Abstract
Whether motion vision uses color contrast is a controversial issue that has been investigated in several species, from insects to humans. We used Drosophila to answer this question, monitoring the optomotor response to moving color stimuli in WT and genetic variants. In the fly eye, a motion channel (outer photoreceptors R1–R6) and a color channel (inner photoreceptors R7 and R8) have been distinguished. With moving bars of alternating colors and high color contrast, a brightness ratio of the two colors can be found, at which the optomotor response is largely missing (point of equiluminance). Under these conditions, mutant flies lacking functional rhodopsin in R1–R6 cells do not respond at all. Furthermore, genetically eliminating the function of photoreceptors R7 and R8 neither alters the strength of the optomotor response nor shifts the point of equiluminance. We conclude that the color channel (R7/R8) does not contribute to motion detection as monitored by the optomotor response.
Keywords: apparent motion, equiluminance, motion detection, opsin mutants, R7/R8 system
The role of color vision in motion detection is still a matter of debate (for reviews, see refs. 1–3). In human vision, it was widely believed that color and motion are processed by parallel pathways (4). More recently, however, the complete segregation of motion detection and color vision came into question (for a review, see ref. 2), because motion of the patterns of two equiluminant colors could still be detected. Thus, either the color vision system contributes to motion detection, or a residual difference in luminance could not have been eliminated experimentally (for a review, see ref. 1).
In insects, it was first shown for the fleshfly Phormia that motion vision as monitored by the optomotor response depends on luminance contrast (5). Also in the honey bee, for which color vision is well established (6), the optomotor response has been shown to be largely insensitive to color contrast (7). The same was found for the landing response (8). Similar results were reported in vertebrate systems. In goldfish, which have a tetrachromatic color vision system (9), only L-cones (red) contribute to the optomotor response (10). In zebrafish larvae, both M- and L-cones seem to mediate motion responses, although only luminance information appears to be used (11). These studies clearly show that luminance contrast plays a major role in motion detection, as it does in humans. However, at the point of equiluminance, residual responses are frequently observed (5, 10), leaving the possibility that moving color contrast can, after all, elicit motion responses. So far, it was not possible to selectively switch off the color channel. Here, we used genetic intervention and the detailed knowledge of the Drosophila retina to address whether the color vision system contributes to motion detection.
Drosophila has been shown to possess color vision (12, 13). As a prerequisite for this quality, an animal needs at least two types of photoreceptors differing in spectral sensitivity (for a review, see ref. 14). The Drosophila compound eyes are equipped with photoreceptors with at least five different spectral sensitivities. Each eye consists of ≈700 ommatidia (15). Like in other Diptera, each ommatidium contains eight photoreceptors (R1–R8) (for a review, see ref. 15). The outer photoreceptors (R1–R6) express a rhodopsin (Rh1) that shows a broad spectral sensitivity peaking in the blue as well as in the UV due to the presence of a sensitizing pigment (16). These cells project to the lamina, the first neuropil of the optic lobe. R7 photoreceptors express one of two UV-sensitive opsins, Rh3 or Rh4, whereas R8 photoreceptors express either the blue-sensitive opsin Rh5 or the green-sensitive opsin Rh6 (for a review, see ref. 17). Only two types of ommatidia exist in the main part of the retina. In one type, referred to as pale (p), Rh3 is always combined with Rh5 (short wavelength pair). In the other type, referred to as yellow (y), Rh4 is always combined with Rh6 (long wavelength pairs). Approximately 70% of the ommatidia are of the “yellow” type. All R7 and R8 cells bypass the lamina and project directly to the second neuropil of the optic lobe, the medulla.
The R1–R6 photoreceptor channel mediates motion detection in visual course control and landing, as well as phototaxis and tropotaxis (18, 19). With only one spectral type of photoreceptor, the R1–R6 channel is achromatic. The R7 and R8 photoreceptor channel mediates basic orientation responses (e.g., turning toward large objects) but does not mediate responses to moving luminance contrast (18, 19). This channel is, however, thought to be crucial for color vision and the detection of e-vector orientation (for reviews, see refs. 15 and 20). Little is known about the interaction of the two channels. In previous studies, it could not be excluded that R7/R8 might contribute color contrast sensitivity to the intact motion detection system because equiluminant color contrast was not tested; secondly, R8 photoreceptors could not be manipulated. Here, we show that, in Drosophila, motion detection as monitored by the optomotor response is indeed fully separated from color vision.
Results
Impairment of the R1–R6 System Causes Motion Blindness.
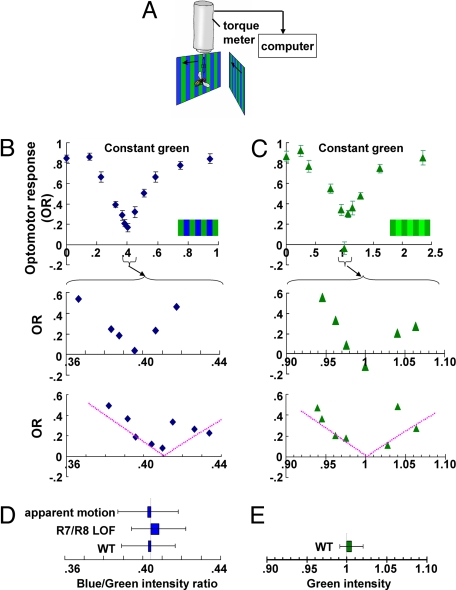
Like other animals, Drosophila uses visual motion to stabilize its gaze and orientation in space (for a review, see ref. 20). To quantitatively assess motion vision, we recorded the optomotor response, measured as the fly's yaw torque during stationary flight in response to rotatory large-field motion (Fig. 1A) (20, 21). The optomotor response belongs to a system of control mechanisms counteracting involuntary changes of flight direction caused, for instance, by air turbulences or the different efficiency of the two wings. To measure the optomotor response, tethered individual flies were attached to a torque meter, and two liquid crystal display computer screens were placed frontolaterally at an angle of ±45° with respect to the fly's longitudinal body axis (Fig. 1A). Computer-generated patterns of vertical stripes were moved from front to back on one screen and at the same time from back to front on the other (and vice versa), thus simulating rotary motion around the fly. The fly's intended turns followed the movement.
Fig. 1.
Motion vision is insensitive to color contrast. (A) Experimental setup. Moving stripes are presented to the fly on two identical screens that are placed in frontal-lateral position. The optomotor response (OR) is measured by recording the fly's yaw torque during stationary flight. (B) Blue/green experiments. OR of WT flies to moving striped patterns where green intensity was kept constant (“constant green,” 3.2 × 1012 quanta cm−2·s−1), whereas blue intensity varied from 0 (black) to the same intensity as the green (1). Relative OR is normalized to the maximal response in each individual fly. Error bars are SEMs. (Top) WT flies (n = 23). (Middle) Single fly's OR goes to zero near the POE. A total of 20 flies exhibited this behavior. (Bottom) Single fly OR in which the response was positive at all points measured. A total of 10 flies behaved similarly. The dotted lines indicate the standard intensity–response function (template). (C) Green/green experiments. OR of WT flies to moving stripes of a single color. Fixed intensity is as in Fig. 1B (3.2 × 1012 quanta cm−2·s−1), and the x axis shows the intensity ratio of the other stripes. (Top) Average OR of 13 flies. (Middle) Representative example of a single fly. (Bottom) Another example of a single fly with the template (dotted line) overlaid. (D) POEs estimated for single flies in blue/green experiments. The template was fitted to the data from individual flies to estimate the POE in each fly. Error bars are SDs. WT (n = 16); R7/R8 LOF mutants (sev LY3,rh52,rh61 and sal, n = 26); WT in apparent motion paradigm (n = 12). (E) POEs for green/green experiments (13 WT flies), estimated from template fitting. Error bars are SDs.
As mentioned above, the R1–R6 system mediates optomotor responses under achromatic stimulus conditions (19). We confirmed the previous report testing ninaE17 flies (22, 23) in our setup with black and white stripes [supporting information (SI) Fig. 5]. In this mutant allele of the rh1 opsin gene, rh1 expression is lost and the rhabdomeres of R1–R6 degenerate (24), whereas expression of opsins Rh3–Rh6 in R7/R8 is unaffected (S.Y. and C.D., unpublished results). The optomotor response is zero, whereas orientation to brightness differences and large landmarks is retained (25). We confirmed that phototaxis was retained in ninaE17 flies (data not shown). The R7/R8 channel alone does not provide any motion sensitivity in the optomotor response.
This finding, however, does not exclude that, with a properly operating motion channel, the R7/R8 channel would contribute to motion vision. With the R1–R6 system operating, color contrast might add to or enhance luminance contrast. Moreover, photoreceptors R7/R8 could modify the R1–R6 system independently of color vision, resulting in a change of spectral sensitivity of the R1–R6 system. Therefore, we directly measured the optomotor response to color contrast.
No Optomotor Response to Color Contrast.
To investigate whether an optomotor response could be elicited not only by luminance contrast but also by color contrast, we recorded the flies' optomotor responses to patterns of alternating blue and green stripes of varied luminance contrast (Fig. 1A). We first measured the spectral profile of each color from the monitor that displayed the moving stripes. The peak wavelength of the green stimulus produced by the monitor was 544 nm, whereas the blue stimulus had a relatively broad profile peaking at 437 nm (SI Fig. 6). We fixed the intensity of one of the stimuli (e.g., green) to an arbitrary value (3.2 × 1012 quanta cm−2·s−1). The value was chosen such that even 1% of this intensity was sufficient to elicit a full-sized optomotor response in this setup. We monitored the optomotor response while varying the intensity of the other color (Fig. 1B; blue/green experiment). The curve representing the strength of the optomotor response as a function of intensity of the blue stripes had the shape of a trough: At a certain point, the response was minimal (blue intensity = 1.3 × 1012 quanta cm−2·s−1). At this point of equiluminance (POE), flies acted as if there were hardly any motion, although the moving stimuli exhibited strong color contrast to the human eye. Taking into account the Drosophila color vision system (13) and the spectral composition and intensity of the two colors, the moving bars should have exhibited strong color contrast for the fly as well. This indicates that the optomotor response was mostly dependent on luminance contrast (Fig. 1B Top), whereas color contrast contributed only little if anything at all.
We determined the POEs for the blue and green stimuli in reciprocal experiments. If the intensity of blue was kept constant at the average POE measured in the previous experiment and that of green varied instead (green/blue experiment), we obtained a similar intensity–response curve, and the POE occurred at the green intensity corresponding to the reference in the first experiment (constant blue: 1.3 × 1012 quanta cm−2·s−1; green: 3.2 × 1012 quanta cm−2·s−1) (SI Fig. 7). At the POE for WT flies, the intensity of the blue stimulus was ≈40% of the green intensity. Moreover, we determined the POE for the same blue/green colors but with different intensities. A similar ratio was obtained (blue, 3.0 × 1012 quanta cm−2·s−1; green, 7.4 × 1012 quanta cm−2·s−1; blue/green ratio = 40%: blue, 2.7 × 1011 quanta cm−2·s−1; green, 6.9 × 1011 quanta cm−2 s−1; blue/green ratio = 39%).
Next, we tested whether the experimentally obtained POE was in agreement with a “theoretical POE” quantitatively estimated from the spectral sensitivity of photoreceptors R1–R6. Taking the spectral emission profiles of each color, we multiplied the intensity at each spectral wavelength with the relative sensitivity of Rh1 (sensitivity values according to R. Hardie, personal communication; and refs. 15, 26, and 27) and integrated the values for the entire wavelength range. At the mean POE, we calculated a blue/green ratio of r = 0.96 ± 0.10, indicating that the blue and green stimuli provided approximately the same stimulation for the R1–R6 system. This result is consistent with Rh1 providing the bulk of the pattern contrast discrimination between the green and blue stripes.
The average curve of WT flies indicates a small but significant response at the average POE (Fig. 1B Top, “residual response”), which could represent some contribution of color contrast to motion detection. To address this possibility, we investigated individual flies and narrowed down the POE by performing a more detailed analysis in the close vicinity of the trough. The individual POEs were slightly different from fly to fly. Moreover, at the individual POEs, residual responses went down to zero in 20 of the 30 flies tested. An example of a single fly is shown in Fig. 1B Middle. This strongly suggested that color contrast did not contribute to the optomotor response in these flies. More likely, each fly was indeed motion blind at its POE, but in 10 of the flies none of the predetermined intensity ratios exactly matched the individual POE (Fig. 1B Bottom).
Comparing the POE of Individual Flies.
If only luminance contrast was effective and all flies had a POE at exactly the same intensity ratio, then the intensity–response curve to a blue/green grating should match that obtained by using a striped pattern without color contrast. To obtain such an intensity–response curve, a stripe pattern consisting of only one color (green with the peak wavelength at 544 nm) but at two differently controlled intensities was used (green/green experiment). The same intensity of green as in Fig. 1B was chosen as the fixed intensity (3.2 × 1012 quanta cm−2·s−1). As expected, we obtained a mean intensity–response curve with a trough where the varied intensity exactly matched the reference intensity (Fig. 1C Top); that is, when there was no motion [luminance contrast m = 0; m = (I1 − I2)/I1, where I1 and I2 are intensities and I1 > I2], the optomotor response was zero. A pattern contrast of m = 0.025 was sufficient to elicit ≈15% of the maximal response, whereas a contrast difference of m = 0.2 elicited ≈50% of the maximal response (Fig. 1C Top), indicating that motion could already be detected by the fly at very low luminance contrast.
To test the hypothesis that in the blue/green experiment (Fig. 1B Top) the optomotor response at the mean POE was not zero because each fly had a slightly different POE, we estimated POEs of individual flies as best as possible. We first derived the mean intensity–response function of the green/green experiment in the vicinity of the trough, which we refer to as “template” (Fig. 1C Bottom, dotted line). This function could then be used to estimate the POE for a particular fly, even if no measured data point resulted in a zero response for that fly. We fitted the above template to the data points of individual flies of the blue/green experiment by shifting it along the intensity axis. As a control, the template was also fitted to the data of individual flies in the green/green experiment (Fig. 1E). The average of the estimated POEs was very close to the mean POE in the respective experiment (Fig. 1 D and E). As would be expected if individual flies indeed differed in their POEs, the scatter of individual POEs was distinctly larger in the blue/green experiments (SD = ±3.3%) (Fig. 1D) compared with the green/green experiments (SD = ±1.4%) (Fig. 1E).
Taking into account this scatter, we estimated how much each fly would respond at the intensity of the mean POE according to the standard intensity–response function (template; see previous paragraph). The mean response at the mean POE calculated from individual flies was very similar to the residual response actually measured at the mean POE (see Fig. 3C), suggesting that the residual response likely results from the scatter of individual POEs.
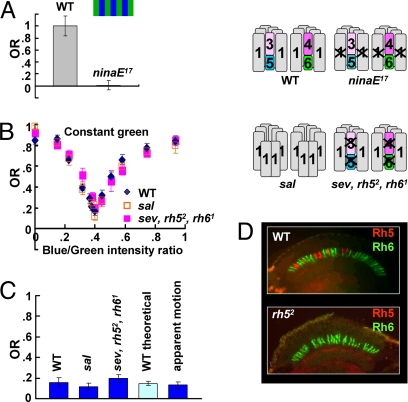
Fig. 3.
R7/R8 system does not contribute to motion detection. (A) Color contrast does not elicit a response in ninaE mutant flies (n = 17) compared with WT (n = 17). Conditions are identical to those in Fig. 1B. OR was normalized to the averaged WT response. In the pictograms, opsins (1–6 refer to rh1–rh6, respectively) expressed in ommatidia of each genotype are shown. (B) OR of sev LY3,rh52,rh61 (n = 20) and sal mutant flies (n = 18). The pictograms for each genotype are shown. (C) The residual response observed at the “trough” for WT (n = 24), sal (n = 17), and sevLY3,rh52,rh61 (n = 20) in the blue/green experiments and for WT flies in the absolute values (see Fig. 2C, n = 10) in the apparent motion paradigm are shown. Additionally, theoretical response for WT flies (n = 16) is shown (see Results). Error bars are SEMs. (D) Absence of Rh5 staining in rh52 mutant was confirmed. Cryosections of WT (Upper) and rh52 mutant (Lower) adult heads were costained with anti-Rh5 (red) and anti-Rh6 (green) antibodies.
Measuring the POE Without Interference by Color Contrast.
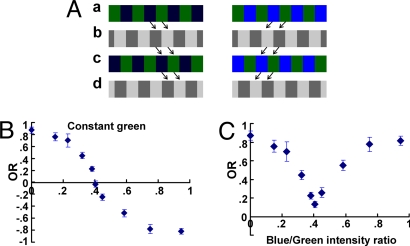
If the residual response at the mean POE were due to color contrast, it should be eliminated if the POE could be measured under conditions when no response should be elicited by color contrast alone. Therefore, we applied a special “apparent-motion paradigm” to Drosophila that had originally been developed to determine the POE in human vision (28). The stimulus consists of loops of four frames that are shown sequentially at 250-ms intervals (Fig. 2A): The first one (Fig. 2Aa) displays green and blue stripes (similar to the previous experiment). Then, bright and dark gray stripes are shown (Fig. 2Ab). They are displaced by half a stripe width (quarter period) compared with Fig. 2Aa. The third (Fig. 2Ac) and fourth (Fig. 2Ad) frames are the same patterns as Fig. 2 Aa and Ab, respectively, but again displaced in the same direction as before by half a stripe width. The loop is closed, and a new loop starts with the first frame (Fig. 2Aa).
Fig. 2.
Color contrast does not contribute to motion detection. (A) Apparent motion paradigm. Sequential presentation of blue/green striped patterns (a and c) and bright/dark striped patterns (b and d) produce an impression of motion (indicated by arrows between the patterns). (B) OR of WT flies (n = 14) measured in the apparent motion paradigm. As in Fig. 1B, green intensity was kept constant (constant green, 3.2 × 1012 quanta cm−2·s−1), and the intensity of blue was varied. (C) For the response of each individual fly in B, the absolute values of the OR were calculated. Note that the averaged curve of the (n = 14) absolute profiles does not go to zero. Error bars are SEMs.
In this paradigm, chromatic (blue and green) stripes and achromatic (bright and dark) stripes are shown alternately, giving a sensation of motion (Fig. 2A). If blue appears darker to the fly than green, apparent motion is clockwise, and the fly responds with a clockwise yaw torque response (Fig. 2A Left). If blue appears brighter than green, apparent motion is counterclockwise, and the fly responds with counterclockwise yaw torque (Fig. 2A Right). Unlike in the first paradigm, the pattern with color contrast does not itself move, as it is the luminance contrast of two colors that gives the observers the sensation of motion. Therefore, the response depends only on the luminance difference of blue and green, and any contribution from color contrast is excluded. The optomotor response crosses zero and reverses its direction after passing through the POE, instead of decreasing and increasing, as in the previous experiments. Importantly, the POE measured in the apparent-motion paradigm was identical to the one obtained by the previous procedure (compare Fig. 1B Top and Fig. 2B; at the POE, blue/green ratio = 0.40).
The curves of the individual flies constituting the mean curve of Fig. 2B crossed zero at slightly different intensities (data not shown), indicating the different individual POEs. Plotting the responses in absolute values generated a mean curve (Fig. 2C) similar to that obtained in the first paradigm (Fig. 1B Top). We calculated the average of the absolute values of the individual responses at the intensity of the mean POE. This residual response was distinctly different from zero and about as large as that in the previous experiment (Figs. 2C and 3C). The mean response at the mean POE was zero in Fig. 2B, because some flies had not passed their POE (giving positive value) and some already had (negative value). Consistently, the average of the estimated POEs and the scatter of individual POEs in the apparent motion paradigm were very close to those in the first paradigm (Fig. 1D). Because these results were obtained without a possible contribution of color contrast, they further support the hypothesis that, also in the first paradigm, the residual response at the mean POE derived from individual differences in POEs, rather than from color contrast.
No Response to Color Contrast in the R1–R6 Mutant.
To see whether color contrast could elicit a response in mutant flies lacking R1–R6 function, we tested ninaE17 flies. No response could be detected when green and blue stripes were presented (green, 3.2 × 1012 quanta cm−2·s−1; blue, 2.8 × 1012 quanta cm−2·s−1) (Fig. 3A). This finding suggests that, in the absence of the motion channel, color contrast does not serve motion vision, but it does not rule out the possibility that, in the presence of the intact R1–R6 system, photoreceptors R7/R8 might still contribute to a small degree.
No Contribution of Color Contrast by the R7/R8 System.
If the residual response at the POE were due to color contrast, it should vanish in flies lacking color vision. We therefore genetically manipulated the R7/R8 system. We generated flies expressing rhodopsin Rh1 in all photoreceptors of the compound eyes, including photoreceptors R7/R8. The spalt (sal) gene complex encodes two transcription factors that are required for inner photoreceptor differentiation. In sal mutant eyes, all eight photoreceptors express Rh1 (Fig. 3B Right) (29). Because loss of function (LOF) of the sal complex leads to early lethality (30), we used the EGUF/hid system (31) to make whole mutant eyes in otherwise phenotypically WT animals. We used the same setup as described in Fig. 1 A and B to measure the intensity–response curve of flies with a sal mutant retina in blue/green experiments. These flies were indistinguishable from WT (Fig. 3B).
Next, we investigated the optomotor response at the mean POE of flies lacking the function of both R7 and R8 photoreceptors. To impair R7 function, we used the mutant sevenlessLY3 (sevLY3) lacking R7 photoreceptors (32). To block R8 function, we generated a mutant of the rh5 opsin gene that is expressed in R8 photoreceptors of p ommatidia. The mutant (rh52) was generated by P-element excision, and loss of rh5 expression was confirmed by the sequence and immunostaining (Fig. 3D). The rh52 mutant was then combined with the sevLY3 mutant and the rh61 mutant, a functional null allele of the rh6 opsin gene expressed in the R8 photoreceptors of the y ommatidia (33). The sevLY3, rh52, rh61 triple-mutant flies have no R7 cells and express no functional R8 opsins (Fig. 3B Right); therefore, color vision should be completely abolished. It should be noted that R8 cells still express rh3 in the dorsal margin area, but this part of the eye is not involved in color vision (for a review, see ref. 34). We measured the intensity–response curve of the sevLY3,rh52,rh61 triple-mutant flies. Their behavior was again indistinguishable from that of WT (Fig. 3B). At every point tested, the response was not significantly different from WT flies. In particular, the response at the mean POE of these flies was still positive (Fig. 3 B and C). Thus, the residual average optomotor response at the mean POE in the experiment of Fig. 1B cannot be attributed to the R7/R8 system.
No Modulatory Effect of R7/R8 on POE.
Besides potentially providing color contrast, R7 and/or R8 might modulate the R1–R6 channel. For instance, the signal of R8 cells might attenuate or amplify the signal of R1–R6. The resulting action spectrum of the motion channel then would be different from both R1–R6 and R8 spectral sensitivities, and therefore the R8 photoreceptors would shift the POE to an intensity different from that of R1–R6 alone. Our genetic intervention in the R7/R8 system did not reveal any such effect. The mean POEs in WT and in the R7/R8 LOF mutants (sevLY3,rh52,rh61 and sal) are essentially the same (Fig. 3B). We estimated the POE for individual mutant flies (Fig. 1D, R7/R8 LOF), just as we did for WT flies (Fig. 1D), and again there was no significant difference between WT and mutant flies.
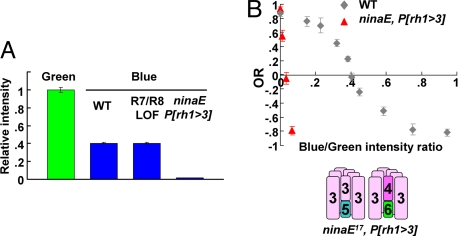
As a control to show that the POE indeed reflects the spectral sensitivity of photoreceptors R1–R6, flies carrying a different opsin in these cells were tested. We used P[Rh1+3],ninaE17 flies, in which the UV-sensitive Rh3 opsin, which is normally expressed in a subset of R7 photoreceptors, is now expressed under the control of the rh1 promoter in a ninaE17 background, thus replacing Rh1 with Rh3 (Fig. 4B) (35). In this situation, the mean POE was drastically shifted such that, compared with WT flies (Fig. 4B), much less blue light (5.4 × 1010 ± 2 × 1010 quanta cm−2·s−1) was required to balance the standard green intensity (3.2 × 1012 quanta cm−2·s−1) (Fig. 4A), giving a blue/green ratio of 0.017 compared with 0.40 for WT flies.
Fig. 4.
Shifting the POE. (A) Relative intensity for blue and green at the POE in each genotype: WT, R7/8 LOF (sev LY3,rh52,rh61 and sal) and ninaE17 P[Rh1+3]. (B) Expression of rh3 in R1–R6 rescues the optomotor response of ninaE mutant but shifts the POE toward the left. NinaE17 P[Rh1+3] flies (n = 13) were tested in the apparent motion paradigm, as in Fig. 2A. The results of WT flies from Fig. 2B were overlaid as controls. Error bars are SEMs.
Discussion
In this study, we show that color contrast does not elicit an optomotor response in Drosophila. Moving colored stimuli do not elicit any response in mutants impaired in the motion channel (R1–R6 system). More significantly, by using new R7/R8 mutant flies we conclusively showed that photoreceptors R7/R8 do not contribute to the motion detection system, even in the presence of a functional R1–R6 system. In principle, the R7/R8 system could contribute to motion vision in two ways: by detecting color contrast of the moving patterns or by modulating the sensitivity of the R1–R6 system. The first possibility would give rise to a “residual response,” even if the two colors were equiluminant for R1–R6, whereas the latter would shift the POE. Both possibilities were rejected. Although we did find a residual response, we concluded that this was due to scatter in the individual POEs and not to color contrast. Two findings led to this conclusion. First, by measuring the POE with a procedure that was insensitive to color contrast, we observed a very similar residual response. Second, genetic manipulation of the R7/R8 channel did not diminish the residual response. These manipulations also excluded a significant modulatory influence of the R7/R8 cells on R1–R6. No shift of the mean POE was observed when these cells were eliminated, functionally impaired, or had their opsins exchanged. By contrast, genetic manipulation of the R1–R6 opsin did change the POE.
Theoretically, the spectral sensitivity of R1–R6 could vary either between individual flies or within each fly (e.g., individual R1–R6 photoreceptors having slightly different POEs). We estimated the POEs of individual flies. The variance in the POEs of the blue/green experiment was distinctly larger than in the green/green experiment, as would be expected if there were interindividual variability in the spectral sensitivity of R1–R6 photoreceptors. This does not, however, rule out the alternative possibility of variability among the spectral sensitivities of R1–R6 photoreceptors within a fly. Such a hypothesis has been advanced in human vision: Perception of motion of equiluminant color stimuli could be due to individual retinal neurons having slightly different POEs. In this case, subjects would not be motion blind at any intensity ratio, even without color contrast being involved.
The finding that motion detection is independent of color contrast is somewhat counterintuitive. Color is thought to increase the salience of objects, such as red fruits in the green foliage of trees. The discovery of “motion standstill,” the effect that a moving heterochromatic pattern appeared to stand still if colors were adjusted in luminance (4), therefore attracted much attention and led to the idea that in humans, motion and color information are processed independently (for a review, see ref. 1). It was suggested that color blindness enhances sensitivity of motion detection (36). Indeed, motion is processed largely by luminance mechanisms. Recently, however, motion standstill has been reinvestigated more systematically, and the complete separation of color and motion processing has been questioned (37–39). Whether the new effects are genuinely dependent on chromatic mechanisms or caused by “luminance artifacts” (e.g., artifacts caused by wavelength-dependent delays in the luminance pathway) remains to be investigated (40–42). Physiologic studies have identified V1 primary cortical cells that respond more efficiently to moving stripes with equiluminant color contrast than to ones with achromatic luminance contrast (43).
In lower vertebrates and insects, the motion channel depends on only one type of photoreceptor: a blue receptor in Drosophila (present work), a green receptor in honey bee (7), and L-cones in goldfish (10) and adult zebrafish (44). By contrast, in photopic human vision, cone cells serve both motion and color vision, but the two visual qualities are segregated into separate pathways by neural processing in the retina. In this case, the separation may not be as complete as in the cases of insects and fish.
On the other hand, so far there is no report showing that a photoreceptor type is not used in color vision. Also, in lower vertebrates and insects, the photoreceptors of the achromatic motion channel might still be required for color vision. Green photoreceptors of the honey bee and L-cones of fish are involved in both motion and color vision (14, 45, 46). Whether these are the same or different photoreceptor cells sharing the same spectral properties, however, is unknown. In goldfish, D1-dopamin receptor antagonists impaired red–green color discrimination ability, presumably by eliminating the contribution of the L-cones (47), whereas the D2-dopamin receptor antagonist impaired motion detection (48). In bees, photoreceptor types were recently reinvestigated (49, 50), and eight (R1–R8) of nine photoreceptors in each ommatidium were characterized. Of these, six project to the lamina [short visual fibers (SVFs)] and express a green opsin. The other two project to the medulla [long visual fibers (LVFs)] and express either blue or UV opsin, depending on the ommatidial subtype (49). This arrangement is much more similar to that in Drosophila than had previously been assumed, with honey bee SVFs sharing similarities with R1–R6, and LVFs with R7/R8. Therefore, it would not be surprising if R1–R6 photoreceptors were also involved in color vision.
Our results in the fly demonstrate that color is strictly excluded from processing directional motion information, which suggests two separate functional pathways. Whether, inversely, the motion detection system is involved in color vision in Drosophila remains to be determined. The monopolar neuron L3 has been proposed to integrate R1–R6 input into the color system (19, 51, 52), as it projects to the same layers as R8 in the medulla. The powerful genetic tools that can be applied to the eye and optic lobes have made Drosophila an ideal system to unravel the functional organization of the peripheral visual system.
Materials and Methods
Fly Genetics.
Fly stocks were raised according to standard procedures. Females 2–5 days old were used. Genotypes of the flies used in this study are as follows: WT Berlin, rh52 (this study), sevenless (sevLY3), rh61 (33), ninaE17, and ninaE17 P[Rh1+3] (rh1 promoter driving expression of rh3) (35), a kind gift from C. Zucker. The whole-eye mutant for sal complex was generated as described previously (29). For details, see SI Materials and Methods.
Generation of rh52 Mutant Flies.
We generated rh5 mutants by excision of two P-elements, rh5pGT1 (BG01539) and EP574, located ≈1.2 kb upstream or downstream of the rh5 ORF, respectively. The two chromosomes with the P-element insertions were crossed into the same flies, together with transposase (w-;rh5pGT1/ EP574;Δ2–3, Sb/+). Males from this cross were further crossed with yw; Sp/CyO;Tm2/Tm6B virgin flies. The progeny were screened for loss of w+ eye color. Approximately 200 lines were established, and only homozygous viable flies were screened for loss of rh5 ORF by PCR. Two alleles, rh51 and rh52, were identified. Because rh51 mutant flies had wing defects, only rh52 flies were characterized further. The sequencing confirmed the removal of the entire ORF of rh5 in the rh52 mutant, without deletion of neighboring genes.
Immunostaining.
Cryosections of adult heads were performed as previously described (33). Primary antibodies used were as follows: anti-Rh5 mouse monoclonal 1:100 (53), anti-Rh6 rabbit 1:1,000 (54). Secondary antibodies used were as follows: Alexa Fluor 488 coupled made in goat anti-rabbit and Cy5 coupled made in donkey anti-mouse (Jackson Immunochemicals).
Behavioral Assays.
The torque meter and preparation of tethered flies have been previously described (20, 21). Two identical screens (FlexScan L768; Eizo) were placed symmetrically at a frontal-lateral position from the fly, as shown in Fig. 1A. Yaw torque was recorded continuously while the pattern rotated clockwise for 30 s and counterclockwise for 30 s, and this procedure was repeated. The software programs were developed by Reinhard Wolf and Andreas Eckart. For details, see SI Materials and Methods.
Spectral Measurement of the Visual Stimuli.
A spectroradiometer USB2000 (OceanOptics) was calibrated by a standard black body lamp (LS1-CAL; OceanOptics). The results were acquired and analyzed by software OOIIrad2beta (OceanOptics) (SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank Drs. Charles Zuker (University of California, San Diego) for the fly ninaE17 P[Rh1+3], Steven Britt (University of Colorado, Denver) for anti-Rh5 antibody, Roger Hardie (University of Cambridge, Cambridge, U.K.) for sharing spectral sensitivity data for rh1 and rh3, Andreas Eckart (University of Würzburg, Würzburg, Germany) for writing software programs, Daniel Vasiliauskas for help with rhodopsin gene expression, and our colleagues in the M.H. and C.D. laboratories for helpful discussions. This work was supported by Sonderforschungsbereich 554 (to R.W. and M.H.) and by National Institutes of Health Grant EY13012 (to C.D.). S.Y. was supported by a Human Frontier Science Program fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711484105/DC1.
References
- 1.Cropper SJ, Wuerger SM. The perception of motion in chromatic stimuli. Behav Cognit Neurosci Rev. 2005;4:192–217. doi: 10.1177/1534582305285120. [DOI] [PubMed] [Google Scholar]
- 2.Gegenfurtner KR, Hawken MJ. Interaction of motion and color in the visual pathways. Trends Neurosci. 1996;19:394–401. doi: 10.1016/S0166-2236(96)10036-9. [DOI] [PubMed] [Google Scholar]
- 3.Derrington AM. Vision: Can colour contribute to motion? Curr Biol. 2000;10:R268–R270. doi: 10.1016/s0960-9822(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran VS, Gregory RL. Does colour provide an input to human motion perception? Nature. 1978;275:55–56. doi: 10.1038/275055a0. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser W. Towards the Question of the Ability to Distinguish Spectral Colours: An Investigation of an Optomotor Response in the Flesh Fly, Phormia regina MEIG. (Translated from German) J Comp Physiol. 1968;61:71–102. [Google Scholar]
- 6.Frisch Kv. The Color Sense and the Sense for Shape in the Bee. (Translated from German) Zool J Physiol. 1914;37:1–238. [Google Scholar]
- 7.Kaiser W, Liske E. Optomotor Reactions of Stationary Flying Bees during Stimulation with Spectral Lights. (Translated from German) J Comp Physiol. 1974;89:391–408. [Google Scholar]
- 8.Tinbergen J, Abeln RG. Spectral sensitivity of the landing blowfly. J Comp Physiol. 1983;150:319–328. [Google Scholar]
- 9.Neumeyer C, Arnold K. Tetrachromatic color vision in the goldfish becomes trichromatic under white adaptation light of moderate intensity. Vision Res. 1989;29:1719–1727. doi: 10.1016/0042-6989(89)90154-5. [DOI] [PubMed] [Google Scholar]
- 10.Schaerer S, Neumeyer C. Motion detection in goldfish investigated with the optomotor response is “color blind”. Vision Res. 1996;36:4025–4034. doi: 10.1016/s0042-6989(96)00149-6. [DOI] [PubMed] [Google Scholar]
- 11.Orger MB, Baier H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis Neurosci. 2005;22:275–281. doi: 10.1017/S0952523805223039. [DOI] [PubMed] [Google Scholar]
- 12.Menne D, Spatz HC. Colour vision in Drosophila melanogaster. J Comp Physiol. 1977;114:301–312. [Google Scholar]
- 13.Hernandez de Salomon C, Spatz HC. Colour vision in Drosophila melanogaster: Wavelength discrimination. J Comp Physiol. 1983;150:31–37. [Google Scholar]
- 14.Menzel R. Handbook of Sensory Physiology: Vision in Invertebrates. Berlin: Springer; 1979. pp. 504–580. [Google Scholar]
- 15.Hardie RC. Functional Organization of the Fly Retina, Progress in Sensory Physiology. Vol 5. Berlin: Springer; 1985. pp. 1–79. [Google Scholar]
- 16.Kirschfeld K, Franceschini N, Minke B. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 17.Cook T, Desplan C. Photoreceptor subtype specification: From flies to humans. Semin Cell Dev Biol. 2001;12:509–518. doi: 10.1006/scdb.2001.0275. [DOI] [PubMed] [Google Scholar]
- 18.Heisenberg M, Buchner E. The role of retinula cell types in visual behavior of Drosophila melanogaster. J Comp Physiol. 1977;117:127–162. [Google Scholar]
- 19.Rister J, et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Heisenberg M, Wolf R. Vision in Drosophila. Berlin: Springer; 1984. pp. 1–250. [Google Scholar]
- 21.Götz KG. Optomotor investigation of the visual system of some eye mutants of the fruit fly Drosophila. (Translated from German) Kybernetik. 1964;2:77–92. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- 22.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 23.O'Tousa JE, et al. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 24.O'Tousa JE, Leonard DS, Pak WL. Morphological defects in oraJK84 photoreceptors caused by mutation in R1–R6 opsin gene of Drosophila. J Neurogenet. 1989;6:41–52. doi: 10.3109/01677068909107099. [DOI] [PubMed] [Google Scholar]
- 25.Strauss R, Renner M, Gotz K. Task-specific association of photoreceptor systems and steering parameters in Drosophila. J Comp Physiol A. 2001;187:617–632. doi: 10.1007/s003590100234. [DOI] [PubMed] [Google Scholar]
- 26.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- 27.Salcedo E, et al. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anstis S, Cavanagh P. In: Color Vision: Physiology and Psychophysics. Mollon J, Sharpe LT, editors. London: Academic; 1983. pp. 66–77. [Google Scholar]
- 29.Mollereau B, et al. Two-step process for photoreceptor formation in Drosophila. Nature. 2001;412:911–913. doi: 10.1038/35091076. [DOI] [PubMed] [Google Scholar]
- 30.Jürgens G. Head and tail development of the Drosophila embryo involves spalt, a novel homeotic gene. EMBO J. 1988;7:189–196. doi: 10.1002/j.1460-2075.1988.tb02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris WA, Stark WS, Walker JA. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976;256:415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 34.Labhart T, Meyer EP. Detectors for polarized skylight in insects: A survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech. 1999;47:368–379. doi: 10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Feiler R, et al. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: Visual physiology and photochemistry of transgenic animals. J Neurosci. 1992;12:3862–3868. doi: 10.1523/JNEUROSCI.12-10-03862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan MV. Shouldn't directional movement detection necessarily be “colour-blind”? Vision Res. 1985;25:997–1000. doi: 10.1016/0042-6989(85)90210-x. [DOI] [PubMed] [Google Scholar]
- 37.Cavanagh P, Anstis S. The contribution of color to motion in normal and color-deficient observers. Vision Res. 1991;31:2109–2148. doi: 10.1016/0042-6989(91)90169-6. [DOI] [PubMed] [Google Scholar]
- 38.Metha AB, Mullen KT. Temporal mechanisms underlying flicker detection and identification for red-green and achromatic stimuli. J Opt Soc Am A. 1996;13:1969–1980. doi: 10.1364/josaa.13.001969. [DOI] [PubMed] [Google Scholar]
- 39.Cavanagh P, et al. Complete sparing of high-contrast color input to motion perception in cortical color blindness. Nat Neurosci. 1998;1:242–247. doi: 10.1038/688. [DOI] [PubMed] [Google Scholar]
- 40.Mullen KT, Yoshizawa T, Baker CL., Jr Luminance mechanisms mediate the motion of red-green isoluminant gratings: The role of “temporal chromatic aberration.”. Vision Res. 2003;43:1235–1247. doi: 10.1016/s0042-6989(03)00115-9. [DOI] [PubMed] [Google Scholar]
- 41.Wandell BA, et al. Color signals in human motion-selective cortex. Neuron. 1999;24:901–909. doi: 10.1016/s0896-6273(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 42.Baraas RC. Perception of chromatic motion requires luminance interaction. Perception. 2005;34:1025–1028. doi: 10.1068/p5176. [DOI] [PubMed] [Google Scholar]
- 43.Vidyasagar TR, Kulikowski JJ, Lipnicki DM, Dreher B. Convergence of parvocellular and magnocellular information channels in the primary visual cortex of the macaque. Eur J Neurosci. 2002;16:945–956. doi: 10.1046/j.1460-9568.2002.02137.x. [DOI] [PubMed] [Google Scholar]
- 44.Krauss A, Neumeyer C. Wavelength dependence of the optomotor response in zebrafish (Danio rerio) Vision Res. 2003;43:1273–1282. doi: 10.1016/s0042-6989(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 45.Daumer K. Stimulus metric investigations of color vision in bees. (Translated from German) Z vergl Physiol. 1956;38:413–478. [Google Scholar]
- 46.Neumeyer C. On spectral sensitivity in the goldfish: Evidence for neural interactions between different “cone mechanisms.”. Vision Res. 1984;24:1223–1231. doi: 10.1016/0042-6989(84)90177-9. [DOI] [PubMed] [Google Scholar]
- 47.Mora-Ferrer C, Neumeyer C. Reduction of red–green discrimination by dopamine D1 receptor antagonists and retinal dopamine depletion. Vision Res. 1996;36:4035–4044. doi: 10.1016/s0042-6989(96)00173-3. [DOI] [PubMed] [Google Scholar]
- 48.Mora-Ferrer C, Gangluff V. D2-dopamine receptor blockade impairs motion detection in goldfish. Vis Neurosci. 2000;17:177–186. doi: 10.1017/s0952523800171196. [DOI] [PubMed] [Google Scholar]
- 49.Wakakuwa M, Kurasawa M, Giurfa M, Arikawa K. Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften. 2005;92:464–467. doi: 10.1007/s00114-005-0018-5. [DOI] [PubMed] [Google Scholar]
- 50.Spaethe J, Briscoe AD. Molecular characterization and expression of the UV opsin in bumblebees: Three ommatidial subtypes in the retina and a new photoreceptor organ in the lamina. J Exp Biol. 2005;208:2347–2361. doi: 10.1242/jeb.01634. [DOI] [PubMed] [Google Scholar]
- 51.Strausfeld NJ, Lee JK. Neuronal basis for parallel visual processing in the fly. Vis Neurosci. 1991;7:13–33. doi: 10.1017/s0952523800010919. [DOI] [PubMed] [Google Scholar]
- 52.Anderson JC, Laughlin SB. Photoreceptor performance and the co-ordination of achromatic and chromatic inputs in the fly visual system. Vision Res. 2000;40:13–31. doi: 10.1016/s0042-6989(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 53.Chou WH, et al. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 54.Tahayato A, et al. Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Dev Cell. 2003;5:391–402. doi: 10.1016/s1534-5807(03)00239-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.