit model for Thailand wave II were used to predict HPAI H5N1 virus presence in Thailand waves I and III and Vietnam waves I, II, and III. The model performance was estimated by using the AUC and ROC plot, by bootstrapping data to force an equivalent number of positive and negatives in the test sets.
Finally, we used the model based on Thailand wave II HPAI data to predict HPAI H5N1 virus presence probability for the whole of the Mekong region and for Indonesia, at a spatial resolution of 500 m, using topographic data from the STRM V3 (29, 32), human population data from the Global Rural-Urban Mapping Project (33, 34), and poultry population data from the Global Livestock Production and Health Atlas database (35). Poultry data were included for Cambodia (chicken: province level; ducks: province level; except for the provinces Ratana Kiri and Svay Rieng for which the information was missing and replaced by the national average duck density), Laos (chickens: province level; ducks: province level), and Indonesia [chicken: Province level, ducks: district level for Java and Sumatra (36), province level elsewhere], and 500-m rice cropping intensity predictions.
Acknowledgments.
The Food and Agricultural Organization thanks the studied countries for sharing their information. This study was supported by the Food and Agricultural Organization and National Institutes of Health Fogarty International Center Grant R01TW00786901 (through the National Institutes of Health National Science Foundation Ecology of Infectious Diseases Program).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710581105/DCSupplemental.
References
- 1.Li KS, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007;51:161–166. doi: 10.1637/7602-041306R.1. [DOI] [PubMed] [Google Scholar]
- 3.World Organization for Animal Health. Update on Avian Influenza in Animals (Type H5) Paris: World Organization for Animal Health; 2007. [Google Scholar]
- 4.WHO. Confirmed Human Cases of Avian Influenza A(H5N1) Geneva: WHO; 2006. [Google Scholar]
- 5.Food Agriculture Organization. A Global Strategy for the Progressive Control of Highly Pathogenic Avian Influenza (HPAI) Rome: Food and Agriculture Organization; 2005. [Google Scholar]
- 6.Webster RG. The importance of animal influenza for human disease. Vaccine. 2002;20:S16–S20. doi: 10.1016/s0264-410x(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick AM, et al. Predicting the global spread of H5N1 avian influenza. Proc Natl Acad Sci USA. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulse-Post DJ, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci USA. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturm-Ramirez KM, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert M, et al. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims LD. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 2007;51:174–181. doi: 10.1637/7637-042806R.1. [DOI] [PubMed] [Google Scholar]
- 12.Tiensin T, et al. Geographic and temporal distribution of highly pathogenic avian influenza A virus (H5N1) in Thailand, 2004–2005: An overview. Avian Dis. 2007;51:182–188. doi: 10.1637/7635-042806R.1. [DOI] [PubMed] [Google Scholar]
- 13.Wallace RG, Hodac H, Lathrop RH, Fitch WM. A statistical phylogeography of influenza A H5N1. Proc Natl Acad Sci USA. 2007;104:4473–4478. doi: 10.1073/pnas.0700435104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer D, Minh P, Martin V, Epprecht M, Otte J. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet J. 2007;174:302–309. doi: 10.1016/j.tvjl.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert M, et al. Avian influenza, domestic ducks and rice agriculture in Thailand. Agric Ecosyst Environ. 2007;119:409–415. doi: 10.1016/j.agee.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teo SS. Evaluation of different duck varieties for the control of the golden apple snail (Pomacea canaliculata) in transplanted and direct seeded rice. Crop Protection. 2001;20:599–604. [Google Scholar]
- 17.Xiao X, et al. Mapping paddy rice agriculture in South and Southeast Asia using multi-temporal MODIS images. Remote Sens Environ. 2006;100:95–113. [Google Scholar]
- 18.Xiao XM, et al. Mapping paddy rice agriculture in southern China using multi-temporal MODIS images. Remote Sens Environ. 2005;95:480–492. [Google Scholar]
- 19.Tiensin T, et al. Highly pathogenic avian influenza H5N1, Thailand, 2004. Emerg Infect Dis. 2005;11:1664–1672. doi: 10.3201/eid1111.050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster RG, et al. Changing epidemiology and ecology of highly pathogenic avian H5N1 influenza viruses. Avian Dis. 2007;51:269–272. doi: 10.1637/7641-050206R.1. [DOI] [PubMed] [Google Scholar]
- 21.Songserm T, et al. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GJD, et al. Emergence and predominance of an H5N1 influenza variant in China. Proc Natl Acad Sci USA. 2006;103:16936–16941. doi: 10.1073/pnas.0608157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halvorson DA, Kelleher CJ, Senne DA. Epizootiology of avian influenza: effect of season on incidence in sentinel ducks and domestic turkeys in Minnesota. Appl Environ Microbiol. 1985;49:914–919. doi: 10.1128/aem.49.4.914-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stallknecht DE, Shane SM. Hosts range of avian influenza virus in free-living birds. Vet Res Commun. 1988;12:124–141. doi: 10.1007/BF00362792. [DOI] [PubMed] [Google Scholar]
- 25.Linser W. Analysis and Suggestions to Improve the Capacity of the MODIS Rice Algorithm to Identify Rice Planting Patterns in Java, Indonesia: Report to the Animal Health Division, Food and Agriculture Organization. Rome: Food and Agriculture Organization; 2006. [Google Scholar]
- 26.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- 27.Thailand Environment Institute. Bangkok: Thailand Environment Research Institute; 1996. Thailand on a Disk: Digital National Database for Use with PC Arc/Info and/or ArcView. [Google Scholar]
- 28.General Statistics Office. Rural Agriculture and Fisheries Census Hanoi, Vietnam. Hanoi, Vietnam: Department of Agriculture, Forestry, and Fisheries Statistics, General Statistics Office; 2001. [Google Scholar]
- 29.Farr TG, et al. The shuttle radar topography mission. Rev Geophys. 2007;45 doi: 10.1029/2005RG000183. [DOI] [Google Scholar]
- 30.Augustin NH, Mugglestone MA, Buckland ST. An autologistic model for the spatial distribution of wildlife. J Appl Ecol. 1996;33:339–347. [Google Scholar]
- 31.McPherson JM, Jetz W, Rogers DJ. The effects of species' range sizes on the accuracy of distribution models: Ecological phenomenon or statistical artefact? J Appl Ecol. 2004;41:811–823. [Google Scholar]
- 32.Jarvis A, Reuter HI, Nelson A, Guevara E. Hole-Filled Seamless SRTM Data V3. Cali, Columbia: International Center for Tropical Agriculture; 2006. [Google Scholar]
- 33.Balk DL, et al. Determining global population distribution: Methods, applications, and data. Adv Parasitol. 2006;62:119–156. doi: 10.1016/S0065-308X(05)62004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay SI, Noor AM, Nelson A, Tatem AJ. The accuracy of human population maps for public health application. Trop Med Int Health. 2005;10:1073–1086. doi: 10.1111/j.1365-3156.2005.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Food Agricultural Organization. Global Livestock Production and Health Atlas. Rome: Food and Agricultural Organization; 2007. [Google Scholar]
- 36.Direktorat Jendral Peternakan. Statistik Peternakan. Jakarta, Indonesia: Direktorat Jendral Peternakan; 2006. [Google Scholar]